Dual Studies of Photo Degradation and Adsorptions of Congo Red in Wastewater on Graphene–Copper Oxide Heterostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterizations
3. Results and Discussion
3.1. XRD Investigations
3.2. Raman Spectroscopy
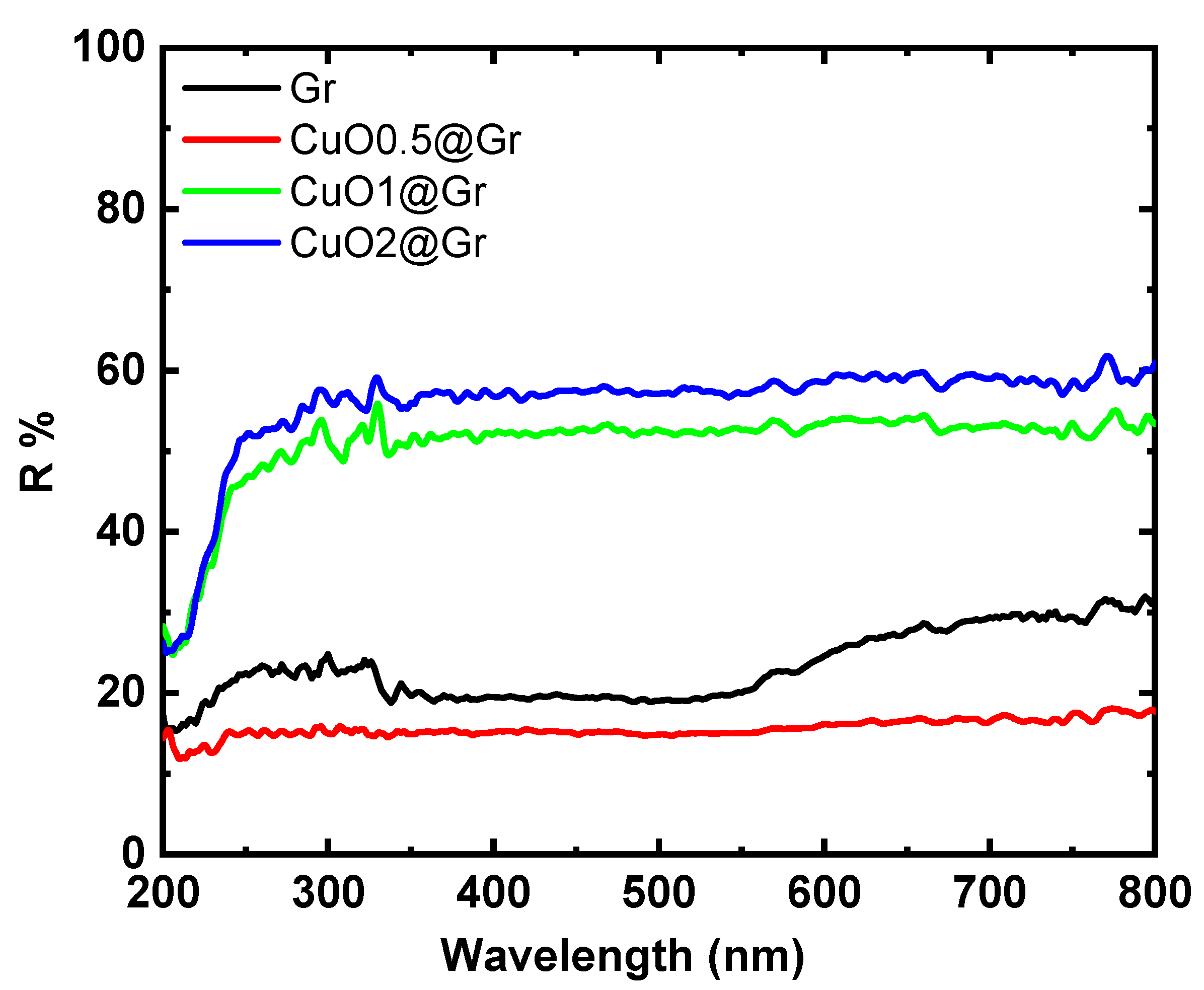
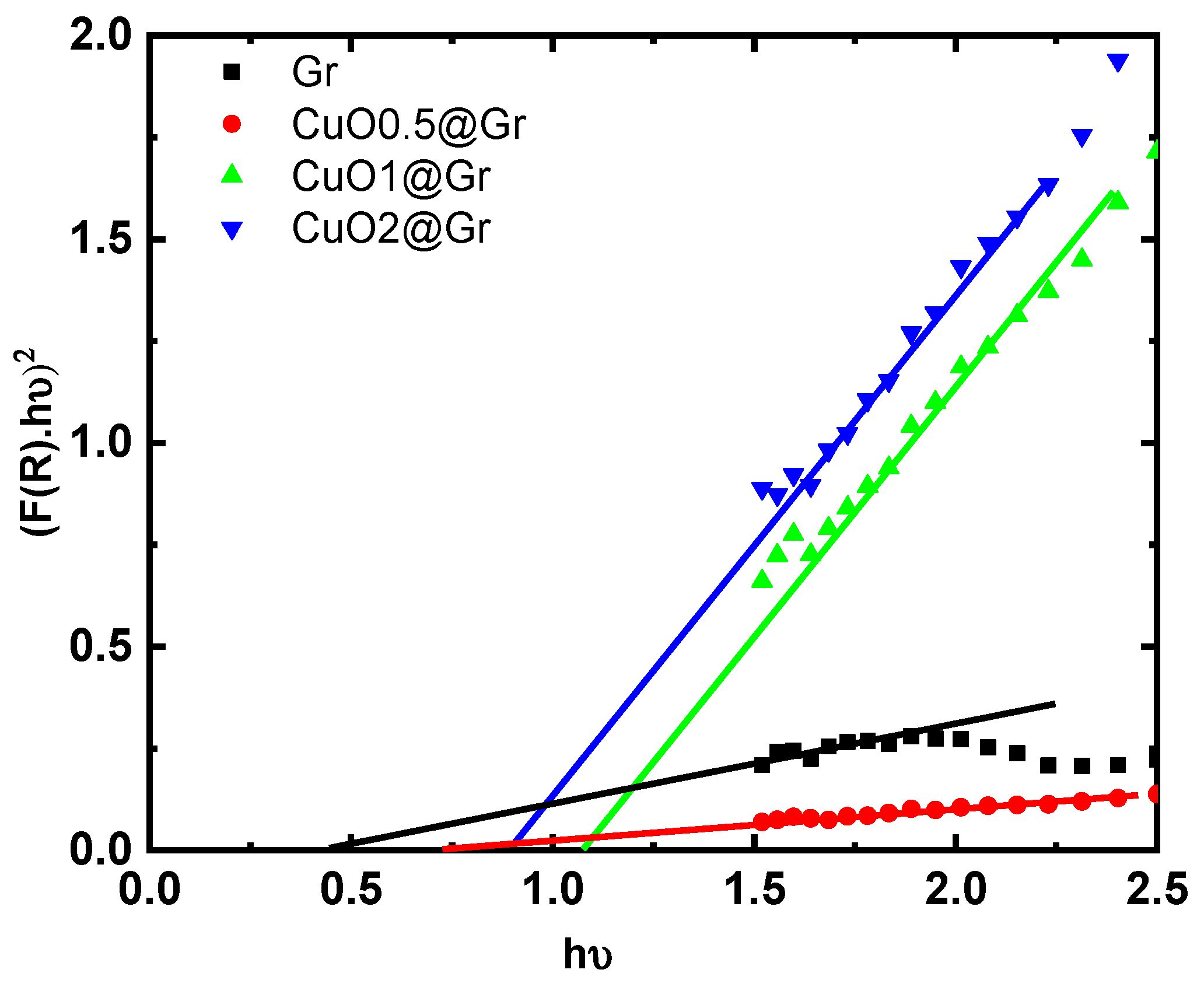
3.3. Optical Investigations
3.4. Catalyst Studies
3.4.1. Photocatalytic Studies
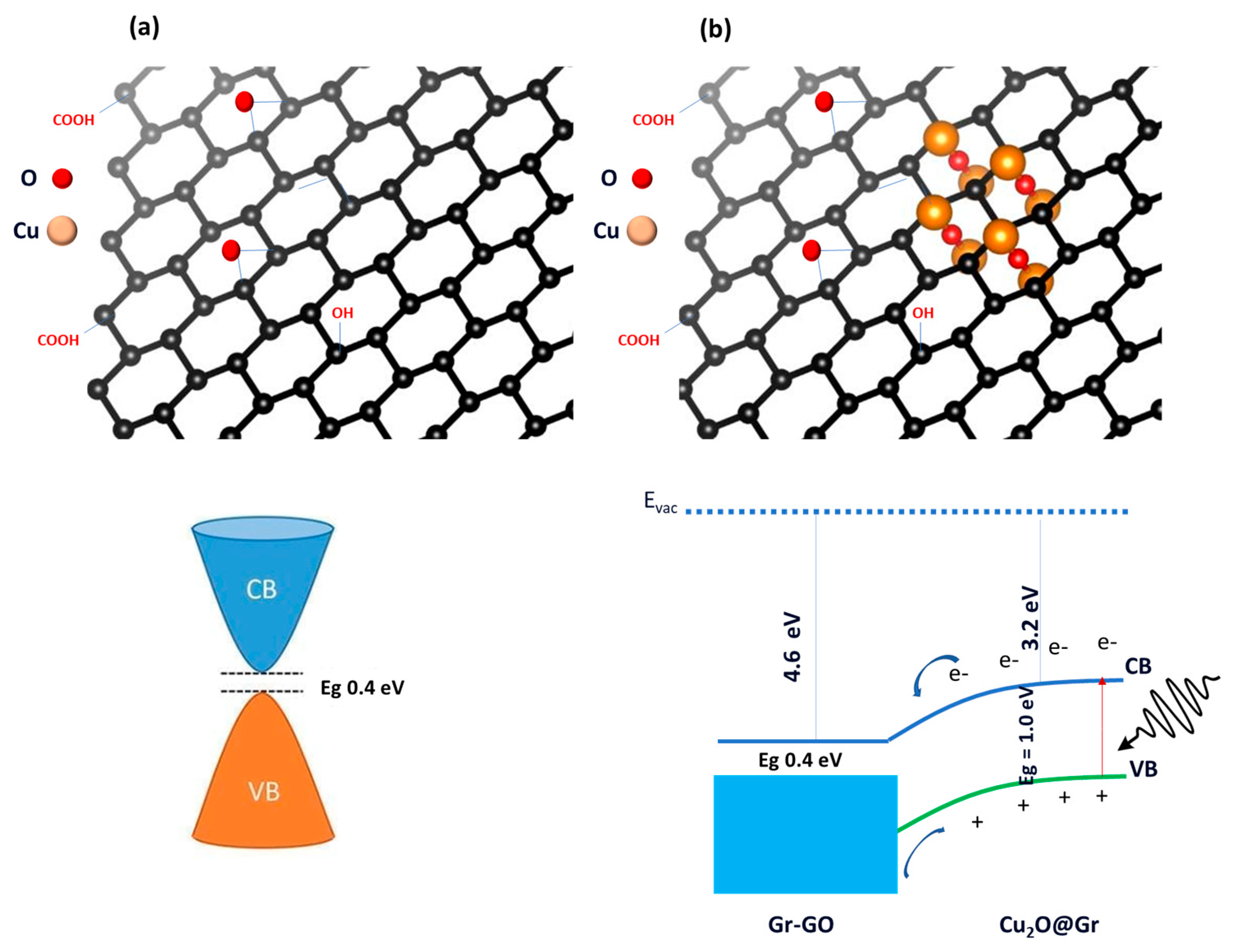
3.4.2. Photocatalysis Mechanism
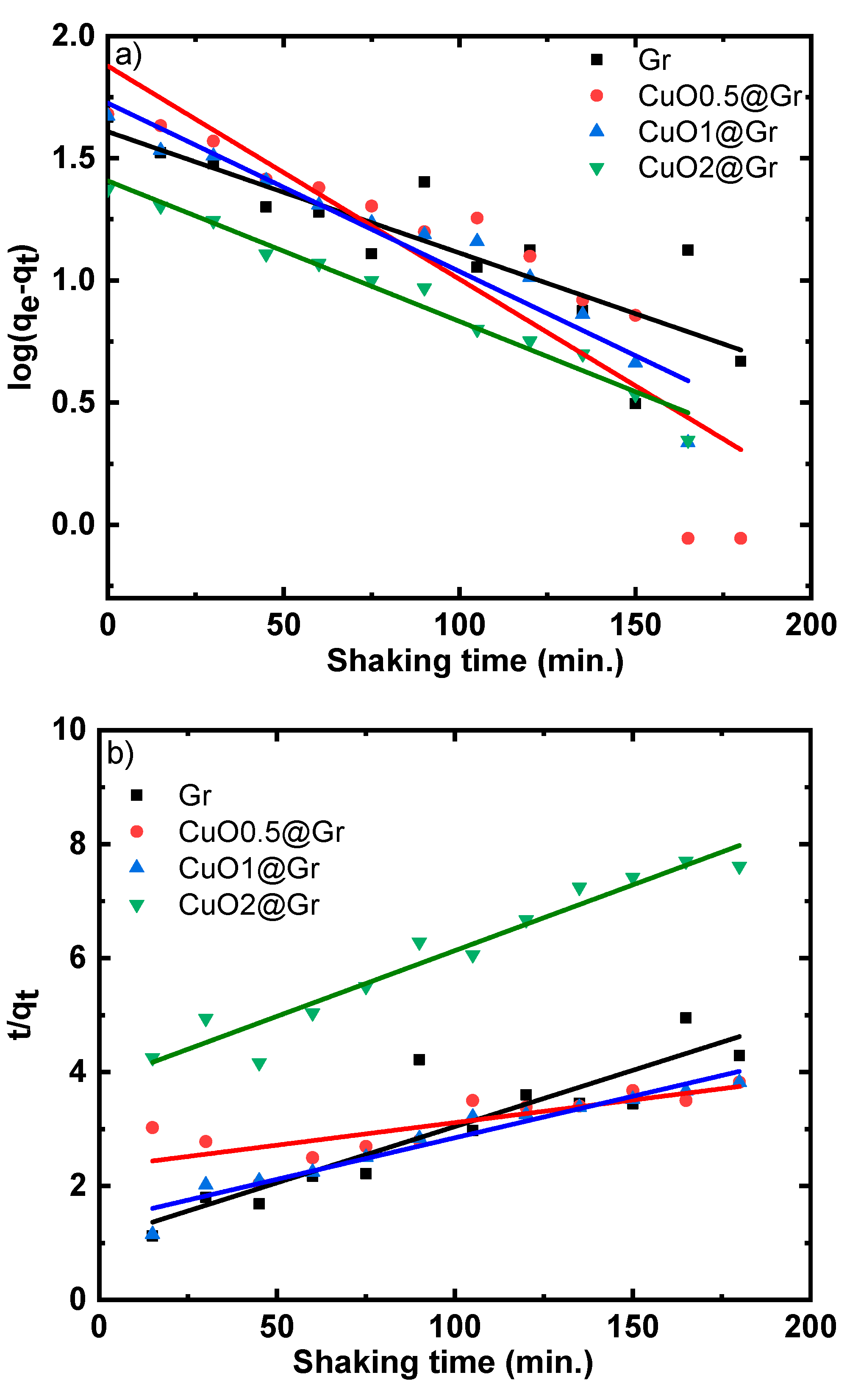
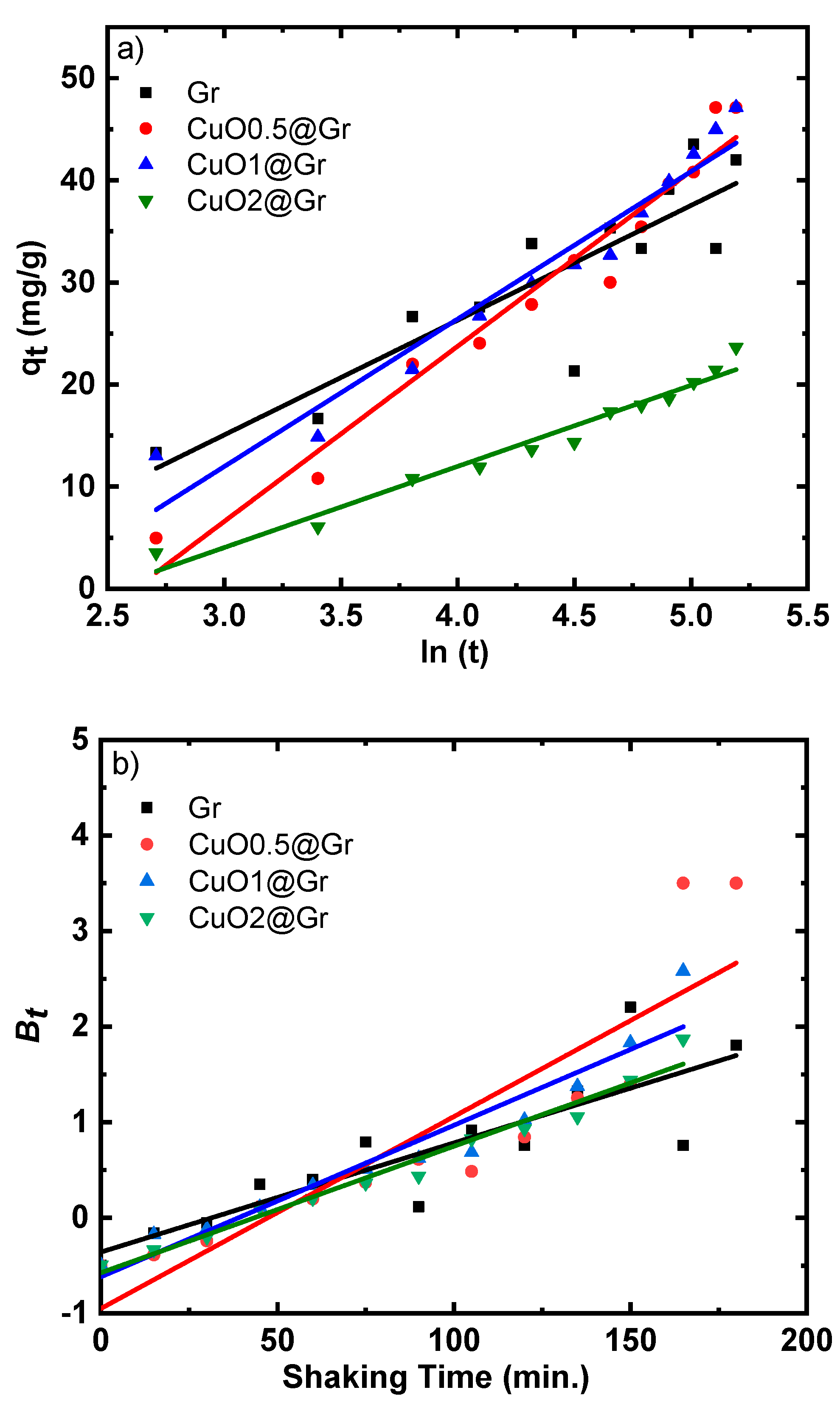
3.4.3. Adsorption Studies
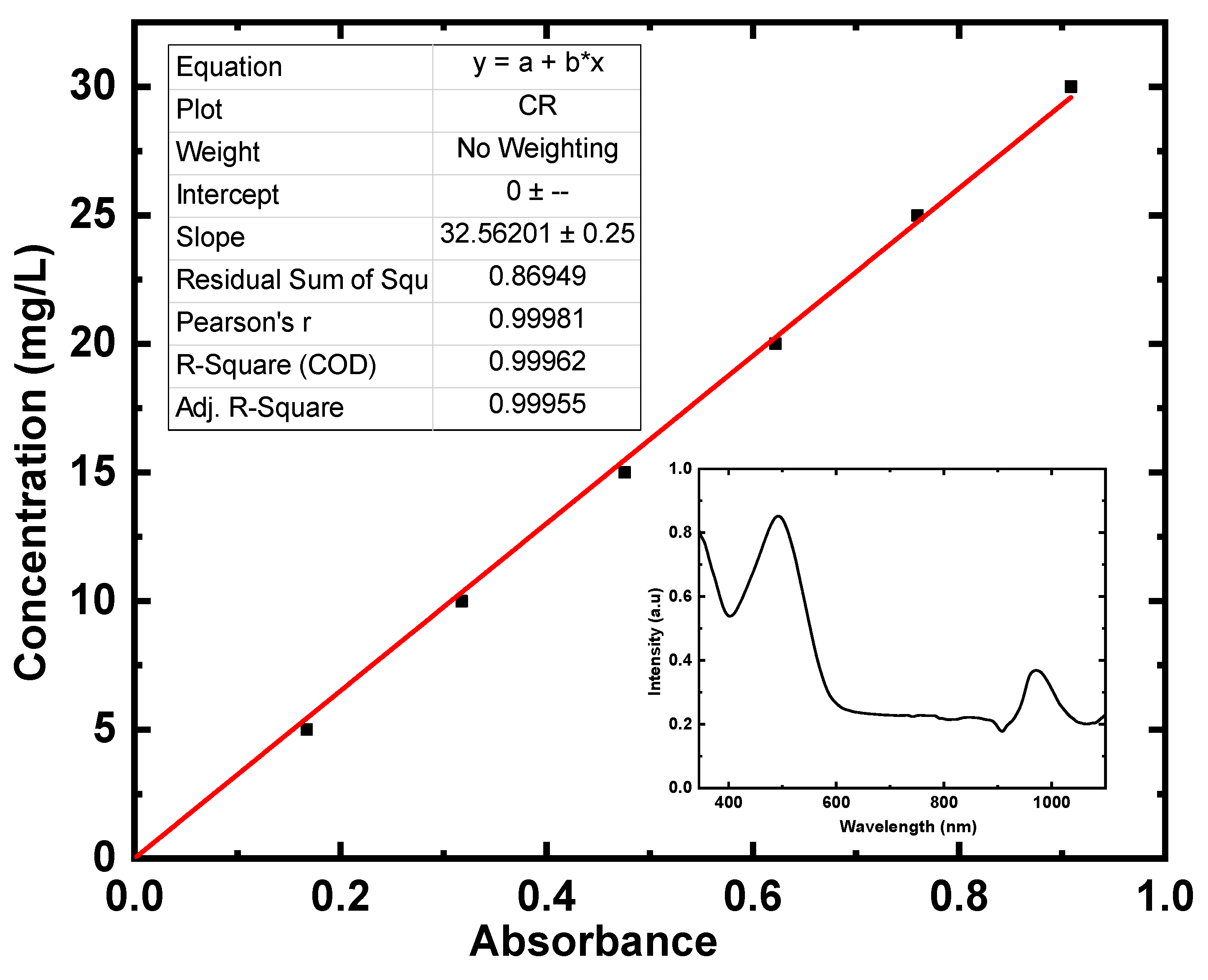
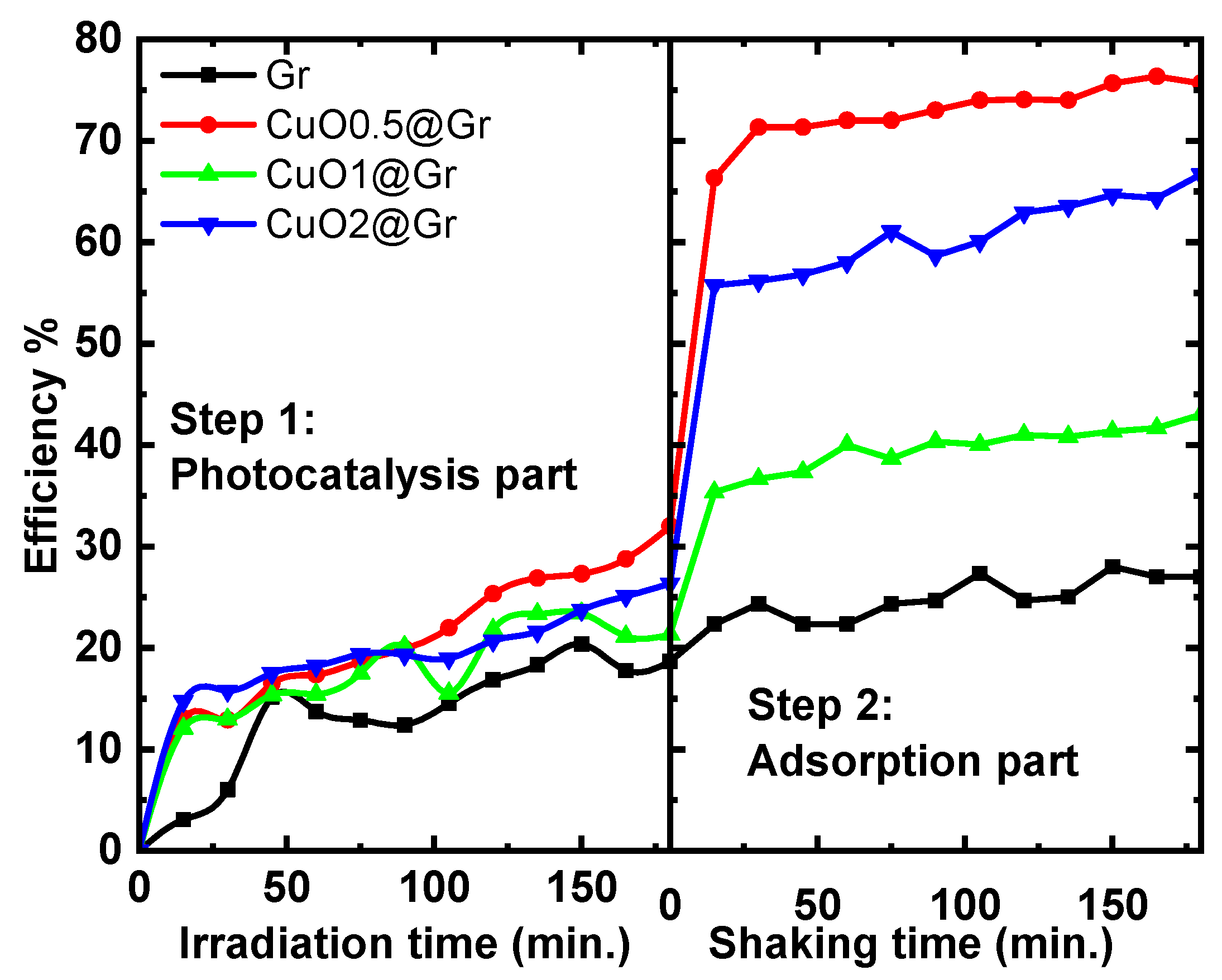
3.4.4. Dual Studies of Photo Degradation and Adsorptions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, A.; Altaf, S.; Ali, S.; Ikram, M.; Li, G. Recent advances in carbonaceous sustainable nanomaterials for wastewater treatments. Sustain. Mater. Technol. 2022, 32, e00406. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, Z.; Zhang, J.; Wei, Z.; Guo, Q.; Jin, H.; Tang, H.; Li, S.; Pan, X.; Su, Z.; et al. Recent advances in solar-driven CO2 reduction over g-C3N4-based photocatalysts. Carbon Energy 2022, 5, e205. [Google Scholar]
- Pang, X.; Xue, S.; Zhou, T.; Xu, Q.; Lei, W. 2D/2D nanohybrid of Ti3C2 MXene/WO3 photocatalytic membranes for efficient water purification. Ceram. Int. 2022, 48, 3659–3668. [Google Scholar] [CrossRef]
- Rizzo, L.; Selcuk, H.; Nikolaou, A.D.; Meriç Pagano, S.; Belgiorno, V. A comparative evaluation of ozonation and heterogeneous photocatalytic oxidation processes for reuse of secondary treated urban wastewater. Desalin. Water Treat. 2014, 52, 1414–1421. [Google Scholar] [CrossRef]
- Jamal, N.; Radhakrishnan, A.; Raghavan, R.; Bhaskaran, B.; Access, O. Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation. Main Group Met. Chem. 2020, 43, 84–91. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Wang, M.; Zhang, X.; Liao, L.; Fang, T.; Li, B. Photocatalytic degradation of Congo red by using the Cu2O/α-Fe2O3 composite catalyst. Desalin. Water Treat. 2021, 215, 222–231. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting graphene oxide–years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C. 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A review of the mechanical and thermal properties of graphene and its hybrid polymer nanocomposites for structural applications. J. Mater. Sci. 2019, 54, 5992–6026. [Google Scholar] [CrossRef]
- Stanford, M.G.; Yang, K.; Chyan, Y.; Kittrell, C.; Tour, J.M. Laser-Induced Graphene for Flexible and Embeddable Gas Sensors. ACS Nano 2019, 13, 3474–3482. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5–12. [Google Scholar] [CrossRef]
- Zheng, X.G.; Xu, C.N.; Tomokiyo, Y.; Tanaka, E.; Yamada, H.; Soejima, Y. Observation of charge stripes in cupric oxide. Phys. Rev. Lett. 2000, 85, 5170. [Google Scholar] [CrossRef]
- O’keeffe, M.; Stone, F.S. The magnetic susceptibility of cupric oxide. J. Phys. Chem. Solids 1962, 23, 261–266. [Google Scholar] [CrossRef]
- Arbuzova, T.I.; Smolyak, I.B.; Naumov, S.V.; Samokhvalov, A.A. Effect of doping on the magnetic properties of the low-dimensional antiferromagnet CuO. Phys. Solid State 1998, 40, 1702–1705. [Google Scholar] [CrossRef]
- Muraleedharan, K.; Subramaniam, C.; Venkataramani, N.; Rao, T.G.; Srivastava, C.; Sankaranarayanan, V.; Srinivasan, R. On the magnetic susceptibility of CuOx. Solid State Commun. 1990, 76, 727–730. [Google Scholar] [CrossRef]
- Rao, G.N.; Yao, Y.D.; Chen, J.W. Superparamagnetic behavior of antiferromagnetic CuO nanoparticles. IEEE Trans. Magn. 2005, 41, 3409–3411. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Abdel-Rahim, M.A. CuO nanoparticles synthesized by microwave-assisted method for methane sensing. Opt. Quantum Electron. 2016, 48, 2–12. [Google Scholar] [CrossRef]
- Li, Y.; Xie, W.; Hu, X.; Shen, G.; Zhou, X.; Xiang, Y.; Zhao, X.; Fang, P. Comparison of dye photodegradation and its coupling with light-to-electricity conversion over TiO2 and ZnO. Langmuir 2010, 26, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.M.; Hamad, D.; Aljaafari, A.; Abdel-Latief, A.Y.; Abdel-Rahim, M.A. Preparation and Characterization of Developed CuxSn1−xO2 Nanocomposite and Its Promising Methane Gas Sensing Properties. Sensors 2019, 19, 2257. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, P.E.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Kelly, J.J. Charge carrier dynamics in illuminated, particulate ZnO electrodes. J. Phys. Chem. B 2000, 104, 7686–7693. [Google Scholar] [CrossRef]
- Kotan, G.; Kardaş, F.; Yokuş, A.; Akyıldırım, O.; Saral, H.; Eren, T.; Yola, M.L.; Atar, N. A novel determination of curcumin via Ru@Au nanoparticle decorated nitrogen and sulfur-functionalized reduced graphene oxide nanomaterials. Anal. Methods 2016, 8, 401–408. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N.; Eren, T.; Karimi-Maleh, H.; Wang, S. Correction: Sensitive and selective determination of aqueous triclosan based on gold nanoparticles on polyoxometalate/reduced graphene oxide nanohybrid. RSC Adv. 2015, 5, 72590–72591. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kalaivani, S.; Joice, J.A.I.; Sivakumar, T. Photocatalytic activity of multielement doped TiO2 in the degradation of congo red. Appl. Surf. Sci. 2012, 258, 2515–2521. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Rong, X.; Zhu, X.; Zhang, X. Preparation of hierarchical micro/nanostructured Bi2S3-WO3 composites for enhanced photocatalytic performance. J. Alloys Compd. 2016, 685, 812–819. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Gong, Z.; Tao, J.; Sun, Z.; Lv, J.; Chen, X.; Jiang, X.; He, G.; Wang, P.; et al. Enhanced charge collection and photocatalysis performance of CdS and PbS nanoclusters co-sensitized TiO2 porous film. J. Alloys Compd. 2015, 649, 190–195. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.-T.; Hong, L.-F.; Ji, X.-Y.; Lin, Z.-D.; Li, Z.-S.; Pan, W.-G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Li, F.; Cheng, L.; Fan, J.; Xiang, Q. Steering the behavior of photogenerated carriers in semiconductor photocatalysts: A new insight and perspective. J. Mater. Chem. A 2021, 9, 23765–23782. [Google Scholar] [CrossRef]
- Rashad, M.; Helali, S.; Issa, S.; Al-Ghamdi, S.; Alsharif, M.; Alzahrani, A.O.; Sobhi, M.; Ene, A.; Abd-Elnaiem, A.M. Adsorption Study of Congo Red Dye from Synthetic Wastewater at Different Concentrations Using Zinc Sulfide Nanoparticles. Materials 2022, 15, 5048. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Zhang, H.; Baeyens, J. Adsorption of Congo red dye on FexCo3−xO4 nanoparticles. J. Environ. Manag. 2019, 238, 473–483. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Awada, C. Synergistic Effect of NiO-Ga2O2-Graphene Heterostructures on Congo Red Photodegradation in Water. Separations 2022, 9, 201. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.; Jung, W. Facile and safe graphene preparation on solution based platform Journal of Industrial and Engineering Chemistry Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Panzeri, G.; Cristina, M.; Jagadeesh, M.S.; Bussetti, G.; Magagnin, L. Modification of large area Cu2O/CuO photocathode with CuS non-noble catalyst for improved photocurrent and stability. Sci. Rep. 2020, 10, 18730. [Google Scholar] [CrossRef] [PubMed]
- Pitigala, D. Electronic and structural properties of Cu2O polycrystalline thin films grown on adhesive copper tape Electronic and structural properties of Cu2O polycrystalline thin films grown on adhesive copper tape. In Proceedings of the 35th Technical Sessions of the Institute of Physics Sri Lanka, Colombo, Sri Lanka, 8–10 October 2019. [Google Scholar]
- Trusovas, R.; Ratautas, K.; Raciukaitis, G.; Barkauskas, J.; Stankevičienė, I.; Niaura, G.; Mažeikienė, R. Reduction of graphite oxide to graphene with laser irradiation. Carbon 2013, 52, 574–582. [Google Scholar] [CrossRef]
- Petridis, C.; Lin, Y.-H.; Savva, K.; Eda, G.; Kymakis, E.; Anthopoulos, T.D.; Stratakis, E. Post-fabrication, in situ laser reduction of graphene oxide devices. Appl. Phys. Lett. 2013, 102, 093115. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Varghese, B.; Tang, L.A.L.; Tan, C.K.; Sow, C.; Loh, K.P. Microstructuring of graphene oxide nanosheets using direct laser writing. Adv. Mater. 2010, 22, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.A.; Shepperd, K.R.; Orlando, T.M. Formation of graphene features from direct laser-induced reduction of graphite oxide. J. Phys. Chem. Lett. 2010, 1, 2633–2636. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Kumar, S.; Melaibari, A.; Hasan, P.M.Z.; Aljaafari, A. Monitoring Food Spoilage Based on a Defect-Induced Multiwall Carbon Nanotube Sensor at Room Temperature: Preventing Food Waste. ACS Omega 2020, 5, 30531–30537. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon N. Y. 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Saber, O.; Ahmed, F.; Aljaafari, A.; Kumar, S. Growth of defect-induced carbon nanotubes for low-temperature fruit monitoring sensor. Chemosensors 2021, 9, 131. [Google Scholar] [CrossRef]
- Kottappara, R.; Palantavida, S.; Pillai, S.C.; Vijayan, B.K. Composition tuning in copper oxide decorated reduced graphene oxide yields efficient photo- and reduction catalysts. Surf. Interfaces 2021, 22, 100792. [Google Scholar] [CrossRef]
- Jahan, F.; Islam, M.H.; Smith, B.E. Band gap and refractive index determination of Mo-black coatings using several techniques. Sol. Energy Mater. Sol. Cells 1995, 37, 283–293. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Y.; Yan, X. Electronic structure and optical properties of Graphene Monoxide. arXiv 2012, arXiv:1209.0555. [Google Scholar]
- Rashad, M.; Shaalan, N.M.; Abd-Elnaiem, A.M. Degradation enhancement of methylene blue on ZnO nanocombs synthesized by thermal evaporation technique. Desalin. Water Treat. 2016, 57, 26267–26273. [Google Scholar] [CrossRef]
- Ramar, V.; Balasubramanian, K. Reduced graphene oxide/WO3 nanorod composites for photocatalytic degradation of methylene blue under sunlight irradiation. ACS Appl. Nano Mater. 2021, 4, 5512–5521. [Google Scholar] [CrossRef]
- Langmuir, I. Part II.—“Heterogeneous reactions”. Chemical reactions on surfaces. Trans. Faraday Soc. 1922, 17, 607–620. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at well-defined surfaces. Surf. Sci. 1994, 299/300, 742–754. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Zhang, H.; Wang, H.; Cai, H.; Zhang, Y.; Yang, Z. Electron transfer mechanism of graphene/Cu heterostructure for improving the stability of triboelectric nanogenerators. Nano Energy 2020, 70, 104540. [Google Scholar] [CrossRef]
- Meyer, J.; Kidambi, P.R.; Bayer, B.C.; Weijtens, C.; Kuhn, A.; Centeno, A.; Pesquera, A.; Zurutuza, A.; Robertson, J.; Hofmann, S. Metal oxide induced charge transfer doping and band alignment of graphene electrodes for efficient organic light emitting diodes. Sci. Rep. 2014, 4, 5380. [Google Scholar] [CrossRef]
- Brandt, R.E.; Young, M.; Park, H.H.; Dameron, A.; Chua, D.; Lee, Y.S.; Teeter, G.; Gordon, R.G.; Buonassisi, T. Band offsets of n-type electron-selective contacts on cuprous oxide (Cu2O) for photovoltaics. Appl. Phys. Lett. 2014, 105, 263901. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Economou, N.A.; Trivich, D. Heterojunction solar cells on cuprous oxide. Sol. Cells 1981, 3, 73–80. [Google Scholar] [CrossRef]
- Asiri, A.M.; Al-Amoudi, M.S.; Al-Talhi, T.A.; Al-Talhi, A.D. Photodegradation of Rhodamine 6G and phenol red by nanosized TiO2 under solar irradiation. J. Saudi Chem. Soc. 2011, 15, 121–128. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Bhavsar, K.S.; Labhane, P.K.; Murade, V.D.; Dhake, R.B.; Sonawane, G.H. A photocatalyst: Zinc sulfides nanospheres immobilized on activated carbon for the abatement of aquatic organic pollutants. Inorg. Chem. Commun. 2021, 133, 108958. [Google Scholar] [CrossRef]
- Al-Aoh, H.A.; Maah, M.J.; Yahya, R.; Abas, M.R.B. A comparative investigation on adsorption performances of activated carbon prepared from coconut husk fiber and commercial activated carbon for acid red 27 dye. Asian J. Chem. 2013, 25, 9582. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, G.; Özer, A. Biosorption of Acid Red 274 (AR 274) on Dicranella varia: Determination of equilibrium and kinetic model parameters. Process. Biochem. 2005, 40, 3559–3568. [Google Scholar] [CrossRef]
- Rashad, M.; L-Aoh, H.A.A. Promising adsorption studies of bromophenol blue using copper oxide nanoparticles. Desalination Water Treat. 2019, 139, 360–368. [Google Scholar] [CrossRef]







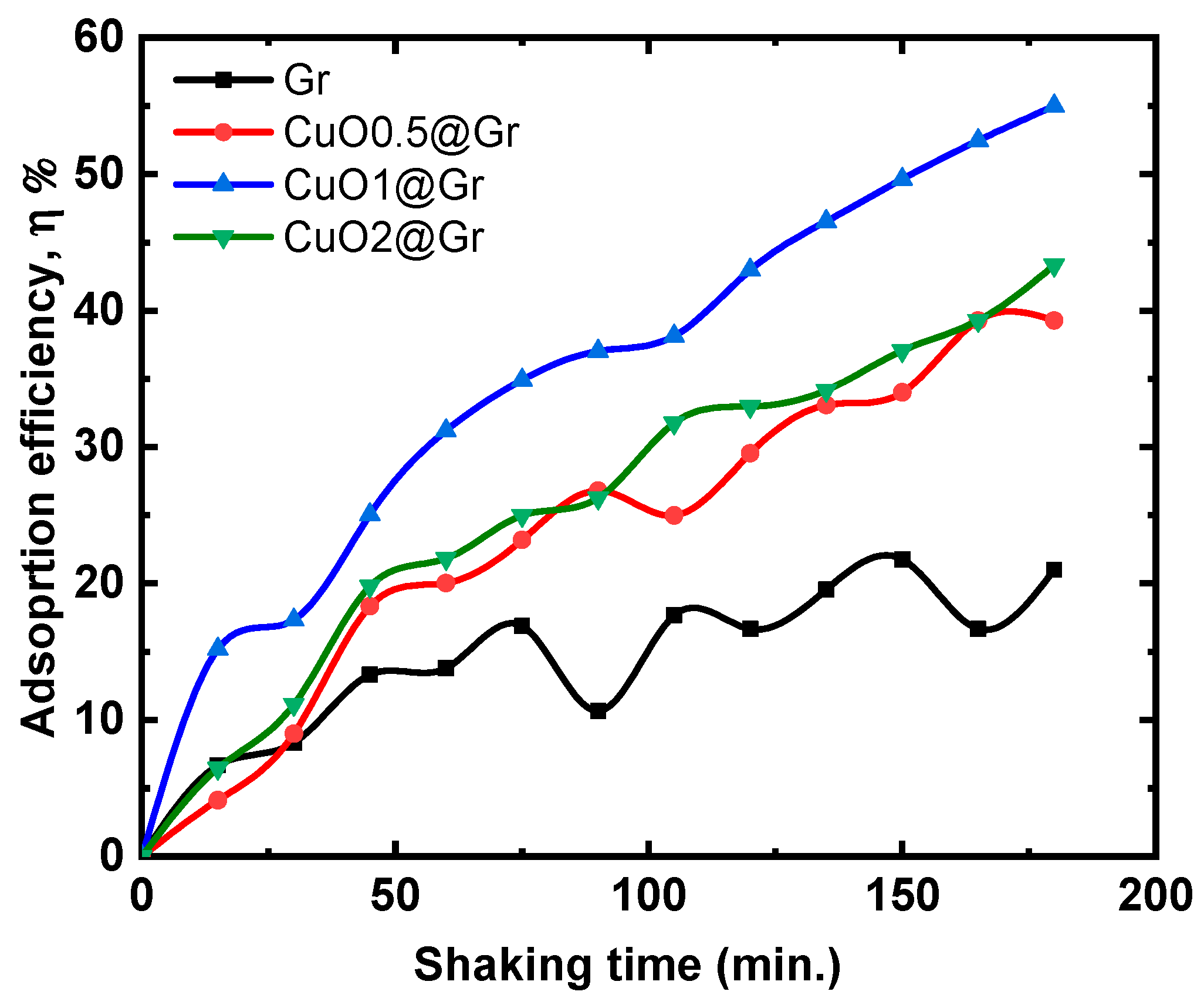
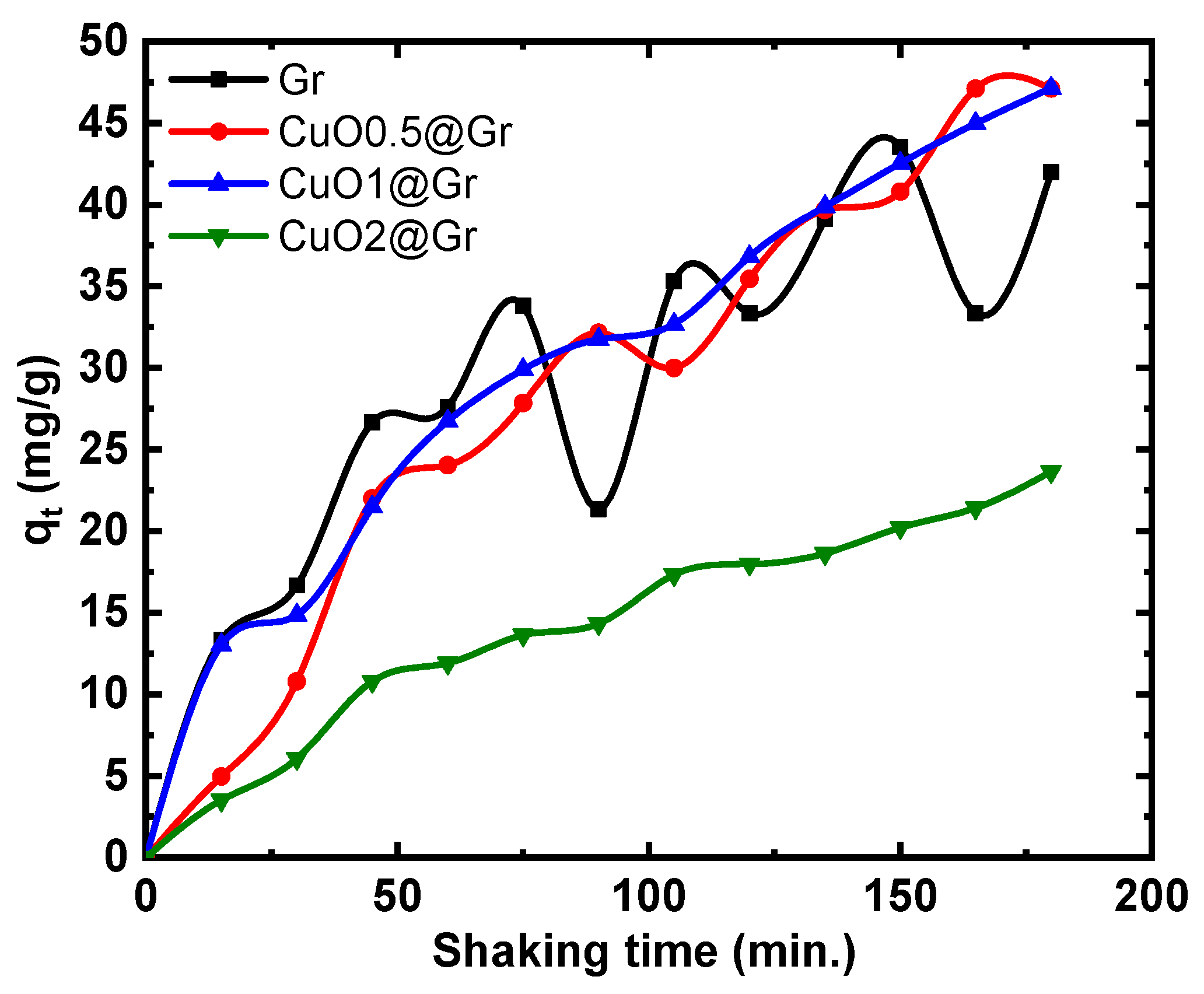

| Sample | ID/G | I2D/G | Peak Position cm−1 | FWHM | |||
|---|---|---|---|---|---|---|---|
| D | G | 2D | D | G | |||
| Gr | 0.31 | 0.47 | 1351.3 | 1582 | 2716 | 29.2 | 28.3 |
| CuO0.5@Gr | 0.33 | 0.17 | 1350.0 | 1580.5 | 2715 | 31.3 | 30.9 |
| CuO1.0@Gr | 0.40 | 0.13 | 1349.7 | 1580.4 | 2714 | 37.5 | 27.1 |
| CuO2.0@Gr | 0.84 | 0.19 | 1344.9 | 1580.3 | 2712 | 82.8 | 59.9 |
| Sample | k | K | R2 |
|---|---|---|---|
| Gr | 0.70549 | 0.001517 | 0.79 |
| CuO0.5@Gr | 0.6867 | 0.00249 | 0.92 |
| CuO1.0@Gr | 0.35066 | 0.00305 | 0.73 |
| CuO2.0@Gr | 0.31863 | 0.00353 | 0.76 |
| Adsorbent | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Intraparticle Diffusion Kinetic Model | Elovic Kinetic Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe,exp | qe,cal | qe(exp) − qe(cal) | k1 | R2 | qe,cal | qe(exp) − qe(cal) | k2x 10−4 | R2 | kdiff | C | R2 | α | β | R2 | |
| Gr | 46.60 | 40.64 | 5.96 | 0.011 | 0.74 | 50.66 | 4.060 | 3.6 | 0.80 | 2.99 | 2.068 | 0.87 | 2.14 | 0.089 | 0.77 |
| CuO0.5@Gr | 48.00 | 75.47 | 27.47 | 0.020 | 0.80 | 126.26 | 78.26 | 0.27 | 0.63 | 3.82 | −5.42 | 0.96 | 1.25 | 0.058 | 0.96 |
| CuO1.0@Gr | 47.14 | 53.20 | 6.06 | 0.016 | 0.92 | 68.59 | 21.45 | 1.53 | 0.94 | 3.55 | −1.55 | 0.99 | 1.65 | 0.069 | 0.94 |
| CuO2.0@Gr | 23.64 | 25.57 | 1.93 | 0.013 | 0.97 | 43.34 | 19.70 | 1.39 | 0.94 | 1.82 | −2.04 | 0.97 | 0.66 | 0.126 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashad, M.; Helali, S.; Shaalan, N.M.; Albalawi, A.E.; Alatawi, N.S.; Al-Faqiri, B.; Al-Belwi, M.M.; Alsharari, A.M. Dual Studies of Photo Degradation and Adsorptions of Congo Red in Wastewater on Graphene–Copper Oxide Heterostructures. Materials 2023, 16, 3721. https://doi.org/10.3390/ma16103721
Rashad M, Helali S, Shaalan NM, Albalawi AE, Alatawi NS, Al-Faqiri B, Al-Belwi MM, Alsharari AM. Dual Studies of Photo Degradation and Adsorptions of Congo Red in Wastewater on Graphene–Copper Oxide Heterostructures. Materials. 2023; 16(10):3721. https://doi.org/10.3390/ma16103721
Chicago/Turabian StyleRashad, Mohamed, Saloua Helali, Nagih M. Shaalan, Aishah E. Albalawi, Naifa S. Alatawi, Bassam Al-Faqiri, Mohammed M. Al-Belwi, and Abdulrhman M. Alsharari. 2023. "Dual Studies of Photo Degradation and Adsorptions of Congo Red in Wastewater on Graphene–Copper Oxide Heterostructures" Materials 16, no. 10: 3721. https://doi.org/10.3390/ma16103721
APA StyleRashad, M., Helali, S., Shaalan, N. M., Albalawi, A. E., Alatawi, N. S., Al-Faqiri, B., Al-Belwi, M. M., & Alsharari, A. M. (2023). Dual Studies of Photo Degradation and Adsorptions of Congo Red in Wastewater on Graphene–Copper Oxide Heterostructures. Materials, 16(10), 3721. https://doi.org/10.3390/ma16103721







