Nanostructured TiO2 Arrays for Energy Storage
Abstract
1. Introduction
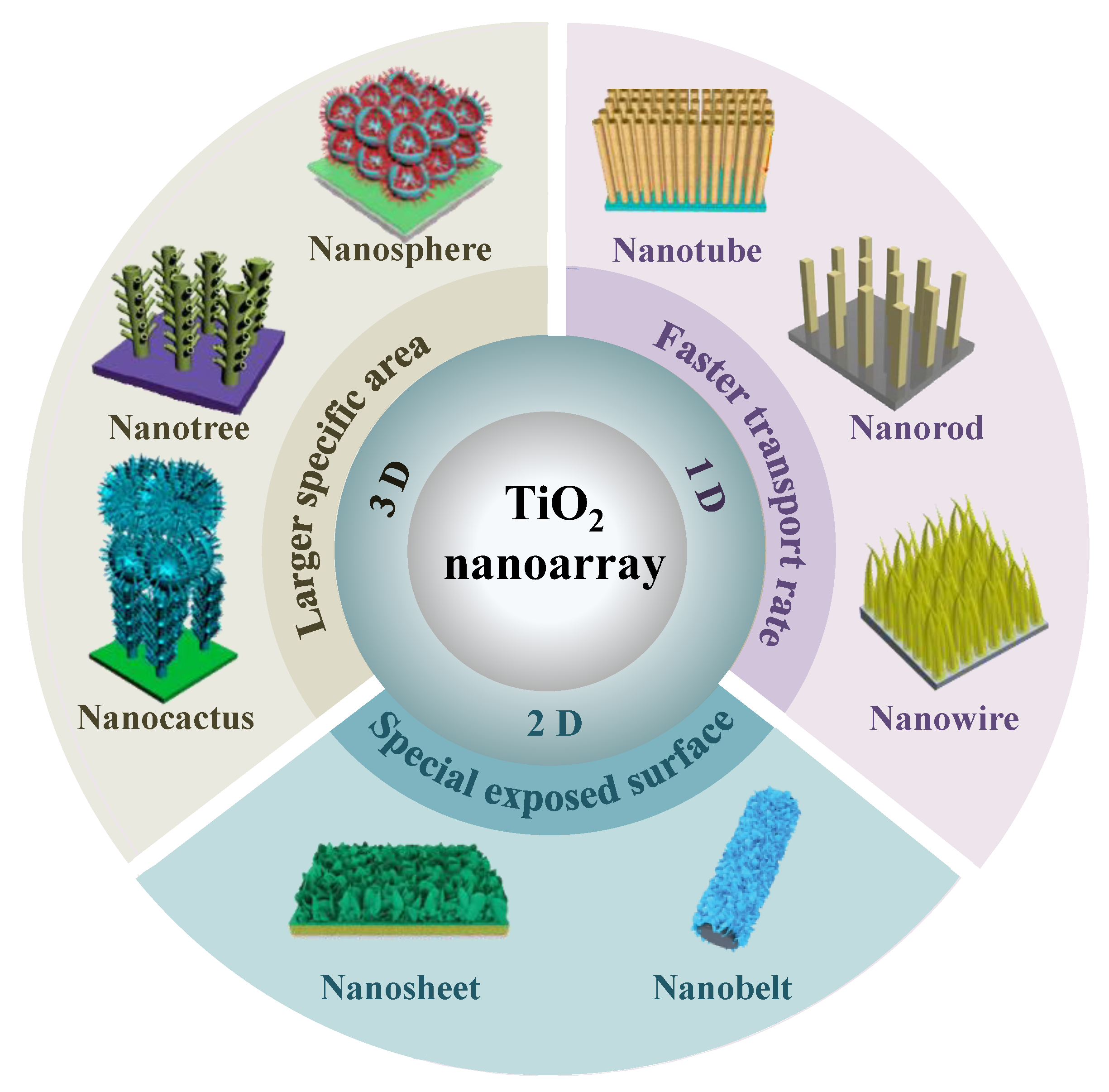
2. Morphology of Nanostructured TiO2 Nanoarrays
2.1. 1D Nanostructured Arrays
- (1)
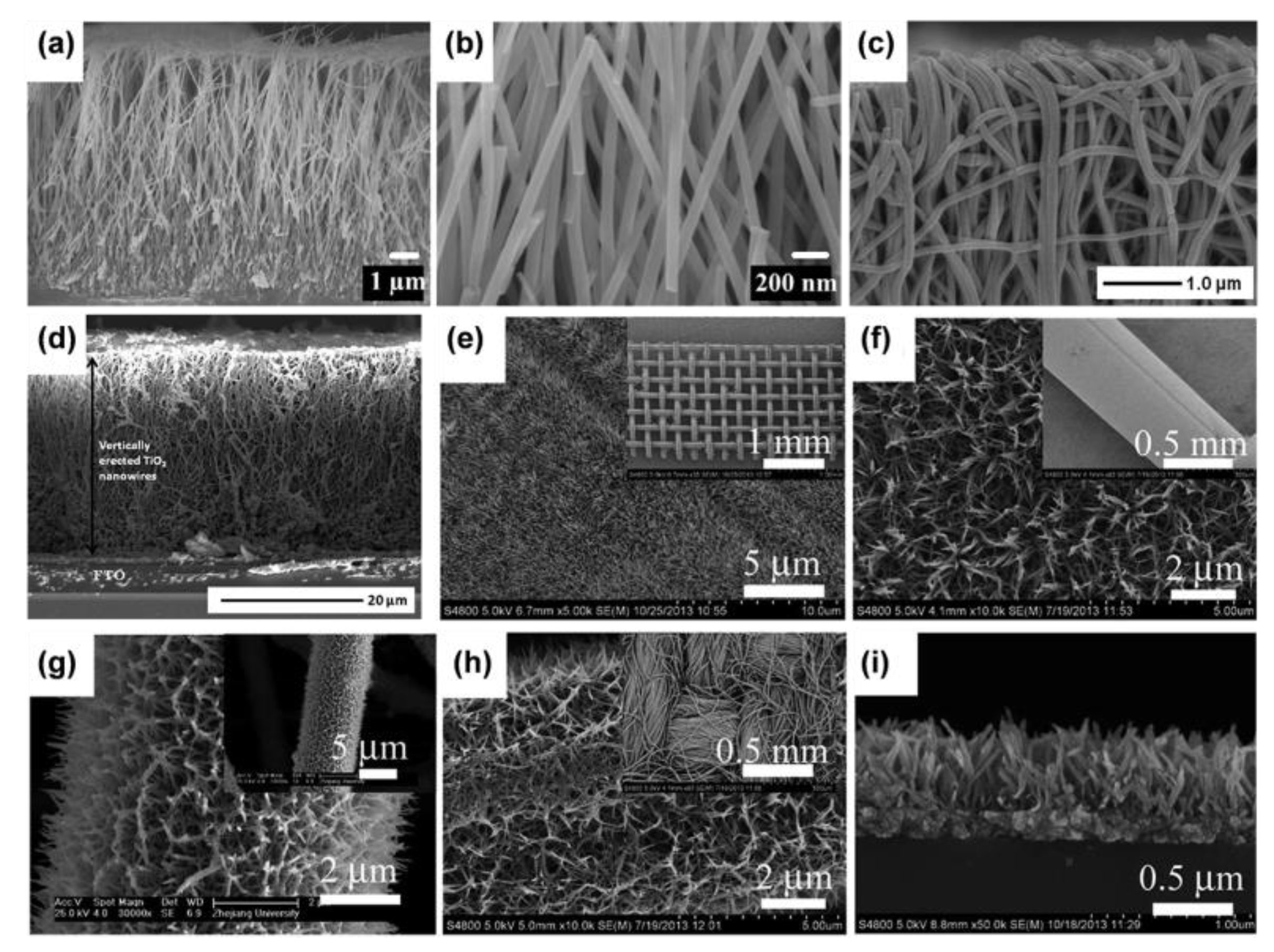
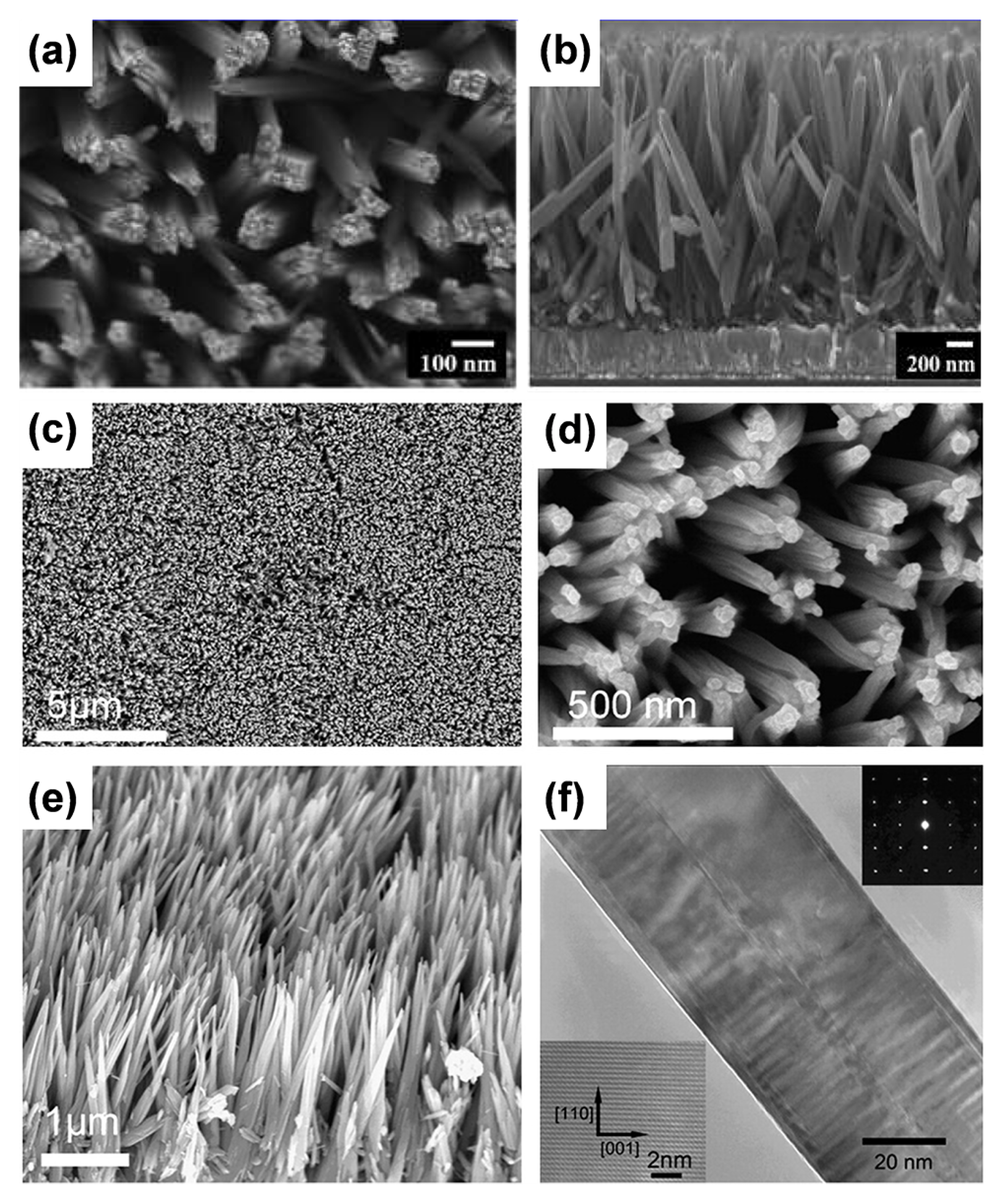
- Nanowire arrays
- (2)
- Nanorod arrays
- (3)
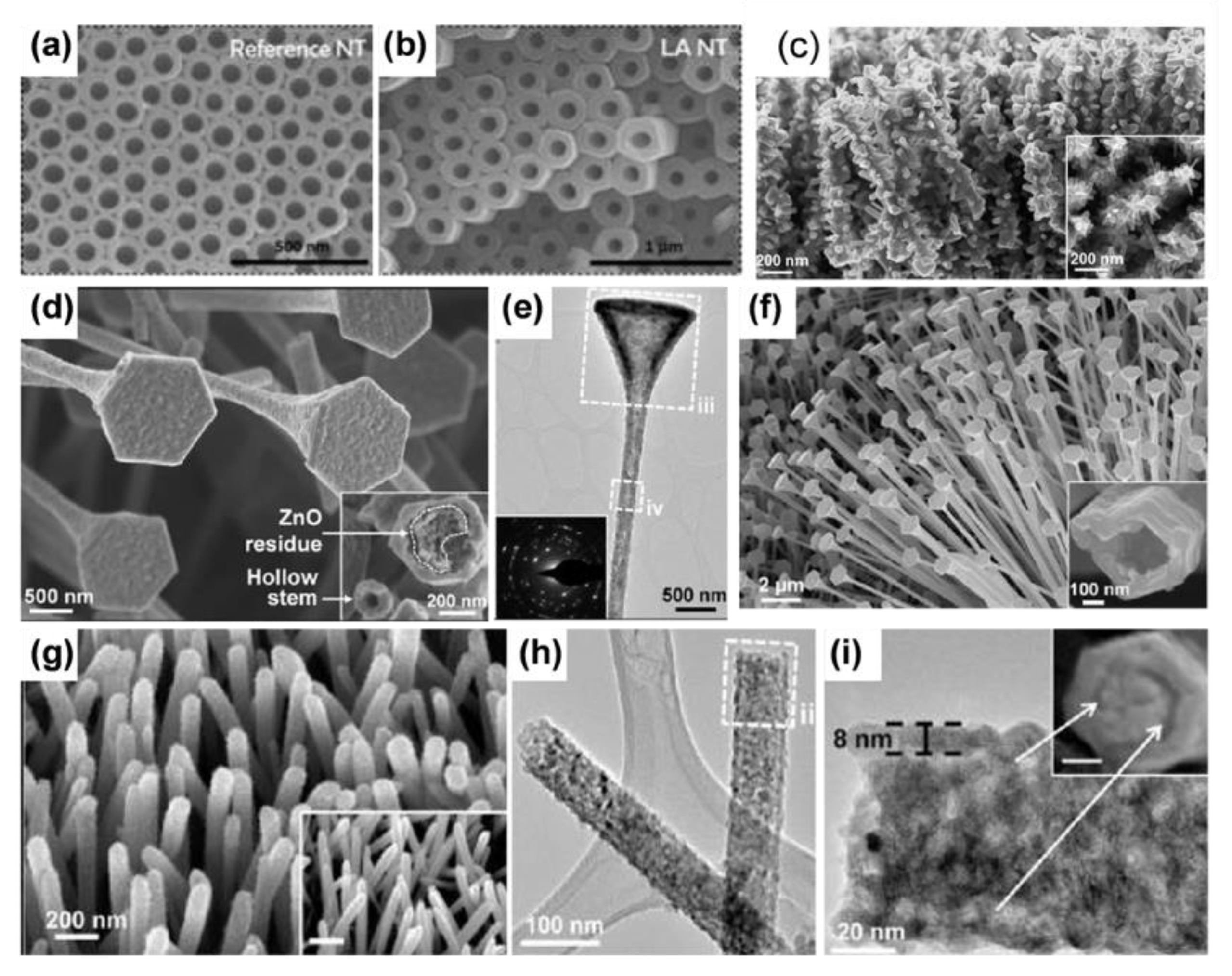
- Nanotube arrays
2.2. 2D Nanostructured Arrays
- (1)
- Nanosheets arrays
- (2)
- Nanobelt arrays
2.3. 3D Nanostructured Arrays
3. Energy Storage Applications of Nanostructured TiO2 Arrays
3.1. Lithium-Ion Batteries
3.2. Sodium-Ion Batteries
3.3. Supercapacitors
3.4. Other Batteries
4. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-Cleaning Applications of TiO2 by Photo-Induced Hydrophilicity and Photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured Titanium Oxide Hybrids-Based Electrochemical Biosensors for Healthcare Applications. Colloids Surf. B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Henderson, M.A. A Surface Science Perspective on TiO2 Photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Zhan, X.; Wu, J.; Wei, S.; Guo, Z. Advanced Titania Nanostructures and Composites for Lithium Ion Battery. J. Mater. Sci. 2012, 47, 2519–2534. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-Dimensional Titanium Dioxide Nanomaterials: Nanowires, Nanorods, and Nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and Lithium Electrochemical Reactivity of Lithium Titanites and Titanium Oxides: A Review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-Phase Synthesis of Titanium Dioxide Nanoparticles and Nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium Dioxide Crystals with Tailored Facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A Review of Photocatalysis Using Self-Organized TiO2 Nanotubes and Other Ordered Oxide Nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Feng, H.-L.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Recent Advances in Hierarchical Three-Dimensional Titanium Dioxide Nanotree Arrays for High-Performance Solar Cells. J. Mater. Chem. A 2017, 5, 12699–12717. [Google Scholar] [CrossRef]
- Cao, X.; Wu, J.-M.; Zhang, Z.; Qin, J.; Fu, Z.; Hai, J.; Wang, Z.; Ye, Z.; Wen, W. Electrochemical Lithium Intercalation in Titania Nanowire Arrays for Boosting Photocatalytic Activity. J. Electron. Mater. 2023, 52, 23–30. [Google Scholar] [CrossRef]
- Wu, J.-M.; Zhang, T.-W.; Zeng, Y.-W.; Hayakawa, S.; Tsuru, K.; Osaka, A. Large-Scale Preparation of Ordered Titania Nanorods with Enhanced Photocatalytic Activity. Langmuir 2005, 21, 6995–7002. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-Dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Zhuo, Y.; Wu, C.; Han, S.; Chi, B.; Pu, J.; Jin, T.; Jian, L. Oriented Nanostructured Titanates Array from Low Concentration Alkaline Solution via Hydrothermal Process. J. Nanosci. Nanotechnol. 2011, 11, 2298–2304. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Geng, D.; Wang, J.; Zhang, L.; Andrew, T.L.; Arnold, M.S.; Wang, X. Development of Lead Iodide Perovskite Solar Cells Using Three-Dimensional Titanium Dioxide Nanowire Architectures. ACS Nano 2015, 9, 564–572. [Google Scholar] [CrossRef]
- Fang, D.; Huang, K.; Liu, S.; Li, Z. Electrochemical Properties of Ordered TiO2 Nanotube Loaded with Ag Nano-Particles for Lithium Anode Material. J. Alloys Compd. 2008, 464, L5–L9. [Google Scholar] [CrossRef]
- Yan, D.; Yu, C.; Zhang, X.; Li, J.; Li, J.; Lu, T.; Pan, L. Enhanced Electrochemical Performances of Anatase TiO2 Nanotubes by Synergetic Doping of Ni and N for Sodium-Ion Batteries. Electrochim. Acta 2017, 254, 130–139. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wu, J.; Shu, X.; Yu, C.; Cui, J.; Qin, Y.; Zhang, Y.; Ajayan, P.M.; Wu, Y. Remarkable Supercapacitive Performance of TiO2 Nanotube Arrays by Introduction of Oxygen Vacancies. Chem. Eng. J. 2017, 313, 1071–1081. [Google Scholar] [CrossRef]
- Lu, Z.; Yip, C.-T.; Wang, L.; Huang, H.; Zhou, L. Hydrogenated TiO2 Nanotube Arrays as High-Rate Anodes for Lithium-Ion Microbatteries. ChemPlusChem 2012, 77, 991–1000. [Google Scholar] [CrossRef]
- Bi, Z.; Paranthaman, M.P.; Menchhofer, P.A.; Dehoff, R.R.; Bridges, C.A.; Chi, M.; Guo, B.; Sun, X.-G.; Dai, S. Self-Organized Amorphous TiO2 Nanotube Arrays on Porous Ti Foam for Rechargeable Lithium and Sodium Ion Batteries. J. Power Sources 2013, 222, 461–466. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.-M.; Guo, T.-T.; Tang, M.-Z.; Wen, W. A Facile Solution Route to Deposit TiO2 Nanowire Arrays on Arbitrary Substrates. Nanoscale 2014, 6, 3046. [Google Scholar] [CrossRef]
- Lei, B.-X.; Zheng, X.-F.; Qiao, H.; Li, Y.; Wang, S.-N.; Huang, G.-L.; Sun, Z.-F. A Novel Hierarchical Homogeneous Nanoarchitecture of TiO2 Nanosheets Branched TiO2 Nanosheet Arrays for High Efficiency Dye-Sensitized Solar Cells. Electrochim. Acta 2014, 149, 264–270. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.; Wang, X.; Zhang, L.; Wang, D.; Fu, Z.; Lin, Y.; Xie, T. Branched Hierarchical Photoanode of Anatase TiO2 Nanotubes on Rutile TiO2 Nanorod Arrays for Efficient Quantum Dot-Sensitized Solar Cells. J. Mater. Chem. A 2015, 3, 4445–4452. [Google Scholar] [CrossRef]
- Zhou, Q.; Fang, Z.; Li, J.; Wang, M. Applications of TiO2 Nanotube Arrays in Environmental and Energy Fields: A Review. Microporous Mesoporous Mater. 2015, 202, 22–35. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of Oriented Single-Crystalline Rutile TiO2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, X.; Lin, G.; Peng, Y.; Chao, J.; Yi, L.; Huang, X.; Li, C.; Liao, W. Structure Engineering in Hexagonal Tungsten Trioxide/Oriented Titanium Dioxide Nanorods Arrays with High Performances for Multi-Color Electrochromic Energy Storage Device Applications. Chem. Eng. J. 2021, 420, 1385–8947. [Google Scholar] [CrossRef]
- Boercker, J.E.; Enache-Pommer, E.; Aydil, E.S. Growth Mechanism of Titanium Dioxide Nanowires for Dye-Sensitized Solar Cells. Nanotechnology 2008, 19, 095604. [Google Scholar] [CrossRef]
- Ji, Q.; Hou, Y.; Wei, S.; Liu, Y.; Du, P.; Luo, L.; Li, W.P. Excellent Energy Storage Performance in Bilayer Composites Combining Aligned TiO2 Nanoarray and Random TiO2 Nanowires with Poly(vinylidene fluoride). J. Phys. Chem. 2020, 124, 2864–2871. [Google Scholar] [CrossRef]
- Liu, B.; Boercker, J.E.; Aydil, E.S. Oriented Single Crystalline Titanium Dioxide Nanowires. Nanotechnology 2008, 19, 505604. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Lei, B.-X.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Oriented Hierarchical Single Crystalline Anatase TiO2 Nanowire Arrays on Ti-Foil Substrate for Efficient Flexible Dye-Sensitized Solar Cells. Energy Env. Sci 2012, 5, 5750–5757. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Lei, B.-X.; Luo, Q.-P.; Yu, X.-Y.; Wu, W.-Q.; Su, C.-Y.; Kuang, D.-B. Hierarchical TiO2 Flowers Built from TiO2 Nanotubes for Efficient Pt-Free Based Flexible Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2012, 14, 13175. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Xu, Y.-F.; Rao, H.-S.; Su, C.-Y.; Kuang, D.-B. A Double Layered TiO2 Photoanode Consisting of Hierarchical Flowers and Nanoparticles for High-Efficiency Dye-Sensitized Solar Cells. Nanoscale 2013, 5, 4362. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2–B Nanowires as Negative Electrodes for Rechargeable Lithium Batteries. J. Power Sources 2005, 146, 501–506. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Zhang, W.F. Preparation and Electrochemical Characterization of TiO2 Nanowires as an Electrode Material for Lithium-Ion Batteries. Electrochim. Acta 2008, 53, 7863–7868. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Thavasi, V.; Subodh, G.M.; Ramakrishna, S. A First Report on the Fabrication of Vertically Aligned Anatase TiO2 Nanowires by Electrospinning: Preferred Architecture for Nanostructured Solar Cells. Energy Environ. Sci. 2011, 4, 2807. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-Based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Feng, X.; Shankar, K.; Varghese, O.K.; Paulose, M.; Latempa, T.J.; Grimes, C.A. Vertically Aligned Single Crystal TiO2 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis Details and Applications. Nano Lett. 2008, 8, 3781–3786. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, X.; Guan, F.; Li, K.; Wang, D.; Chen, L.; Feng, X. Length-Independent Charge Transport of Well-Separated Single-Crystal TiO2 Long Nanowire Arrays. Chem. Sci. 2018, 9, 7400–7404. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zheng, D.; Ye, M.; Xiao, J.; Guo, W.; Lai, Y.; Sun, L.; Lin, C.; Zuo, J. Optimized Porous Rutile TiO2 Nanorod Arrays for Enhancing the Efficiency of Dye-Sensitized Solar Cells. Energy Environ. Sci. 2013, 6, 1615. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Lei, B.-X.; Wang, Y.-F.; Liu, J.-M.; Su, C.-Y.; Kuang, D.-B. Hydrothermal Fabrication of Quasi-One-Dimensional Single-Crystalline Anatase TiO2 Nanostructures on FTO Glass and Their Applications in Dye-Sensitized Solar Cells. Chem. Eur. J. 2011, 17, 1352–1357. [Google Scholar] [CrossRef]
- Albu, S.P.; Roy, P.; Virtanen, S.; Schmuki, P. Self-Organized TiO2 Nanotube Arrays: Critical Effects on Morphology and Growth. Isr. J. Chem. 2010, 50, 453–467. [Google Scholar] [CrossRef]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and Thermal Parameters for Rutile and Anatase. Acta Crystallogr. B 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Dong, S.; Wang, H.; Gu, L.; Zhou, X.; Liu, Z.; Han, P.; Wang, Y.; Chen, X.; Cui, G.; Chen, L. Rutile TiO2 Nanorod Arrays Directly Grown on Ti Foil Substrates towards Lithium–Ion Micro-Batteries. Thin Solid Films 2011, 519, 5978–5982. [Google Scholar] [CrossRef]
- Li, J.; Cao, K.; Li, Q.; Xu, D. Tetragonal Faceted-Nanorods of Anatase TiO2 with A Large Percentage of Active {100} Facets and Their Hierarchical Structure. CrystEngComm 2012, 14, 83–85. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent Progress in Design, Synthesis, and Applications of One-Dimensional TiO2 Nanostructured Surface Heterostructures: A Review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Yang, C.; Xiaoli, C. Titanium Dioxide Anode Materials for Lithium-ion Batteries. Prog. Chem. 2021, 33, 1249–1269. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core-Shell Structured Titanium Dioxide Nanomaterials for Solar Energy Utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef]
- Arul, R.; Dong, J.; Simpson, M.C.; Gao, W. LIPSS-Sticks: Laser Induced Double Self Organization Enhances the Broadband Light Absorption of TiO2 Nanotube Arrays. Adv. Photonics Res. 2021, 2, 2000133. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, W.; Gao, X.; Li, X. Sol–Gel Assisted ZnO Nanorod Array Template to Synthesize TiO2 Nanotube Arrays. Nanotechnology 2006, 17, 4695–4698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhuge, F.; Wang, Y.; Zhang, J.; Gan, L.; Zhou, X.; Li, H.; Zhai, T. Decorating Perovskite Quantum Dots in TiO2 Nanotubes Array for Broadband Response Photodetector. Adv. Funct. Mater. 2017, 27, 1703115. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 Nanotubes: Self-Organized Electrochemical Formation, Properties and Applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nguyen, N.T.; Özkan, S.; Schmuki, P. Anodic TiO2 Nanotube Layers: Why Does Self-Organized Growth Occur—A Mini Review. Electrochem. Commun. 2014, 46, 157–162. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Schmuki, P. High-Aspect-Ratio TiO2 Nanotubes by Anodization of Titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Jiang, H.B.; Cuan, Q.; Wen, C.Z.; Xing, J.; Wu, D.; Gong, X.Q.; Li, C.; Yang, H.G. Anatase TiO2 Crystals with Exposed High-Index Facets. Angew. Chem. Int. Ed. Engl. 2011, 50, 3764–3768. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef]
- Bauer, S.; Kleber, S.; Schmuki, P. TiO2 Nanotubes: Tailoring the Geometry in H3PO4/HF Electrolytes. Electrochem. Commun. 2006, 8, 1321–1325. [Google Scholar] [CrossRef]
- Lan, M.-Y.; Liu, C.-P.; Huang, H.-H.; Chang, J.-K.; Lee, S.-W. Diameter-Sensitive Biocompatibility of Anodic TiO2 Nanotubes Treated with Supercritical CO2 Fluid. Nanoscale Res. Lett. 2013, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 Μm Long Anodic TiO2 Nanotubes with Hexagonal Self-Ordering. Phys. Status Solidi RRL—Rapid Res. Lett. 2007, 1, R65–R67. [Google Scholar] [CrossRef]
- Ni, J.; Noh, K.; Frandsen, C.J.; Kong, S.D.; He, G.; Tang, T.; Jin, S. Preparation of near Micrometer-Sized TiO2 Nanotube Arrays by High Voltage Anodization. Mater. Sci. Eng. C 2013, 33, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Schmuki, P. TiO2 Nanotubes Grown in Different Organic Electrolytes: Two-Size Self-Organization, Single vs. Double-Walled Tubes, and Giant Diameters. Phys. Status Solidi RRL—Rapid Res. Lett. 2010, 4, 215–217. [Google Scholar] [CrossRef]
- Enachi, M.; Tiginyanu, I.; Sprincean, V.; Ursaki, V. Self-Organized Nucleation Layer for the Formation of Ordered Arrays of Double-Walled TiO2 Nanotubes with Temperature Controlled Inner Diameter. Phys. Status Solidi RRL—Rapid Res. Lett. 2010, 4, 100–102. [Google Scholar] [CrossRef]
- Ruff, T.; Hahn, R.; Schmuki, P. From Anodic TiO2 Nanotubes to Hexagonally Ordered TiO2 Nanocolumns. Appl. Surf. Sci. 2011, 257, 8177–8181. [Google Scholar] [CrossRef]
- Banerjee, S.; Misra, M.; Mohapatra, S.K.; Howard, C.; Mohapatra, S.K.; Kamilla, S.K. Formation of Chelating Agent Driven Anodized TiO2 Nanotubular Membrane and Its Photovoltaic Application. Nanotechnology 2010, 21, 145201. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ultrafast Growth of Highly Ordered Anodic TiO2 Nanotubes in Lactic Acid Electrolytes. J. Am. Chem. Soc. 2012, 134, 11316–11318. [Google Scholar] [CrossRef]
- Ali, G.; Kim, H.J.; Kim, J.J.; Cho, S.O. Controlled Fabrication of Porous Double-Walled TiO2 Nanotubes Via Ultraviolet-Assisted Anodization. Nanoscale 2014, 6, 3632–3637. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverría, F.E. TiO2 Nanotubes Produced on Curved Titanium Surfaces Using Aqueous Electrolytes with Carboxymethyl Cellulose. Physica E 2021, 125, 114391. [Google Scholar] [CrossRef]
- Sander, M.S.; Côté, M.J.; Gu, W.; Kile, B.M.; Tripp, C.P. Template-Assisted Fabrication of Dense, Aligned Arrays of Titania Nanotubes with Well-Controlled Dimensions on Substrates. Adv. Mater. 2004, 16, 2052–2057. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania Nanotubes Prepared by Chemical Processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshikawa, S. Synthesis and Thermal Analyses of TiO2-Derived Nanotubes Prepared by the Hydrothermal Method. J. Mater. Res. 2004, 19, 982–985. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Harada, M.; Yoshikawa, S. Formation of Titania Nanotubes with High Photo-Catalytic Activity. Chem. Lett. 2000, 29, 942–943. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Okada, I.; Yoshikawa, S. Formation of Titania Nanotubes and Applications for Dye-Sensitized Solar Cells. J. Electrochem. Soc. 2003, 150, G488. [Google Scholar] [CrossRef]
- Hoyer, P. Formation of a Titanium Dioxide Nanotube Array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Chakarvarti, S.K.; Vetter, J. Template Synthesisda Membrane Based Technology for Generation of Nano-/Micro Materials: A Review. Radiat. Meas. 1998, 29, 149–159. [Google Scholar] [CrossRef]
- Heo, S.Y.; Kim, D.J.; Jeon, H.; Jung, B.; Kang, Y.S.; Kim, J.H. Vertically Aligned Anatase TiO2 Nanotubes on Transparent Conducting Substrates Using Polycarbonate Membranes. RSC Adv. 2013, 3, 13681. [Google Scholar] [CrossRef]
- Zhang, M.; Bando, Y.; Wada, K. Sol-Gel Template Preparation of TiO2 Nanotubes and Nanorods. J. Mater. Sci. Lett. 2001, 20, 167–170. [Google Scholar] [CrossRef]
- Lakshmi, B.B.; Dorhout, P.K.; Martin, C.R. Sol−Gel Template Synthesis of Semiconductor Nanostructures. Chem. Mater. 1997, 9, 857–862. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, X.; Kvit, A.; Wang, X. Evolution of Hollow TiO2 Nanostructures via the Kirkendall Effect Driven by Cation Exchange with Enhanced Photoelectrochemical Performance. Nano Lett. 2014, 14, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Song, T.; Lee, E.-K.; Devadoss, A.; Jeon, Y.; Ha, J.; Chung, Y.-C.; Choi, Y.-M.; Jung, Y.-G.; Paik, U. Dominant Factors Governing the Rate Capability of a TiO2 Nanotube Anode for High Power Lithium Ion Batteries. ACS Nano 2012, 6, 8308–8315. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Chang, C.; Chen, W.; Chen, Y.; Chen, Y.; Chen, Y.; Ding, F.; Fan, C.; Jin Fan, H.; Fan, Z.; Gong, C.; et al. Recent Progress on Two-Dimensional Materials. Acta Phys. Chim. Sin. 2021, 37, 2108017. [Google Scholar] [CrossRef]
- ten Elshof, J.E.; Yuan, H.; Gonzalez Rodriguez, P. Two-Dimensional Metal Oxide and Metal Hydroxide Nanosheets: Synthesis, Controlled Assembly and Applications in Energy Conversion and Storage. Adv. Energy Mater. 2016, 6, 1600355. [Google Scholar] [CrossRef]
- Xu, H.; Reunchan, P.; Ouyang, S.; Tong, H.; Umezawa, N.; Kako, T.; Ye, J. Anatase TiO2 Single Crystals Exposed with High-Reactive {111} Facets Toward Efficient H2 Evolution. Chem. Mater. 2013, 25, 405–411. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Zhou, C.; Wang, W. Anatase TiO2 Single Crystals with Exposed 001 and 110 Facets: Facile Synthesis and Enhanced Photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; He, D.; Yang, J.; Zhu, K.; Feng, X. Oriented Assembled TiO2 Hierarchical Nanowire Arrays with Fast Electron Transport Properties. Nano Lett. 2014, 14, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 Single Crystals with A Large Percentage of Reactive Facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Zhang, Y.; Jia, Y.; Yang, J.; Sun, P.; Liu, Y. Hydrothermal Growth of Layered Titanate Nanosheet Arrays on Titanium Foil and Their Topotactic Transformation to Heterostructured TiO2 Photocatalysts. J. Phys. Chem. C 2011, 115, 22276–22285. [Google Scholar] [CrossRef]
- Feng, S.; Yang, J.; Zhu, H.; Liu, M.; Zhang, J.; Wu, J.; Wan, J. Synthesis of Single Crystalline Anatase TiO2(001) Tetragonal Nanosheet-Array Films on Fluorine-Doped Tin Oxide Substrate: Rapid Communications of the American Ceramic Society. J. Am. Ceram. Soc. 2011, 94, 310–315. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and Crystal Chemistry of the Hybrid Perovskite (CH3NH3)PbI3 for Solid-State Sensitised Solar Cell Applications. J. Mater. Chem. A 2013, 1, 5628. [Google Scholar] [CrossRef]
- Jiang, C.; Wei, M.; Qi, Z.; Kudo, T.; Honma, I.; Zhou, H. Particle Size Dependence of the Lithium Storage Capability and High Rate Performance of Nanocrystalline Anatase TiO2 Electrode. J. Power Sources 2007, 166, 239–243. [Google Scholar] [CrossRef]
- Zhong, D.; Jiang, Q.; Huang, B.; Zhang, W.-H.; Li, C. Synthesis and Characterization of Anatase TiO2 Nanosheet Arrays on FTO Substrate. J. Energy Chem. 2015, 24, 626–631. [Google Scholar] [CrossRef]
- Gan, X.; Gao, X.; Qiu, J.; He, P.; Li, X.; Xiao, X. TiO2 Nanorod-Derived Synthesis of Upstanding Hexagonal Kassite Nanosheet Arrays: An Intermediate Route to Novel Nanoporous TiO2 Nanosheet Arrays. Cryst. Growth Des. 2012, 12, 289–296. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.; Jiang, Y.; Yu, S.; Bai, J.; Cao, M.; Cui, J. Anatase TiO2 Ultrathin Nanobelts Derived from Room-Temperature-Synthesized Titanates for Fast and Safe Lithium Storage. Sci. Rep. 2015, 5, 11804. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, D.; Luo, J.; Chen, S.; Zhang, D.; Qiu, K.; Qi, X.; Zhang, H.; Li, C.M.; Yu, T. Hierarchical TiO2 Nanobelts@MnO2 Ultrathin Nanoflakes Core–Shell Array Electrode Materials for Supercapacitors. RSC Adv. 2013, 3, 14413. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, J.; Zhou, W.; Qi, X.; Zhang, H.; Yu, D.Y.W.; Li, C.M.; Fan, H.J.; Yu, T. Controlled Synthesis of Hierarchical Graphene-Wrapped TiO2 @Co3O4 Coaxial Nanobelt Arrays for High-Performance Lithium Storage. J. Mater. Chem. A 2013, 1, 273–281. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Deng, C.; Yan, M.; Shi, W. MOF-Derived 3D Hierarchical Nanoarrays Consisting of NiCoZn-S Nanosheets Coupled with Granular NiCo2S4 Nanowires for High-Performance Hybrid Supercapacitors. J. Mater. Chem. A 2019, 7, 26131–26138. [Google Scholar] [CrossRef]
- Luo, W.; Zeng, W.; Quan, H.; Pan, M.; Wang, Y.; Chen, D. Carbon Dots Decorated NiCo Hydroxycarbonate Hierarchical Nanoarrays on Carbon Cloth with High Areal Capacitance as Pseudocapacitor Electrode. J. Alloys Compd. 2021, 868, 159048. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Meng, L.; Wang, L.; Liu, Y.; Li, W.; Sun, W.; Li, G. Morphology Engineering of Nickel Molybdate Hydrate Nanoarray for Electrocatalytic Overall Water Splitting: From Nanorod to Nanosheet. RSC Adv. 2018, 8, 35131–35138. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yan, L.; Huang, H.; Yan, F.; Liang, X.; Xu, S.; Lan, Z.; Zhou, W.; Guo, J. The Hetero-Structured Nanoarray Construction of Co3O4 Nanowires Anchored on Nanoflakes as a High-Performance Electrode for Supercapacitors. Appl. Surf. Sci. 2021, 538, 147932. [Google Scholar] [CrossRef]
- Pan, Z.; Qiu, Y.; Yang, J.; Liu, M.; Zhou, L.; Xu, Y.; Sheng, L.; Zhao, X.; Zhang, Y. Synthesis of Three-Dimensional Hyperbranched TiO2 Nanowire Arrays with Significantly Enhanced Photoelectrochemical Hydrogen Production. J. Mater. Chem. A 2015, 3, 4004–4009. [Google Scholar] [CrossRef]
- Yang, X.; Zhuang, J.; Li, X.; Chen, D.; Ouyang, G.; Mao, Z.; Han, Y.; He, Z.; Liang, C.; Wu, M.; et al. Hierarchically Nanostructured Rutile Arrays: Acid Vapor Oxidation Growth and Tunable Morphologies. ACS Nano 2009, 3, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hai, J.; Wu, J.-M.; Kobayashi, H.; Sun, T.; Zhang, Z.; Geng, C.; Zhang, Z.; Wen, W. Surface Diffusion Enhancement of Titania by Surface Fluorine Modification to Boost Energy Storage Capacities. Appl. Surf. Sci. 2023, 612, 155843. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 Nanorods for Photoelectrochemical Hydrogen Production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Li, L.; Su, J.; Tu, F.; Liu, N.; Gao, Y. A General Method for Preparing Anatase TiO2 Treelike-Nanoarrays on Various Metal Wires for Fiber Dye-Sensitized Solar Cells. Sci. Rep. 2014, 4, 4420. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Rao, H.-S.; Feng, H.-L.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A Family of Vertically Aligned Nanowires with Smooth, Hierarchical and Hyperbranched Architectures for Efficient Energy Conversion. Nano Energy 2014, 9, 15–24. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Feng, H.-L.; Rao, H.-S.; Xu, Y.-F.; Kuang, D.-B.; Su, C.-Y. Maximizing Omnidirectional Light Harvesting in Metal Oxide Hyperbranched Array Architectures. Nat. Commun. 2014, 5, 3968. [Google Scholar] [CrossRef]
- Geng, C.; Sun, T.; Wang, Z.; Wu, J.-M.; Gu, Y.-J.; Kobayashi, H.; Yang, P.; Hai, J.; Wen, W. Surface-Induced Desolvation of Hydronium Ion Enables Anatase TiO2 as an Efficient Anode for Proton Batteries. Nano Lett. 2021, 21, 7021–7029. [Google Scholar] [CrossRef]
- Wen, W.; Yao, J.-C.; Gu, Y.-J.; Sun, T.-L.; Tian, H.; Zhou, Q.-L.; Wu, J.-M. Balsam-Pear-like Rutile/Anatase Core/Shell Titania Nanorod Arrays for Photoelectrochemical Water Splitting. Nanotechnology 2017, 28, 465602. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.K.; Chi, W.S.; Jeon, H.; Kim, S.J.; Kim, J.H. High Efficiency Solid-State Dye-Sensitized Solar Cells Assembled with Hierarchical Anatase Pine Tree-like TiO2 Nanotubes. Adv. Funct. Mater. 2014, 24, 379–386. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.-M.; Jiang, Y.-Z.; Lai, L.-L.; Song, J. Pseudocapacitance-Enhanced Li-Ion Microbatteries Derived by a TiN@TiO2 Nanowire Anode. Chem 2017, 2, 404–416. [Google Scholar] [CrossRef]
- Qin, J.; Cao, X.; Huang, H.; Fu, Z.; Wu, J.-M.; Zhang, P.; Ye, Z.; Wen, W. Modulation of Titania Nanoflower Arrays Transformed from Titanate Nanowire Arrays to Boost Photocatalytic Cr(ⅤⅠ) Detoxification. New J. Chem. 2022, 46, 21599–21604. [Google Scholar] [CrossRef]
- Wen, W.; Hai, J.; Yao, J.; Gu, Y.-J.; Kobayashi, H.; Tian, H.; Sun, T.; Chen, Q.; Yang, P.; Geng, C.; et al. Univariate Lattice Parameter Modulation of Single-Crystal-like Anatase TiO2 Hierarchical Nanowire Arrays to Improve Photoactivity. Chem. Mater. 2021, 33, 1489–1497. [Google Scholar] [CrossRef]
- Potapenko, D.V.; Li, Z.; Kysar, J.W.; Osgood, R.M. Nanoscale Strain Engineering on The Surface of A Bulk TiO2 Crystal. Nano Lett 2014, 14, 6185–6189. [Google Scholar] [CrossRef]
- Wen, W.; Yao, J.; Tan, H.; Wu, J.-M. Hollow TiN Nanotrees Derived from a Surface-Induced Kirkendall Effect and Their Application in High-Power Supercapacitors. J. Mater. Chem. A 2019, 7, 21378–21385. [Google Scholar] [CrossRef]
- Che, G.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R. Carbon Nanotubule Membranes for Electrochemical Energy Storage and Production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Nishizawa, M.; Mukai, K.; Kuwabata, S.; Martin, C.R.; Yoneyama, H. Template Synthesis of Polypyrrole-Coated Spinel LiMn2O4 Nanotubules and Their Properties as Cathode Active Materials for Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1923–1927. [Google Scholar] [CrossRef]
- Sides, C.R.; Martin, C.R. Nanostructured Electrodes and the Low-Temperature Performance of Li-Ion Batteries. Adv. Mater. 2005, 17, 125–128. [Google Scholar] [CrossRef]
- Wei, T.-T.; Wang, F.-F.; Li, X.-Z.; Zhang, J.-H.; Zhu, Y.-R.; Yi, T.-F. Towards High-Performance Battery Systems By Regulating Morphology of TiO2 Materials. Sustain. Mater. Technol. 2021, 30, 2214–2232. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 Nanowire Arrays for Lithium Ion Batteries with High Capacity and Rate Capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Zhang, X.F.; Cui, Y. High Capacity Li Ion Battery Anodes Using Ge Nanowires. Nano Lett. 2008, 8, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Wang, Y.-G.; Xia, Y.-Y. Ti-Based Compounds as Anode Materials for Li-Ion Batteries. Energy Environ. Sci. 2012, 5, 6652. [Google Scholar] [CrossRef]
- Sun, C.H.; Yang, X.H.; Chen, J.S.; Li, Z.; Lou, X.W.; Li, C.; Smith, S.C.; Lu, G.Q.; Yang, H.G. Higher Charge/Discharge Rates of Lithium-Ions across Engineered TiO2 Surfaces Leads to Enhanced Battery Performance. Chem. Commun. 2010, 46, 6129. [Google Scholar] [CrossRef]
- Nuspl, G.; Yoshizawa, K.; Yamabe, T. Lithium Intercalation in TiO2 Modifications. J. Mater. Chem. 1997, 7, 2529–2536. [Google Scholar] [CrossRef]
- Kavan, L.; Rathouský, J.; Grätzel, M.; Shklover, V.; Zukal, A. Surfactant-Templated TiO2(Anatase): Characteristic Features of Lithium Insertion Electrochemistry in Organized Nanostructures. J. Phys. Chem. B 2000, 104, 12012–12020. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, L.; Qiu, Z.; Huang, J. Template-Free Synthesis of Mesoporous Spinel Lithium Titanate Microspheres and Their Application in High-Rate Lithium Ion Batteries. J. Mater. Chem. 2009, 19, 5980. [Google Scholar] [CrossRef]
- Sudant, G.; Baudrin, E.; Larcher, D.; Tarascon, J.-M. Electrochemical Lithium Reactivity with Nanotextured Anatase-Type TiO2. J. Mater. Chem. 2005, 15, 1263–1269. [Google Scholar] [CrossRef]
- Kubiak, P.; Geserick, J.; Hüsing, N.; Wohlfahrt-Mehrens, M. Electrochemical Performance of Mesoporous TiO2 Anatase. J. Power Sources 2008, 175, 510–516. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Wark, M.; Brezesinski, T.; Smarsly, B.M.; Rathouský, J. Highly Organized Mesoporous TiO2 Films with Controlled Crystallinity: A Li-Insertion Study. Adv. Funct. Mater. 2007, 17, 123–132. [Google Scholar] [CrossRef]
- Gligor, F.; Deleeuw, S. Lithium Diffusion in Rutile Structured Titania. Solid State Ion. 2006, 177, 2741–2746. [Google Scholar] [CrossRef]
- Johnson, O.W. One-Dimensional Diffusion of Li in Rutile. Phys. Rev. 1964, 136, A284–A290. [Google Scholar] [CrossRef]
- Baudrin, E.; Cassaignon, S.; Koelsch, M.; Jolivet, J.; Dupont, L.; Tarascon, J. Structural Evolution during the Reaction of Li with Nano-Sized Rutile Type TiO2 at Room Temperature. Electrochem. Commun. 2007, 9, 337–342. [Google Scholar] [CrossRef]
- Jiang, C.; Honma, I.; Kudo, T.; Zhou, H. Nanocrystalline Rutile TiO2 Electrode for High-Capacity and High-Rate Lithium Storage. Electrochem. Solid-State Lett. 2007, 10, A127. [Google Scholar] [CrossRef]
- Reddy, M.A.; Kishore, M.S.; Pralong, V.; Caignaert, V.; Varadaraju, U.V.; Raveau, B. Room Temperature Synthesis and Li Insertion into Nanocrystalline Rutile TiO2. Electrochem. Commun. 2006, 8, 1299–1303. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, D.; Wang, H.; Wang, H. Electrochemical Properties of Rutile TiO2 Nanorod Array in Lithium Hydroxide Solution. Nanoscale Res. Lett. 2016, 11, 448. [Google Scholar] [CrossRef]
- Guan, D.; Cai, C.; Wang, Y. Amorphous and Crystalline TiO2 Nanotube Arrays for Enhanced Li-Ion Intercalation Properties. J. Nanosci. Nanotechnol. 2011, 11, 3641–3650. [Google Scholar] [CrossRef]
- Cava, B.R.J.; Murphy, D.W.; Zahurak, S. The Crystal Structures of the Lithium-Inserted Metal Oxides Li0.5TiO2 Anatase, LiTi2O4 Spinel, and Li2Ti2O4. J. Solid State Chem. 1984, 53, 64–75. [Google Scholar] [CrossRef]
- Yiping, T.; Xiaoxu, T.; Guangya, H.; Huazhen, C.; Guoqu, Z. Synthesis of Dense Nanocavities inside TiO2 Nanowire Array and Its Electrochemical Properties as a Three-Dimensional Anode Material for Li-Ion Batteries. Electrochim. Acta 2012, 78, 154–159. [Google Scholar] [CrossRef]
- Liu, S.; Jia, H.; Han, L.; Wang, J.; Gao, P.; Xu, D.; Yang, J.; Che, S. Nanosheet-Constructed Porous TiO2-B for Advanced Lithium Ion Batteries. Adv. Mater. 2012, 24, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Bui, V.K.H.; Lee, Y.C. Recent Advances in Hierarchical Anode Designs of TiO2-Bnanostructures for Lithium-ion Batteries. Int. J. Energy Res. 2021, 45, 17532–17562. [Google Scholar] [CrossRef]
- Liu, G.; Wu, H.-H.; Meng, Q.; Zhang, T.; Sun, D.; Jin, X.; Guo, D.; Wu, N.; Liu, X.; Kim, J.-K. Role of the anatase/TiO2(B) heterointerface for ultrastable high-rate lithium and sodium energy storage performance. Nanoscale Horiz. 2020, 5, 150–162. [Google Scholar] [CrossRef]
- Shen, W.; Huo, X.; Zhang, M.; Guo, M. Synthesis of Oriented Core/Shell Hexagonal Tungsten Oxide/Amorphous Titanium Dioxide Nanorod Arrays and its Electrochromic-Pseudocapacitive Properties. Appl. Surf. Sci. 2020, 515, 146034. [Google Scholar] [CrossRef]
- Huang, M.; Xi, B.; Shi, N.; Wei, R.; Li, H.; Feng, J.; Xiong, S. Systematic Study of Alkali Cations Intercalated Titanium Dioxide Effect on Sodium and Lithium Storage. Small 2020, 16, e2001391. [Google Scholar] [CrossRef]
- Liu, B.; Deng, D.; Lee, J.Y.; Aydil, E.S. Oriented Single-Crystalline TiO2 Nanowires on Titanium Foil for Lithium Ion Batteries. J. Mater. Res. 2010, 25, 1588–1594. [Google Scholar] [CrossRef]
- Tang, Y.; Hong, L.; Wu, Q.; Li, J.; Hou, G.; Cao, H.; Wu, L.; Zheng, G. TiO2(B) Nanowire Arrays on Ti Foil Substrate as Three-Dimensional Anode for Lithium-Ion Batteries. Electrochim. Acta 2016, 195, 27–33. [Google Scholar] [CrossRef]
- Fu, S.; Chen, J.; Wang, X.; He, Q.; Tong, S.; Wu, M. Free-Standing Crystalline@Amorphous Core-Shell Nanoarrays for Efficient Energy Storage. Small 2020, 16, e2000040. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Yang, X.; Lu, F.; He, S.; Yang, J.; Fan, H.J.; Wu, M. Ultrathin Anatase TiO2 Nanosheets Embedded with TiO2-B Nanodomains for Lithium-Ion Storage: Capacity Enhancement by Phase Boundaries. Adv. Energy Mater. 2015, 5, 1401756. [Google Scholar] [CrossRef]
- Wang, R.; Xue, X.; Lu, W.; Liu, H.; Lai, C.; Xi, K.; Che, Y.; Liu, J.; Guo, S.; Yang, D. Tuning and Understanding the Phase Interface of TiO2 Nanoparticles for More Efficient Lithium Ion Storage. Nanoscale 2015, 7, 12833–12838. [Google Scholar] [CrossRef] [PubMed]
- Zhukovskii, Y.F.; Balaya, P.; Kotomin, E.A.; Maier, J. Evidence for Interfacial-Storage Anomaly in Nanocomposites for Lithium Batteries from First-Principles Simulations. Phys. Rev. Lett. 2006, 96, 058302. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Wu, J.-M.; Jiang, Y.-Z.; Bai, J.-Q.; Lai, L.-L. Titanium Dioxide Nanotrees for High-Capacity Lithium-Ion Microbatteries. J. Mater. Chem. A 2016, 4, 10593–10600. [Google Scholar] [CrossRef]
- Cheah, S.K.; Perre, E.; Rooth, M.; Fondell, M.; Hårsta, A.; Nyholm, L.; Boman, M.; Gustafsson, T.r.; Lu, J.; Simon, P. Self-Supported Three-Dimensional Nanoelectrodes for Microbattery Applications. Nano Lett. 2009, 9, 3230–3233. [Google Scholar] [CrossRef]
- Wang, W.; Tian, M.; Abdulagatov, A.; George, S.M.; Lee, Y.C.; Yang, R. Three-Dimensional Ni/TiO2 Nanowire Network for High Areal Capacity Lithium Ion Microbattery Applications. Nano Lett 2012, 12, 655–660. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Xie, G.; Zheng, Y.; Yu, J.; Gao, L.; Liu, B. Construction of Rutile-TiO2 Nanoarray Homojuction for Non-Contact Sensing of TATP Under Natural Light. Coat. 2020, 10, 409. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, I.; Joo, J.B.; Zaera, F.; Yin, Y. Core–Shell Nanostructured Catalysts. Acc. Chem. Res. 2013, 46, 1816–1824. [Google Scholar] [CrossRef]
- Liu, R.; Shen, Z.; Wan, Z.; Zhu, L.; Chen, J.; Dong, C.; Chen, W.; Cao, W.; Chen, B.; Yuan, X.; et al. Nanoarray Heterojunction and Its Efficient Solar Cells without Negative Impact of Photogenerated Electric Field. Commun. Phys. 2021, 4, 177. [Google Scholar] [CrossRef]
- Ying, J.Y.; Sun, T. Research Needs Assessment on Nanostructured Catalysts. J. Electroceram. 1997, 1, 219–238. [Google Scholar] [CrossRef]
- Cai, H.; Yan, S.; Dong, X.; Cao, F.; Wang, G. Significantly Enhanced Energy Storage Performance By Constructing TiO2 Nanowire Arrays In Pbzro3-Based Antiferroelectric Films. Ceram. Int. 2020, 46, 6436–6442. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Hoang, S.; Suib, S.L.; Gao, P.-X. Solvent Effects on the Heterogeneous Growth of TiO2 Nanostructure Arrays By Solvothermal Synthesis. Catal. Today 2021, 360, 275–283. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Jiang, B.; Sun, Y.; Gong, Z.; Zhang, N.; Hou, S.; Li, J.; Yang, N. Superhydrophilic and Underwater Superoleophobic Ti Foam with Robust Nanoarray Structures of TiO2 For Effective Oil-In-Water Emulsion Separation. Sep. Purif. Technol. 2020, 252, 117437. [Google Scholar] [CrossRef]
- Hu, C.; Chen, L.; Hu, Y.; Chen, A.; Chen, L.; Jiang, H.; Li, C. Light-Motivated SnO2/TiO2 Heterojunctions Enabling the Breakthrough in Energy Density for Lithium-ion Batteries. Adv Mater 2021, 33, e2103558. [Google Scholar] [CrossRef]
- Jamil, M.; Wei, S.; Taylor, M.P.; Chen, J.J.J.; Kennedy, J.V. Hybrid Anode Materials for Rechargeable Batteries-A Review of Sn/TiO2 Based Nanocomposites. Energy Rep. 2021, 7, 2836–2848. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Doanh, T.T.; Vinh, L.Q.; Thu, V.T.H. A Hybrid Ag/TiO2 Nanoarray-Based in Situ Charge Transfer Toward Multi-Functional Active-Platform. Ceram. Int. 2021, 47, 27524–27534. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, M.; Zhou, J.; Chen, H.; Cong, G.; Liu, H.; Ji, M.; Zhu, C.; Xu, J. A One-Pot Synthesis of Nitrogen Doped Porous Mxene/TiO2 Heterogeneous Film for High-Performance Flexible Energy Storage. Chem. Eng. J. 2021, 426, 130765. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Wang, C.; Jiang, Z.X.; Zhang, L.S.; Gu, L.L.; Wang, K.X.; Gao, J.; Zhu, X.D.; Wu, G. Rational Design of Mxene@TiO2 Nanoarray Enabling Dual Lithium Polysulfide Chemisorption Towards High-Performance Lithium-Sulfur Batteries. Nanoscale 2020, 12, 16678–16684. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Vacandio, F.; Sougrati, M.T.; Martinez, H.; Jumas, J.C.; Knauth, P.; Djenizian, T. Effect of Sn-Doping on the Electrochemical Behaviour of TiO2 Nanotubes As Potential Negative Electrode Materials for 3D Li-ion Micro Batteries. J. Power Sources 2013, 224, 269–277. [Google Scholar] [CrossRef]
- Luo, J.; Xia, X.; Luo, Y.; Guan, C.; Liu, J.; Qi, X.; Ng, C.F.; Yu, T.; Zhang, H.; Fan, H.J. Rationally Designed Hierarchical TiO2 @Fe2O3 Hollow Nanostructures for Improved Lithium Ion Storage. Adv. Energy Mater. 2013, 3, 737–743. [Google Scholar] [CrossRef]
- Eustache, E.; Tilmant, P.; Morgenroth, L.; Roussel, P.; Patriarche, G.; Troadec, D.; Rolland, N.; Brousse, T.; Lethien, C. Silicon-Microtube Scaffold Decorated with Anatase TiO2 As A Negative Electrode for A 3D Litium-Ion Microbattery. Adv. Energy Mater. 2014, 4, 1301612. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, P.; Zhang, Y.; Garcia, B.B.; Zhang, Q.; Guo, Q.; Champion, R.; Cao, G. TiO2 Nanotube Arrays Annealed in N2 for Efficient Lithium-Ion Intercalation. J. Phys. Chem. C 2008, 112, 11175–11180. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xiao, P.; Garcia, B.B.; Zhang, Q.; Zhou, X.; Jeong, Y.-H.; Cao, G. TiO2 Nanotube Arrays Annealed in CO Exhibiting High Performance for Lithium Ion Intercalation. Electrochim. Acta 2009, 54, 6816–6820. [Google Scholar] [CrossRef]
- Li, Y.; Lv, X.; Li, J. High Performance Binderless TiO2 Nanowire Arrays Electrode for Lithium-Ion Battery. Appl. Phys. Lett. 2009, 95, 113102. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Knauth, P.; Lavela, P.; Tirado, J.L.; Djenizian, T. TiO2 Nanotubes Manufactured By Anodization of Ti Thin Films for On-Chip Li-ion 2D Microbatteries. Electrochim. Acta 2009, 54, 4262–4268. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Djenizian, T.; Lavela, P.; Tirado, J.L.; Knauth, P. Alternative Li-Ion Battery Electrode Based on Self-Organized Titania Nanotubes. Chem. Mater. 2009, 21, 63–67. [Google Scholar] [CrossRef]
- Xiong, H.; Yildirim, H.; Shevchenko, E.V.; Prakapenka, V.B.; Koo, B.; Slater, M.D.; Balasubramanian, M.; Sankaranarayanan, S.K.R.S.; Greeley, J.P.; Tepavcevic, S.; et al. Self-Improving Anode for Lithium-Ion Batteries Based on Amorphous to Cubic Phase Transition in TiO2 Nanotubes. J. Phys. Chem. C 2012, 116, 3181–3187. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.S.; Wei, X.; Lou, X.W.; Liu, X.-W. Sandwich-Like, Stacked Ultrathin Titanate Nanosheets for Ultrafast Lithium Storage. Adv. Mater. 2011, 23, 998–1002. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Chabot, V.; Gu, M.; Wang, C.; Xiao, X.; Chen, Z. Dual Phase Li4Ti5O12–TiO2 Nanowire Arrays as Integrated Anodes for High-Rate Lithium-Ion Batteries. Nano Energy 2014, 9, 383–391. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, J.; Jiang, J.; Zhou, W.; Yang, H.; Qi, X.; Zhang, H.; Fan, H.J.; Yu, D.Y.W.; Li, C.M.; et al. Seed-Assisted Synthesis of Highly Ordered TiO2@α-Fe2O3 Core/Shell Arrays on Carbon Textiles for Lithium-Ion Battery Applications. Energy Environ. Sci. 2012, 5, 6559. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Lavela, P.; Knauth, P.; Tirado, J.L.; Djenizian, T. Nanoarchitectured TiO2/SnO: A Future Negative Electrode for High Power Density Li-Ion Microbatteries? Chem. Mater. 2010, 22, 1926–1932. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Wang, H.; Zuo, W.; Li, Y.; Liu, J. Fabrication and Shell Optimization of Synergistic TiO2-MoO3 Core-Shell Nanowire Array Anode for High Energy and Power Density Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3524–3533. [Google Scholar] [CrossRef]
- Du, G.; Guo, Z.; Zhang, P.; Li, Y.; Chen, M.; Wexler, D.; Liu, H. SnO2 Nanocrystals on Self-Organized TiO2 Nanotube Array as Three-Dimensional Electrode for Lithium Ion Microbatteries. J. Mater. Chem. 2010, 20, 5689. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Wang, L.; Du, Z.; Fang, H.; Ling, Y.; Huang, Z. Coaxial SnO2@TiO2 Nanotube Hybrids: From Robust Assembly Strategies to Potential Application in Li+ Storage. J. Mater. Chem. 2012, 22, 11151. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Lebouin, C.; Knauth, P.; Djenizian, T. The Electrochemical Behaviour of TiO2 Nanotubes with Co3O4 or NiO Submicron Particles: Composite Anode Materials for Li-Ion Micro Batteries. Electrochim. Acta 2013, 88, 814–820. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Bae, J.-Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 Hollow Nanofibers as an Anode Material for High Power Lithium Ion Batteries. Energy Environ. Sci. 2011, 4, 4532. [Google Scholar] [CrossRef]
- Zeng, L.; Zheng, C.; Xia, L.; Wang, Y.; Wei, M. Ordered Mesoporous TiO2–C Nanocomposite as an Anode Material for Long-Term Performance Lithium-Ion Batteries. J. Mater. Chem. A 2013, 1, 4293. [Google Scholar] [CrossRef]
- Wang, B.; Xin, H.; Li, X.; Cheng, J.; Yang, G.; Nie, F. Mesoporous CNT@ TiO2-C Nanocable with Extremely Durable High Rate Capability for Lithium-Ion Battery Anodes. Sci. Rep. 2014, 4, 3729. [Google Scholar] [CrossRef]
- Zhu, C.; Xia, X.; Liu, J.; Fan, Z.; Chao, D.; Zhang, H.; Fan, H.J. TiO2 Nanotube @ SnO2 Nanoflake Core–Branch Arrays for Lithium-Ion Battery Anode. Nano Energy 2014, 4, 105–112. [Google Scholar] [CrossRef]
- Hou, X.; Wang, X.; Liu, B.; Wang, Q.; Wang, Z.; Chen, D.; Shen, G. SnO2@TiO2 Heterojunction Nanostructures for Lithium-Ion Batteries and Self-Powered UV Photodetectors with Improved Performances. ChemElectroChem 2014, 1, 108–115. [Google Scholar] [CrossRef]
- Jeun, J.-H.; Park, K.-Y.; Kim, D.-H.; Kim, W.-S.; Kim, H.-C.; Lee, B.-S.; Kim, H.; Yu, W.-R.; Kang, K.; Hong, S.-H. SnO2@TiO2 Double-Shell Nanotubes for a Lithium Ion Battery Anode with Excellent High Rate Cyclability. Nanoscale 2013, 5, 8480. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Wu, X. Synthesis and Lithium Storage Properties of NiO@TiO2 Nanotube Heterojunction Arrays. Chem. Lett. 2011, 40, 1428–1430. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Q.; Kim, J.-H.; Pesaran, A.A.; Frank, A.J. Pseudocapacitive Lithium-Ion Storage in Oriented Anatase TiO2 Nanotube Arrays. J. Phys. Chem. C 2012, 116, 11895–11899. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L.V.; Yang, Z.; Liu, J. Reversible Sodium Ion Insertion in Single Crystalline Manganese Oxide Nanowires with Long Cycle Life. Adv. Mater. 2011, 23, 3155–3160. [Google Scholar] [CrossRef]
- Longoni, G.; Pena Cabrera, R.L.; Polizzi, S.; D’Arienzo, M.; Mari, C.M.; Cui, Y.; Ruffo, R. Shape-Controlled TiO2 Nanocrystals for Na-Ion Battery Electrodes: The Role of Different Exposed Crystal Facets on the Electrochemical Properties. Nano Lett. 2017, 17, 992–1000. [Google Scholar] [CrossRef]
- Bella, F.; Muñoz-García, A.B.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Unveiling the Controversial Mechanism of Reversible Na Storage in TiO2 Nanotube Arrays: Amorphous versus Anatase TiO2. Nano Res. 2017, 10, 2891–2903. [Google Scholar] [CrossRef]
- Bella, F.; Muñoz-García, A.B.; Colò, F.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Combined Structural, Chemometric, and Electrochemical Investigation of Vertically Aligned TiO2 Nanotubes for Na-Ion Batteries. ACS Omega 2018, 3, 8440–8450. [Google Scholar] [CrossRef]
- Wei, W.; Valvo, M.; Edström, K.; Nyholm, L. A Facile Vapor-Phase Hydrothermal Method for Direct Growth of Nanotube Anodes for Sodium-Ion Batteries. ChemElectroChem 2018, 5, 674–684. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Wang, H.; Liang, F.; Chen, R.; Wu, R. Highly Ordered Three-Dimensional TiO2@C Nanotube Arrays as Freestanding Electrode for Sodium-Ion Battery. Mater. Lett. 2017, 207, 149–152. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Wen, L.; Wang, C.; Zhao, H.; Mi, Y.; Liang, L.; Fu, Q.; Wu, M.; Lei, Y. Highly Ordered Three-Dimensional Ni-TiO2 Nanoarrays as Sodium Ion Battery Anodes. Chem. Mater. 2015, 27, 4274–4280. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Dew Luna, B.; Manthiram, A. TiO2-B Nanowire Arrays Coated with Layered MoS2 Nanosheets for Lithium and Sodium Storage. J. Mater. Chem. A 2016, 4, 801–806. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Yuan, Y.; Ma, L.; Jiang, Y.; Li, L.; Lu, J. Boosting Sodium Storage in TiO2 Nanotube Arrays through Surface Phosphorylation. Adv. Mater. 2018, 30, 1704337. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-Supported Nanotube Arrays of Sulfur-Doped TiO2 Enabling Ultrastable and Robust Sodium Storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef]
- Xiong, H.; Slater, M.D.; Balasubramanian, M.; Johnson, C.S.; Rajh, T. Amorphous TiO2 Nanotube Anode for Rechargeable Sodium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Raj, C.C.; Prasanth, R. Review—Advent of TiO2 Nanotubes as Supercapacitor Electrode. J. Electrochem. Soc. 2018, 165, E345–E358. [Google Scholar] [CrossRef]
- He, Z.-K.; Lu, Y.; Zhao, C.; Zhao, J.; Gao, Z.; Song, Y.-Y. Surface-Charge Regulated TiO2 Nanotube Arrays As Scaffold for Constructing Binder-Free High-Performance Supercapacitor. Appl. Surf. Sci. 2021, 567, 150832. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, T.-W.; Park, J.H. Controlled TiO2 Nanotube Arrays as an Active Material for High Power Energy-Storage Devices. J. Electrochem. Soc. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Konstantinov, K.; Liu, H.K. A Highly Ordered Titania Nanotube Array as a Supercapacitor Electrode. Phys. Chem. Chem. Phys. 2011, 13, 5038. [Google Scholar] [CrossRef] [PubMed]
- Raj, C.C.; Sundheep, R.; Prasanth, R. Enhancement of Electrochemical Capacitance by Tailoring the Geometry of TiO2 Nanotube Electrodes. Electrochim. Acta 2015, 176, 1214–1220. [Google Scholar] [CrossRef]
- Heng, I.; Low, F.W.; Lai, C.W.; Juan, J.C.; Tiong, S.K. Hybrid Graphene Titanium Nanocomposites and Their Applications in Energy Storage Devices: A Review. J. Electron. Mater. 2020, 49, 1777–1786. [Google Scholar] [CrossRef]
- Chowdhury, R.; Saini, S.K.; Roy, J. Bio-Fabrication of TiO2 Nanomaterials and Their Applications in Electronics Devices. J. Electron. Mater. 2021, 50, 6087–6101. [Google Scholar] [CrossRef]
- Ates, M.; Kuzgun, O.; Candan, I. Supercapacitor Performances of Titanium–Polymeric Nanocomposites: A Review Study. Iran. Polym. J. 2022, 31, 31–57. [Google Scholar] [CrossRef]
- Ramadoss, A.; Kim, G.-S.; Kim, S.J. Fabrication of Reduced Graphene Oxide/ TiO2 Nanorod/Reduced Graphene Oxide Hybrid Nanostructures as Electrode Materials for Supercapacitor Applications. CrystEngComm 2013, 15, 10222. [Google Scholar] [CrossRef]
- Ke, Q.; Liao, Y.; Yao, S.; Song, L.; Xiong, X. A Three-Dimensional TiO2/Graphene Porous Composite with Nano-Carbon Deposition for Supercapacitor. J. Mater. Sci. 2016, 51, 2008–2016. [Google Scholar] [CrossRef]
- Guo, Q.; Li, J.; Zhang, B.; Nie, G.; Wang, D. High-Performance Asymmetric Electrochromic-Supercapacitor Device Based on Poly(Indole-6-Carboxylicacid)/TiO2 Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 6491–6501. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Gan, J.; Tong, Y.; Li, Y. Hydrogenated TiO2 Nanotube Arrays for Supercapacitors. Nano Lett. 2012, 12, 1690–1696. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Xu, J.; Lu, L.; Fan, Z.; Chen, X.; Song, Y.; Li, D. Enhanced Supercapacitance in Anodic TiO2 Nanotube Films by Hydrogen Plasma Treatment. Nanotechnology 2013, 24, 455401. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Enhancing the Capacitance of TiO2 Nanotube Arrays by a Facile Cathodic Reduction Process. J. Power Sources 2013, 239, 128–131. [Google Scholar] [CrossRef]
- Wu, H.; Li, D.; Zhu, X.; Yang, C.; Liu, D.; Chen, X.; Song, Y.; Lu, L. High-Performance and Renewable Supercapacitors Based on TiO2 Nanotube Array Electrodes Treated by an Electrochemical Doping Approach. Electrochim. Acta 2014, 116, 129–136. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.; Lee, J.; Kim, J.; Yoon, J. Capacitive and Oxidant Generating Properties of Black-Colored TiO2 Nanotube Array Fabricated by Electrochemical Self-Doping. ACS Appl. Mater. Interfaces 2015, 7, 7486–7491. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Kim, S.-J. Blue TiO2 Nanosheets as a High-Performance Electrode Material for Supercapacitors. J. Colloid Interface Sci. 2019, 536, 62–70. [Google Scholar] [CrossRef]
- Kim, J.-H.; Zhu, K.; Yan, Y.; Perkins, C.L.; Frank, A.J. Microstructure and Pseudocapacitive Properties of Electrodes Constructed of Oriented NiO-TiO2 Nanotube Arrays. Nano Lett. 2010, 10, 4099–4104. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Shaikh, J.S.; Bhat, T.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Hydrothermally Grown 3D Hierarchical TiO2 Based on Electrochemically Anodized 1D TiO2 Nanostructure for Supercapacitor. Appl. Phys. A 2018, 124, 592. [Google Scholar] [CrossRef]
- Qorbani, M.; Khajehdehi, O.; Sabbah, A.; Naseri, N. Ti-Rich TiO2 Tubular Nanolettuces by Electrochemical Anodization for All-Solid-State High-Rate Supercapacitor Devices. ChemSusChem 2019, 12, 4064–4073. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Y.; Kang, W.; Li, C.; Lin, D.; Wang, X.; Chen, Z.; Wu, M.; Pan, D. Reduction Mechanism and Capacitive Properties of Highly Electrochemically Reduced TiO2 Nanotube Arrays. Electrochim. Acta 2015, 161, 40–47. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Enhanced Electrochemical Performance of Manganese Dioxide Spheres Deposited on a Titanium Dioxide Nanotube Arrays Substrate. J. Power Sources 2014, 272, 866–879. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Li, Y.-H.; Guo, J.; Gao, Z.-D.; Li, Y. Facile Method to Synthesize a Carbon Layer Embedded into Titanium Dioxide Nanotubes with Metal Oxide Decoration for Electrochemical Applications. J. Mater. Chem. A 2015, 3, 23754–23759. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, C.; Zhou, L.; Liu, Y.; Huang, H. Supercapacitor Application of Nickel Oxide–Titania Nanocomposites. Compos. Sci. Technol. 2009, 69, 2108–2114. [Google Scholar] [CrossRef]
- Ray, R.S.; Sarma, B.; Misra, M. Random Shaped ZnO Supported on a Porous Substrate as Supercapacitor. Mater. Lett. 2015, 155, 102–105. [Google Scholar] [CrossRef]
- Sarkar, A.; Singh, A.K.; Sarkar, D.; Khan, G.G.; Mandal, K. Three-Dimensional Nanoarchitecture of BiFeO3 Anchored TiO2 Nanotube Arrays for Electrochemical Energy Storage and Solar Energy Conversion. ACS Sustain. Chem. Eng. 2015, 3, 2254–2263. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, D.; Yang, M.; Schmuki, P. Vertically Aligned Mixed V2O5–TiO2 Nanotube Arrays for Supercapacitor Applications. Chem. Commun. 2011, 47, 7746. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Li, Z.; Yang, X. Design and Synthesis of MWNTs-TiO2 Nanotube Hybrid Electrode and Its Supercapacitance Performance. J. Power Sources 2015, 283, 397–407. [Google Scholar] [CrossRef]
- Siuzdak, K.; Bogdanowicz, R.; Sawczak, M.; Sobaszek, M. Enhanced Capacitance of Composite TiO2 Nanotube/Boron-Doped Diamond Electrodes Studied by Impedance Spectroscopy. Nanoscale 2015, 7, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiong, M.; Qu, D.; Liu, D.; Zhang, Z.; Xie, Z.; Wei, X.; Tu, W.; Qu, D. Enhanced Supercapacitive Performance on TiO2@C Coaxial Nano-Rod Array through a Bio-Inspired Approach. Nano Energy 2015, 15, 75–82. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Z.; Li, H.; Li, Q.; Zhang, Y. Preparation of Highly Capacitive Polyaniline/Black TiO2 Nanotubes as Supercapacitor Electrode by Hydrogenation and Electrochemical Deposition. Electrochim. Acta 2015, 166, 174–182. [Google Scholar] [CrossRef]
- Ambade, R.B.; Ambade, S.B.; Shrestha, N.K.; Nah, Y.-C.; Han, S.-H.; Lee, W.; Lee, S.-H. Polythiophene Infiltrated TiO2 Nanotubes as High-Performance Supercapacitor Electrodes. Chem. Commun. 2013, 49, 2308. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ma, Y.; Cao, Y.; Hu, Z.; Zhang, X.; Fu, J.; Huo, K.; Chu, P.K. MnO2–TiO2/C Nanocomposite Arrays for High-Performance Supercapacitor Electrodes. Thin Solid Films 2015, 584, 61–65. [Google Scholar] [CrossRef]
- Yang, F.; Yao, J.; Liu, F.; He, H.; Zhou, M.; Xiao, P.; Zhang, Y. Ni–Co Oxides Nanowire Arrays Grown on Ordered TiO2 Nanotubes with High Performance in Supercapacitors. J. Mater. Chem. A 2013, 1, 594–601. [Google Scholar] [CrossRef]
- Gobal, F.; Faraji, M. Electrodeposited Polyaniline on Pd-Loaded TiO2 Nanotubes as Active Material for Electrochemical Supercapacitor. J. Electroanal. Chem. 2013, 691, 51–56. [Google Scholar] [CrossRef]
- Xie, S.; Gan, M.; Ma, L.; Li, Z.; Yan, J.; Yin, H.; Shen, X.; Xu, F.; Zheng, J.; Zhang, J.; et al. Synthesis of Polyaniline-Titania Nanotube Arrays Hybrid Composite via Self-Assembling and Graft Polymerization for Supercapacitor Application. Electrochim. Acta 2014, 120, 408–415. [Google Scholar] [CrossRef]
- Zhi, J.; Zhao, W.; Lin, T.; Huang, F. Boosting Supercapacitor Performance of TiO2 Nanobelts by Efficient Nitrogen Doping. ChemElectroChem 2017, 4, 2328–2335. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, X.; Yang, J.; Li, X.; Liu, Z.; Loh, X.J.; Wang, J. Aqueous Rechargeable Multivalent Metal-Ion Batteries: Advances and Challenges. Adv. Energy Mater. 2021, 11, 2100608. [Google Scholar] [CrossRef]
- Novák, P.; Scheifele, W.; Haas, O. Magnesium Insertion Batteries-an Alternative to Lithium? J. Power Sources 1995, 54, 479–482. [Google Scholar] [CrossRef]
- Li, C.; Hou, C.-C.; Chen, L.; Kaskel, S.; Xu, Q. Rechargeable Al-Ion Batteries. EnergyChem 2021, 3, 100049. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.J.; Yan, N.F.; Pan, G.L.; Li, G.R.; Gao, X.P. Aluminum Storage Behavior of Anatase TiO2 Nanotube Arrays in Aqueous Solution for Aluminum Ion Batteries. Energy Environ. Sci. 2012, 5, 9743. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, L.; Li, Z.; Dou, H.; Zhang, X. Design Strategies and Research Progress for Water-in-Salt Electrolytes. Energy Storage Mater. 2022, 44, 10–28. [Google Scholar] [CrossRef]
- Chen, M.; Feng, G.; Qiao, R. Water-in-Salt Electrolytes: An Interfacial Perspective. Curr. Opin. Colloid Interface Sci. 2020, 47, 99–110. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, Y.; Zhu, X.; Li, X.; Yue, J.; Ma, X.; Gu, L.; Hu, Y.-S.; Li, H.; Huang, X.; et al. TiO2(B) Anode for High-Voltage Aqueous Li-Ion Batteries. Energy Storage Mater. 2021, 42, 438–444. [Google Scholar] [CrossRef]
- Yan, L.; Huang, J.; Guo, Z.; Dong, X.; Wang, Z.; Wang, Y. Solid-State Proton Battery Operated at Ultralow Temperature. ACS Energy Lett. 2020, 5, 685–691. [Google Scholar] [CrossRef]
- Donald, W.A.; Leib, R.D.; Demireva, M.; O’Brien, J.T.; Prell, J.S.; Williams, E.R. Directly Relating Reduction Energies of Gaseous Eu(H2O)n3+ ,n = 55–140, to Aqueous Solution: The Absolute SHE Potential and Real Proton Solvation Energy. J. Am. Chem. Soc. 2009, 131, 13328–13337. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, J.; Dong, X.; Xia, Y.; Yan, L.; Wang, Z.; Wang, Y. An Organic/Inorganic Electrode-Based Hydronium-Ion Battery. Nat. Commun. 2020, 11, 959. [Google Scholar] [CrossRef]
- Jiang, H.; Shin, W.; Ma, L.; Hong, J.J.; Wei, Z.; Liu, Y.; Zhang, S.; Wu, X.; Xu, Y.; Guo, Q. A High-Rate Aqueous Proton Battery Delivering Power Below− 78 °C via an Unfrozen Phosphoric Acid. Adv. Energy Mater. 2020, 10, 2000968. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Yan, X.; Xiao, Z.; Liu, C.; Zhang, Y.; Yang, X. Regulating Fast Anionic Redox for High-Voltage Aqueous Hydrogen-Ion-Based Energy Storage. Angew. Chem. 2019, 131, 211–216. [Google Scholar] [CrossRef]
- Liang, G.; Mo, F.; Yang, Q.; Huang, Z.; Li, X.; Wang, D.; Liu, Z.; Li, H.; Zhang, Q.; Zhi, C. Commencing an Acidic Battery Based a Copper Anode with Ultrafast Proton-Regulated Kinetics and Superior Dendrite-Free Property. Adv. Mater. 2019, 31, 1905873. [Google Scholar] [CrossRef]
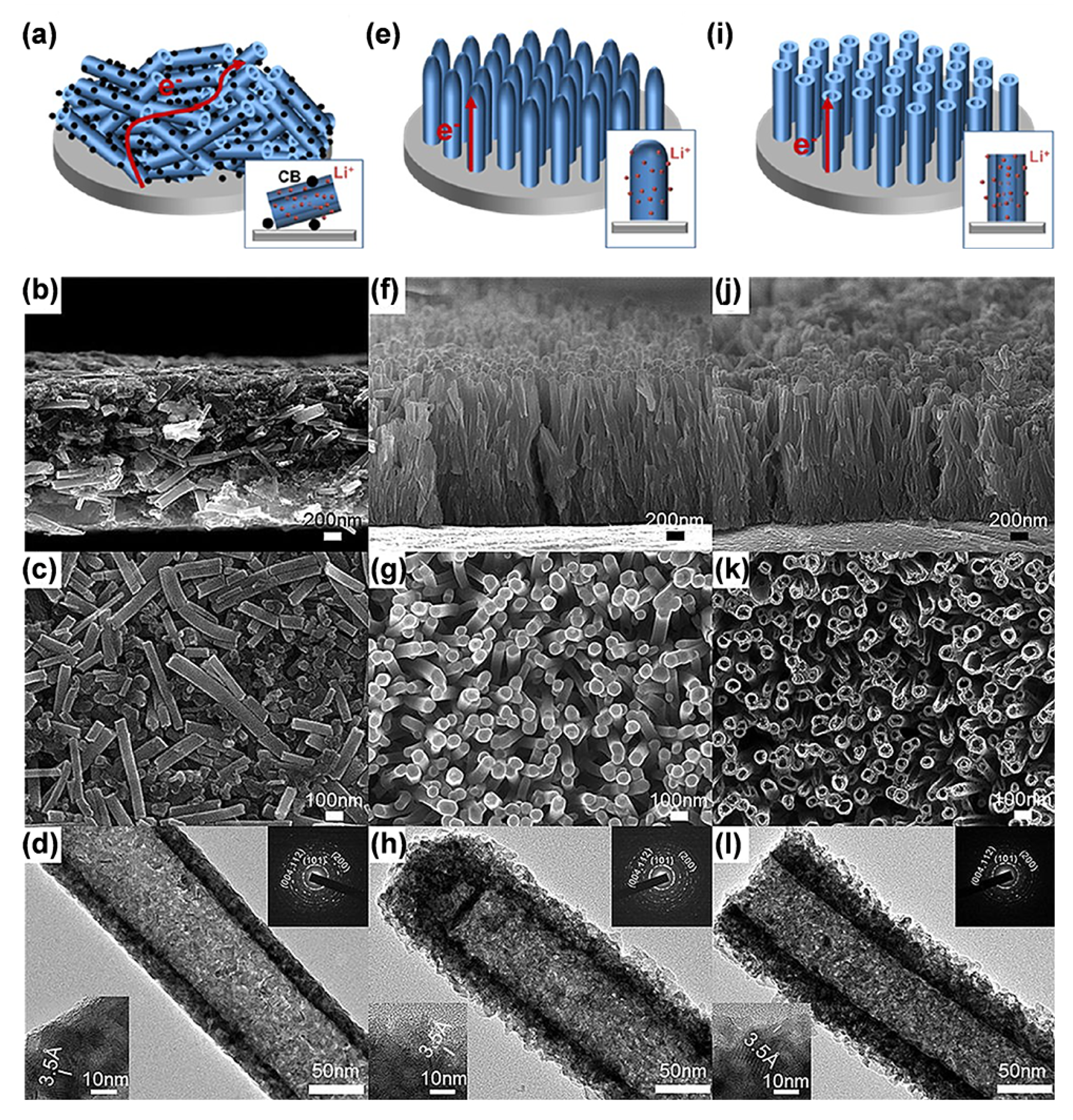
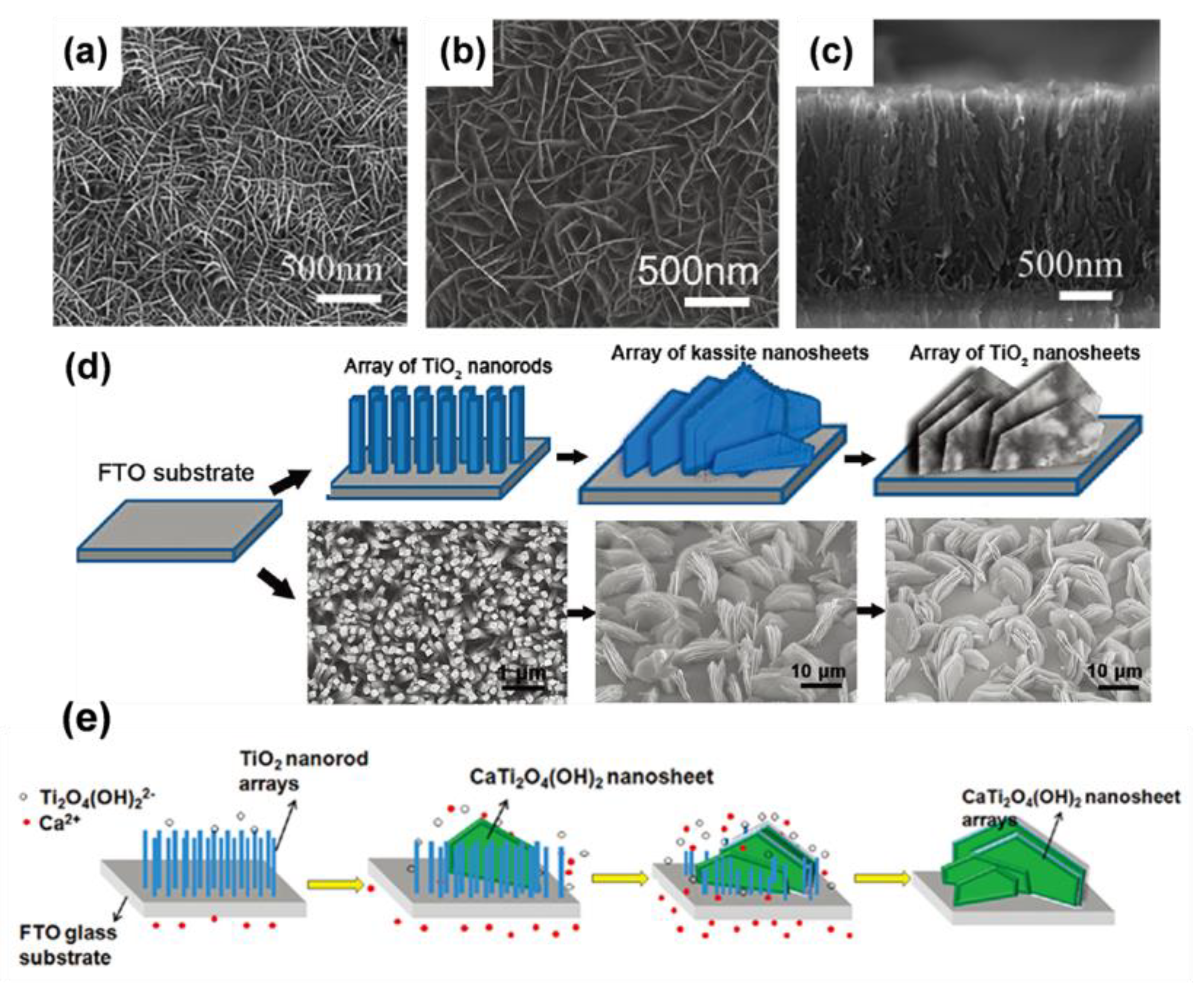
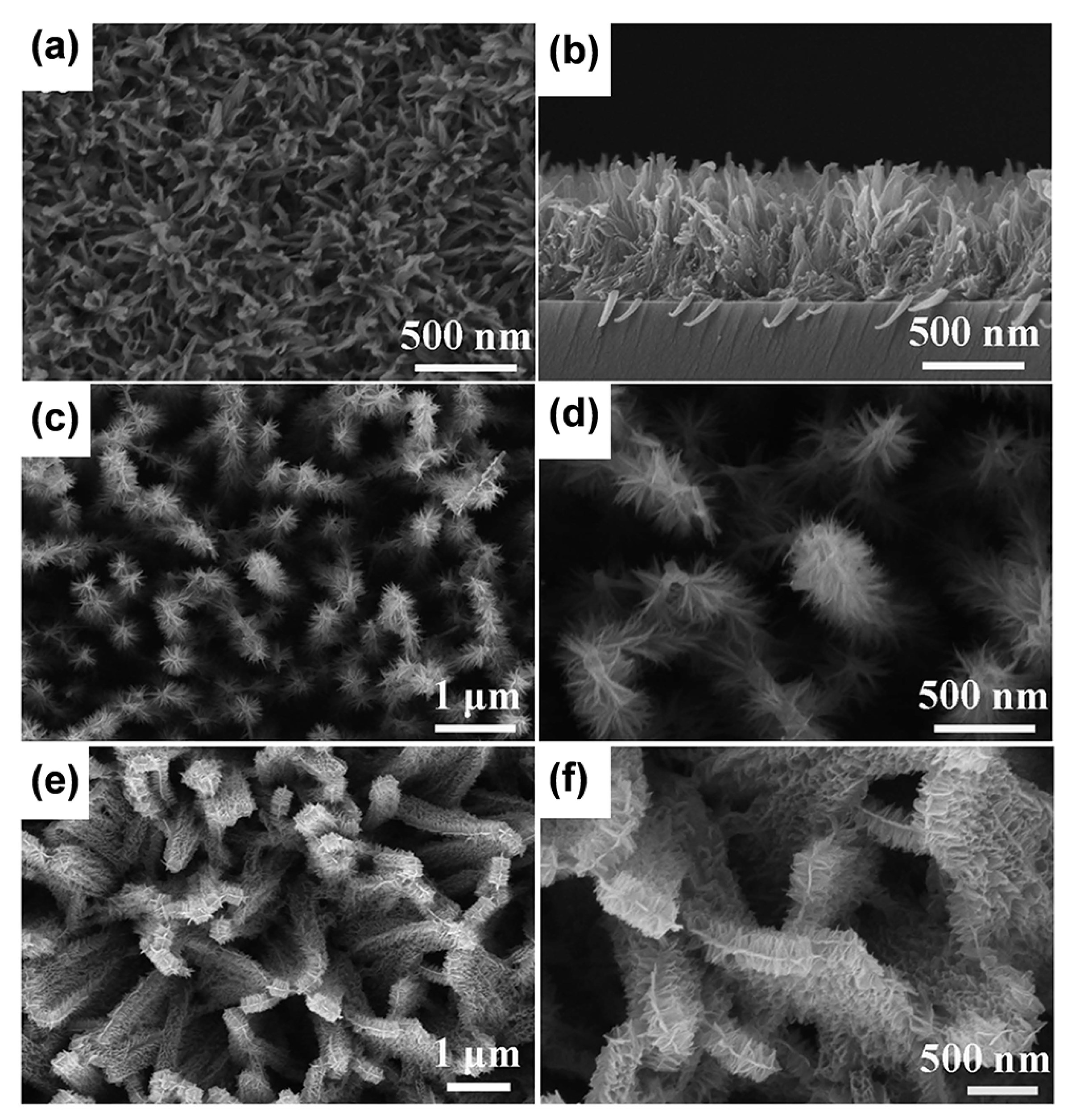


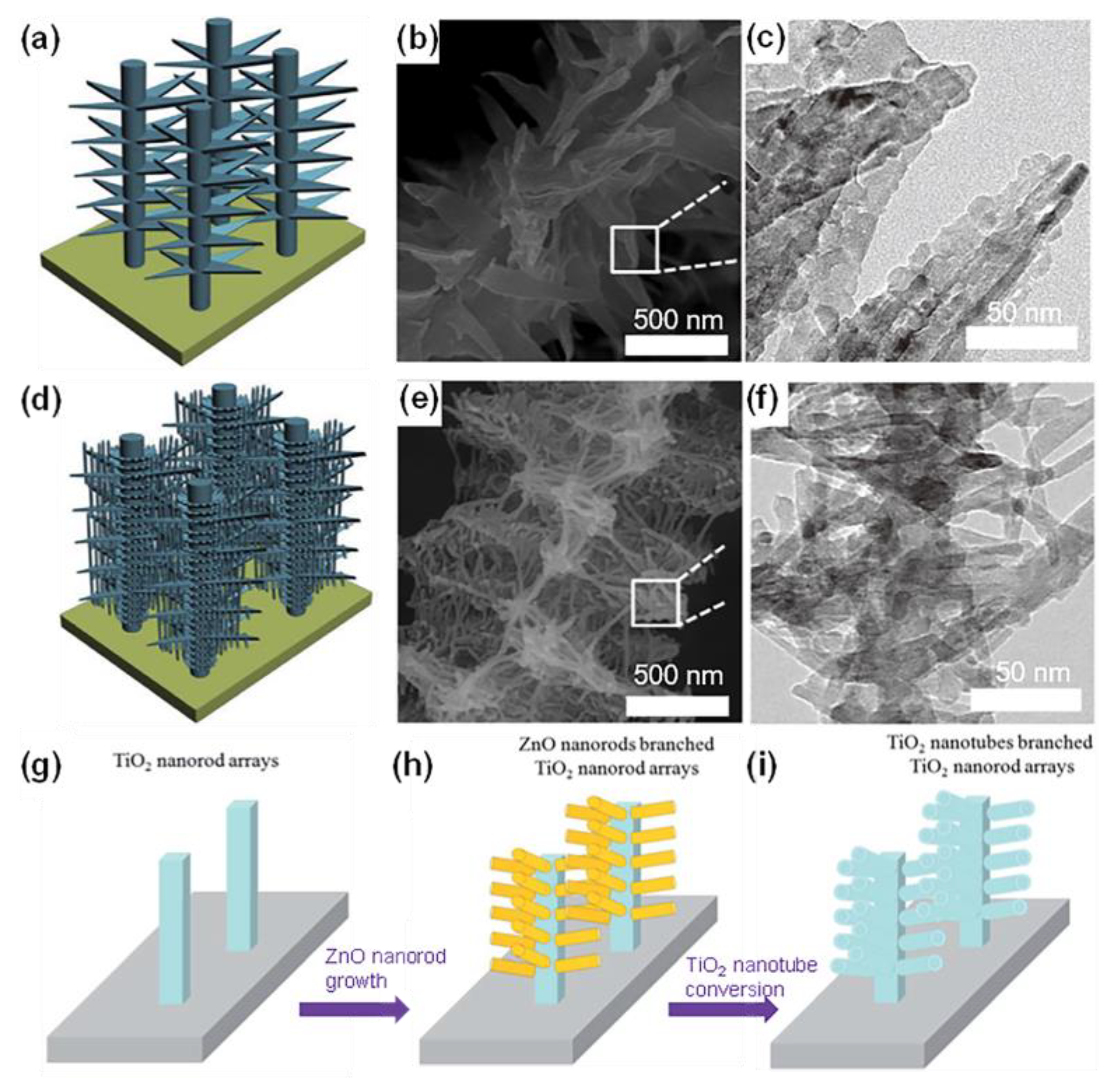
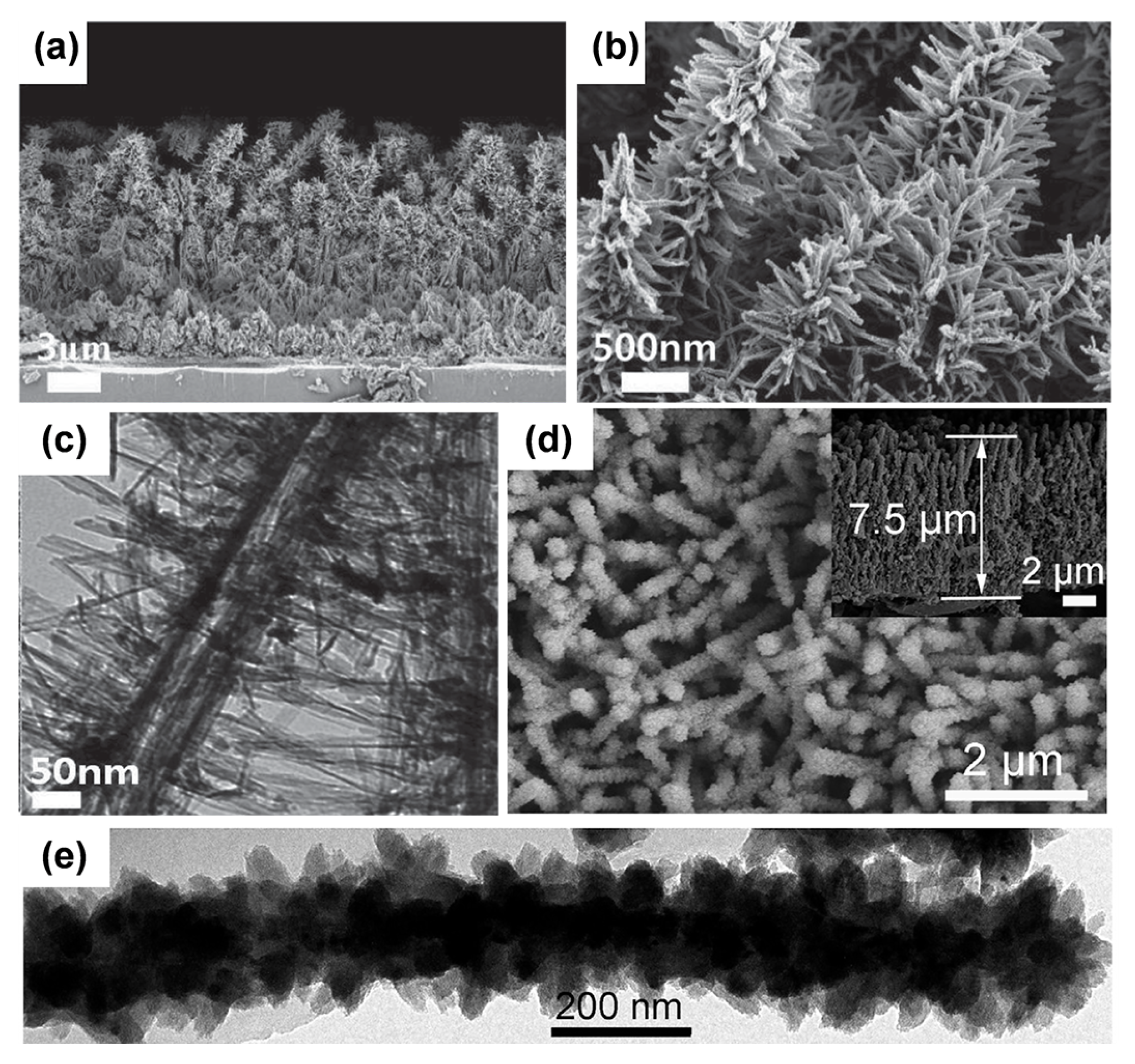

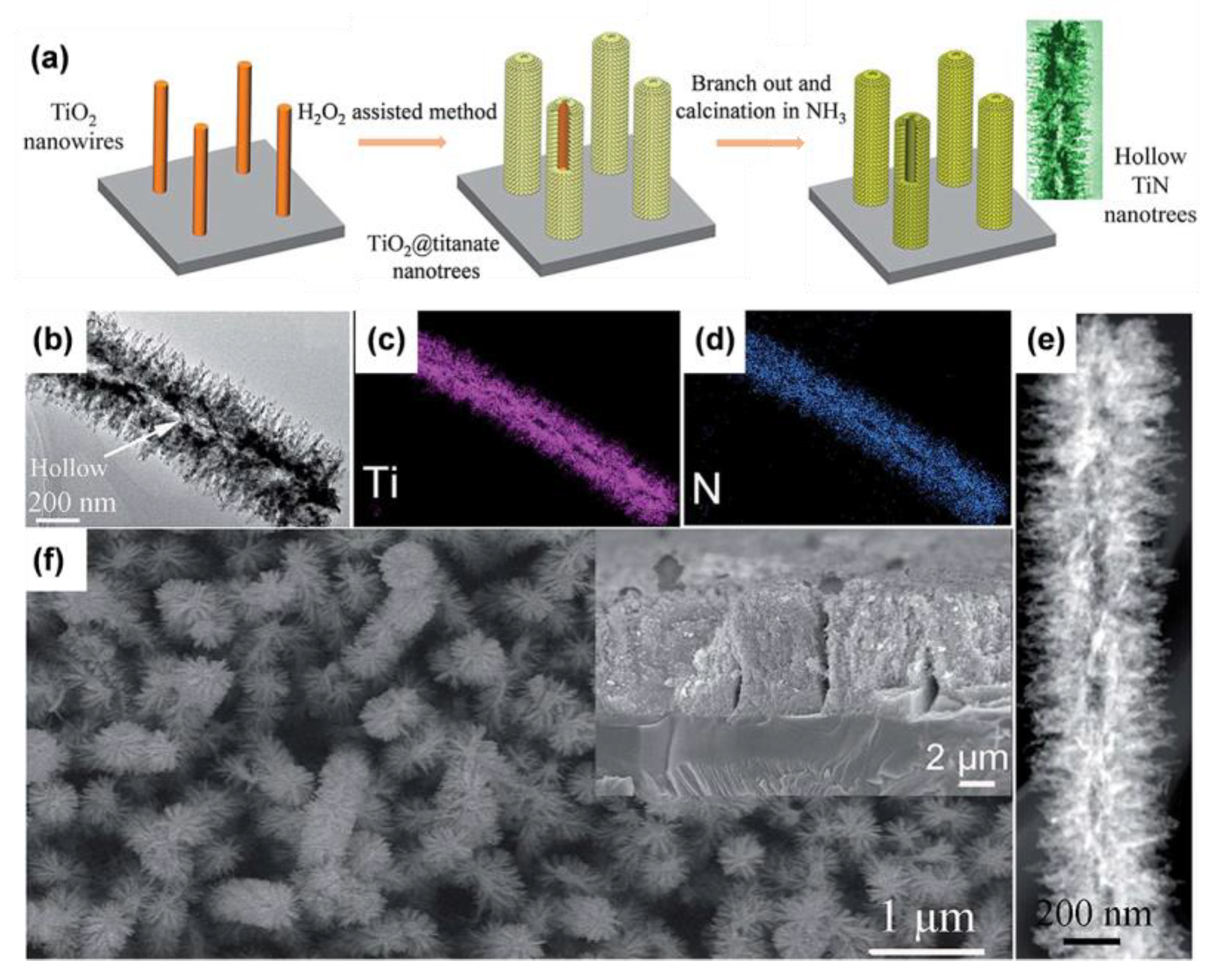
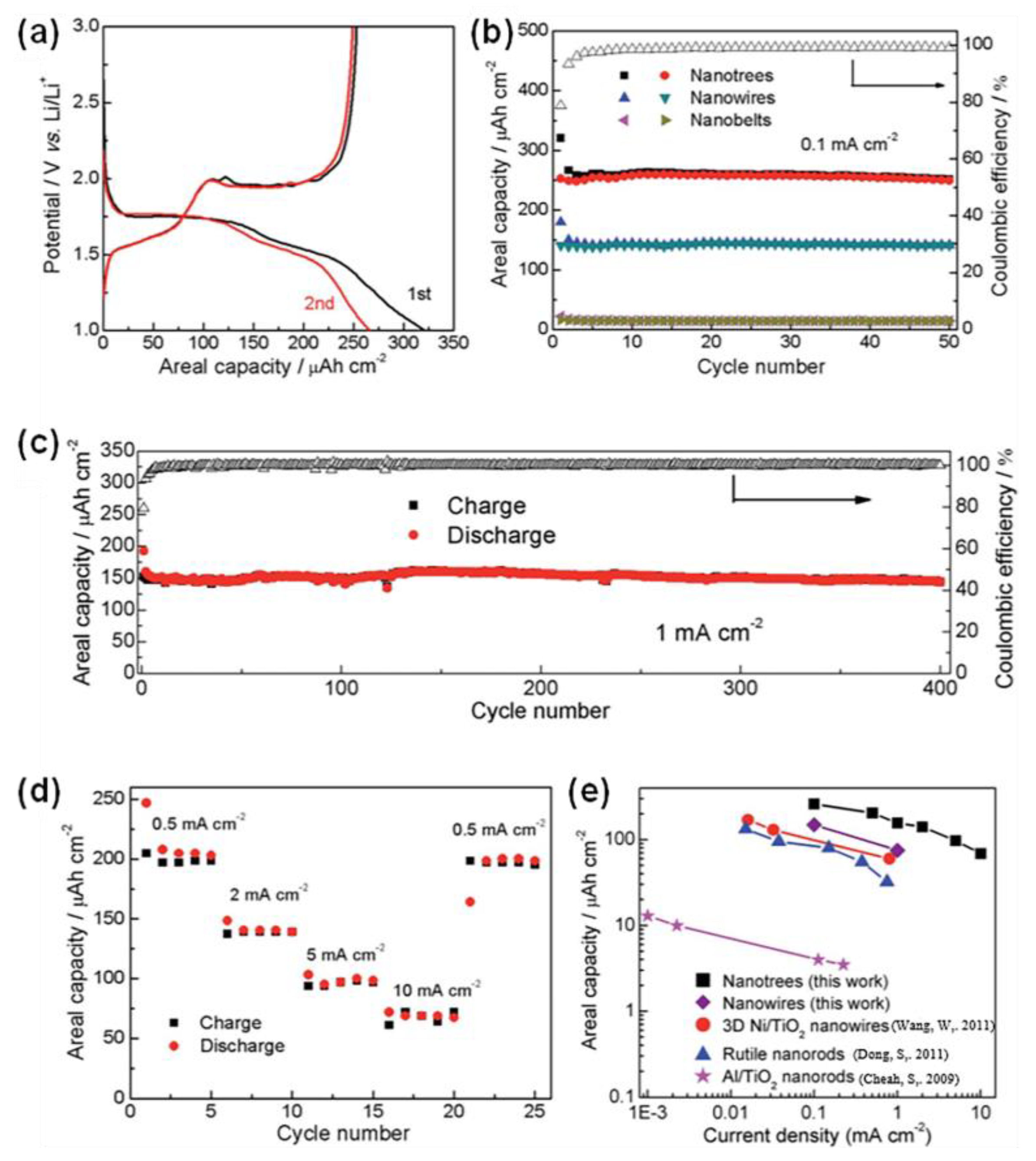


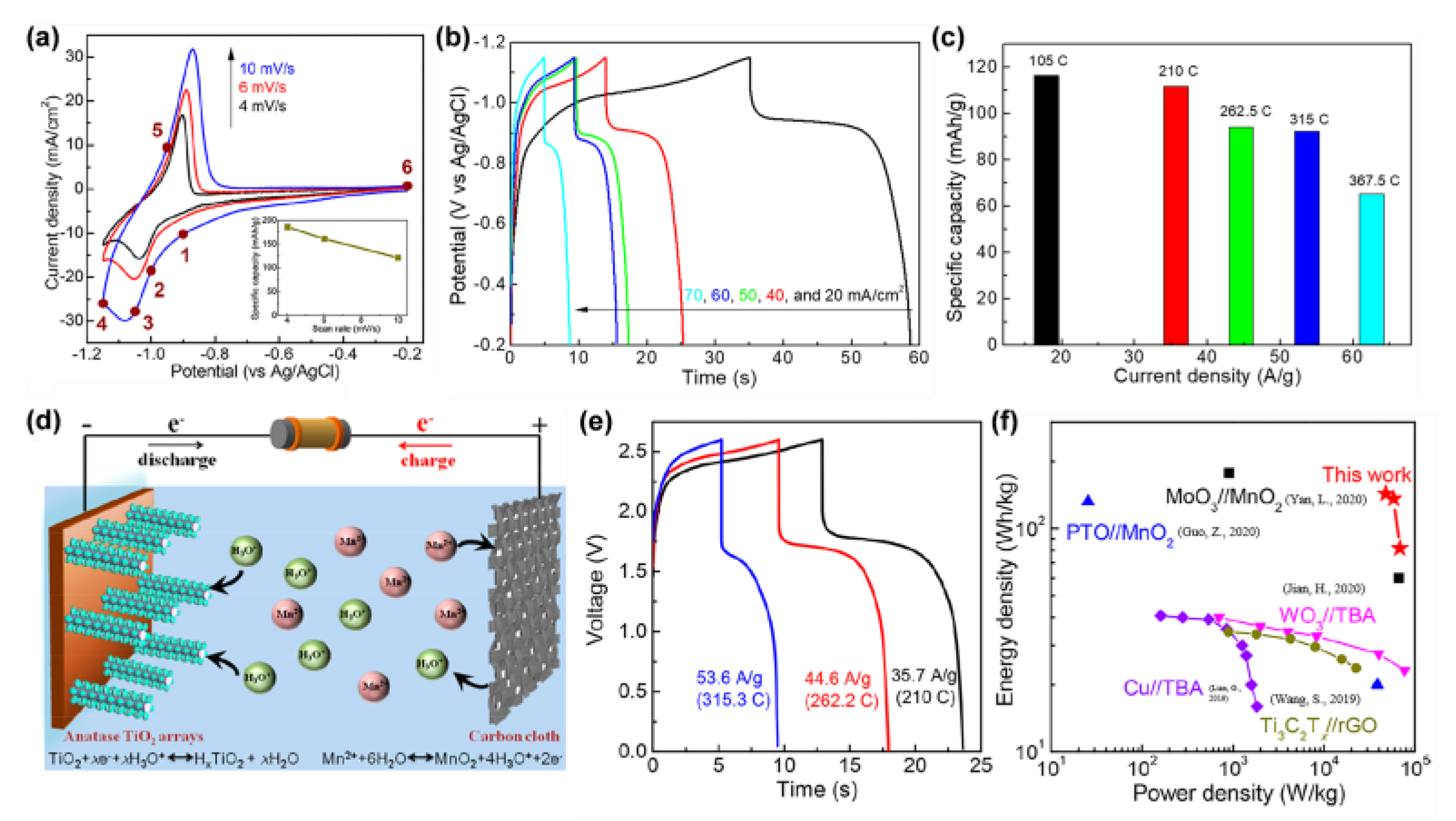
| Sample | Synthesis Method | Crystal Structure | Morphology | Ref. |
|---|---|---|---|---|
| Oriented single crystalline TiO2 nanowire arrays | Alkali hydrothermal and ion-exchange reaction | Anatase | Diameter: 105 nm and length: 12–16 μm | [31] |
| Mesoporous TiO2 nanowire arrays | Alkali hydrothermal and ion-exchange reaction | Anatase | Diameter: 20 nm and length: 7 μm | [29] |
| Oriented single-crystalline TiO2 nanorod arrays | Hydrothermal method | Rutile | Diameter: 90 nm, length: 1.9 μm | [27] |
| Ordered TiO2 nanorod arrays | Chemical oxidation with 30 mass % H2O2 solution | Anatase and rutile | Diameter: 20–30 nm and length: 150 nm | [14] |
| TiO2 nanotube arrays | Electrochemical deposition | Anatase | Tube length: 8 μm, inner diameter: 70 nm, and wall thickness: 25 nm | [76] |
| Sealed TiO2 nanotube arrays | Template assisted methods | Anatase | Tube length: 2 μm, inner diameter: 80 nm, and wall thickness: 20 nm | [82] |
| Long and hollow TiO2 nanofiber arrays with uniform, circular cross-sections | Electrospinning | Anatase | Tube length: 4 μm, inner diameter: 200 nm, and wall thickness: 50 nm | [83] |
| Layered TiO2 nanosheet arrays | Hydrothermal treatment | TiO2(B)/Anatase | Length: 6 μm and thickness: 10 nm | [90] |
| Nanoporous TiO2 nanosheet arrays | Hydrothermal treatment with diluted HNO3 aqueous solution | Rutile | Diameter: 12 μm, thickness: 200–300 nm and a smooth surface | [95] |
| TiO2 ultrathin nanobelt arrays | H2O2-asisted dissolution/precipitation process | Anatase | Thickness: 1–2 nm and a high specific surface area: 193 cm3 g−1 | [96] |
| Oriented assembled TiO2 hierarchical nanowire arrays | Solvothermal method for trunks and hydrothermal treatment for branches | Anatase | A length of branches: 70 nm and an average thickness: 3 μm | [88] |
| Hierarchically tunable TiO2 nanoarrays | Acid vapor oxidation | Rutile | Branches diameter: 100 nm, length: 300 nm and trees reach 3 μm in height and have a diameter of 280 nm | [104] |
| TiO2 nanowire arrays with F-decorated TiO2 nanoparticles | H2O2-asisted dissolution/precipitation process | Anatase | Diameter: 100 nm and the thickness: 2.3 μm | [105] |
| Branched hierarchical TiO2 nanotubes on TiO2 nanorod arrays | Hydrothermal method and sol–gel method | Anatase for trunks and rutile for branches | Branches diameter: 100 nm, length: 500 nm and nanorods reach 1 μm in height and have a diameter of 50–100 nm | [25] |
| Single-crystal-like TiO2 hierarchical nanowire arrays | Hydrothermal synthesis and solvothermal method | Anatase | Diameter: 130–180 nm and the size of nanoparticles is 10–20 nm | [115] |
| Types of TiO2 Nanostructured Arrays | Current Density | Specific Capacitance | Cycle Number and Retention | Ref. |
|---|---|---|---|---|
| TiO2 nanowire arrays | 70 mA g−1 | 320 mAh g−1 | 230 mAh g−1 (20th) | [174] |
| Amorphous TiO2 nanotube on Si | 5 μA cm−2 | 196 μAh cm−2 | 56 μAh cm−2 (50th) | [175] |
| Crystalline TiO2 nanotube on Si | 5 μA cm−2 | 165 μAh cm−2 | 40 μAh cm−2 (50th) | [175] |
| Amorphous TiO2 nanotube on Ti foil | 5 μA cm−2 | 129 μAh cm−2 | 37 μAh cm−2 (50th) | [175] |
| Crystalline TiO2 nanotube on Ti foil | 5 μA cm−2 | 83 μAh cm−2 | 29 μAh cm−2 (50th) | [175] |
| TiN@TiO2 nanowire arrays | 0.11 mA cm−2 | 0.156 mAh cm−2 | 0.151 mAh cm−2 (300th) | [113] |
| Rutile TiO2 nanorod arrays | 15 μA cm−2 | 133 μAh cm−2 | 130 μAh cm−2 (50th) | [46] |
| Hydrogenated TiO2 nanotube arrays | 200 μA cm−2 | 0.2 mAh cm−2 | 0.18 mAh cm−2 (100th) | [21] |
| TiO2 nanotube arrays annealed in N2 | 320 mA g−1 | 240 mAh g−1 | 170 mAh g−1 (50th) | [172] |
| Self-organized TiO2 nanotubes | 5 µA cm−2 | 0.14 mAh cm−2 | 0.07 mAh cm−2 (50th) | [176] |
| TiO2 nanotube arrays annealed in CO | 320 mA g−1 | 223 mAh g−1 | 179 mAh g−1 (50th) | [173] |
| Self-organized amorphous TiO2 nanotube arrays | 10 μA cm−2 | 103 μAh cm−2 | 101μAh cm−2 (100th) | [22] |
| TiO2 nanotubes (from Amorphous to Cubic Phase) | 7 A g−1 | 230 mAh g−1 | 220 mAh g−1 (600th) | [177] |
| TiO2 nanotrees | 1.0 mA cm−2 | 159 mAh cm−2 | 152 mAh cm−2 (400th) | [154] |
| Sandwich-like, stacked TiO2 nanosheets | 10 C | 175 mAh g−1 | 160 mAh g−1 (150th) | [178] |
| Dual-phase Li4Ti5O12-TiO2 nanowire arrays | 10 C | 135.5 mAh g−1 | 129.3 mAh g−1 (100th) | [179] |
| TiO2@α-Fe2O3 core/shell arrays | 120 mA g−1 | 475 mAh g−1 | 480 mAh g−1 (150th) | [180] |
| Sn/SnO@TiO2 nanowire arrays | 50 μA cm−2 | 140 μAh cm−2 | 120 μAh cm−2 (50th) | [169] |
| Sn-doping TiO2 nanotube arrays | 70 μA cm−2 | 70 μAh cm−2 | 62 μAh cm−2 (50th) | [181] |
| TiO2-MoO3 Core-Shell nanowire array | 250 mA g−1 | 600 mAh g−1 | 500 mAh g−1 (100th) | [182] |
| SnO2 nanocrystals@TiO2 nanotubes | 20 μA cm −2 | 55 μAh cm−2 | 35 μAh cm−2 (100th) | [183] |
| Coaxial SnO2@TiO2 nanotube array | 100 μA cm−2 | 225 μAh cm−2 | 150 μAh cm−2 (50th) | [184] |
| TiO2 nanotubes with Co3O4/NiO particles | 70 μAh cm−2 | 110 μAh cm−2 | 103 μAh cm−2 (25th) | [185] |
| Nitridated TiO2 hollow nanofibers | 0.2 C | 180 mAh g−1 | 170 mAh g−1 (100th) | [186] |
| Sealed TiO2 nanotubes array | 0.2 C | 190 mAh g−1 | 185 mAh g−1 (100th) | [82] |
| unsealed TiO2 nanotubes array | 0.2 C | 195 mAh g−1 | 190 mAh g−1 (100th) | [82] |
| randomly oriented TiO2 nanotubes | 0.2 C | 140 mAh g−1 | 139 mAh g−1 (100th) | [82] |
| Ordered mesoporous TiO2-C nanocomposite | 1 C | 175 mAh g−1 | 166 mAh g−1 (900th) | [187] |
| Mesoporous CNT@TiO2-C nanocable | 50 C | 150 mAh g−1 | 127 mAh g−1 (2000th) | [188] |
| TiO2@SnO2 nanoflake nanotube arrays | 1.6 A g−1 | 620 mAh g−1 | 530 mAh g−1 (50th) | [189] |
| SnO2@TiO2 heterojunction nanotubes | 20 μA cm−2 | 50 μAh cm−2 | 35 μAh cm−2 (30th) | [190] |
| SnO2@TiO2 hollow microtubes (array) | 200 mA g−1 | 900 mAh g−1 | 800 mAh g−1 (100th) | [36] |
| SnO2@TiO2 double-shell nanotubes (array) | 1500 mA g−1 | 250 mAh g−1 | 232 mAh g−1 (30th) | [191] |
| NiO@TiO2 nanotube heterojunction arrays | 0.02 mA cm−2 | 325 μAh cm−2 | 275 μAh cm−2 (10th) | [192] |
| Anatase TiO2 ultrathin nanobelts | 1 C | 204 mAh g−1 | 198 mAh g−1 (60th) | [96] |
| Oriented anatase TiO2 nanotube arrays | 0.25 C | 250 mAh g−1 | 190 mAh g−1(10th) | [193] |
| Types of TiO2 Nanostructured Arrays | Current Density | Specific Capacitance | Cycle Number and Retention | Ref. |
|---|---|---|---|---|
| Sulfur-doped TiO2 nanotube arrays | 10 C | 140 mAh g−1 | 130 mAh g−1 (4400th) | [206] |
| Ni/N-doped anatase TiO2 nanotube | 50 mA g−1 | 310 mAh g−1 | 303 mAh g−1 (500th) | [19] |
| Amorphous TiO2 nanotube | 0.05 A g−1 | 100 mAh g−1 | 140 mAh g−1 (50th) | [207] |
| Crystalline (anatase) TiO2 nanotubular arrays | 0.1 mA cm–2 | 125 μAh cm–2 | 115 μAh cm–2 (50th) | [199] |
| Monolithic anatase TiO2 nanotube arrays | C/5 | 161 mAh g−1 | 156 mAh g−1 (350th) | [201] |
| Ni-TiO2 core-shell nanoarrays | 50 mA g−1 | 250 mAh g−1 | 200 mAh g−1 (100th) | [203] |
| TiO2-B/MoS2 nanoarrays | C/10 | 350 mAh g−1 | 191 mAh g−1 (100th) | [204] |
| Surface phosphorylated TiO2 nanotube arrays | 67 mA g−1 | 334 mAh g−1 | 270 mAh g−1 (100th) | [205] |
| Types of TiO2 Nanostructured Arrays | Current Density | Specific Capacitance | Cycle Number and Retention | Ref. |
|---|---|---|---|---|
| 3D-1D TiO2 microflowers | 5 mV s−1 | 66.50 F g−1 | 54.09 F g−1 (2000th) | [227] |
| Oriented NiO-TiO2 nanotube arrays | 0.4 mA cm−2 | 2.6 F cm−2 | 3.0 F cm−2 (500th) | [226] |
| Highly ordered TiO2 nanotube array | 1 mV s−1 | 911 μF cm−2 | 600 μF cm−2 (500th) | [211] |
| The reduced MnCo2O4 TiO2 nanotube arrays by introduction of oxygen vacancies | 1 mA cm−2 | 20 mF cm−2 | 18 mF cm−2 (5000th) | [20] |
| Pristine TiO2 nanotube arrays | 50 mV s−1 | 2.4 mF cm−2 | 2.0 mF cm−2 (1000th) | [210] |
| Plasma treatment TiO2 nanotube arrays | 2 mA cm−2 | 7.22 mF cm−2 | 7.0 mF cm−2 (10000th) | [220] |
| Electrochemical reduction TiO2 nanotube arrays | 0.01 mA cm−2 | 4 mF cm−2 | 3.8 mF cm−2 (5000th) | [223] |
| Hydrogenation TiO2 nanotube arrays | 10 mVs−1 | 24 mF cm−2 | 8 mF cm−2 (1000th) | [229] |
| Black TiO2 nanotube arrays | 10 mV s−1 | 20 mF cm−2 | 18 mF cm−2 (100th) | [224] |
| MnO2/TiO2 nanotube arrays | 100 mV s−1 | 1.8 mF cm−2 | 1.7 mF cm−2 (100th) | [221] |
| MnO2/TiO2 nanotube arrays | 100 mV s−1 | 101 mF cm−2 | 95 mF cm−2 (100th) | [230] |
| RuO2/TiO2 nanotube arrays | 5 mV s−1 | 31.82 F g−1 | 28 F g−1 (100th) | [231] |
| NiO/TiO2 nanotube arrays | 0.5 mA cm−2 | 72.7 mF cm−2 | 60.2 mF cm−2 (100th) | [232] |
| ZnO/TiO2 nanotube arrays | 20 mV s−1 | 302 F g−1 | 278 F g−1 (100th) | [233] |
| MoO3/TiO2 nanotube arrays | 5 mV s−1 | 209.6 mF cm−2 | 201.5 mF cm−2 (100th) | [233] |
| BiFeO3/TiO2 nanotube arrays | 1.1 A g−1 | 440 F g−1 | 423 F g−1 (100th) | [234] |
| V2O5/TiO2 nanotube arrays | 0.2 mA cm−2 | 220 F g−1 | 210 F g−1 (100th) | [235] |
| MWCNT/TiO2 nanotube arrays | 0.1 mA cm−2 | 4.4 mF cm−2 | 3.9 mF cm−2 (100th) | [236] |
| BDD/TiO2 nanotube arrays | 10 mV s−1 | 7.46 mF cm−2 | 7.01 mF cm−2 (100th) | [237] |
| C Nanorod/TiO2 nanotube arrays | 0.2 mA cm−2 | 40.75 mF cm−2 | 35.7 mF cm−2 (100th) | [238] |
| PANI/TiO2 nanotube arrays | 0.6 A g−1 | 993 F g−1 | 863 F g−1 (100th) | [239] |
| PTh/TiO2 nanotube arrays | 2 A g−1 | 640 F g−1 | 580 F g−1 (1000th) | [240] |
| MnO2/TiO2/CNT nanotube arrays | 2.6 A g−1 | 580 F g−1 | 550 F g−1 (100th) | [241] |
| Ni-Co/TiO2 nanotube arrays | 2.5 A g−1 | 2353 F g−1 | 2153 F g−1 (3000th) | [242] |
| Pd/PANI/TiO2 nanotube arrays | 2.0 A g−1 | 1060 F g−1 | 980 F g−1 (100th) | [243] |
| PANI/APTES/TiO2 nanotube arrays | 0.5 A g−1 | 380 F g−1 | 340 F g−1 (1000th) | [244] |
| Nitrogen doping TiO2 nanobelts | 1 A g−1 | 216 F g−1 | 198 F g−1 (10000th) | [245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, P.; Zheng, Z.; Gu, Y.; Geng, C.; Guo, Z.; Qin, J.; Wen, W. Nanostructured TiO2 Arrays for Energy Storage. Materials 2023, 16, 3864. https://doi.org/10.3390/ma16103864
Si P, Zheng Z, Gu Y, Geng C, Guo Z, Qin J, Wen W. Nanostructured TiO2 Arrays for Energy Storage. Materials. 2023; 16(10):3864. https://doi.org/10.3390/ma16103864
Chicago/Turabian StyleSi, Pingyun, Zhilong Zheng, Yijie Gu, Chao Geng, Zhizhong Guo, Jiayi Qin, and Wei Wen. 2023. "Nanostructured TiO2 Arrays for Energy Storage" Materials 16, no. 10: 3864. https://doi.org/10.3390/ma16103864
APA StyleSi, P., Zheng, Z., Gu, Y., Geng, C., Guo, Z., Qin, J., & Wen, W. (2023). Nanostructured TiO2 Arrays for Energy Storage. Materials, 16(10), 3864. https://doi.org/10.3390/ma16103864






