Construction of Bio-TiO2/Algae Complex and Synergetic Mechanism of the Acceleration of Phenol Biodegradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and TiO2 Biosynthesis
2.2. Microalgae Culture and Growth Conditions
2.3. Characterization Methods
2.4. Photocatalytic Degradation of Phenol
2.5. Electrochemical Characteristic Experiments
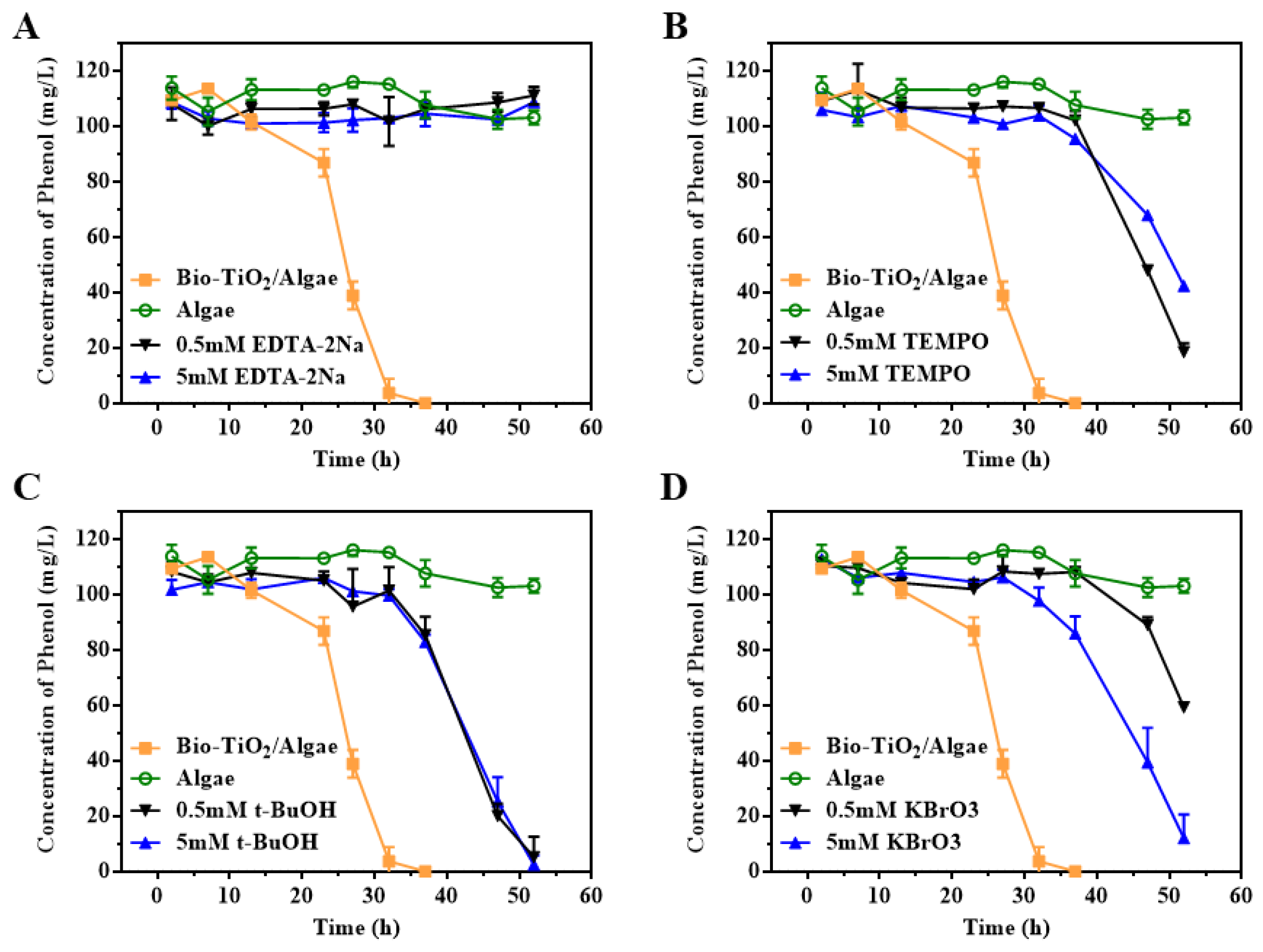
2.6. Free Radical Capture Experiments
3. Results and Discussion
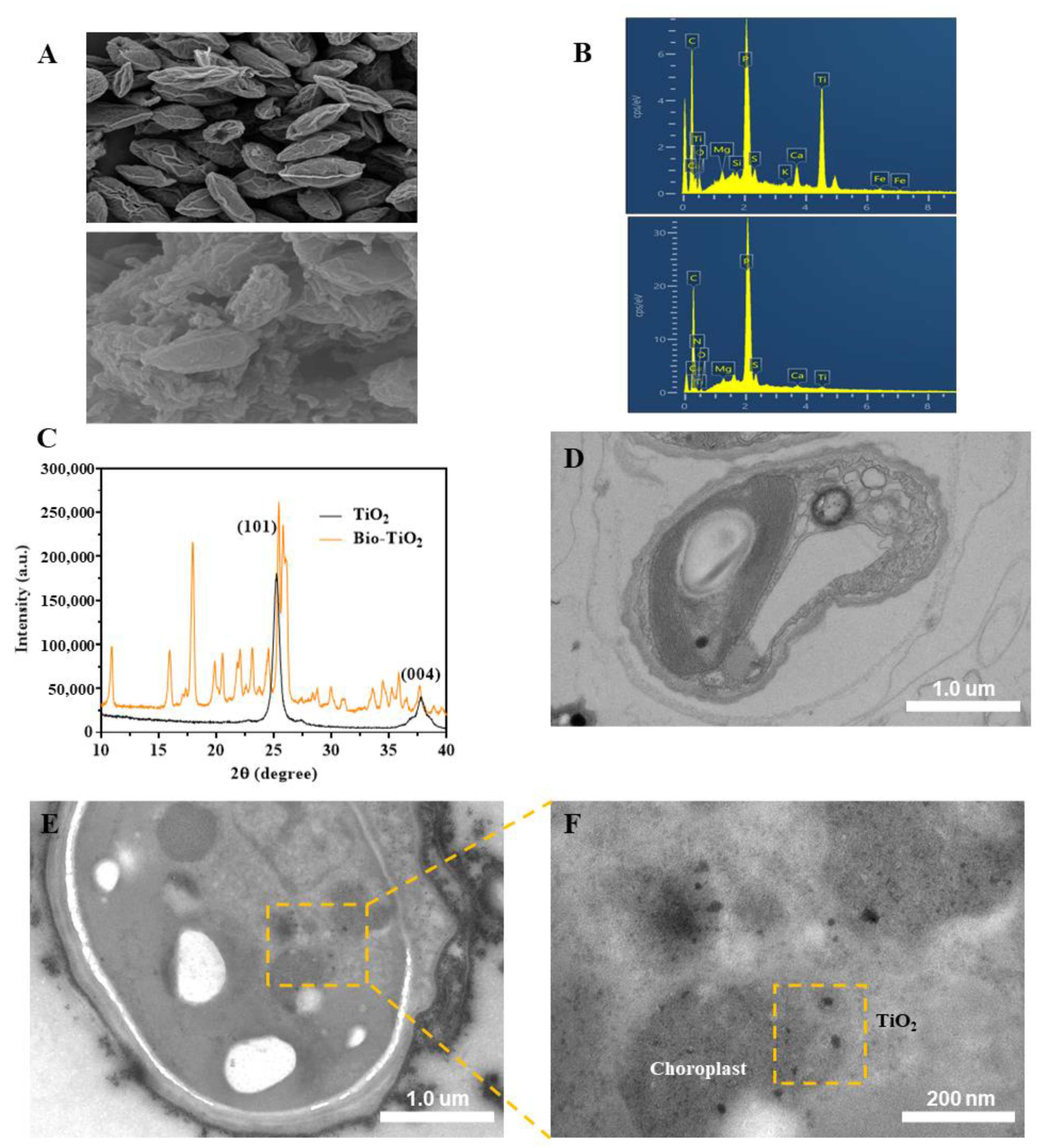
3.1. Synthesis and Characterization of bio-TiO2 NPs and Bio-TiO2/Algae Complex
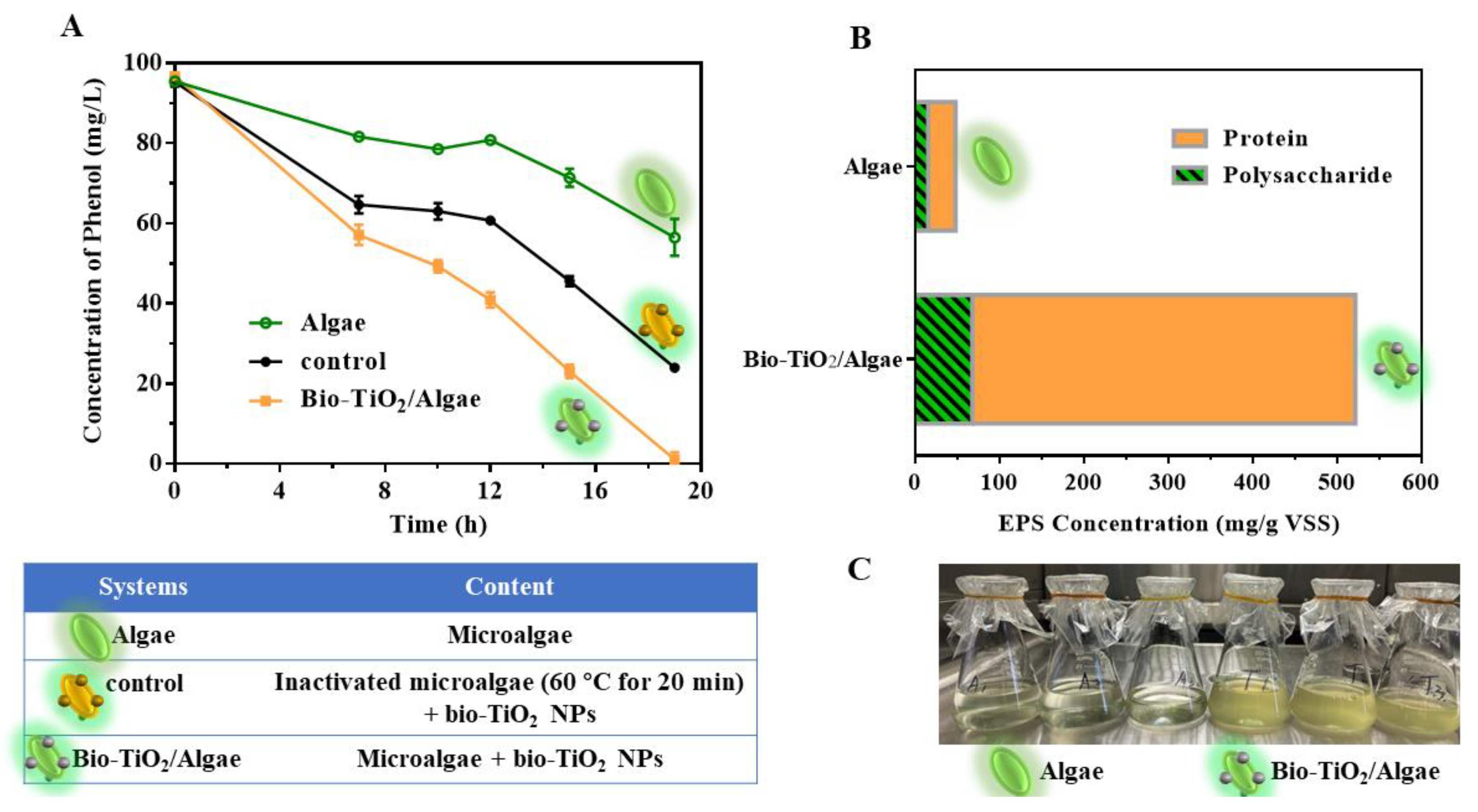
3.2. Phenol Degradation Promotion by Bio-TiO2/Algae Complex
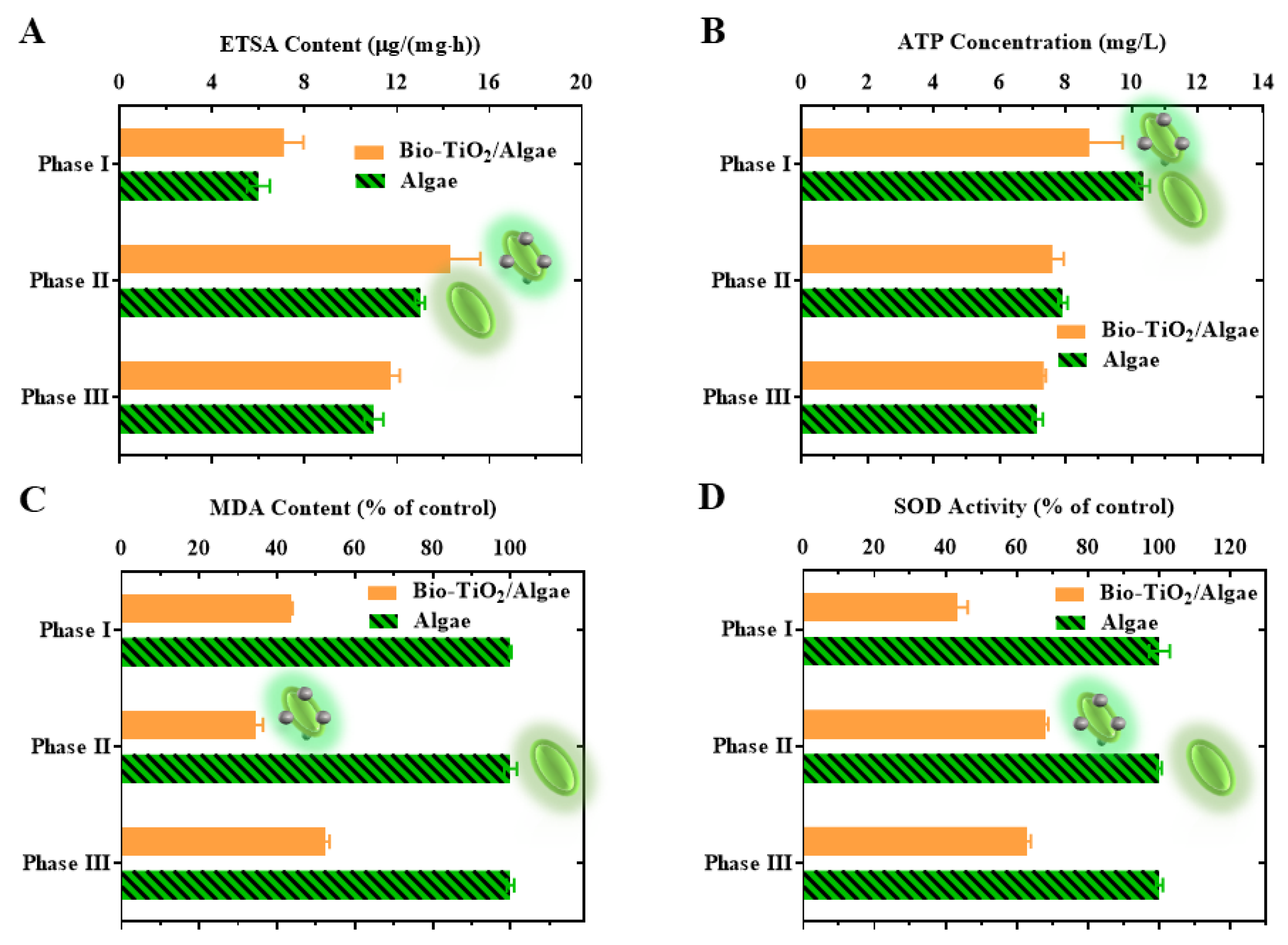
3.3. Metabolic Activity Analysis of Microalgae in Bio-TiO2/Algae Complex
3.3.1. EPS Excretion in Bio-TiO2/Algae Complex
3.3.2. Analysis of ETSA in Bio-TiO2/Algae Complex
3.3.3. Measurement of ATP Level in Bio-TiO2/Algae Complex
3.4. Stress Response Analysis of Microalgae in Bio-TiO2/Algae Complex
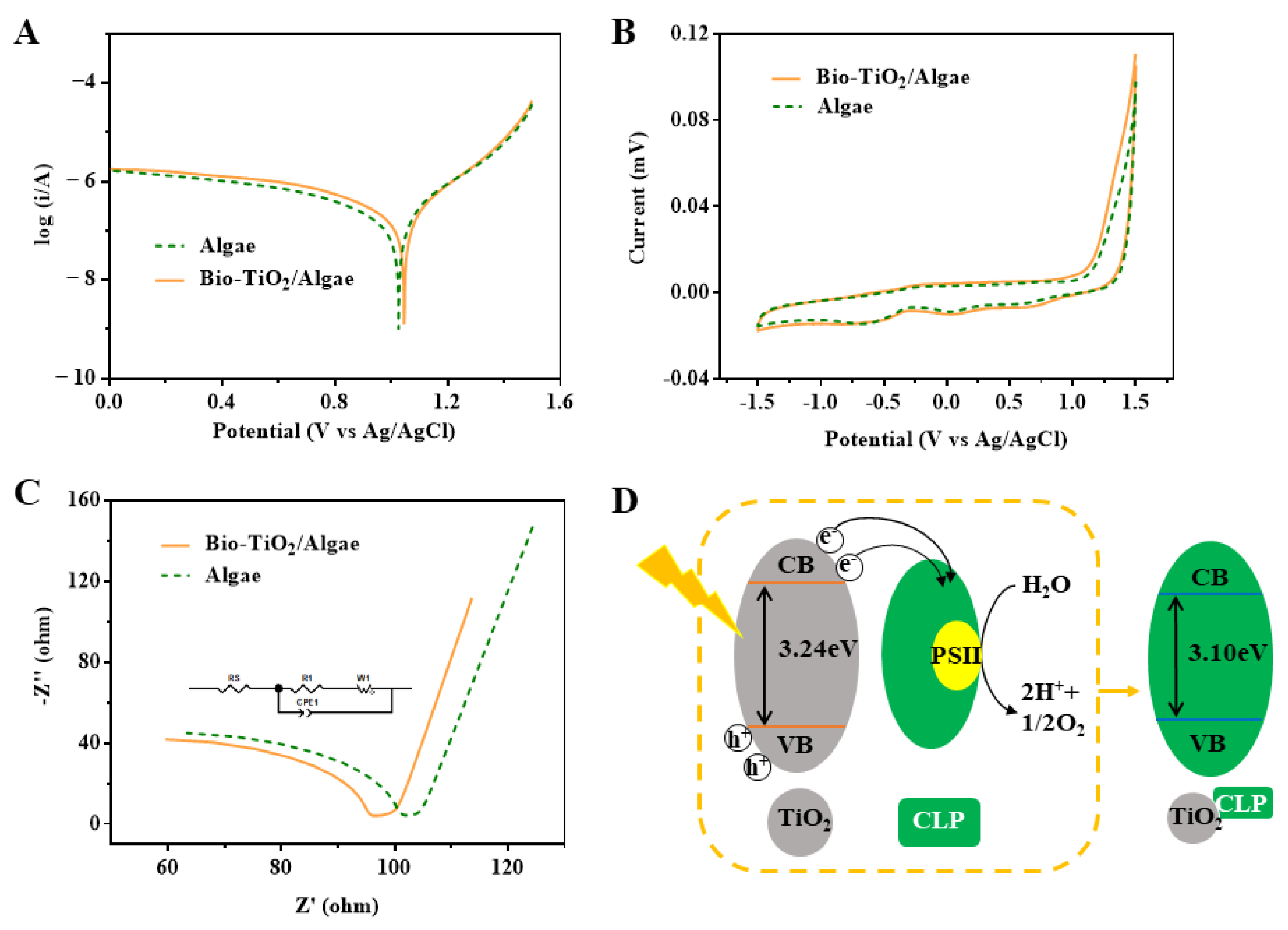
3.5. Variations of Extracellular Electron Transfer Behaviors in Bio-TiO2/Algae Complex
3.6. Effect of Photoelectron and Photohole on the Degradation of Phenol by Bio-TiO2/Algae Complex
3.7. Synergetic Mechanism of Phenol Degradation by Bio-TiO2/Algae Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priyadharshini, D.S.; Bakthavatsalam, A.K. Optimization of phenol degradation by the microalga Chlorella pyrenoidosa using Plackett-Burman Design and Response Surface Methodology. Bioresour. Technol. 2016, 207, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol Env. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol removal from industrial wastewaters: A short review. Desalination Water Treat 2014, 53, 2215–2234. [Google Scholar] [CrossRef]
- Park, H.S.; Koduru, J.R.; Choo, K.H.; Lee, B. Activated carbons impregnated with iron oxide nanoparticles for enhanced removal of bisphenol A and natural organic matter. J. Hazard. Mater. 2015, 286, 315–324. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2013, 91, 1–18. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, K.; Zhou, H.; Hu, Y.; Sergei, P.; Wu, H.; Wei, C. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater. J. Env. Manag. 2018, 224, 376–386. [Google Scholar] [CrossRef]
- Ucun, H.; Yildiz, E.; Nuhoglu, A. Phenol biodegradation in a batch jet loop bioreactor (JLB): Kinetics study and pH variation. Bioresour. Technol. 2010, 101, 2965–2971. [Google Scholar] [CrossRef]
- Escobedo-Morales, G.; Hernandez-Beltran, J.U.; Nagamani, B.; Hernandez-Almanza, A.Y.; Luevanos-Escareno, M.P. Immobilized enzymes and cell systems: An approach to the removal of phenol and the challenges to incorporate nanoparticle-based technology. World J. Microbiol. Biotechnol. 2022, 38, 42. [Google Scholar] [CrossRef]
- Li, N.; Jiang, J.; Chen, D.; Xu, Q.; Li, H.; Lu, J. A reusable immobilization matrix for the biodegradation of phenol at 5000 mg/L. Sci. Rep. 2015, 5, 8628. [Google Scholar] [CrossRef] [PubMed]
- Kotresha, D.; Vidyasagar, G.M. Phenol degradation in a packed bed reactor by immobilized cells of Pseudomonas aeruginosa MTCC 4997. Biocatal. Agric. Biotechnol. 2017, 10, 386–389. [Google Scholar] [CrossRef]
- Dong, R.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Removal of phenol from aqueous solution using acid-modified Pseudomonas putida-sepiolite/ZIF-8 bio-nanocomposites. Chemosphere 2020, 239, 124708. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Env. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Papazi, A.; Assimakopoulos, K.; Kotzabasis, K. Bioenergetic strategy for the biodegradation of p-cresol by the unicellular green alga Scenedesmus obliquus. PLoS ONE 2012, 7, e51852. [Google Scholar] [CrossRef]
- Al-Khalid, T.; El-Naas, M.H. Aerobic Biodegradation of Phenols: A Comprehensive Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1631–1690. [Google Scholar] [CrossRef]
- Osundeko, O.; Dean, A.P.; Davies, H.; Pittman, J.K. Acclimation of microalgae to wastewater environments involves increased oxidative stress tolerance activity. Plant Cell Physiol. 2014, 55, 1848–1857. [Google Scholar] [CrossRef]
- Papry, R.I.; Miah, S.; Hasegawa, H. Integrated environmental factor-dependent growth and arsenic biotransformation by aquatic microalgae: A review. Chemosphere 2022, 303, 135164. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, L.X.; Liu, Y.S.; Zhao, J.L.; He, L.Y.; Ying, G.G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Env. Int. 2021, 155, 106594. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Shang, Q.; Guo, Y. Copper doping and organic sensitization enhance photocatalytic activity of titanium dioxide: Efficient degradation of phenol and tetrabromobisphenol A. Sci. Total Env. 2020, 716, 137144. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Wang, C.Y. K4Nb6O17/Fe3N/alpha-Fe2O3/C3N4 as an enhanced visible light-driven quaternary photocatalyst for acetamiprid photodegradation, CO2 reduction, and cancer cells treatment. Appl. Surf. Sci. 2021, 544, 148939. [Google Scholar] [CrossRef]
- Padervand, M.; Heidarpour, H.; Goshadehzehn, M.; Hajiahmadi, S. Photocatalytic degradation of 3-methyl-4-nitrophenol over Ag/AgCl-decoratecli[MOYI]-coated/ZnO nanostructures: Material characterization, photocatalytic performance, and in-vivo toxicity assessment of the photoproducts. Env. Technol. Innov. 2021, 21, 101212. [Google Scholar]
- Padervand, M.; Rhimi, B.; Wang, C.Y. One-pot synthesis of novel ternary Fe3N/Fe2O3/C3N4 photocatalyst for efficient removal of rhodamine B and CO2 reduction. J. Alloy. Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Saqib, N.U.; Adnan, R.; Shah, I. A mini-review on rare earth metal-doped TiO2 for photocatalytic remediation of wastewater. Env. Sci. Pollut. Res. Int. 2016, 23, 15941–15951. [Google Scholar] [CrossRef]
- Smirnova, N.; Petrik, I.; Vorobets, V.; Kolbasov, G.; Eremenko, A. Sol-gel Synthesis, Photo- and Electrocatalytic Properties of Mesoporous TiO2 Modified with Transition Metal Ions. Nanoscale Res. Lett. 2017, 12, 239. [Google Scholar] [CrossRef]
- An, N.; Ma, Y.; Liu, J.; Ma, H.; Yang, J.; Zhang, Q. Enhanced visible-light photocatalytic oxidation capability of carbon-doped TiO2 via coupling with fly ash. Chin. J. Catal. 2018, 39, 1890–1900. [Google Scholar] [CrossRef]
- Pan, X.; Dong, W.; Zhang, J.; Xie, Z.; Li, W.; Zhang, H.; Zhang, X.; Chen, P.; Zhou, W.; Lei, B. TiO2/Chlorophyll S-Scheme Composite Photocatalyst with Improved Photocatalytic Bactericidal Performance. ACS Appl. Mater. Interfaces 2021, 13, 39446–39457. [Google Scholar] [CrossRef]
- Pan, X.; Li, D.; Fang, Y.; Liang, Z.; Zhang, H.; Zhang, J.Z.; Lei, B.; Song, S. Enhanced Photogenerated Electron Transfer in a Semiartificial Photosynthesis System Based on Highly Dispersed Titanium Oxide Nanoparticles. J. Phys. Chem. Lett. 2020, 11, 1822–1827. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, D.; Zhang, L.; Li, L.; Sun, Q.; Zhang, Y.; Li, J.; Jiang, Z. Biomimetic synthesis of titania nanoparticles induced by protamine. Dalton Trans. 2008, 31, 4165–4171. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Guo, J.; Lu, C.; Li, H.; Song, Y.; Han, Y.; Hou, Y. Effect and mechanism of cyclodextrins on nitrate reduction and bio-activity by S.oneidensis.MR-1. Bioresour. Technol. 2020, 317, 124002. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; Patil, M.K.; Nadagouda, M.N.; Dionysiou, D.D. Hydrothermal synthesis of photoactive nitrogen-and boron-codoped TiO2 nanoparticles for the treatment of bisphenol A in wastewater: Synthesis, photocatalytic activity, degradation byproducts and reaction pathways. Appl. Catal. B Environ. 2019, 241, 598–611. [Google Scholar] [CrossRef]
- Courvoisier, E.; Williams, P.A.; Lim, G.K.; Hughes, C.E.; Harris, K.D. The crystal structure of L-arginine. Chem. Commun. 2012, 48, 2761–2763. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hessou, E.P.; Colombo, E.; Belletti, G.; Moussadik, A.; Lucas, I.T.; Frochot, V.; Daudon, M.; Rouziere, S.; Bazin, D.; et al. Crystalline structures of L-cysteine and L-cystine: A combined theoretical and experimental characterization. Amino Acids 2022, 54, 1123–1133. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.H.; Jin, P.K. Photocatalytic activity and mechanism of bisphenol a removal over TiO2−x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, S.; Wang, L.; Xiao, H. Black NiO-TiO2 nanorods for solar photocatalysis: Recognition of electronic structure and reaction mechanism. Appl. Catal. B Environ. 2018, 224, 705–714. [Google Scholar] [CrossRef]
- Duan, P.; Qi, Y.; Feng, S.; Peng, X.; Wang, W.; Yue, Y.; Shang, Y.; Li, Y.; Gao, B.; Xu, X. Enhanced degradation of clothianidin in peroxymonosulfate/catalyst system via core-shell FeMn @ N-C and phosphate surrounding. Appl. Catal. B Environ. 2020, 267, 118717. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, H.; Buchanan, I.; Mohammed, A.; Liu, Y. Comparison of extracellular polymeric substance (EPS) in nitrification and nitritation bioreactors. Int. Biodeterior. Biodegrad. 2019, 143, 104713. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Guo, J.; Lu, C.; Song, Y.; Han, Y.; Li, H.; Hou, Y. Enhanced denitrification performance and biocatalysis mechanisms of polyoxometalates as environmentally-friendly inorganic redox mediators. Bioresour. Technol. 2019, 291, 121816. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Ng, H.Y. Aerobic granular sludge systems for treating hypersaline pharmaceutical wastewater: Start-up, long-term performances and metabolic function. J. Hazard. Mater. 2021, 412, 125229. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, C.; Lian, J.; Lu, C.; Chen, Z.; Song, Y.; Guo, Y.; Xing, Y. Effect of thiosulfate on rapid start-up of sulfur-based reduction of high concentrated perchlorate: A study of kinetics, extracellular polymeric substances (EPS) and bacterial community structure. Bioresour. Technol. 2017, 243, 932–940. [Google Scholar] [CrossRef]
- Shi, W.; Guo, J.; Lu, C.; Chen, Z.; Li, H.; Song, Y.; Han, Y.; Hou, Y. Non-quinone redox mediators enhanced perchlorate bioreduction: Effect, structure-activity relationship and mechanism. Chem. Eng. J. 2021, 420, 127604. [Google Scholar] [CrossRef]
- Jang, H.J.; Nde, C.; Toghrol, F.; Bentley, W.E. Global transcriptome analysis of the Mycobacterium bovis BCG response to sodium hypochlorite. Appl. Microbiol. Biotechnol. 2009, 85, 127–140. [Google Scholar] [CrossRef]
- Chen, S.; Shi, N.; Huang, M.; Tan, X.; Yan, X.; Wang, A.; Huang, Y.; Ji, R.; Zhou, D.; Zhu, Y.G.; et al. MoS2 Nanosheets-Cyanobacteria Interaction: Reprogrammed Carbon and Nitrogen Metabolism. ACS Nano 2021, 15, 16344–16356. [Google Scholar] [CrossRef]
- Yang, F.; Liu, C.; Gao, F.; Su, M.; Wu, X.; Zheng, L.; Hong, F.; Yang, P. The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol. Trace Elem. Res. 2007, 119, 77–88. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Feng, Q.; Sun, Y.; Li, A.; Lin, X.; Lu, T.; Ding, D.; Shi, M.; Sun, Y.; Yuan, Y. Revealing dual roles of g-C3N4 in Chlorella vulgaris cultivation. J. Hazard. Mater. 2022, 424, 127639. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Guo, J.; Zhang, J.; Hou, Y.; Song, Y.; Lu, C.; Li, H.; Zhong, Y. New insights of simultaneous partial nitritation, anammox and denitrification (SNAD) system to Zn(II) exposure: Focus on affecting the regulation of quorum sensing on extracellular electron transfer and microbial metabolism. Bioresour. Technol. 2022, 346, 126602. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Lu, C.; Guo, J.; Li, H.; Song, Y.; Han, Y.; Hou, Y. Effect and ameliorative mechanisms of polyoxometalates on the denitrification under sulfonamide antibiotics stress. Bioresour. Technol. 2020, 305, 123073. [Google Scholar] [CrossRef]
- Ye, J.; Hu, A.; Ren, G.; Zhou, T.; Zhang, G.; Zhou, S. Red mud enhances methanogenesis with the simultaneous improvement of hydrolysis-acidification and electrical conductivity. Bioresour. Technol. 2018, 247, 131–137. [Google Scholar] [CrossRef]
- He, Y.; Guo, J.; Song, Y.; Chen, Z.; Lu, C.; Han, Y.; Li, H.; Hou, Y.; Zhao, R. Acceleration mechanism of bioavailable Fe() on Te(IV) bioreduction of Shewanella oneidensis MR-1: Promotion of electron generation, electron transfer and energy level. J. Hazard. Mater. 2021, 403, 123728. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Du, Y.; Guo, J.; Hou, Y.-N.; Song, Y.; Lu, C.; Han, Y.; Li, H. A light-switch for efficient decolorization of azo dye wastewater based on bacteria–semiconductor interaction. Environ. Sci. Nano 2022, 9, 1819–1830. [Google Scholar] [CrossRef]
- Grabowska, E.; Reszczynska, J.; Zaleska, A. Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef]
- Nazos, T.T.; Mavroudakis, L.; Pergantis, S.A.; Ghanotakis, D.F. Biodegradation of phenol by Chlamydomonas reinhardtii. Photosynth. Res. 2020, 144, 383–395. [Google Scholar] [CrossRef]
- Nazos, T.T.; Ghanotakis, D.F. Biodegradation of phenol by alginate immobilized Chlamydomonas reinhardtii cells. Arch. Microbiol. 2021, 203, 5805–5816. [Google Scholar] [CrossRef]
- Lindner, A.V.; Pleissner, D. Utilization of phenolic compounds by microalgae. Algal Res. 2019, 42, 101602. [Google Scholar] [CrossRef]
- Mishra, A.; Kavita, K.; Jha, B. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr. Polym. 2011, 83, 852–857. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, J.; Song, Y.; Chen, Z.; Lu, C.; Han, Y.; Li, H.; Hou, Y.; He, Y. Mediated electron transfer efficiencies of Se(IV) bioreduction facilitated by meso-tetrakis (4-sulfonatophenyl) porphyrin. Int. Biodeterior. Biodegrad. 2020, 147, 104838. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, M.; Lu, T.; Ding, D.; Sun, Y.; Yuan, Y. Bio-removal of PtCl62− complex by Galdieria sulphuraria. Sci. Total Environ. 2021, 796, 149021. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Gu, Y.J. Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells. Processes 2021, 9, 133. [Google Scholar] [CrossRef]
- Luo, L.; Luo, S.; Wang, H.; Hu, K.; Lin, X.; Liu, L.; Yan, B. Effect of nano-TiO2 on humic acid utilization from piggery biogas slurry by microalgae. Bioresour. Technol. 2021, 337, 125414. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Hsu, Y.-J. Au@Cu 7 S 4 yolk@shell nanocrystal-decorated TiO2 nanowires as an all-day-active photocatalyst for environmental purification. Nano Energy 2017, 31, 286–295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Guo, X.; Yang, H.; Zhang, D.; Jiang, X. Construction of Bio-TiO2/Algae Complex and Synergetic Mechanism of the Acceleration of Phenol Biodegradation. Materials 2023, 16, 3882. https://doi.org/10.3390/ma16103882
Guo J, Guo X, Yang H, Zhang D, Jiang X. Construction of Bio-TiO2/Algae Complex and Synergetic Mechanism of the Acceleration of Phenol Biodegradation. Materials. 2023; 16(10):3882. https://doi.org/10.3390/ma16103882
Chicago/Turabian StyleGuo, Jinxin, Xiaoman Guo, Haiyan Yang, Daohong Zhang, and Xiaogeng Jiang. 2023. "Construction of Bio-TiO2/Algae Complex and Synergetic Mechanism of the Acceleration of Phenol Biodegradation" Materials 16, no. 10: 3882. https://doi.org/10.3390/ma16103882
APA StyleGuo, J., Guo, X., Yang, H., Zhang, D., & Jiang, X. (2023). Construction of Bio-TiO2/Algae Complex and Synergetic Mechanism of the Acceleration of Phenol Biodegradation. Materials, 16(10), 3882. https://doi.org/10.3390/ma16103882







