Failure Mechanism and Optimization of Metal-Supported Solid Oxide Fuel Cells
Abstract
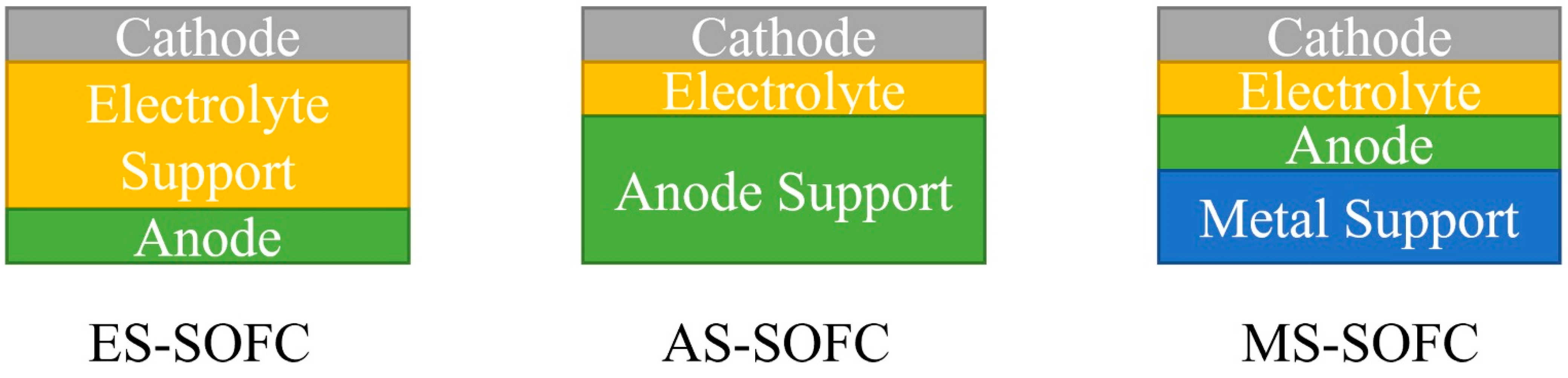
1. Introduction
2. Failure Analysis
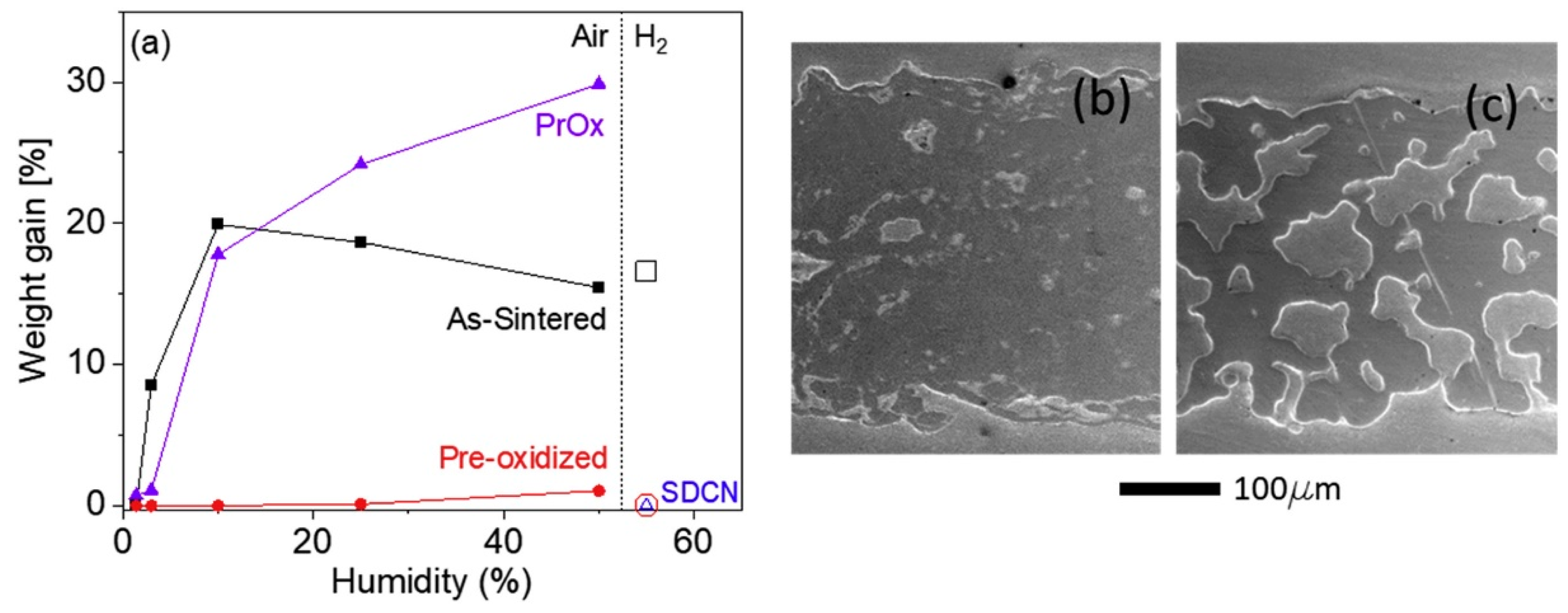
2.1. High-Temperature Oxidation Problem
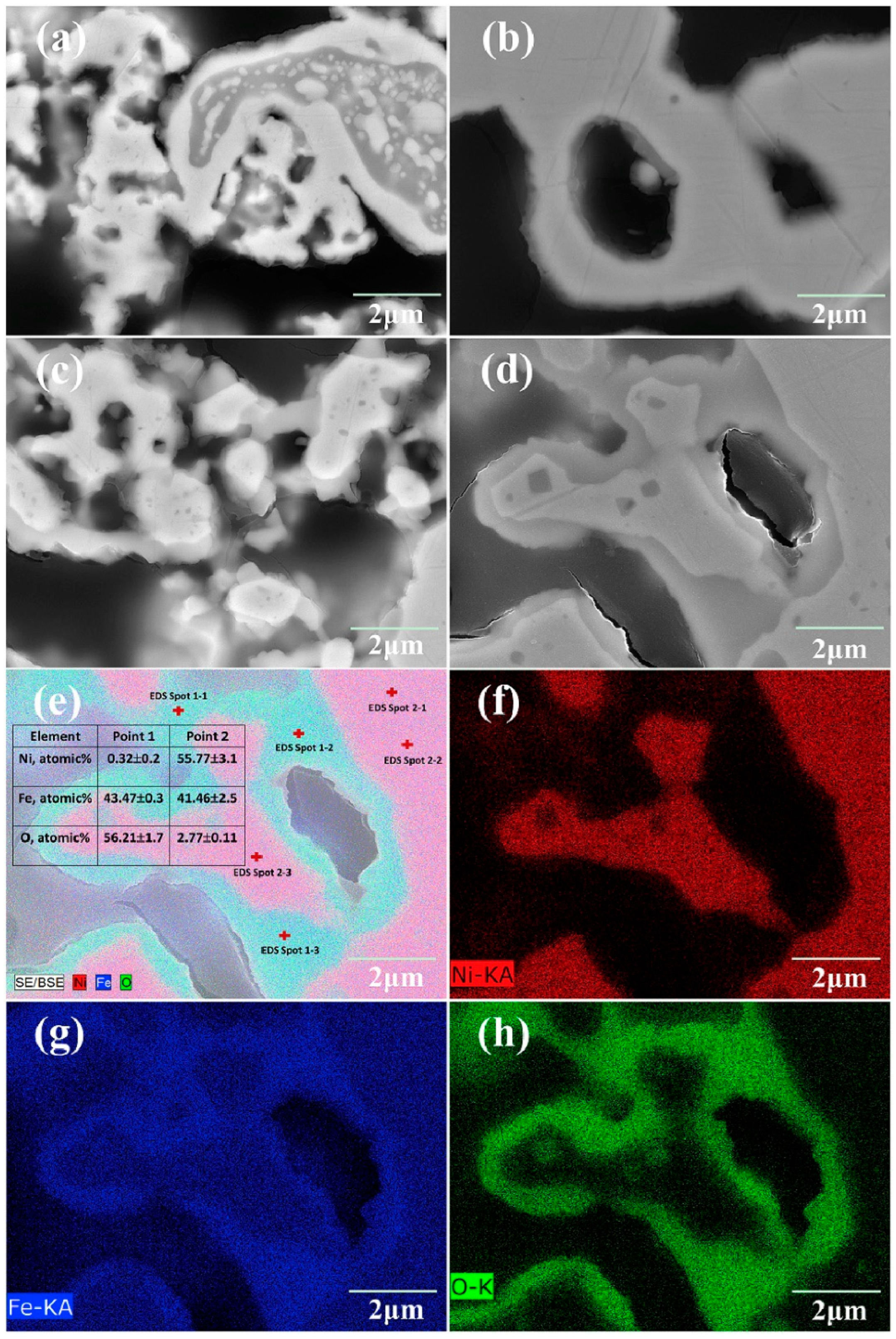
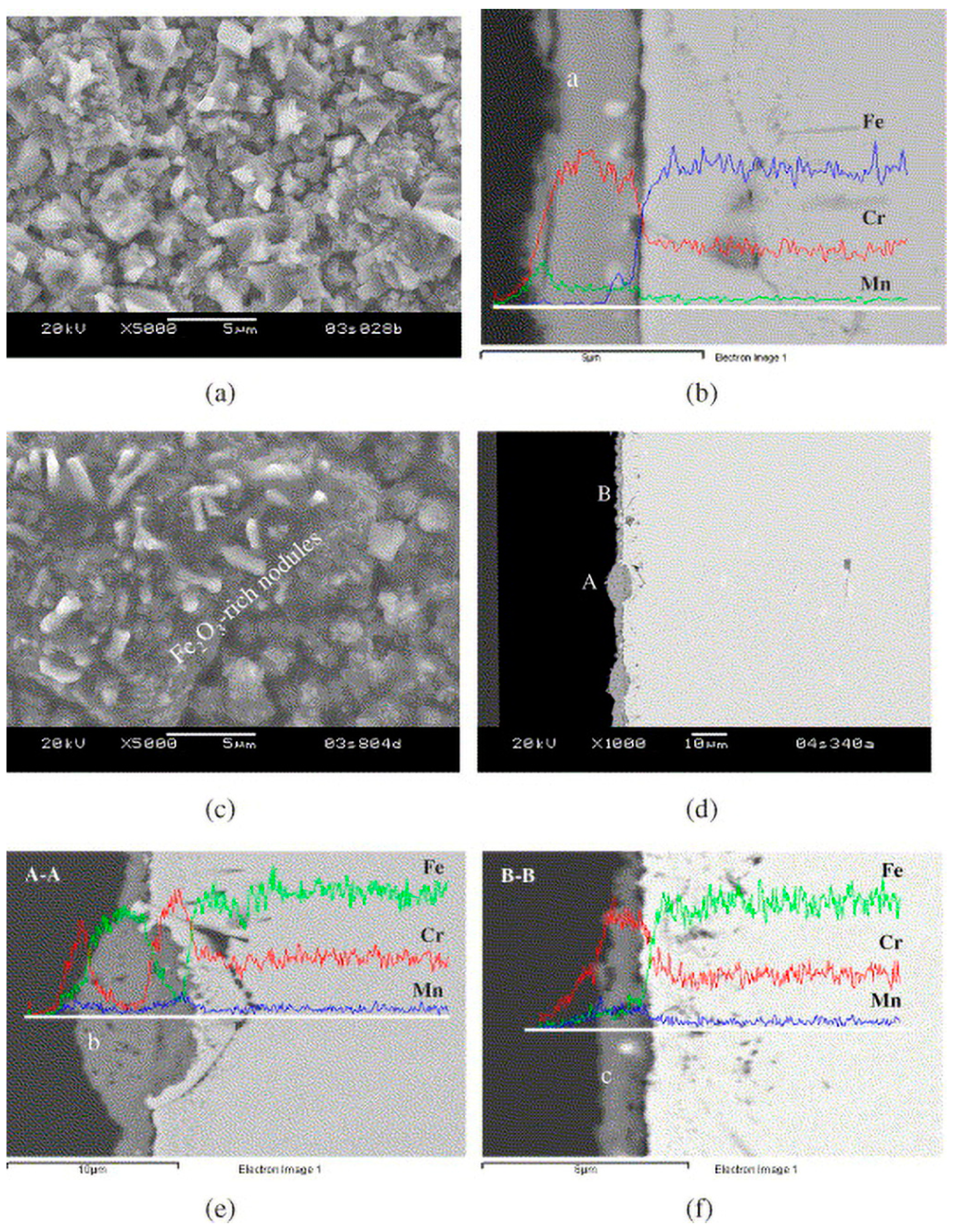
2.2. Cation Interdiffusion
2.3. Heat Matching Problems
2.4. Electrolyte Defects
3. Optimization
3.1. Infiltration Method
3.2. Thermal Spraying Method
3.3. Sintering Aids Method
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brueckner, M.; Pforr, C. Global Environmental Issues; Springer: Berlin, Heidelberg, 2010. [Google Scholar]
- Rubin, E.; Rao, A. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar]
- Haszeldine, R. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Yu, X.; Cai, N. Thermal shock resistance and failure probability analysis on solid oxide electrolyte direct flame fuel cells. J. Power Sources 2014, 255, 377–386. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Shen, F.; Wang, R.; Robinson, I.; Lau, G.; Tucker, M. Progress in metal-supported solid oxide fuel cells and electrolyzers with symmetric metal supports and infiltrated electrodes. ECS Trans. 2019, 91, 877–885. [Google Scholar] [CrossRef]
- Shimura, T.; Jiao, Z.; Hara, S.; Shikazono, N. Quantitative analysis of solid oxide fuel cell anode microstructure change during redox cycles. J. Power Sources 2014, 267, 58–68. [Google Scholar] [CrossRef]
- Wain-Martin, A.; Moran-Ruiz, A.; Laguna-Bercero, M.; Campana, R.; Larrañaga, A.; Slater, P.; Arriortua, M. SOFC cathodic layers using wet powder spraying technique with self synthesized nanopowders. Int. J. Hydrogen Energy 2019, 44, 7555–7563. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, S.; Shin, H.; Bae, J. Development and evaluation of 3-Layer metal supported solid oxide fuel cell short stack. ECS Trans. 2017, 78, 2045–2050. [Google Scholar] [CrossRef]
- Sudireddy, B.; Nielsen, J.; Persson, Å.; Thydén, K.; Brodersen, K.; Ramousse, S.; Neagu, D.; Stefan, E.; Irvine, J.; Geisler, H.; et al. Development of robust metal-supported SOFCs and stack components in EU METSAPP Consortium. Fuel Cells 2017, 17, 508–516. [Google Scholar] [CrossRef]
- Tucker, M. Dynamic-temperature operation of metal-supported solid oxide fuel cells. J. Power Sources 2018, 395, 314–317. [Google Scholar] [CrossRef]
- Tucker, M.; Ying, A. Metal-supported solid oxide fuel cells operated in direct-flame configuration. Int. J. Hydrogen Energy 2017, 42, 24426–24434. [Google Scholar] [CrossRef]
- Tucker, M. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Shen, F.; Hu, B.; Tucker, M. Enhanced long term durability of metal-supported solid oxide electrolysis cells by advanced coatings. ECS Meet. Abstr. 2020, MA2020-02, 2659. [Google Scholar] [CrossRef]
- Shen, F.; Welander, M.; Tucker, M. Oxidation of porous stainless steel supports for metal-supported solid oxide electrolysis cells. Int. J. Hydrogen Energy 2023, 48, 12168–12175. [Google Scholar] [CrossRef]
- Xu, N.; Chen, M.; Han, M. Oxidation behavior of a Ni-Fe support in SOFC anode atmosphere. J. Alloys Compd. 2018, 765, 757–763. [Google Scholar] [CrossRef]
- Cho, H.; Choi, G. Fabrication and characterization of Ni-supported solid oxide fuel cell. Solid State Ion. 2009, 180, 792–795. [Google Scholar] [CrossRef]
- Tsai, C.; Hwang, C.; Chang, C.; Wu, S.; Lin, H.; Shiu, W.; Lin, J.; Yang, S.; Fu, C.; Yang, C. Performances of plasma sprayed metal-supported solid oxide fuel cell and stack. Fuel Cells 2018, 18, 800–808. [Google Scholar] [CrossRef]
- Kirdyashkin, A.; Kitler, V.; Maznoy, A.; Solov’ev, A.; Kovalchuk, A.; Ionov, I. Development of new Ni-Al porous alloys for metal-supported solid oxide fuel cells. Adv. Mater. Res. 2014, 1040, 19–23. [Google Scholar] [CrossRef]
- Bae, J. A novel metal supported SOFC fabrication method developed in KAIST: A sinter-joining method. J. Korean Ceram. Soc. 2016, 53, 478–482. [Google Scholar] [CrossRef]
- Franco, T.; Haydn, M.; Weber, A.; Schafbauer, W.; Blum, L.; Packbier, U.; Roehrens, D.; Menzler, N.; Rechberger, J.; Venskutonis, A.; et al. The status of metal-supported SOFC development and industrialization at Plansee. ECS Trans. 2013, 57, 471–480. [Google Scholar] [CrossRef]
- Tucker, M. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Jia, L.; Zhang, Q.; Jiang, S.; Chi, B.; Pu, J.; Jian, L.; Yan, D. Porous Ni–Fe alloys as anode support for intermediate temperature solid oxide fuel cells: I. Fabrication, redox and thermal behaviors. J. Power Sources 2015, 277, 474–479. [Google Scholar] [CrossRef]
- Zhu, J.; Geng, S.; Lu, Z.; Porter, W. Evaluation of binary Fe–Ni alloys as intermediate temperature SOFC. J. Electrochem. Soc. 2007, 154, B1288–B1294. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.; Singh, P.; Stevenson, J. Effects of water vapor on oxidation behavior of ferritic stainless steels under solid oxide fuel cell interconnect exposure conditions. Solid State Ion. 2005, 176, 1495–1503. [Google Scholar] [CrossRef]
- Yang, Z.; Walker, M.; Singh, P.; Stevenson, J.; Norby, T. Oxidation behavior of ferritic stainless steels under SOFC interconnect exposure conditions. J. Electrochem. Soc. 2004, 151, B669–B678. [Google Scholar] [CrossRef]
- Chandra-Ambhorn, S.; Wouters, Y.; Antoni, L.; Toscan, F.; Galerie, A. Adhesion of oxide scales grown on ferritic stainless steels in solid oxide fuel cell temperature and atmosphere conditions. J. Power Sources 2007, 171, 688–695. [Google Scholar] [CrossRef]
- Molin, S.; Kusz, B.; Gazda, M.; Jasinski, P. Evaluation of porous 430 L stainless steel for SOFC operation at intermediate temperatures. J. Power Sources 2008, 181, 31–37. [Google Scholar] [CrossRef]
- Reisert, M.; Berova, V.; Aphale, A.; Singh, P.; Tucker, M. Oxidation of porous stainless steel supports for metal-supported solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 30882–30897. [Google Scholar] [CrossRef]
- Knibbe, R.; Wang, H.; Blennow, P.; Thydén, K.; Persson, Å.; Mikkelsen, L.; Klemensø, T. Oxidation in ceria infiltrated metal supported SOFCs—A TEM investigation. J. Power Sources 2013, 228, 75–82. [Google Scholar] [CrossRef]
- Dheeradhada, V.; Cao, H.; Alinger, M. Oxidation of ferritic stainless steel interconnects: Thermodynamic and kinetic assessment. J. Power Sources 2011, 196, 1975–1982. [Google Scholar] [CrossRef]
- Żurek, J.; Wessel, E.; Niewolak, L.; Schmitz, F.; Kern, T.; Singheiser, L.; Quadakkers, W. Anomalous temperature dependence of oxidation kinetics during steam oxidation of ferritic steels in the temperature range 550–650 °C. Corros. Sci. 2004, 46, 2301–2317. [Google Scholar] [CrossRef]
- Visco, S.; Jacobson, C.; Jonghe, L. LBNL Activity Overview. In Proceedings of the SECA Core Technology Program Review, Sacramento, CA, USA, 19–20 February 2003. [Google Scholar]
- Brandner, M.; Bram, M.; Sebold, D.; Uhlenbruck, S.; Ertl, S.; Höfler, T.; Wetzel, F.; Buchkremer, H.; Stöver, D. Inhibition of diffusion between metallic substrates and Ni-YSZ anodes during sintering. ECS Proc. Vol. 2005, 2005-07, 1235–1243. [Google Scholar] [CrossRef]
- Hilpert, K.; Das, D.; Miller, M.; Peck, D.; Weiß, R. Chromium vapor species over solid oxide fuel cell interconnect materials and their potential for degradation processes. J. Electrochem. Soc. 1996, 143, 3642–3647. [Google Scholar] [CrossRef]
- Fergus, J. Effect of cathode and electrolyte transport properties on chromium poisoning in solid oxide fuel cells. Int. J. Hydrogen Energy 2007, 32, 3664–3671. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, X. Chromium deposition and poisoning of cathodes of solid oxide fuel cells—A review. Int. J. Hydrogen Energy 2014, 39, 505–531. [Google Scholar] [CrossRef]
- Simner, S.; Anderson, M.; Xia, G.; Yang, Z.; Pederson, L.; Stevenson, J. SOFC performance with Fe-Cr-Mn alloy interconnect. J. Electrochem. Soc. 2005, 152, A740–A745. [Google Scholar] [CrossRef]
- Huang, Y.; Hussain, A.; Pellegrinelli, C.; Xiong, C.; Wachsman, E. Chromium poisoning effects on surface exchange kinetics of La0.6Sr0.4Co0.2Fe0.8O3-δ. ACS Appl. Mater. Interfaces 2017, 9, 16660–16668. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemenso, T.; Persson, A.; Brodersen, K.; Srivastava, A.; Frandsen, H.; Lundberg, M.; Ramousse, S.; Mogensen, M. Development of planar metal supported SOFC with novel cermet anode. ECS Trans. 2009, 25, 701–710. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, C.; Chen, T.; Meng, X.; Ye, X.; Li, J.; Wang, S.; Zhan, Z. Evaluation of Ni and Ni-Ce0.8Sm0.2O2-δ (SDC) impregnated 430 L anodes for metal-supported solid oxide fuel cells. J. Power Sources 2014, 267, 117–122. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, X.; Liu, X.; Pan, X.; Li, J.; Ye, X.; Nie, H.; Xia, C.; Wang, S.; Zhan, Z. Novel architectured metal-supported solid oxide fuel cells with Mo-doped SrFeO3-δ electrocatalysts. J. Power Sources 2014, 267, 148–154. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Jia, L.; Chi, B.; Pu, J.; Li, J. High performance and carbon-deposition resistance metal-supported solid oxide fuel cell with a Nickel-Manganese spinel modified anode. Mater. Today Energy 2020, 17, 100473. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Li, C.; Yang, X.; Jia, L.; Li, J. A direct CH4 metal-supported solid oxide fuel cell with an engineered Ni/Gd-doped CeO2 anode containing Ni and MnO nanoparticles. Compos. Part B Eng. 2022, 229, 109462. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Liu, C.; Yang, X.; Li, C.; Jia, L.; Li, J. A direct-methane solid oxide fuel cell with a functionally engineered Ni-Fe metal support. J. Power Sources 2022, 537, 231533. [Google Scholar] [CrossRef]
- Soloviev, A.; Sochugov, N.; Ionov, I.; Kirdyashkin, A.; Kitler, V.; Maznoy, A.; Sigfusson, T. Solid oxide fuel cell on porous Ni-Al support. In Proceedings of the International Forum on Strategic Technology, Tomsk, Russia, 18–21 September 2012. [Google Scholar]
- Irvine, J.; Connor, P. Solid Oxide Fuels Cells: Facts and Figures; Green Energy & Technology; Springer: London, UK, 2014. [Google Scholar]
- Kuhna, B.; Jimenez, C.; Niewolaka, L.; Hüttel, T.; Beck, T.; Hattendorf, H.; Singheiser, L.; Quadakkers, W. Effect of Laves phase strengthening on the mechanical properties of high Cr ferritic steels for solid oxide fuel cell interconnect application. Mater. Sci. Eng. A 2011, 528, 5888–5899. [Google Scholar] [CrossRef]
- Boccaccini, D.; Frandsen, H.; Sudireddy, B.; Blennow, P.; Persson, Å.; Kwok, K.; Hendriksen, P. Creep behaviour of porous metal supports for solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 21569–21580. [Google Scholar] [CrossRef]
- Esposito, L.; Boccaccini, D.; Pucillo, G.; Frandsen, H. Secondary creep of porous metal supports for solid oxide fuel cells by a CDM approach. Mater. Sci. Eng. A 2017, 691, 155–161. [Google Scholar] [CrossRef]
- Reolon, R.; Sanna, S.; Xu, Y.; Lee, I.; Bergmann, C.; Pryds, N.; Esposito, V. Effects of accelerated degradation on metal supported thin film-based solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 7887–7896. [Google Scholar] [CrossRef]
- Kim, K.; Park, B.; Kim, S.; Lee, Y.; Bae, H.; Choi, G. Micro solid oxide fuel cell fabricated on porous stainless steel: A new strategy for enhanced thermal cycling ability. Sci. Rep. 2016, 6, 22443. [Google Scholar] [CrossRef]
- Haydn, M.; Ortner, K.; Franco, T.; Uhlenbruck, S.; Menzler, N.; Stöver, D.; Bräuer, G.; Venskutonis, A.; Sigl, L.; Buchkremer, H.; et al. Multi-layer thin-film electrolytes for metal supported solid oxide fuel cells. J. Power Sources 2014, 256, 52–60. [Google Scholar] [CrossRef]
- Stange, M.; Denonville, C.; Larring, Y.; Brevet, A.; Montani, A.; Sicardy, O.; Mougin, J.; Larsson, P. Improvement of corrosion properties of porous alloy supports for solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 12485–12495. [Google Scholar] [CrossRef]
- Nielsen, J.; Sudireddy, B.; Hagen, A.; Persson, Å. Performance factors and sulfur tolerance of metal supported solid oxide fuel cells with nanostructured Ni:GDC infiltrated anodes. J. Electrochem. Soc. 2016, 163, F574–F580. [Google Scholar] [CrossRef]
- Nielsen, J.; Persson, S.; Muhl, T.; Brodersen, K. Towards high power density metal supported solid oxide fuel cell for mobile applications. J. Electrochem. Soc. 2018, 165, F90–F96. [Google Scholar] [CrossRef]
- Nielsen, J.; Persson, Å.; Sudireddy, B.; Irvine, J.; Thydén, K. Infiltrated La0.4Sr0.4Fe0.03Ni0.03Ti0.94O3 based anodes for all ceramic and metal supported solid oxide fuel cells. J. Power Sources 2017, 372, 99–106. [Google Scholar] [CrossRef]
- Tucker, M.; Sholklapper, T. Solvent-Based Infiltration of Porous Structures. U.S. Patent 20110251053, 13 October 2011. [Google Scholar]
- Zhou, Y.; Xie, M.; Yuan, C.; Luo, T.; Ye, X.; Li, J.; Wang, S.; Zhan, Z. SrFe0.75Mo0.25O3-δ impregnated 430 L alloys for efficient fuel oxidation in metal supported solid oxide fuel cells. J. Power Sources 2014, 269, 244–249. [Google Scholar] [CrossRef]
- Stefan, E.; Neagu, D.; Tullmar, P.; Persson, Å.; Sudireddy, B.; Miller, D.; Chen, M.; Irvine, J. Spinel-based coatings for metal supported solid oxide fuel cells. Mater. Res. Bull. 2017, 89, 232–244. [Google Scholar] [CrossRef]
- Tucker, M. Development of high power density metal-supported solid oxide fuel cells. Energy Technol. 2017, 5, 2175–2181. [Google Scholar] [CrossRef]
- Stange, M.; Denonville, C.; Larring, Y.; Haavik, C.; Brevet, A.; Montani, A.; Sicardy, O.; Mougin, J.; Larsson, P. Coating developments for Metal-supported Solid Oxide Fuel Cells. ECS Trans. 2013, 57, 511–520. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.; Karimaghaloo, A.; Lee, M.; Tucker, M. Progress in durability of metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2019, 437, 226935. [Google Scholar] [CrossRef]
- Tucker, M.; Lau, G.; Jacobson, C.; DeJonghe, L.; Visco, S. Stability and robustness of metal-supported SOFCs. J. Power Sources 2008, 175, 447–451. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Cheng, Y.; Shen, F.; Wang, R.; Hu, B.; Tucker, M. Scaleup and manufacturability of symmetric-structured metal-supported solid oxide fuel cells. J. Power Sources 2021, 489, 229439. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Ramousse, S.; Kromp, A.; Leonide, A.; Weber, A. Manufacturing and characterization of metal-supported solid oxide fuel cells. J. Power Sources 2011, 196, 7117–7125. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhou, Y.; Wang, S.; Liu, X.; Meng, X.; Wen, T. Nanostructure Electrodes for Metal-Supported Solid Oxide Fuel Cells. ECS Trans. 2013, 57, 925–931. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, X.; Li, J.; Zhan, Z.; Wang, S. Metal-Supported Solid Oxide Fuel Cells with a Simple Structure. J. Electrochem. Soc. 2014, 161, F332–F336. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, D.; Yuan, C.; Liu, M.; Chen, T.; Wang, S.; Zhan, Z. Infiltrated SmBa0.5Sr0.5Co2O5+δ cathodes for metal–supported solid oxide fuel cells. Electrochim. Acta 2014, 149, 231–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, T.; Li, J.; Yuan, C.; Xin, X.; Chen, G.; Miao, G.; Zhan, W.; Zhan, Z.; Wang, S. Long-term stability of metal-supported solid oxide fuel cells employing infiltrated electrodes. J. Power Sources 2015, 295, 67–73. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, C.; Chen, T.; Liu, M.; Li, J.; Wang, S.; Zhan, Z. Enhanced performance and stability of metal-supported solid oxide fuel cells with (Bi2O3)(0.7)(Er2O3)(0.3)-Ag composite cathode. J. Electrochem. Soc. 2015, 162, F9–F13. [Google Scholar] [CrossRef]
- Tucker, M. Durability of symmetric-structured metal-supported solid oxide fuel cells. J. Power Sources 2017, 369, 6–12. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.; Tucker, M. High performance metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2019, 410–411, 91–98. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Fukuyama, Y.; Tucker, M. Ethanol internal reforming in solid oxide fuel cells: A path toward high performance metal-supported cells for vehicular applications. J. Power Sources 2020, 449, 227598. [Google Scholar] [CrossRef]
- Dewa, M.; Elharati, M.; Hussain, A.; Miura, Y.; Song, D.; Fukuyama, Y.; Furuya, Y.; Dale, N.; Zhang, X.; Marin-Flores, O.; et al. Metal-supported solid oxide fuel cell system with infiltrated reforming catalyst layer for direct ethanol feed operation. J. Power Sources 2022, 541, 231625. [Google Scholar] [CrossRef]
- Blennow, P.; Sudireddy, B.; Persson, A.; Nielsen, J.; Sachitanand, R.; Froitzheim, J. Investigation of Cu-based infiltration coatings for metal-supported SOFC. ECS Trans. 2013, 57, 771–780. [Google Scholar] [CrossRef]
- Dayaghi, A.; Kim, K.; Kim, S.; Sung, Y.; Choi, G. Oxidation of porous stainless-steel coated with donor-doped SrTiO3 in anodic atmosphere of solid oxide fuel cell. J. Power Sources 2017, 360, 488–494. [Google Scholar] [CrossRef]
- Yang, S.; Hwang, C.; Tsai, C.; Chang, C.; Wu, M. Production of metal-supported solid oxide fuel cell using thermal plasma spraying technique. IEEE Trans. Plasma Sci. 2017, 45, 318–322. [Google Scholar] [CrossRef]
- Chang, C.; Hwang, C.; Tsai, C.; Nien, S.; Chuang, C.; Cheng, S.; Wu, S. The Development of Plasma Sprayed Metal-Supported Solid Oxide Fuel Cells at Institute of Nuclear Energy Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hwang, C.; Tsai, C.; Chang, C.; Chuang, C.; Yang, S.; Cheng, S.; Shie, Z.; Lee, R. Recoverable Performance of Plasma-Sprayed Metal-Supported Solid Oxide Fuel Cell; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Li, C.; Li, C.; Yang, G. Development of a Ni/Al2O3 cermet-supported tubular solid oxide fuel cell assembled with different functional layers by atmospheric plasma-spraying. J. Therm. Spray Technol. 2009, 18, 83–89. [Google Scholar] [CrossRef]
- Yao, S.; Li, C.; Tian, J.; Yang, G.; Li, C. Conditions and mechanisms for the bonding of a molten ceramic droplet to a substrate after high-speed impact. Acta Mater. 2016, 119, 9–25. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Wang, Y.; Li, C.; Li, C. Performance and stability of plasma-sprayed 10 × 10 cm2 self-sealing metal-supported solid oxide fuel cells. J. Therm. Spray Technol. 2021, 30, 1059–1068. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Li, C.; Zhang, S.; Yang, G.; Li, C. Study on the fabrication and performance of very low pressure plasma sprayed porous metal supported solid oxide fuel cell. ECS Trans. 2017, 78, 2059–2067. [Google Scholar] [CrossRef]
- Soysal, D.; Ansar, A.; Ilhan, Z.; Costa, R. Nanostructured composite cathodes by suspension plasma spraying for SOFC applications. ECS Trans. 2011, 35, 2233–2241. [Google Scholar] [CrossRef]
- Baek, S.; Jeong, J.; Schlegl, H.; Azad, A.; Park, D.; Baek, U.; Kim, J. Metal-supported SOFC with an aerosol deposited in-situ LSM and 8YSZ composite cathode. Ceram. Int. 2016, 42, 2402–2409. [Google Scholar] [CrossRef]
- Panthi, D.; Hedayat, N.; Yanhai, D. Densification behavior of yttria-stabilized zirconia powders for solid oxide fuel cell electrolytes. J. Adv. Ceram. 2018, 7, 39–49. [Google Scholar] [CrossRef]
- Fu, C.; Liu, Q.; Chan, S.; Ge, X.; Pasciak, G. Effects of transition metal oxides on the densification of thin-film GDC electrolyte and on the performance of intermediate-temperature SOFC. Int. J. Hydrogen Energy 2010, 35, 11200–11207. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. Cheminform 1994, 25, 3801–3803. [Google Scholar] [CrossRef]
- Ishihara, T.; Kilner, J.A.; Honda, M. Oxygen surface exchange and diffusion in the new perovskite oxide ion conductor LaGaO3. J. Am. Chem. Soc. 1997, 119, 2747–2748. [Google Scholar] [CrossRef]
- Toor, S.; Alharbi, A.; Croiset, E. Shrinkage dynamics of stainless steel 430-L and yittrium stabilized zirconia and its application in co-sintering for MS-SOFCs. ECS Trans. 2019, 91, 921–929. [Google Scholar] [CrossRef]
- Satardekar, P.; Montinaro, D.; Sglavo, V. Fe-doped YSZ electrolyte for the fabrication of metal supported-SOFC by co-sintering. Ceram. Int. 2015, 41, 9806–9812. [Google Scholar] [CrossRef]
- Kleinlogel, C.; Gauckler, L. Sintering and properties of nanosized ceria solid solutions. Solid State Ion. 2000, 135, 567–573. [Google Scholar] [CrossRef]
- Seidensticker, J.; Mayo, M. Thermal analysis of 3-mol%-yttria-stabilized tetragonal zirconia powder doped with copper oxide. J. Am. Ceram. Soc. 1996, 79, 401–406. [Google Scholar] [CrossRef]
- Dayaghi, A.; Kim, K.; Kim, S.; Park, J.; Kim, S.; Park, B.; Choi, G. Stainless steel-supported solid oxide fuel cell with La0.2Sr0.8Ti0.9Ni0.1O3-δ/yttria-stabilized zirconia composite anode. J. Power Sources 2016, 324, 288–293. [Google Scholar] [CrossRef]
- Chang, C.; Tsai, C.; Fu, C.; Yang, C.; Yang, S.; Lee, R. Fabrication and characterization of metal-supported solid oxide fuel cell fabricated by atmospheric plasma spraying. ECS Trans. 2019, 91, 855–865. [Google Scholar] [CrossRef]
- Leah, R.; Bone, A.; Lankin, M.; Selcuk, A.; Pierce, R.; Rees, L.; Corcoran, D.; Muhl, P.; Dehaney-Steven, Z.; Brackenbury, C. Low-cost, redox-stable, low-temperature SOFC developed by Ceres Power for multiple applications: Latest development update. ECS Trans. 2013, 57, 461–470. [Google Scholar] [CrossRef]
- Gao, H.; Liu, J.; Chen, H.; Li, S.; He, T.; Ji, Y.; Zhang, J. The effect of Fe doping on the properties of SOFC electrolyte YSZ. Solid State Ion. 2008, 179, 1620–1624. [Google Scholar] [CrossRef]
- Kim, S.; Dayaghi, A.; Kim, K.; Choi, G. Er0.4Bi1.6O3-δ—La0.8Sr0.2MnO3-δ nano-composite as a low-temperature firing cathode of solid oxide fuel cell. J. Power Sources 2017, 344, 218–222. [Google Scholar] [CrossRef]
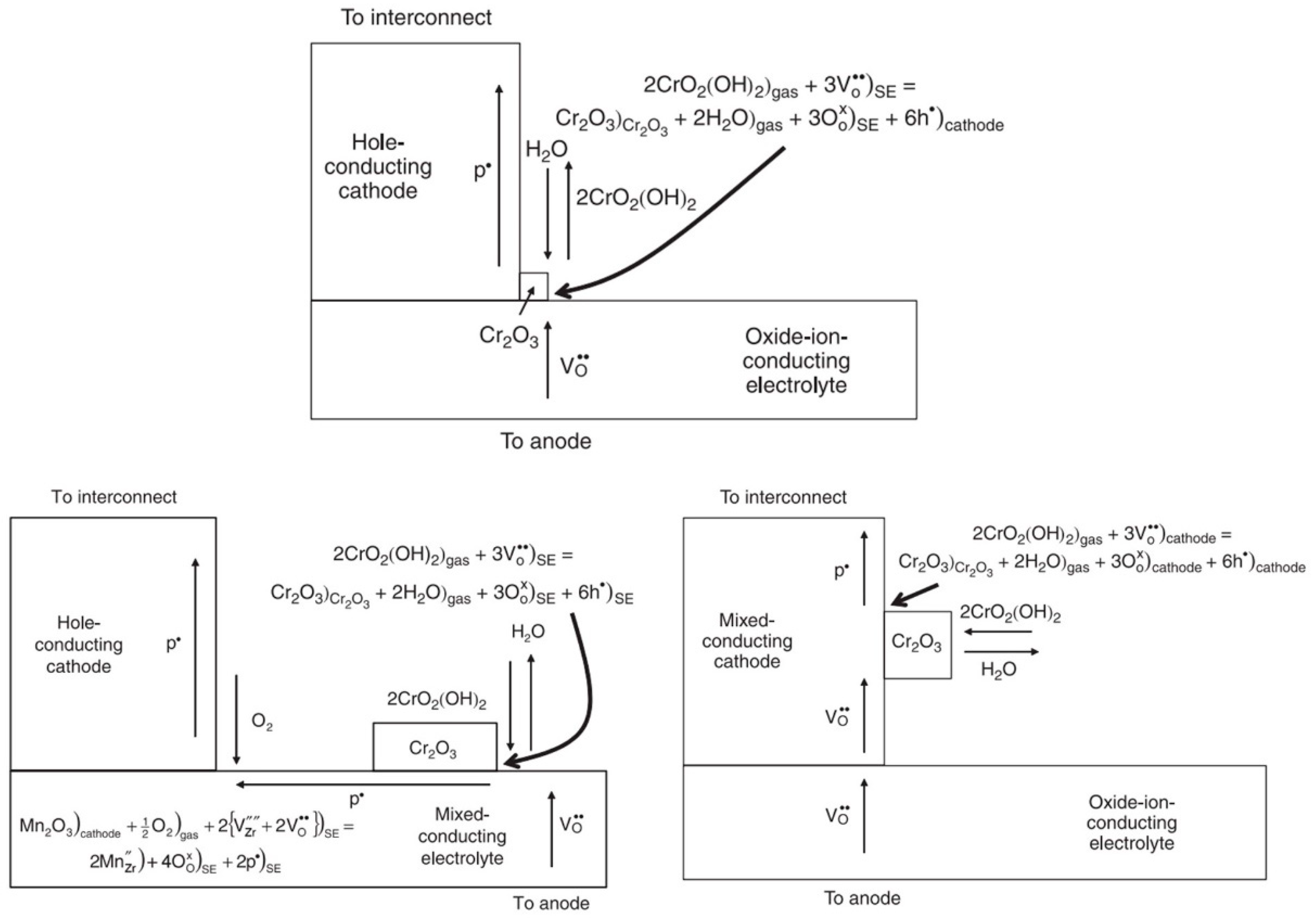
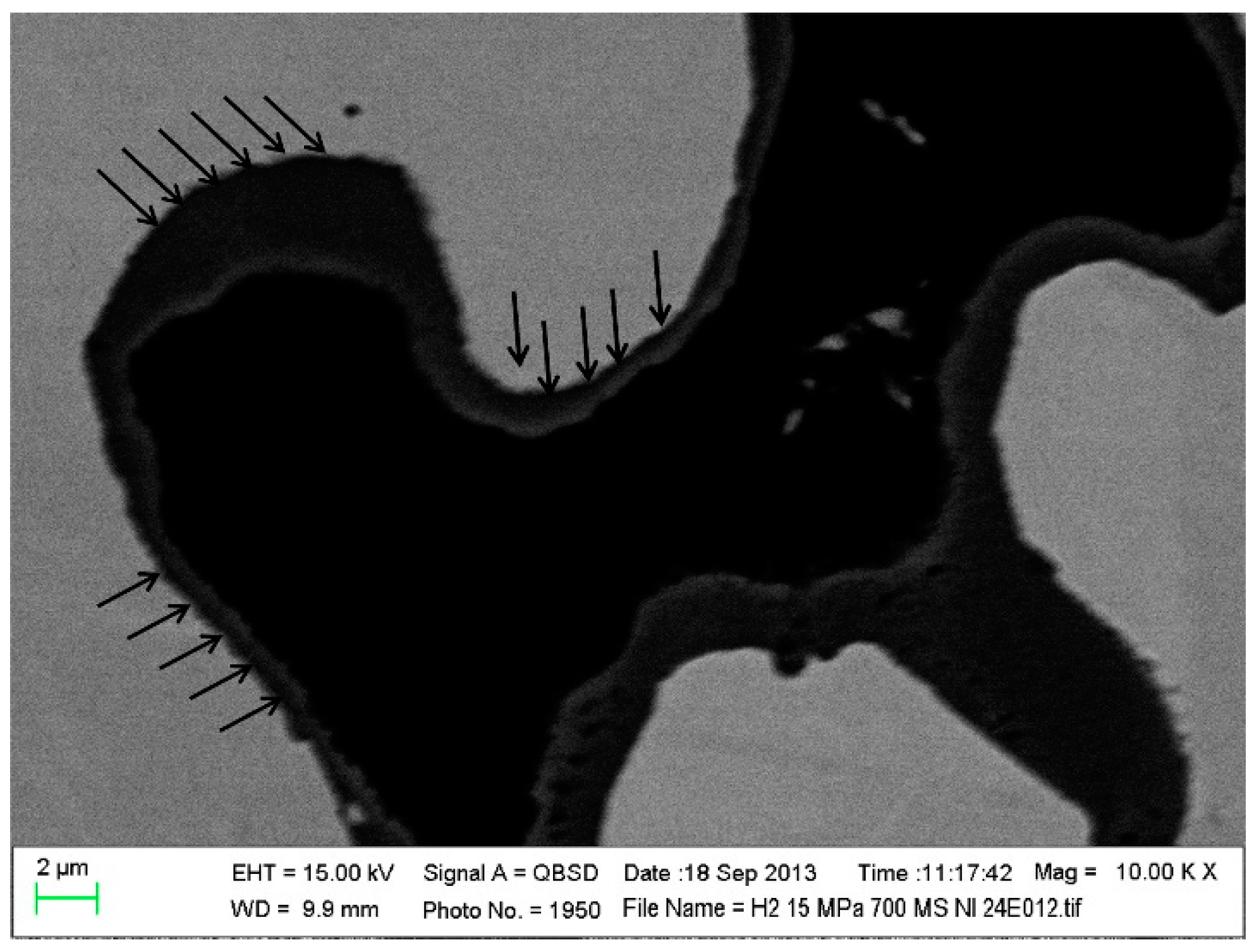
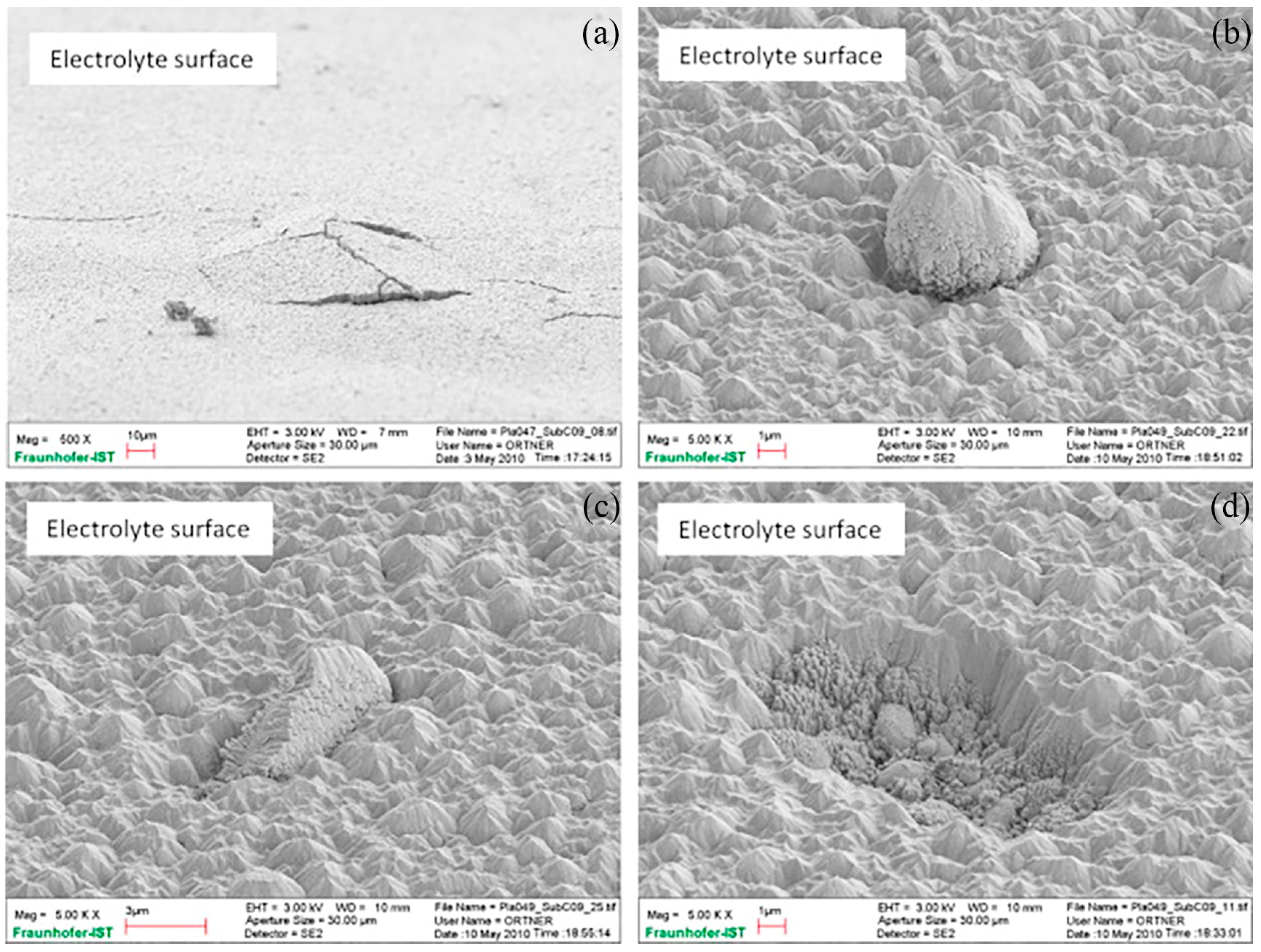

| Cell Substrate | Impregnation Material | Fuel Gas Composition | Operating Temperature (°C) | Open-Circuit Voltage (OCV) (V) | Peak Power (W cm−2) | Durability | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Fe-22Cr/Fe-22Cr + YSZ/ScYSZ/LSCF-GDC/LSC | Ni-GDC/- | H2-4%H2O | 650 | ~1.11 | ~0.43 | <~5% kh−1 | 2011 | [65] |
| 430 L/YSZ/YSZ | Ni/SSC | H2-3%H2O | 750 | 1.0 | 0.38 | Dropped by 64% (12 h) | 2013 | [66] |
| SFMO/SFMO | 800 | ~1.07 | 0.81 | - | 2014 | [58] | ||
| Ni-SDC/LSFSc | 600 | 1.12 | 0.40 | No obvious degradation (190 h) | 2014 | [67] | ||
| 430 L/430 L-YSZ/SSZ/SSZ | Ni-SDC/SBSCO | H2-3%H2O | 700 | ~1.10 | 1.25 | - | 2014 | [68] |
| 430 L/SSZ/SSZ | Ni-SDC/LSFSc | H2-3%H2O | 650 | ~1.10 | 0.53 | 1.3% kh−1 | 2015 | [69] |
| 430 L/SSZ/ESB-Ag-LBSM | Ni-SDC/- | H2-3%H2O | 600 | ~1.10 | 0.46 | No obvious degradation (90 h) | 2015 | [70] |
| FeCr/LSFNT-FeCr-ScYSZ/ScYSZ/GDC/LSC | Ni-GDC/- | H2-20%H2O | 750 | ~1.03 | 1.07 | - | 2017 | [56] |
| P434L/YSZ/YSZ/YSZ/P434L | SDCN/LSM | H2 | 700 | ~1.10 | ~1.20 | 60% kh−1 | 2017 | [71] |
| SDCN/SDCN | ~1.12 | ~0.60 | 54% kh−1 | |||||
| P434L/SCSZ/SCSZ/SCSZ/P434L | SDCN/LSM | H2-3%H2O | 700 | 1.12 | 0.9 | - | 2019 | [72] |
| SDCN/LSF | 1.09 | 0.7 | ||||||
| SDCN/LSC | 1.09 | 1.0 | ||||||
| SDCN/LSCF | 1.10 | 0.8 | ||||||
| SDCN/SSC | 1.10 | 1.0 | ||||||
| SDCN/PrOx | 1.12 | 1.30 | ||||||
| 2xNi-SDCN 40/PrOx | ethanol | 700 | ~1.03 | 1.40 | - | 2020 | [73] | |
| ethanol-water | 1.02 | 1.32 | No carbon deposition | |||||
| 430 L/ScYSZ/ScYSZ/LSC | 5 wt.% Rh-CZ/- | ethanol | 600 | 1.0 | 0.15 | 1.5 mV h−1 (130 h) | 2022 | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, P.; Wu, J.; Li, Z.; Wang, X.; Jia, L. Failure Mechanism and Optimization of Metal-Supported Solid Oxide Fuel Cells. Materials 2023, 16, 3978. https://doi.org/10.3390/ma16113978
Du P, Wu J, Li Z, Wang X, Jia L. Failure Mechanism and Optimization of Metal-Supported Solid Oxide Fuel Cells. Materials. 2023; 16(11):3978. https://doi.org/10.3390/ma16113978
Chicago/Turabian StyleDu, Pengxuan, Jun Wu, Zongbao Li, Xin Wang, and Lichao Jia. 2023. "Failure Mechanism and Optimization of Metal-Supported Solid Oxide Fuel Cells" Materials 16, no. 11: 3978. https://doi.org/10.3390/ma16113978
APA StyleDu, P., Wu, J., Li, Z., Wang, X., & Jia, L. (2023). Failure Mechanism and Optimization of Metal-Supported Solid Oxide Fuel Cells. Materials, 16(11), 3978. https://doi.org/10.3390/ma16113978





