Ni-Al Bronze in Molten Carbonate Manufactured by LPBF: Effect of Porosity Design on Mechanical Properties and Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Fabrication
2.2. Morphological and Chemical Characterization
2.3. Gravimetric Measurements
2.4. Mechanical Characterization
3. Results and Discussion
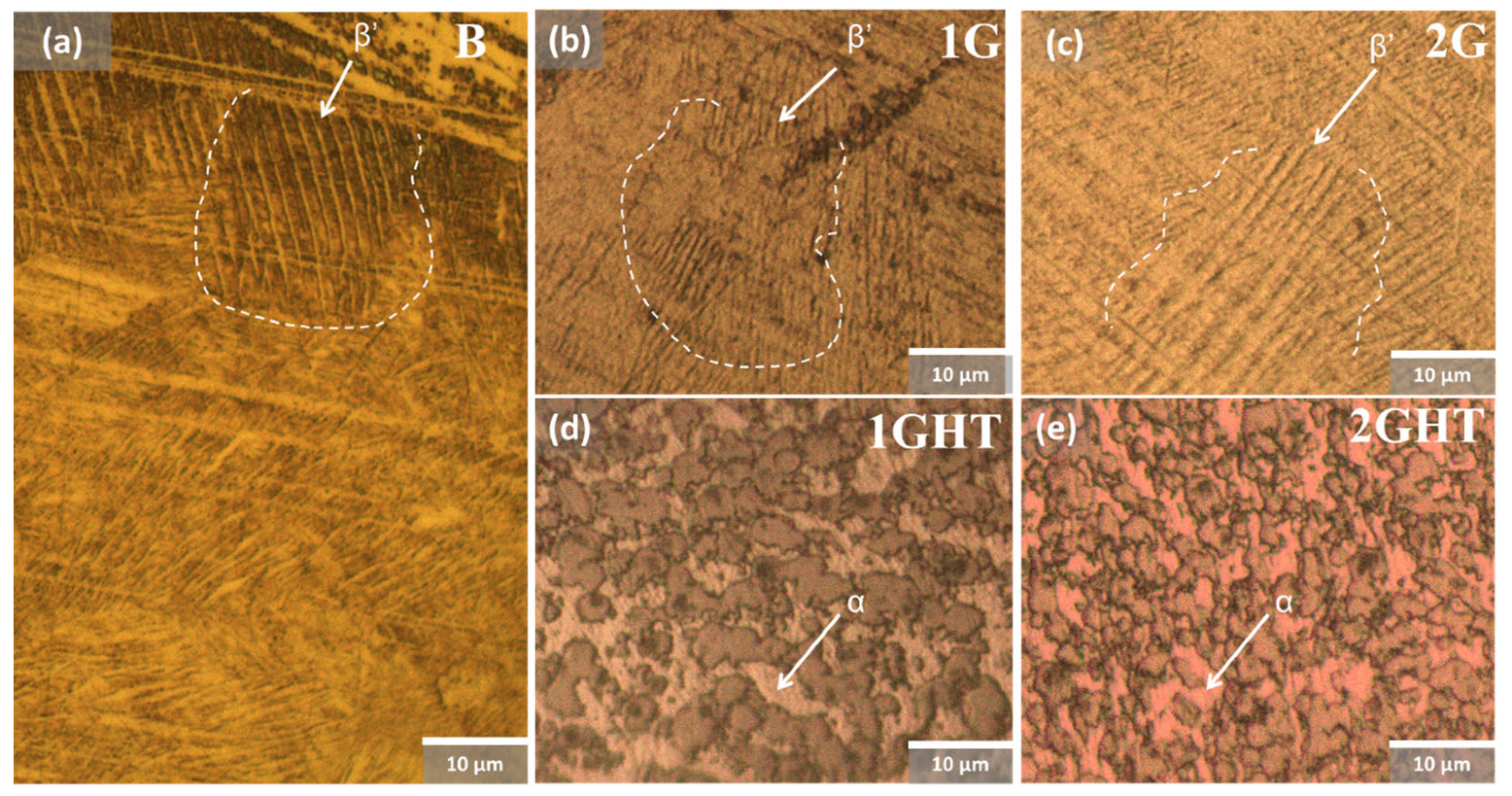
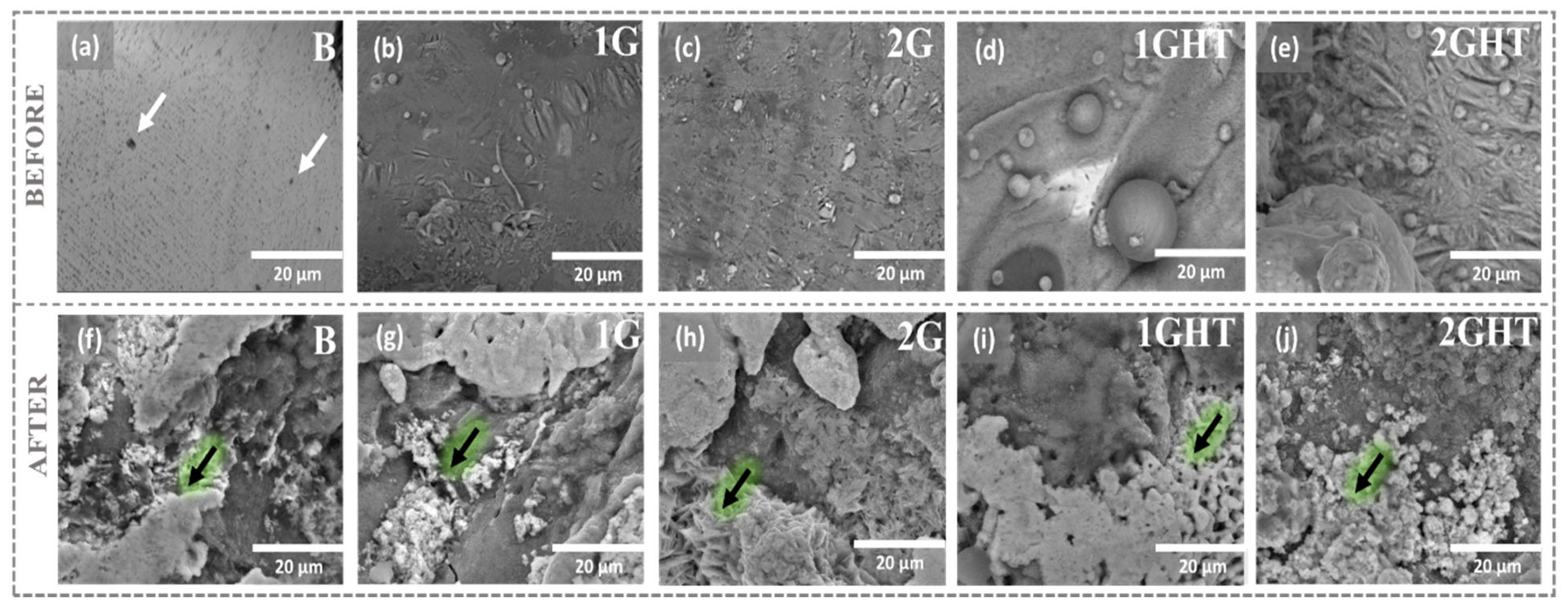
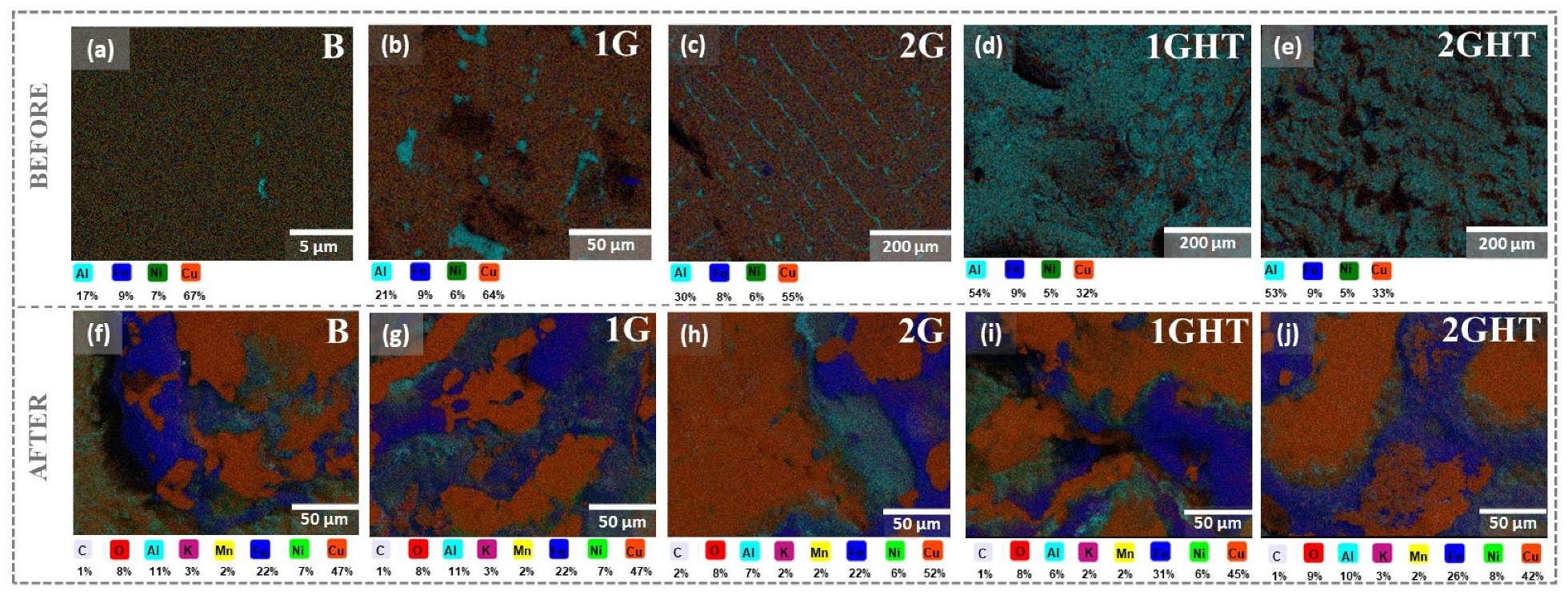
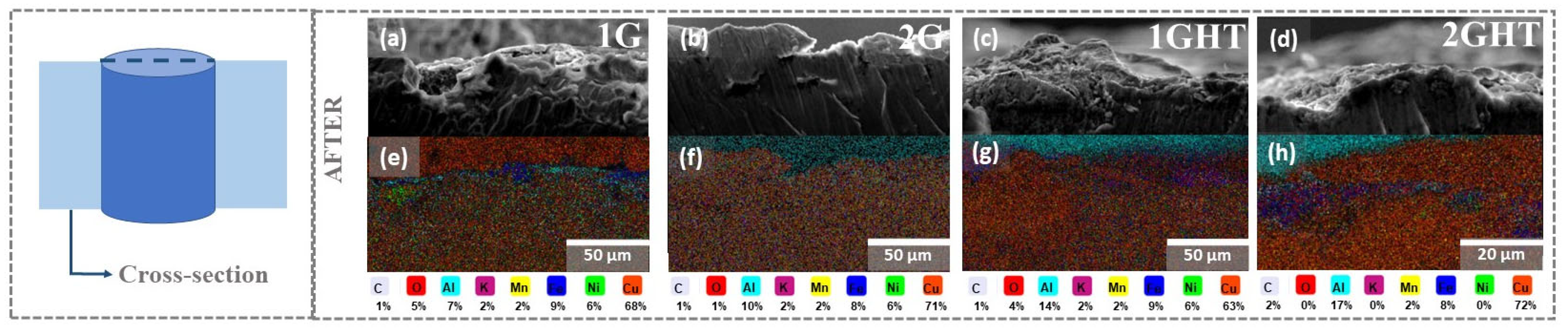
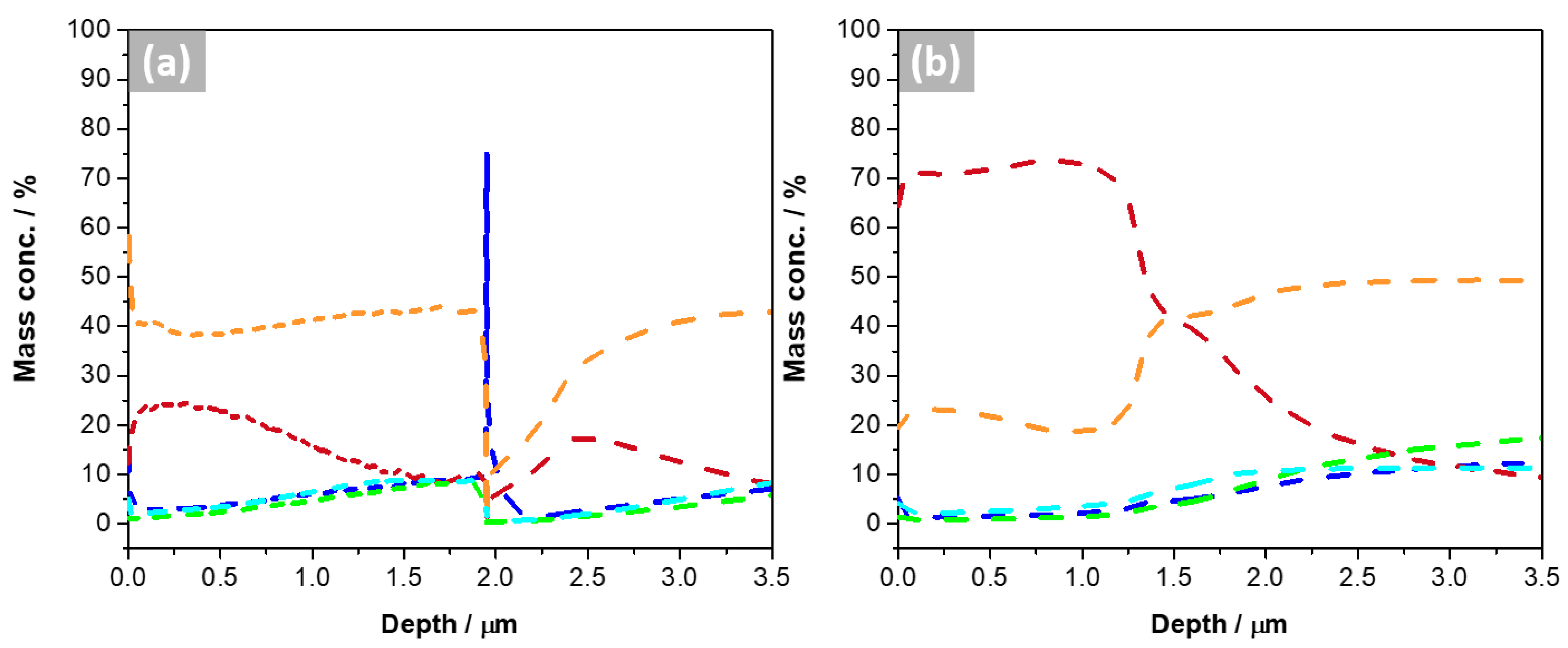
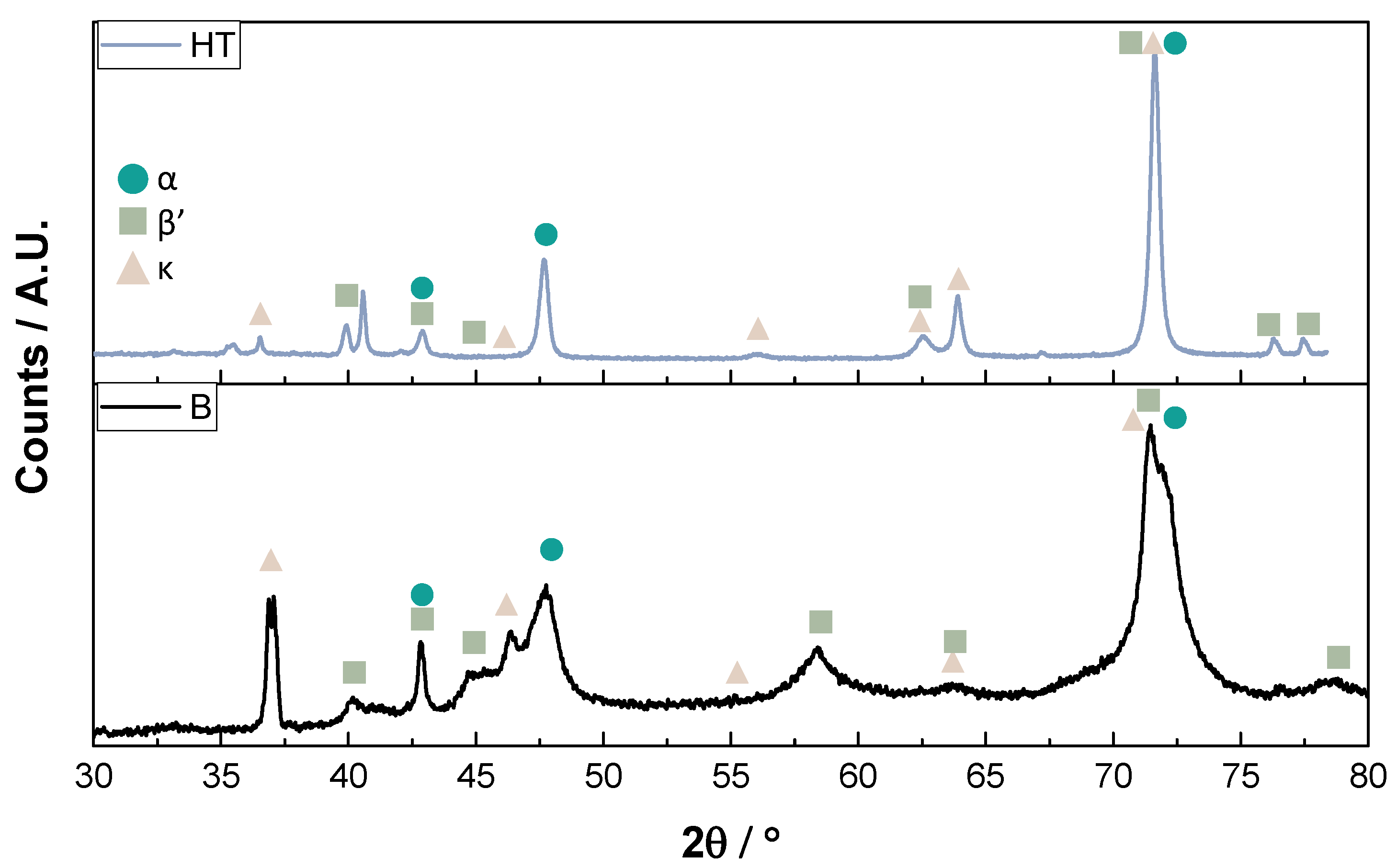
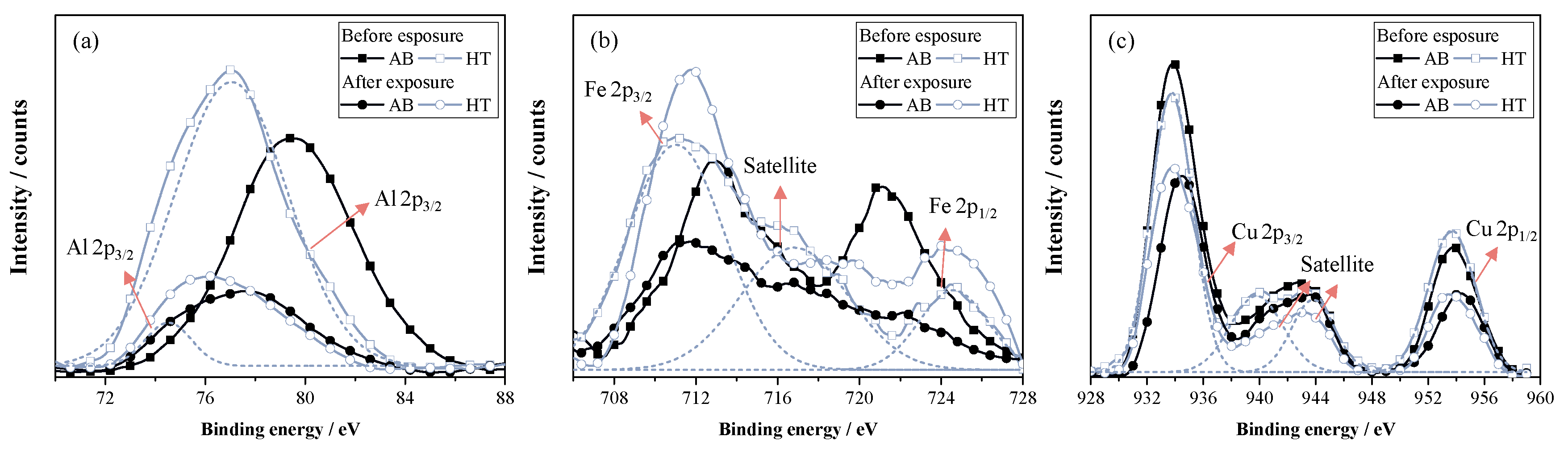
3.1. Microstructural and Chemical Characterization
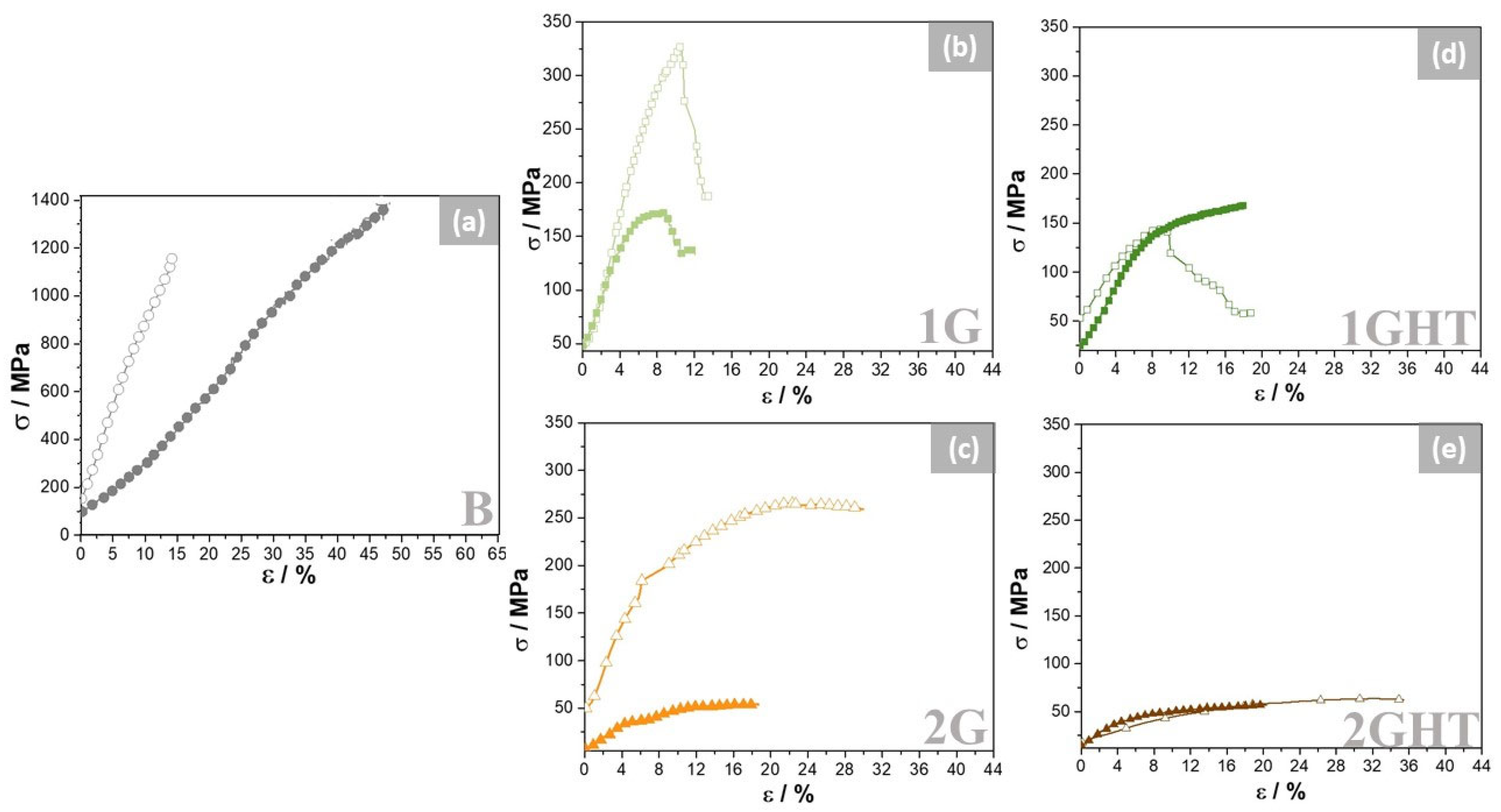
3.2. Mechanical Properties
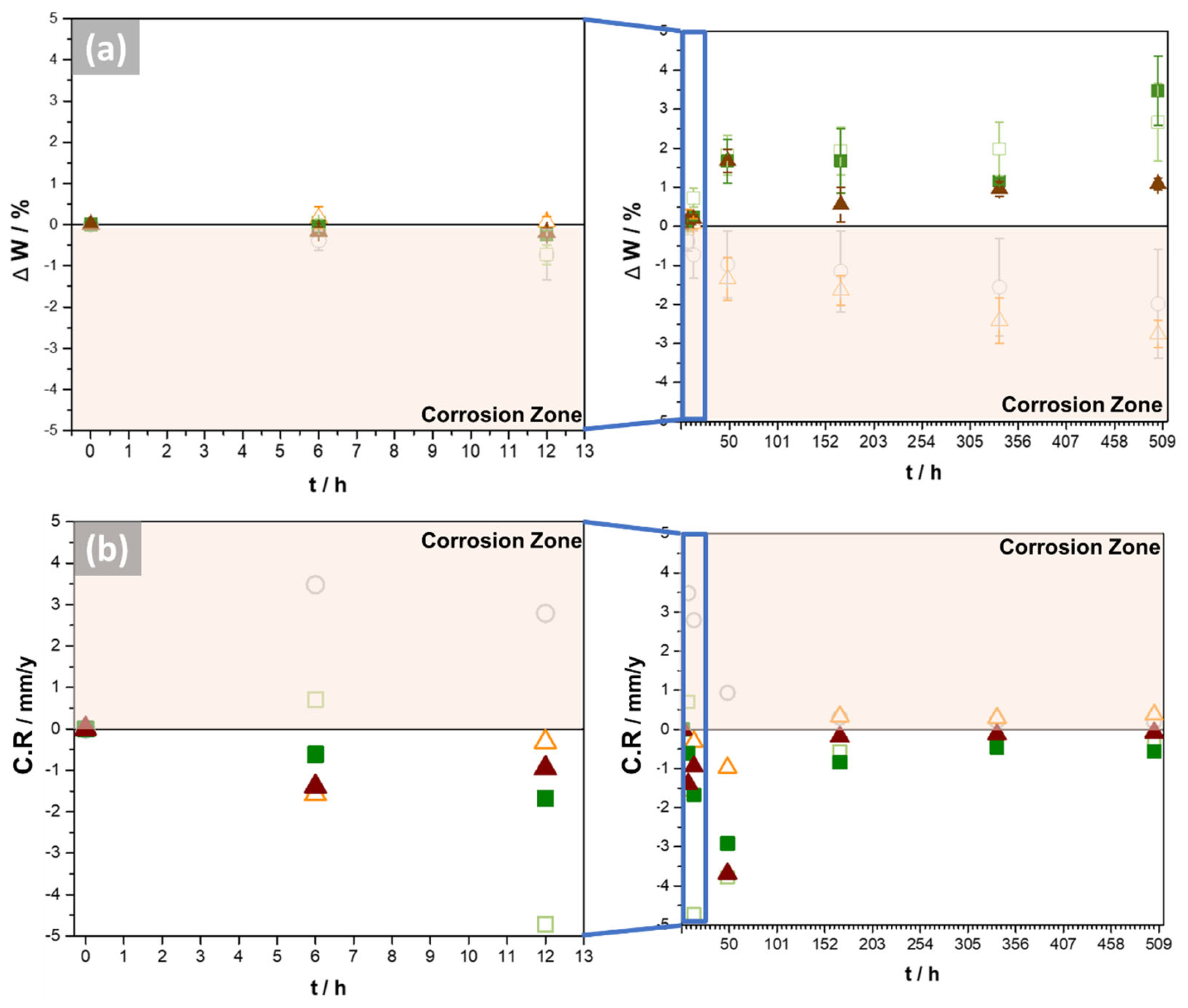
3.3. Gravimetric Measurements
4. Conclusions
- The porosity significantly influenced the mechanical response, decreasing the maximum strength by 27% when the porosity was more than 80%. The differences fall if the porous samples present an external wall, which contributes to the strengthening of the material. Therefore, a control in density gradient could increase the maximum strength of porous samples.
- Microstructurally, the bulk sample in its AB condition shows a great amount of martensite phase due to the quick solidification, which decreases in quantity after annealing, demonstrating a more significant amount of α-phase. During exposure, the phase composition had no effect on mechanical properties since the immersion temperatures contribute to phase homogenization, and these exhibited similar mechanical properties after exposure. From a corrosion point of view, was no evidence of preferential phase corrosion.
- The heat treatment (annealing) performed before exposure produced a thin oxide layer composed principally of Al, which protected the alloy from further corrosion in contact with molten salt. However, it is unknown if the layer can influence its anode functions negatively due to the isolation that it can produce, for which a catalytic study is recommended.
- The molten salt exposure influenced the formation of the corrosion products, composed principally of Cu, Al, and Fe, whose thickness, determined using GD-OES analysis, was approximately 1.5 µm. The 2G sample had the worst performance since it lost mass and had a corrosion rate of 0.37 mm·y−1, followed by the bulk sample that had a corrosion rate of 0.19 mm·y−1. Therefore, it is possible to conclude that the pores and their geometry affected the corrosion, since the oxides formed on the surface of these samples were not sufficiently protective or adherent.
- Regarding the suitability of Ni-Al bronze alloy fabricated through additive manufacturing as an anode for molten salt carbonate, it could be a great candidate due to its low corrosion rate compared to its counterpart and its high strength under compressive loads.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, M. Trends in global energy supply and demand. Dev. Pet. Sci. 2021, 71, 15–42. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrogen Energy 2018, 45, 3396–3406. [Google Scholar] [CrossRef]
- Bie, K.; Fu, P.; Liu, Y.; Muhammad, A. Comparative study on the performance of different carbon fuels in a molten carbonate direct carbon fuel cell with a novel anode structure. J. Power Sources 2020, 460, 228101. [Google Scholar] [CrossRef]
- Wang, F.; Deng, S.; Zhang, H.; Wang, J.; Zhao, J.; Miao, H.; Yuan, J.; Yan, J. A comprehensive review on high-temperature fuel cells with carbon capture. Appl. Energy 2020, 275, 115342. [Google Scholar] [CrossRef]
- Wee, J.-H. Carbon dioxide emission reduction using molten carbonate fuel cell systems. Renew. Sustain. Energy Rev. 2014, 32, 178–191. [Google Scholar] [CrossRef]
- Da Rosa, A.V.; Ordóñez, J.C. Fuel Cells. In Fundamentals of Renewable Energy Processes; Academic Press: Cambridge, MA, USA, 2021; pp. 317–417. [Google Scholar] [CrossRef]
- Hacker, V.; Mitsushima, S. Fuel Cells and Hydrogen: From Fundamentals to Applied Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–276. [Google Scholar] [CrossRef]
- Abdollahipour, A.; Sayyaadi, H. Thermal energy recovery of molten carbonate fuel cells by thermally regenerative electrochemical cycles. Energy 2021, 227, 120489. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Wejrzanowski, T.; Sobczak, P.; Cwieka, K.; Lysik, A.; Skibinski, J.; Oliver, G.J. Insight into cathode microstructure effect on the performance of molten carbonate fuel cell. J. Power Sources 2021, 491, 229562. [Google Scholar] [CrossRef]
- Accardo, G.; Frattini, D.; Moreno, A.; Yoon, S.P.; Han, J.H.; Nam, S.W. Influence of nano zirconia on NiAl anodes for molten carbonate fuel cell: Characterization, cell tests and post-analysis. J. Power Sources 2017, 338, 74–81. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Liang, H.; Li, C. Research on high-temperature compression and creep behavior of porous Cu–Ni–Cr alloy for molten carbonate fuel cell anodes. Mater. Sci. 2015, 33, 356–362. [Google Scholar] [CrossRef]
- Nosewicz, S.; Jurczak, G.; Wejrzanowski, T.; Ibrahim, S.H.; Grabias, A.; Węglewski, W.; Kaszyca, K.; Rojek, J.; Chmielewski, M. Thermal conductivity analysis of porous NiAl materials manufactured by spark plasma sintering: Experimental studies and modelling. Int. J. Heat Mass Transf. 2022, 194, 123070. [Google Scholar] [CrossRef]
- Accardo, G.; Frattini, D.; Yoon, S.P.; Ham, H.C.; Nam, S.W. Performance and properties of anodes reinforced with metal oxide nanoparticles for molten carbonate fuel cells. J. Power Sources 2017, 370, 52–60. [Google Scholar] [CrossRef]
- Frattini, D.; Accardo, G.; Moreno, A.; Yoon, S.P.; Han, J.H.; Nam, S.W. A novel Nickel-Aluminum alloy with Titanium for improved anode performance and properties in Molten Carbonate Fuel Cells. J. Power Sources 2017, 352, 90–98. [Google Scholar] [CrossRef]
- Nguyen, H.V.P.; Song, S.A.; Seo, D.; Park, D.-N.; Ham, H.C.; Oh, I.-H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J. Fabrication of Ni–Al–Cr alloy anode for molten carbonate fuel cells. Mater. Chem. Phys. 2012, 136, 910–916. [Google Scholar] [CrossRef]
- Lee, C.-G.; Hwang, J.-Y.; Lee, S.-Y.; Oh, M.; Kim, D.-H.; Lim, H.-C. Effect of Anode Area on the Cell Performance in a Molten Carbonate Fuel Cell. J. Electrochem. Soc. 2008, 155, A138. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Li, W.; Hu, Y.; Ren, Y.; Qiu, W.; He, J.; Chen, J. Investigation on compressive behavior of Cu-35Ni-15Al alloy at high temperatures. Mater. Sci. 2014, 32, 341–349. [Google Scholar] [CrossRef]
- Hwang, E.; Park, J.; Kim, Y.; Kim, S.; Kang, S. Effect of alloying elements on the copper-base anode for molten carbonate fuel cells. J. Power Sources 1997, 69, 55–60. [Google Scholar] [CrossRef]
- Ren, Y.; Peng, Y.; Chen, J.; Qiu, W.; Li, W.; Li, C.; Niu, Y. Electrochemical Impedance Studies on the Corrosion of Cu-35Ni-10Al Alloy in a Molten (0.62Li, 0.38K)2CO3 Environment. Int. J. Electrochem. Sci. 2019, 14, 6147–6153. [Google Scholar] [CrossRef]
- Meléndez-Ceballos, A.; Albin, V.; Crapart, C.; Lair, V.; Ringuedé, A.; Cassir, M. Influence of Cs and Rb additions in LiK and LiNa molten carbonates on the behaviour of MCFC commercial porous Ni cathode. Int. J. Hydrogen Energy 2017, 42, 1853–1858. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, K.-Y.; Chun, H.-S. Creep characteristics of porous Ni/Ni3Al anodes for molten carbonate fuel cells. J. Power Sources 2001, 99, 26–33. [Google Scholar] [CrossRef]
- Li, G.; Thomas, B.; Stubbins, J.F. Modeling creep and fatigue of copper alloys. Met. Mater. Trans. A 2000, 31, 2491–2502. [Google Scholar] [CrossRef]
- Parameswaran, P.; Antony, G.; Dinesh, S.; Radhakrishnan, K. Experimental study on mechanical and corrosion characteristics of nab alloy with the addition of chromium. Mater. Today Proc. 2018, 5, 8089–8094. [Google Scholar] [CrossRef]
- De Miguel, M.; Lasanta, M.; García-Martín, G.; Díaz, R.; Pérez, F. Temperature effect and alloying elements impact on the corrosion behaviour of the alloys exposed to molten carbonate environments for CSP application. Corros. Sci. 2022, 201, 110274. [Google Scholar] [CrossRef]
- Ceccanti, F.; Giorgetti, A.; Citti, P. A Support Structure Design Strategy for Laser Powder Bed Fused Parts. Procedia Struct. Integr. 2019, 24, 667–679. [Google Scholar] [CrossRef]
- Gera, D.; Santos, J.; Kiminami, C.S.; Gargarella, P. Comparison of Cu–Al–Ni–Mn–Zr shape memory alloy prepared by selective laser melting and conventional powder metallurgy. Trans. Nonferrous Met. Soc. China 2020, 30, 3322–3332. [Google Scholar] [CrossRef]
- ASTM C373-88; Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products. ASTM: West Conshohocken, PA, USA, 2006.
- Scanning Electron Microscope (Quanta 250 FEG). Available online: https://www.bose.res.in/facilities/Technical_Cell/sem.html (accessed on 9 November 2021).
- Cassir, M.; Meléndez-Ceballos, A.; Ringuedé, A.; Lair, V. 3-Molten Carbonate Fuel Cells. In Compendium of Hydrogen Energy; Vezirglu, F., Barbir, A., Basile, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Ricca, C.; Ringuedé, A.; Cassir, M.; Adamo, C.; Labat, F. Mixed lithium-sodium (LiNaCO3) and lithium-potassium (LiKCO3) carbonates for low temperature electrochemical applications: Structure, electronic properties and surface reconstruction from ab-initio calculations. Surf. Sci. 2016, 647, 66–77. [Google Scholar] [CrossRef]
- Lair, V.; Albin, V.; Ringuedé, A.; Cassir, M. Theoretical predictions vs. experimental measurements of the electrical conductivity of molten Li2CO3–K2CO3 modified by additives. Int. J. Hydrogen Energy 2012, 37, 19357–19364. [Google Scholar] [CrossRef]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 2017.
- Hooper, P.A. Melt pool temperature and cooling rates in laser powder bed fusion. Addit. Manuf. 2018, 22, 548–559. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Kelly, P. Crystallography of spheroidite and tempered martensite. Acta Mater. 1998, 46, 4081–4091. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Wang, X.; Liang, B.; Zhang, Y. Microstructure and Mechanical Behavior of Cu–9Al–4Ni-3.5Fe-0.5Mn Alloy Fabricated by Laser Melting Deposition. Mater. Sci. Eng. A 2021, 826, 142006. [Google Scholar] [CrossRef]
- Orzolek, S.M.; Semple, J.K.; Fisher, C.R. Influence of processing on the microstructure of nickel aluminum bronze (NAB). Addit. Manuf. 2022, 56, 102859. [Google Scholar] [CrossRef]
- Culpan, E.A.; Rose, G. Microstructural characterization of cast nickel aluminium bronze. J. Mater. Sci. 1978, 13, 1647–1657. [Google Scholar] [CrossRef]
- Brezina, P. Heat treatment of complex aluminium bronzes. Int. Met. Rev. 1982, 27, 77–120. [Google Scholar] [CrossRef]
- Tavares, S.; Mota, N.; da Igreja, H.; Barbosa, C.; Pardal, J. Microstructure, mechanical properties, and brittle fracture of a cast nickel-aluminum-bronze (NAB) UNS C95800. Eng. Fail. Anal. 2021, 128, 105606. [Google Scholar] [CrossRef]
- Dareh Baghi, A.; Nafisi, S.; Hashemi, R.; Ebendorff-Heidepriem, H.; Ghomashchi, R. Effective Post Processing of SLM Fabricated Ti-6Al-4 V Alloy: Machining vs Thermal Treatment. J. Manuf. Process. 2021, 68, 1031–1046. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. A review on molten salt synthesis of metal oxide nanomaterials: Status, opportunity, and challenge. Prog. Mater. Sci. 2020, 117, 100734. [Google Scholar] [CrossRef]
- Audigié, P.; Encinas-Sánchez, V.; Juez-Lorenzo, M.; Rodríguez, S.; Gutiérrez, M.; Pérez, F.; Agüero, A. High temperature molten salt corrosion behavior of aluminide and nickel-aluminide coatings for heat storage in concentrated solar power plants. Surf. Coat. Technol. 2018, 349, 1148–1157. [Google Scholar] [CrossRef]
- Vossen, J.P.T.; Janssen, A.H.H.; De Wit, J.H.W. Corrosion Behavior of Nickel-Iron Alloys in Molten Carbonate. J. Electrochem. Soc. 1996, 143, 58–66. [Google Scholar] [CrossRef]
- Tang, D.; Zheng, K.; Yin, H.; Mao, X.; Sadoway, D.R.; Wang, D. Electrochemical growth of a corrosion-resistant multi-layer scale to enable an oxygen-evolution inert anode in molten carbonate. Electrochim. Acta 2018, 279, 250–257. [Google Scholar] [CrossRef]
- Zheng, K.; Du, K.; Cheng, X.; Jiang, R.; Deng, B.; Zhu, H.; Wang, D. Nickel-Iron-Copper Alloy as Inert Anode for Ternary Molten Carbonate Electrolysis at 650 °C. J. Electrochem. Soc. 2018, 165, E572–E577. [Google Scholar] [CrossRef]
- Hasegawa, M. Ellingham Diagram. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 507–516. [Google Scholar]
- Spiegel, M.; Biedenkopf, P.; Grabke, H. Corrosion of iron base alloys and high alloy steels in the Li2CO3-K2CO3 eutectic mixture. Corros. Sci. 1997, 39, 1193–1210. [Google Scholar] [CrossRef]
- Luo, J.; Deng, C.K.; Tariq, N.U.H.; Li, N.; Han, R.F.; Liu, H.H.; Wang, J.Q.; Cui, X.Y.; Xiong, T.Y. Corrosion behavior of SS316L in ternary Li2CO3–Na2CO3–K2CO3 eutectic mixture salt for concentrated solar power plants. Sol. Energy Mater. Sol. Cells 2020, 217, 110679. [Google Scholar] [CrossRef]
- Goupil, G.; Bonnefont, G.; Idrissi, H.; Guay, D.; Roué, L. Consolidation of mechanically alloyed Cu–Ni–Fe material by spark plasma sintering and evaluation as inert anode for aluminum electrolysis. J. Alloys Compd. 2013, 580, 256–261. [Google Scholar] [CrossRef]
- Alkelae, F.; Sasaki, S. Microstructures Generated by Nickel Aluminium Bronze Alloy L-Pbfed and Their Effect on Tribological and Mechanical Properties. J. Tribol. 2021, 29, 41–56. [Google Scholar]
- Lv, Y.; Ding, Y.; Han, Y.; Zhang, L.-C.; Wang, L.; Lu, W. Strengthening mechanism of friction stir processed and post heat treated NiAl bronze alloy: Effect of rotation rates. Mater. Sci. Eng. A 2017, 685, 439–446. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N. Metal foams: A design guide. Mater. Des. 2002, 23, 119. [Google Scholar] [CrossRef]
- Förster, W.; Pucklitzsch, T.; Dietrich, D.; Nickel, D. Mechanical performance of hexagonal close-packed hollow sphere infill structures with shared walls under compression load. Addit. Manuf. 2022, 59, 103135. [Google Scholar] [CrossRef]
- Wee, J.-H.; Song, D.-J.; Jun, C.-S.; Lim, T.-H.; Hong, S.-A.; Lim, H.-C.; Lee, K.-Y. Evaluation of Ni–Ni3Al(5wt.%)–Al(3wt.%) as an anode electrode for molten carbonate fuel cell: Part I: Creep and sintering resistance. J. Alloys Compd. 2005, 390, 155–160. [Google Scholar] [CrossRef]
- Choe, H.; Dunand, D. Creep of N1-Al and Ni-Cr-Al Superalloy Foams. 2003, undefined.
- Öztürk, S.; Sünbül, S.E.; Metoğlu, A.; Önal, S.; Için, K. Characterisation of nickel–aluminium bronze powders produced by the planar flow casting method. Mater. Sci. Technol. 2020, 36, 1771–1784. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Noel, J.; Weber, J. Corrosion evaluation of alloys and MCrAlX coatings in molten carbonates for thermal solar applications. Sol. Energy Mater. Sol. Cells 2016, 157, 517–525. [Google Scholar] [CrossRef]
- Karfidov, E.; Nikitina, E.; Erzhenkov, M.; Seliverstov, K.; Chernenky, P.; Mullabaev, A.; Tsvetov, V.; Mushnikov, P.; Karimov, K.; Molchanova, N.; et al. Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators. Materials 2022, 15, 761. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Duan, Y.Y. Corrosion resistance of CU2O and CuO on copper surfaces in aqueous media. Br. Corros. J. 1994, 29, 309–314. [Google Scholar] [CrossRef]

 .
.
 .
.

| ID | Lattice Type | Heat Treated | Image |
|---|---|---|---|
| B | Bulk | No |  |
| 1G | Gyroid + wall | No |  |
| 1GHT | Yes | ||
| 2G | Gyroid | No |  |
| 2GHT | Yes |
| Sample ID | Before Exposure | After Exposure | ||||
|---|---|---|---|---|---|---|
| σUTS (MPa) | Max. ε (%) | % Var. * | σUTS (MPa) | Max. ε (%) | % Var. * | |
| B | 1140.9 ± 27.1 | 20.8 ± 0.5 | 100 | 1415.5 | 59.9 | 100 |
| 1G | 317.7 ± 10.0 | 19.0 ± 0.5 | 27.8 | 177.4 ± 6.5 | 19.1 | 12.5 |
| 2G | 138.1 ± 6.0 | 19.5 ± 5.9 | 12.1 | 50.6 ± 5.7 | 24.6 ± 4.9 | 3.6 |
| 1GHT | 237.7 ± 5.6 | 37.3 ± 0.2 | 20.8 | 168.7 | 29.2 ± 5.3 | 11.9 |
| 2GHT | 96.8 ± 33.0 | 51.8 ± 10.4 | 8.5 | 64.9 ± 10.8 | 26.9 ± 1.5 | 4.6 |
| Elem. | Al | Fe | Cu | |||||||||
| Peak | 74 eV | 76 eV | 712 eV | 724 eV | 933 eV | 954 eV | ||||||
| Exp. | AB | HT | AB | HT | AB | HT | AB | HT | AB | HT | AB | HT |
| Before | 0 | 48.9 | 684.1 | 789.5 | 232.8 | 339.7 | 213.6 | 85.6 | 1145.3 | 1291.6 | 680.2 | 734.4 |
| After | 0 | 142.6 | 279.6 | 393.3 | 131.1 | 335.0 | 27.0 | 116.8 | 954.3 | 1067.6 | 409.5 | 430.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcos, C.; Guerra, C.; Ramos-Grez, J.A.; Sancy, M. Ni-Al Bronze in Molten Carbonate Manufactured by LPBF: Effect of Porosity Design on Mechanical Properties and Oxidation. Materials 2023, 16, 3893. https://doi.org/10.3390/ma16103893
Arcos C, Guerra C, Ramos-Grez JA, Sancy M. Ni-Al Bronze in Molten Carbonate Manufactured by LPBF: Effect of Porosity Design on Mechanical Properties and Oxidation. Materials. 2023; 16(10):3893. https://doi.org/10.3390/ma16103893
Chicago/Turabian StyleArcos, Camila, Carolina Guerra, Jorge A. Ramos-Grez, and Mamié Sancy. 2023. "Ni-Al Bronze in Molten Carbonate Manufactured by LPBF: Effect of Porosity Design on Mechanical Properties and Oxidation" Materials 16, no. 10: 3893. https://doi.org/10.3390/ma16103893
APA StyleArcos, C., Guerra, C., Ramos-Grez, J. A., & Sancy, M. (2023). Ni-Al Bronze in Molten Carbonate Manufactured by LPBF: Effect of Porosity Design on Mechanical Properties and Oxidation. Materials, 16(10), 3893. https://doi.org/10.3390/ma16103893









