Active Food Packaging Made of Biopolymer-Based Composites
Abstract
1. Introduction
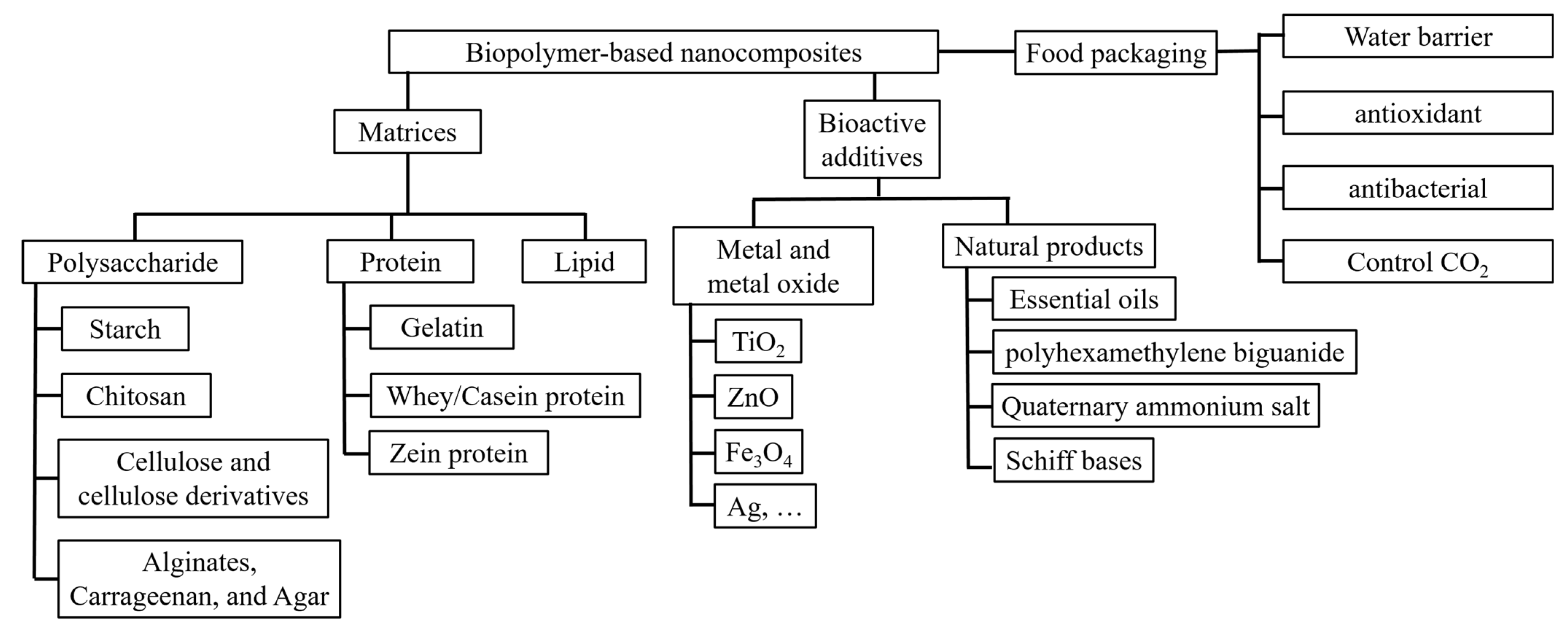
2. Natural Biopolymers Used as the Matrices of Food Packaging
2.1. Polysaccharide-Based Biopolymers
2.1.1. Starch
2.1.2. Chitosan
2.1.3. Cellulose and Cellulose Derivatives
2.1.4. Alginates, Carrageenan, and Agar
2.2. Protein-Based Biopolymers
2.2.1. Gelatin
2.2.2. Whey/Casein Protein
2.2.3. Zein Protein
2.3. Lipid-Based Biopolymers
Waxes
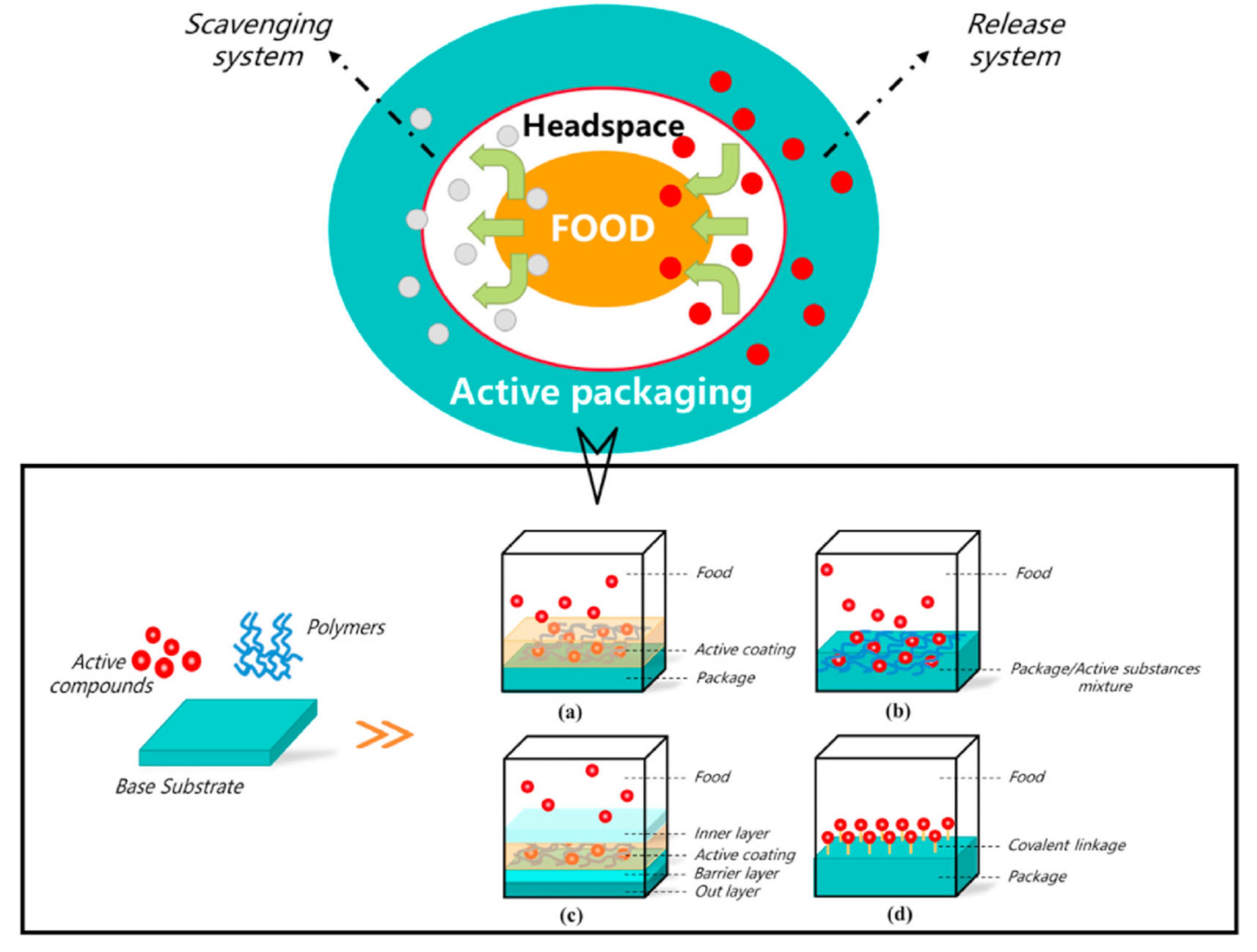
3. Composites with Water and Oxygen Barrier Properties
4. Antioxidant Packaging Systems
Biopolymers as Carriers of Antioxidant Agents
5. Biopolymer-Based Composites with Antimicrobial Properties for Active Food Packaging
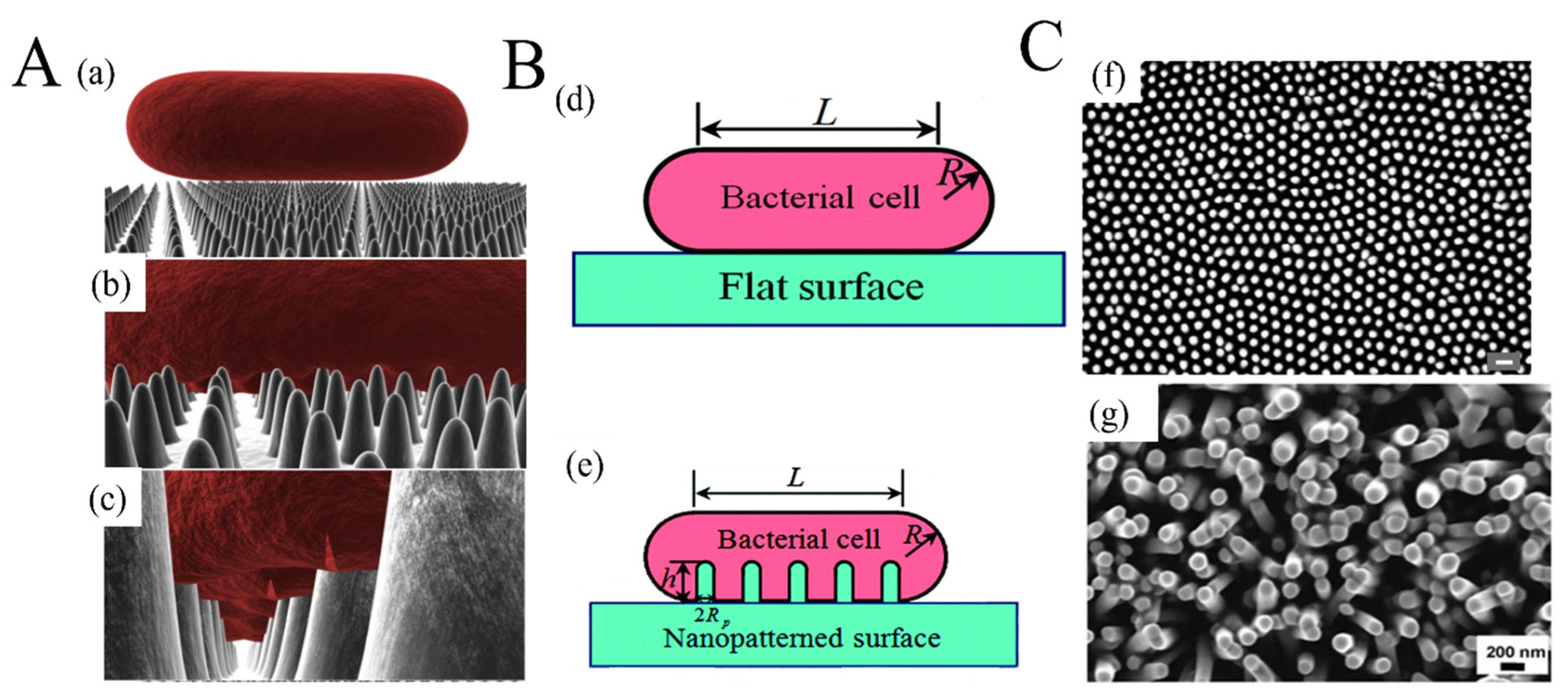
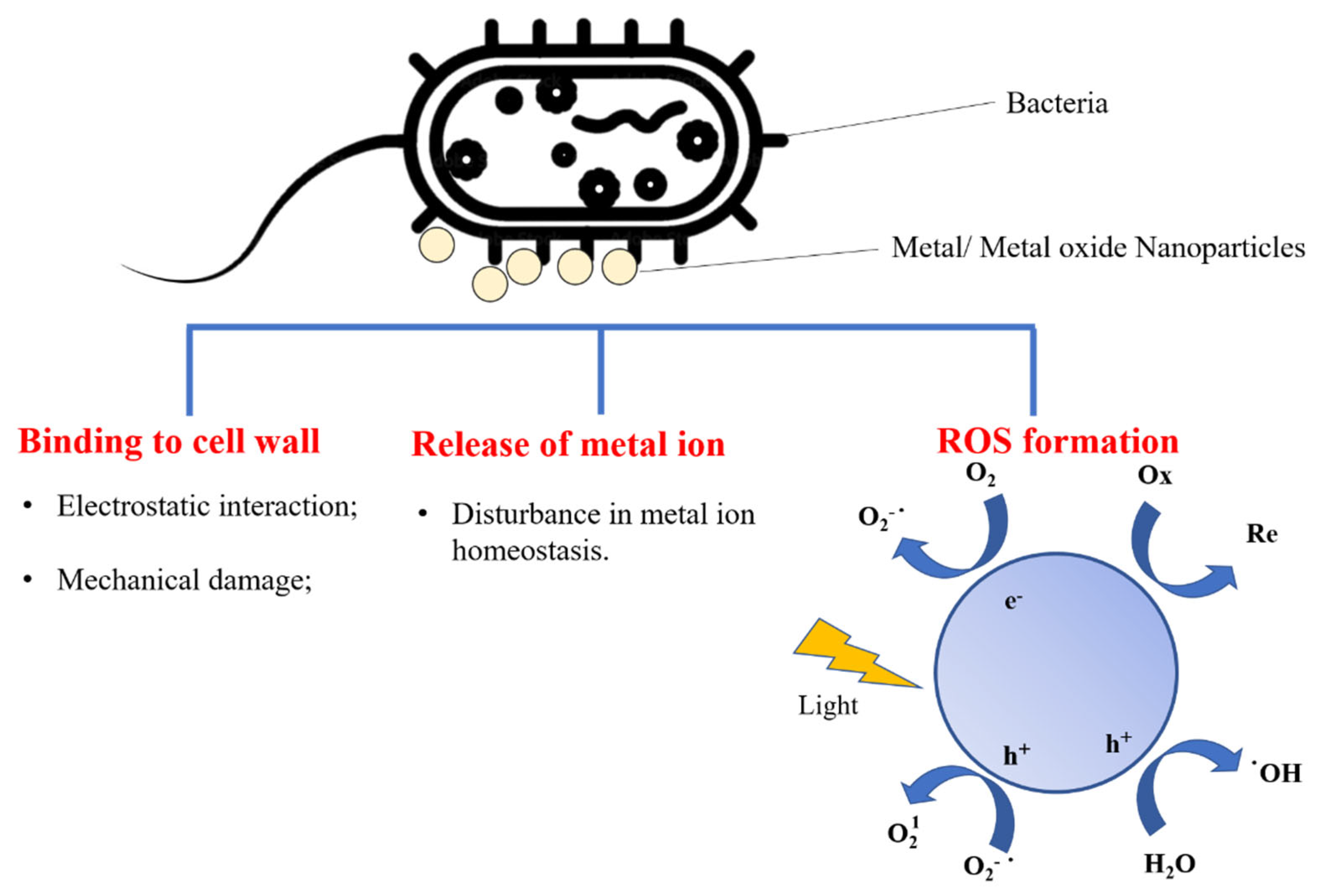
5.1. Metal and Metal Oxide Nanostructures with Antimicrobial Properties Integrated with Biopolymers for Active Food Packaging
| Nanomaterials | Size/Distribution | Density | Matrix | Properties | Targeted Microorganisms | Ref. |
|---|---|---|---|---|---|---|
| ZnO | 35.5–69.7 nm nanoparticles (NPs) | 5 wt% | Chitosan and carboxymethyl cellulose films | Biodegradable coatings | S. aureus | [106] |
| Around 200 nm NPs. | 93.75 μg/cm2, 187.5 μg/cm2 | Poly(vinyl chloride) film | UV irradiation required | E. coli or S. aureus | [107] | |
| <100 nm NPs. | 0.05, 0.1, and 0.2 wt% | Hydroxyethyl cellulose and citric-acid-based biopolymer film | Good swelling abilities and hydrophilicity | E. coli or S. aureus | [108] | |
| 30 nm NPs. | 1–5 wt% | Polyurethane/chitosan composite film | Extended shelf life of food, improved mechanical properties, and reduced oxygen permeability | E. coli or S. aureus | [109] | |
| ZnO nanorods grow less directly (500 nm in diameter, 2–3 μm in length). | Poly(lactic acid) | Biodegradable | E. coli or S. aureus | [119] | ||
| ZnO + Ag | ZnO nanorods with vertical growth (160 nm in diameter, 2 μm in length). | Polydimethylsiloxane | No significant cytotoxicity | E. coli or S. aureus | [113] | |
| ZnO + stearic acid | 250–500 nm NPs. | 2, 5 wt% | Isotactic polypropylene | Higher thermal stability and improved mechanical and impact properties | E. coli | [120] |
| TiO2 | 50–80 nm NPs. | 10 wt% | Chitosan | Enhanced hydrophilicity and better mechanical properties; extended shelf life of fruit | E. coli, S. aureus, Candida albicans, Aspergillus niger | [96] |
| 0.2–0.3 μm | 1 wt% | High-density polyethylene with CaCO3. | Extended shelf life of cheese | Inhibition of lactic acid bacteria and coliforms | [97] | |
| Nanotubes | 0.5–5 wt% | Gelatin film | High UV barrier | E. coli and L. monocytogenes | [98] | |
| 10 nm NPs | 0.75 wt% | Polylactic acid nanofiber/film | Increased antibacterial activity under UV-A irradiation | E. coli, S. aureus | [99] | |
| 12.22 nm | 3.3–10 wt% | Alginate | Highly transparent to light | E. coli, S. aureus | [121] | |
| TiO2+Ag | - | 0.1–0.5%, w/v | Gelatin–chitosan film | Reduced light transmittance; antibacterial ability with or without light | E. coli, S. aureus, Botrytis cinerea | [112] |
| Au-TiO2 | Au NPs with TiO2 as shell 45 nm | 2.5 wt% | Alginate nanocomposite | Excellent visible light absorption; increased production of reactive oxygen species | E. coli, S. aureus | [122] |
| Ag | 13.7 ± 3.5 nm | 0.075–0.3 wt% | Carrageenan | Improved UV shielding properties | E. coli and L. monocytogenes | [123] |
| Fe3O4+Sulfur | 281.4 nm NPs | 0.5–1.0 wt% | Carrageenan | Effectively blocked UV light; improved thermal stability | E. coli and L. monocytogenes | [115] |
| Fe3O4-Ag | - | 2 wt% | [116] | |||
| Fe2O3/TiO2 | 10–27 nm | 5–20 wt% | Chitosan, polyvinyl alcohol | Enhanced antibacterial activity and mechanical properties | E. coli, S. aureus | [124] |
| CuO | - | 2 wt% | Carrageenan | UV barrier and strong antibacterial activity | E. coli and L. monocytogenes | [125] |
5.2. Natural Products with Antimicrobial Activity Integrated with Biopolymers for Active Food Packaging
| Biopolymers | Strategies | Effect | Ref. |
|---|---|---|---|
| Cellulose | Incorporation with rosin | Antibacterial activity against E. coli and B. subtilis | [151] |
| Mixing with lytic bacteriophages | Bacteriophages remained viable for 14 days | [129] | |
| Starch | Addition of essential oil (lemon) | Enhanced antibacterial activity against S. aureus and E. coli | [137] |
| Cinnamon essential oil | Against Salmonella Typhi, S. aureus, and E. coli (suitable for meatball packaging); extended shelf life from 48 h to 96 h for pork | [138] | |
| Lavender essential oil | Against S. aureus and E. coli (not suitable for food with a high-water content) | [139] | |
| Peppermint and lime oil | Inhibited growth of mold; delayed ripening during mangosteen fruit transportation | [140] | |
| Cumin essential oil | Reduced rot lesion on infected pears caused by Alternaria alternata | [141] | |
| Carvacrol and thymol essential oils | Fungistatic effect against Colletotrichum gloeosporioides on mango and papaya; reduced the incidence of anthracnose symptoms; extended shelf life from 4–5 days to 8 and 13 days | [142] | |
| Carrageenan | Encapsulation of orange essential oil and trehalose | Showed resistance to Gram-positive bacteria | [145] |
| Encapsulation of olive leaf extract | Reduced the count of psychrophiles five times further than commercial films for lamb meat packaging | [146] | |
| Fabrication with epsilon-polylysine | Broad antibacterial activity; inhibited growth of A. acidoterrestris in juice | [148] | |
| Starch and cellulose | Polyhexamethylene biguanide (PHMB) | Prolonged shelf life of grapes; inhibition rate against E. coli. reached 100% when PHMB% > 6% | [149] |
| Starch | Better efficacy against B. subtilis than against E. coli | [150] | |
| Chitosan | Formation of quaternary ammonium salt (N,N,N-trimethyl-chitosan chloride) | Higher inhibition efficiency on E. coli | [154] |
| Formation of quaternary ammonium salt (N-(2-hydroxypropyl)-3-trimethylammonium chitosan chlorides) (HTCC) | Inhibition rate of E. coli, S. aureus, and Botrytis cinerea up to 99%; prolonged shelf life of strawberries by over 5 days | [155] | |
| Larger inhibition zone as HTCC content increases; extended shelf life of bananas by over 5 days for | [163] | ||
| Functionalization with cinnamaldehyde | Sustained release of active reagents; enhanced fungicidal effect on R. stolonifer in bread slices | [158] | |
| Modification by salicylaldehyde/TiO2 | Full eradication of S. aureus and P. aeruginosa | [159] | |
| Formation of Schiff bases containing halogenobenzenes | >95% inhibition of Botrytis cinerea | [164] | |
| Formation of Schiff bases containing benzaldehydes | Electron-withdrawing group on aromatic ring decreases the antibacterial activities | [162] | |
| Formation of Schiff bases containing ethyl vanillin | An excellent barrier to UV light; higher inhibitory efficiency against Gram-negative bacteria. | [160] |
6. Active Food Packaging Made of Composites to Control Carbon Dioxide
6.1. CO2 Emitters
6.2. CO2 Absorbers
6.2.1. Physical Adsorption of CO2
6.2.2. Chemical Absorption of CO2
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalyoussef, S.; Feja, K.N. Foodborne illnesses. Adv. Pediatr. 2014, 61, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, S.R.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Comparative studies of microbial and heavy metal safety assessment of the herbs cultivated in hydroponically and regular soil system. J. Food Saf. 2021, 41, e12936. [Google Scholar] [CrossRef]
- Pogreba-Brown, K.; Austhof, E.; Armstrong, A.; Schaefer, K.; Villa Zapata, L.; McClelland, D.J.; Batz, M.B.; Kuecken, M.; Riddle, M.; Porter, C.K. Chronic gastrointestinal and joint-related sequelae associated with common foodborne illnesses: A scoping review. Foodborne Pathog. Dis. 2020, 17, 67–86. [Google Scholar] [CrossRef] [PubMed]
- WHO Foodborne Disease. Available online: https://www.who.int/health-topics/foodborne-diseases#tab=tab_1 (accessed on 2 April 2022).
- WHO Listeriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 2 April 2022).
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B. Chapter 7—Active Packaging. In Nanomaterials for Food Packaging, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 173–202. [Google Scholar]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Ozen, B.F.; Floros, J.D. Effects of emerging food processing techniques on the packaging materials. Trends Food Sci. Technol. 2001, 12, 60–67. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef]
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Yang, W.; Kenny, J.M.; Torre, L.; Puglia, D. Chapter 4—Bio-Based Nanomaterials for Food Packaging. In Nanomaterials for Food Packaging, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 71–110. [Google Scholar]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Islam Sarker, M.Z.; Fazita, M.R.N.; Syakir, M.I.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C. Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 2006, 22, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Bos, H.L.; Van den Oever, M.J.A.; Peters, O.C.J.J. Tensile and compressive properties of flax fibres for natural fibre reinforced composites. J. Mater. Sci. 2002, 37, 1683–1692. [Google Scholar] [CrossRef]
- Zhuang, R.; Beuchat, L.R.; Chinnan, M.S.; Shewfelt, R.L.; Huang, Y.W. Inactivation of Salmonella montevideo on Tomatoes by Applying Cellulose-Based Edible Films. J. Food Prot. 1996, 59, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.B. Edible Food Packaging, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 10–72. [Google Scholar]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Basak, G.; Awasthi, M.G. Materiality of Edible Film Packaging in Muscle Foods: A Worthwhile Conception. J. Package Technol. Res. 2020, 4, 117–132. [Google Scholar] [CrossRef]
- Wanstedti, K.G.; Seideman, S.C.; Donnelly, L.S.; Quenzer, N.M. Sensory Attributes of Precooked, Calcium Alginate-Coated Pork Patties. J. Food Prot. 1981, 44, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A.; Hanna, M.A.; Kurth, L.B. Application of Edible Coatings on Meats, Poultry and Seafoods: A Review. Food Sci. Technol. 1997, 30, 337–350. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Improvement of barrier properties of fish gelatin films promoted by gelatin glycation with lactose at high temperatures. LWT-Food Sci. Technol. 2015, 63, 315–321. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Ambrosio- Martín, J.; Fabra, M.J.; Pérez- Masiá, R.; López-Rubio, A. Nanotechnology in Nutraceuticals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 374–386. [Google Scholar]
- Cao, N.; Yang, X.; Fu, Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, H. Effect of transglutaminase on properties of tilapia scale gelatin films incorporated with soy protein isolate. Food Chem. 2015, 169, 255–260. [Google Scholar] [CrossRef]
- Xu, D.; Chen, T.; Liu, Y. The physical properties, antioxidant and antimicrobial activity of chitosan–gelatin edible films incorporated with the extract from hop plant. Polym. Bull. 2020, 78, 3607–3624. [Google Scholar] [CrossRef]
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk proteins for edible films and coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Fematt-Flores, G.E.; Aguiló-Aguayo, I.; Marcos, B.; Camargo-Olivas, B.A.; Sánchez-Vega, R.; Soto-Caballero, M.C.; Salas-Salazar, N.A.; Flores-Córdova, M.A.; Rodríguez-Roque, M.J. Milk Protein-Based Edible Films: Influence on Mechanical, Hydrodynamic, Optical and Antioxidant Properties. Coatings 2022, 12, 196. [Google Scholar] [CrossRef]
- Ozdemir, M.; Floros, J.D. Optimization of edible whey protein films containing preservatives for water vapor permeability, water solubility and sensory characteristics. J. Food Eng. 2008, 86, 215–224. [Google Scholar] [CrossRef]
- Cagri, A.; Ustunol, Z.; Ryster, E.T. Antimicrobial, Mechanical, and Moisture Barrier Properties of Low pH Whey Protein-based Edible Films Containing p-Aminobenzoic or Sorbic Acids. J. Food Sci. 2001, 66, 865–870. [Google Scholar] [CrossRef]
- Aisha, I.; Abdullahi, Y. Development of whey protein concentrate edible membrane with cinnamon essential oil. J. Adv. Biol. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef]
- Luecha, J.; Sozer, N.; Kokini, J.L. Synthesis and properties of corn zein/montmorillonite nanocomposite films. J. Mater. Sci. 2010, 45, 3529–3537. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, T.; Qiao, M.; Huang, T.S.; Xia, X. The reduction of Salmonella on chicken skin by the combination of sodium dodecyl sulfate with antimicrobial chemicals and coating wax microemulsions. Poult. Sci. 2019, 98, 2615–2621. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bang, I.H.; Kang, J.H.; Min, S.C. Development of a Microbial Decontamination System Combining Washing with Highly Activated Calcium Oxide Solution and Antimicrobial Coating for Improvement of Mandarin Storability. J. Food Sci. 2019, 84, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Donhowe, I.G.; Fennema, O. Water Vapor and Oxygen Permeability of Wax Films. J. Am. Oil Chem. Soc. 1993, 70, 867–873. [Google Scholar] [CrossRef]
- Muthuselvi, R.N.K.P.; Krishnakumare, B.; Minithra, R.; Rameshkumar, D.; Jagathjothi, N.; Easwari, S.; Ramasamy, R.; Suresh, S. Importance of Edible wax coatings in fruits and vegetables. Indian Farmer 2020, 7, 1006–1009. [Google Scholar]
- Butkinaree, S.; Jinkarn, T.; Yoksan, R. Effects of biodegradable coating on barrier properties of paperboard food packaging. J. Met. Mater. Miner. 2008, 18, 219–222. [Google Scholar]
- Chavan, P.; Sinhmar, A.; Sharma, S.; Dufresne, A.; Thory, R.; Kaur, M.; Sandhu, K.S.; Nehra, M.; Nain, V. Nanocomposite Starch Films: A New Approach for Biodegradable Packaging Materials. Starch-Stärke 2022, 74, 2100302. [Google Scholar] [CrossRef]
- Costa, É.K.D.C.; de Souza, C.O.; da Silva, J.B.A.; Druzian, J.I. Hydrolysis of part of cassava starch into nanocrystals leads to increased reinforcement of nanocomposite films. J. Appl. Polym. Sci. 2017, 134, 45311. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of alginates as food packaging materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Rodrigues, J.M.; Valadares, A.C.F.; de Almeida, A.B.; de Lima, T.M.; Takeuchi, K.P.; Alves, C.C.F.; de Figueiredo Sousa, H.A.; da Silva, E.R.; Dyszy, F.H. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Valdés, A.; Beltran, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible films and coatings from whey proteins: A review on formulation, and on mechanical and bioactive properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Lai, W.F. Design of Polymeric Films for Antioxidant Active Food Packaging. Int. J. Mol. Sci. 2021, 23, 12. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety-Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Herskovitz, J.E.; Goddard, J.M. Antioxidant functionalization of biomaterials via reactive extrusion. J. Appl. Polym. Sci. 2021, 138, 50591. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.; Conte-Junior, C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Barba, F.J.; Gomez, B.; Putnik, P.; Bursac Kovacevic, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Kadam, A.A.; Singh, S.; Gaikwad, K.K. Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control 2021, 124, 107877. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Song, L. Characterization of antioxidant and antibacterial gelatin films incorporated with Ginkgo biloba extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef]
- Boeira, C.P.; Alves, J.d.S.; Flores, D.C.B.; Moura, M.R.; Melo, P.T.S.; Rosa, C.S. Antioxidant and antimicrobial effect of an innovative active film containing corn stigma residue extract for refrigerated meat conservation. J. Food Process. Preserv. 2021, 45, e15721. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Yang, J.Y.; Lu, H.B.; Wang, S.S.; Yang, J.; Yang, X.C.; Chai, M.; Li, L.; Cao, J.X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Release of synthetic phenolic antioxidants from extruded poly lactic acid (PLA) film. Food Control 2012, 28, 445–455. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro Bruni, G.; Dos Santos Acunha, T.; de Oliveira, J.P.; Martins Fonseca, L.; Tavares da Silva, F.; Martins Guimaraes, V.; da Rosa Zavareze, E. Electrospun protein fibers loaded with yerba mate extract for bioactive release in food packaging. J. Sci. Food Agric. 2020, 100, 3341–3350. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Guar gum/carboxymethyl cellulose based antioxidant film incorporated with halloysite nanotubes and litchi shell waste extract for active packaging. Int. J. Biol. Macromol. 2022, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocoll. 2018, 75, 13–21. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of biodegradable films with improved antioxidant properties based on the addition of carrageenan containing olive leaf extract for food packaging applications. J. Polym. Environ. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Han, H.-S.; Song, K.B. Noni (Morinda citrifolia) fruit polysaccharide films containing blueberry (Vaccinium corymbosum) leaf extract as an antioxidant packaging material. Food Hydrocoll. 2021, 112, 106372. [Google Scholar] [CrossRef]
- Zhu, X.; Hondroulis, E.; Liu, W.; Li, C.Z. Biosensing approaches for rapid genotoxicity and cytotoxicity assays upon nanomaterial exposure. Small 2013, 9, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Su, Y.P.; Chen, C.-C.; Jia, G.; Wang, H.L.; Wu, J.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Noori Hashemabad, Z.; Shabanpour, B.; Azizi, H.; Ojagh, S.M.; Alishahi, A. Effect of TiO2 nanoparticles on the antibacterial and physical properties of low-density polyethylene film. Polym. Plast. Technol. Eng. 2017, 56, 1516–1527. [Google Scholar] [CrossRef]
- Abutalib, M.; Rajeh, A. Enhanced structural, electrical, mechanical properties and antibacterial activity of Cs/PEO doped mixed nanoparticles (Ag/TiO2) for food packaging applications. Polym. Test. 2021, 93, 107013. [Google Scholar] [CrossRef]
- Enescu, D.; Dehelean, A.; Gonçalves, C.; Cerqueira, M.A.; Magdas, D.A.; Fucinos, P.; Pastrana, L.M. Evaluation of the specific migration according to EU standards of titanium from Chitosan/Metal complexes films containing TiO2 particles into different food simulants. A comparative study of the nano-sized vs micro-sized particles. Food Packag. Shelf Life 2020, 26, 100579. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Gumiero, M.; Peressini, D.; Pizzariello, A.; Sensidoni, A.; Iacumin, L.; Comi, G.; Toniolo, R. Effect of TiO2 photocatalytic activity in a HDPE-based food packaging on the structural and microbiological stability of a short-ripened cheese. Food Chem. 2013, 138, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, F.; Ahmed, S.; Liu, Y. Physico-mechanical and antibacterial properties of PLA/TiO2 composite materials synthesized via electrospinning and solution casting processes. Coatings 2019, 9, 525. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, X.; Zhang, L.; Xu, Q.; Che, Z.; Li, W.; Bai, Y.; Li, K. Effect of TiO2 nanoparticles on the antibacterial and physical properties of polyethylene-based film. Prog. Org. Coat. 2012, 73, 219–224. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO nanostructures in active antibacterial food packaging: Preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [Google Scholar] [CrossRef]
- Wahab, R.; Mishra, A.; Yun, S.-I.; Hwang, I.; Mussarat, J.; Al-Khedhairy, A.A.; Kim, Y.-S.; Shin, H.-S. Fabrication, growth mechanism and antibacterial activity of ZnO micro-spheres prepared via solution process. Biomass Bioenergy 2012, 39, 227–236. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef]
- Zahiri Oghani, F.; Tahvildari, K.; Nozari, M. Novel antibacterial food packaging based on chitosan loaded ZnO nano particles prepared by green synthesis from Nettle leaf extract. J. Inorg. Organomet. Polym. 2021, 31, 43–54. [Google Scholar] [CrossRef]
- Li, X.; Xing, Y.; Li, W.; Jiang, Y.; Ding, Y. Antibacterial and physical properties of poly (vinyl chloride)-based film coated with ZnO nanoparticles. Int. J. Food Sci. Technol. 2010, 16, 225–232. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Indumathi, M.; Rajarajeswari, G. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef]
- Lin, D.; Yang, Y.; Wang, J.; Yan, W.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef]
- Chen, Y.; Tse, W.H.; Chen, L.; Zhang, J. Ag nanoparticles-decorated ZnO nanorod array on a mechanical flexible substrate with enhanced optical and antimicrobial properties. Nanoscale Res. Lett. 2015, 10, 106. [Google Scholar] [CrossRef]
- Kumar, R.; Inbaraj, B.S.; Chen, B. Surface modification of superparamagnetic iron nanoparticles with calcium salt of poly (γ-glutamic acid) as coating material. Mater. Res. Bull. 2010, 45, 1603–1607. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Priyadarshi, R.; Rhim, J.-W. Carrageenan-based antimicrobial films integrated with sulfur-coated iron oxide nanoparticles (Fe3O4@ SNP). ACS Appl. Polym. Mater. 2021, 3, 4913–4923. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Priyadarshi, R.; Rhim, J.-W. Silver ion loaded 3-aminopropyl trimethoxysilane-modified Fe3O4 nanoparticles for the fabrication of carrageenan-based active packaging films. Colloids Surf. B Biointerfaces 2021, 208, 112085. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. 2016, 18, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Akshaykranth, A.; Jayarambabu, N.; Venkatappa Rao, T.; Rakesh Kumar, R.; Srinivasa Rao, L. Antibacterial activity study of ZnO incorporated biodegradable poly (lactic acid) films for food packaging applications. Polym. Bull. 2022, 1–16. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Marra, A.; Strongone, V.; Cimmino, S. Development of antibacterial composite films based on isotactic polypropylene and coated ZnO particles for active food packaging. Coatings 2016, 6, 4. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin–TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, P.; Li, W.; Li, C.; Wang, Y.; Chu, P.K. Degradable and photocatalytic antibacterial Au-TiO2/sodium alginate nanocomposite films for active food packaging. Nanomaterials 2018, 8, 930. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.-W. Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Alghamdi, H.M.; Abutalib, M.; Rajeh, A.; Mannaa, M.A.; Nur, O.; Abdelrazek, E. Effect of the Fe2O3/TiO2 Nanoparticles on the Structural, Mechanical, Electrical Properties and Antibacterial Activity of the Biodegradable Chitosan/Polyvinyl Alcohol Blend for Food Packaging. J. Polym. Environ. 2022, 30, 3865–3874. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Preparation and properties of carbohydrate-based composite films incorporated with CuO nanoparticles. Carbohydr. Polym. 2017, 169, 264–271. [Google Scholar] [CrossRef]
- Tang, X.; Kumar, P.; Alavi, S.; Sandeep, K. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Ji, N.; Ge, S.; Li, M.; Wang, Y.; Xiong, L.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Effect of annealing on the structural and physicochemical properties of waxy rice starch nanoparticles: Effect of annealing on the properties of starch nanoparticles. Food Chem. 2019, 286, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, D.M.; Mendonça, R.C.S.; Soto, M.L.; Cruz, R.S. Acetate cellulose film with bacteriophages for potential antimicrobial use in food packaging. LWT-Food Sci. Technol. 2015, 63, 85–91. [Google Scholar] [CrossRef]
- Jung, J.; Raghavendra, G.M.; Kim, D.; Seo, J. One-step synthesis of starch-silver nanoparticle solution and its application to antibacterial paper coating. Int. J. Biol. Macromol. 2018, 107, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Antibacterial properties of starch-reduced graphene oxide–polyiodide nanocomposite. Food Chem. 2021, 342, 128385. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Júnior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, I.O.; Songsamoe, S.; Khunjan, K.; Matan, N. Effect of tapioca starch coated-rubberwood box incorporated with essential oils on the postharvest ripening and quality control of mangosteen during transportation. Food Control 2021, 126, 108007. [Google Scholar] [CrossRef]
- Oyom, W.; Xu, H.; Liu, Z.; Long, H.; Li, Y.; Zhang, Z.; Bi, Y.; Tahergorabi, R.; Prusky, D. Effects of modified sweet potato starch edible coating incorporated with cumin essential oil on storage quality of ‘early crisp’. LWT 2022, 153, 112475. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch edible films/coatings added with carvacrol and thymol: In vitro and in vivo evaluation against Colletotrichum gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, characterization and antibacterial activity of oxidized κ-carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug delivery systems and other biomedical applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—Structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; Moraes, C.C.D.; Rosa, G.S.D.; Dotto, G.L. Bio-based active packaging: Carrageenan film with olive leaf extract for lamb meat preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A review of property enhancement techniques for carrageenan-based films and coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zeng, X.; Cai, R.; Wang, Z.; Yuan, Y.; Yue, T. One-pot synthesis of magnetic self-assembled carrageenan-ε-polylysine composites: A reusable and effective antibacterial agent against Alicyclobacillus acidoterrestris. Food Chem. 2021, 360, 130062. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, H. Preparation and characterization of a biodegradable starch-based antibacterial film containing nanocellulose and polyhexamethylene biguanide. Food Packag. Shelf Life 2021, 30, 100718. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ward, V.; Mekonnen, T.H. Functionalized starch microparticles for contact-active antimicrobial polymer surfaces. Carbohydr. Polym. 2020, 229, 115422. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid/chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Tănase, E.E.; Popa, V.; Popa, M.E. Sustainable alternative for food packaging: Chitosan biopolymer—A review. AgroLife Sci. J. 2015, 4, 52–61. [Google Scholar]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Abdallah, H.M.; Gamal, H.; Moustafa, E.B.; Goda, E.S. Rational design of biocompatible IPNs hydrogels containing carboxymethyl starch and trimethyl chitosan chloride with high antibacterial activity. Cellulose 2022, 29, 7317–7330. [Google Scholar] [CrossRef]
- Min, T.; Zhu, Z.; Sun, X.; Yuan, Z.; Zha, J.; Wen, Y. Highly efficient antifogging and antibacterial food packaging film fabricated by novel quaternary ammonium chitosan composite. Food Chem. 2020, 308, 125682. [Google Scholar] [CrossRef]
- Rudlong, A.M.; Koga, Y.T.; Goddard, J.M. Advances in Nonfouling and Antimicrobial Coatings: Perspectives for the Food Industry. ACS Food Sci. Technol. 2022, 2, 1401–1416. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; De Benedictis, V.M.; Madaghiele, M.; Corcione, C.E.; Maffezzoli, A. Nanostructured active chitosan-based films for food packaging applications: Effect of graphene stacks on mechanical properties. Measurement 2016, 90, 418–423. [Google Scholar] [CrossRef]
- Montaser, A.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Narasagoudr, S.S.; Hegde, V.G.; Vanjeri, V.N.; Chougale, R.B.; Masti, S.P. Ethyl vanillin incorporated chitosan/poly (vinyl alcohol) active films for food packaging applications. Carbohydr. Polym. 2020, 236, 116049. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, J.; Bai, J. Synthesis and antimicrobial activity of the Schiff base from chitosan and citral. Carbohydr. Res. 2009, 344, 825–829. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.; Yuan, W.; Lin, Q.; Ji, L.; Liu, F. Preparation and antibacterial activity of Schiff bases from O-carboxymethyl chitosan and para-substituted benzaldehydes. Polym. Bull. 2012, 68, 1215–1226. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT-Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int. J. Biol. Macromol. 2021, 179, 292–298. [Google Scholar] [CrossRef]
- Haghighi-Manesh, S.; Azizi, M.H. Active packaging systems with emphasis on its applications in dairy products. J. Food Process Eng. 2017, 40, e12542. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Lee, D.S.; Wang, H.J.; Jaisan, C.; An, D.S. Active food packaging to control carbon dioxide. Packag. Technol. Sci. 2021, 35, 213–227. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, Y.S. Current Scenario of Gas Scavenging Systems Used in Active Packaging—A Review. Korean J. Food Sci. Technol. 2017, 23, 109–117. [Google Scholar]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Moen, B.; Rødbotten, M.; Berget, I.; Pettersen, M.K. Effect of vacuum or modified atmosphere packaging (MAP) in combination with a CO2 emitter on quality parameters of cod loins (Gadus morhua). Food Packag. Shelf Life 2016, 9, 29–37. [Google Scholar] [CrossRef]
- Holck, A.L.; Pettersen, M.K.; Moen, M.H.; Sorheim, O. Prolonged shelf life and reduced drip loss of chicken filets by the use of carbon dioxide emitters and modified atmosphere packaging. J. Food Prot. 2014, 77, 1133–1141. [Google Scholar] [CrossRef]
- Tsironi, T.; Ntzimani, A.; Gogou, E.; Tsevdou, M.; Semenoglou, I.; Dermesonlouoglou, E.; Taoukis, P. Modeling the Effect of Active Modified Atmosphere Packaging on the Microbial Stability and Shelf Life of Gutted Sea Bass. Appl. Sci. 2019, 9, 5019. [Google Scholar] [CrossRef]
- Nugraha, B.; Bintoro, N.; Murayama, H. Influence of CO2 and C2H4 Adsorbents to the Symptoms of Internal Browning on the Packaged ‘Silver Bell’ Pear (Pyrus communis L.). Agric. Agric. Sci. Procedia 2015, 3, 127–131. [Google Scholar] [CrossRef][Green Version]
- Veasna, H.; Hwang, Y.-S.; Choi, J.-M.; Ahn, Y.-J.; Lim, B.-S.; Chun, J.-P. 1-Methylcyclopropene and Carbon Dioxide Absorber Reduce Chilling Injury of Eggplant (Solanum melongena L.) during MAP Storage. J. Bio-Environ. Con. 2012, 21, 50–56. [Google Scholar]
- An, D.S. Effect of Active Master Packaging System on Preservation of Fresh Shiitake Mushrooms in Supply Chain. J. Korean Soc. Food Sci. Nutr. 2016, 45, 402–408. [Google Scholar] [CrossRef]
- Lee, D.S.; Shin, D.H.; Lee, D.U.; Kim, J.C.; Cheigh, H.S. The use of physical carbon dioxide absorbents to control pressure buildup and volume expansion of kimchi packages. J. Food Eng. 2001, 48, 183–188. [Google Scholar] [CrossRef]
- Lee, H.-G.; Jeong, S.; Yoo, S. Development of food packaging materials containing calcium hydroxide and porous medium with carbon dioxide-adsorptive function. Food Packag. Shelf Life 2019, 21, 100352. [Google Scholar] [CrossRef]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S. Carbon dioxide absorbers for food packaging applications. Trends Food Sci. Technol. 2016, 57, 146–155. [Google Scholar] [CrossRef]
- Emanuel, N.; Sandhu, H.K. Food packaging development: Recent perspective. J. Thin Film Coat. Sci. Technol. Appl. 2019, 6, 13–29. [Google Scholar]
- Wang, H.J.; An, D.S.; Rhim, J.-W.; Lee, D.S. A Multi-functional Biofilm Used as an Active Insert in Modified Atmosphere Packaging for Fresh Produce. Packag. Technol. Sci. 2015, 28, 999–1010. [Google Scholar] [CrossRef]
| Biopolymer-Based Matrix | Antioxidant | Effect | Ref. |
|---|---|---|---|
| Gelatin/starch | Corn stigma extract (natural) | Increase in bioactive and antioxidant properties; reduction in lipid oxidation by 60% after the incorporation of the corn stigma extract in the polymer matrix | [74] |
| Chitosan–gum arabic edible film | Cinnamon oil (natural) | Significant enhancement of antioxidant effectiveness; enhancing water barrier properties | [75] |
| Chitosan | Tea polyphenols (natural) | Microbiological shelf-life extension; reduction in lipid oxidation and discoloration; maintaining acceptable sensory quality | [76] |
| Polylactic acid (PLA) | Synthetic phenolic antioxidants (SPA) (synthetic) | The high release rate of the antioxidant agent; reduction in the amounts of directly added antioxidants in foods | [77] |
| Polyvinyl alcohol (PVA)/corn starch | Pineapple peel extract | Improved antioxidant activity of the developed film as compared to the control film | [78] |
| Zein fibers | Yerba mate extract (natural) | Much higher antioxidant activity for zein fibers loaded with a 5 wt% extract | [79] |
| Furcellaran and gelatin (FUR/GEL) | Pu-erh (RTE) and green tea (GTE) extracts | Improved antioxidant activity and antimicrobial properties of FUR/GEL with GTE | [80] |
| Guar gum/carboxymethyl cellulose incorporated with halloysite nanotubes (HNTs) | Litchi shell extract (LSE) | Increased antioxidant activity; increased UV light barrier properties | [81] |
| Licorice residue extract (LRE) | Soy protein isolate (SPI) | Great antioxidant activity; excellent UV barrier properties | [82] |
| Sodium alginate | Essential oils (EO) of R. officinalis L., A. herba-alba Asso, O. basilicum L., and M. pulegium L. | Improved antioxidant activity; decreased moisture thickness and tensile strength; strong antibacterial properties | [83] |
| Polyethylene (PE) films coated with chitosan and the liposome loaded with LEO and silver nanoparticles (PC-Lip/LEO/Ag NPs) | Laurel essential oil (LEO) | Good antioxidant properties and antimicrobial activity; strong antimicrobial activity; extended storage period from 9 days to 15 days at 4 °C | [84] |
| Olive leaf extract | Carrageenan | High antioxidant activity; reduction in tensile strength; high water vapor permeability; good barrier properties | [85] |
| Noni (Morinda citrifolia) fruit polysaccharide (NPS) | Blueberry leaf extract (BLE) | Increased antioxidant activity of the films; greater water vapor permeability | [86] |
| System Type | Food | Strategy | Effect | Ref. |
|---|---|---|---|---|
| CO2 emitter | Cod loins (farmed Atlantic cod, Gadus morhua) | NaHCO3 and citric acid | Improvement of initial freshness; shelf-life extension with reduced microbial growth | [171] |
| CO2 emitter | Chicken | NaHCO3 and citric acid | Reduction in drip loss; the inhibition of microbial growth and avoidance of packaging collapse; extension of sensory and microbial shelf life | [172] |
| CO2 emitter | Gutted sea bass | McAirlaid’s Inc.® (commercial) | Shelf-life extension with reduced microbial growth | [173] |
| CO2 absorber | Pear | Ageless® (commercial) | CO2 levels reduced in the bags during cold storage and preventing the development of internal browning | [174] |
| CO2 absorber | Eggplant | Lipmen® (commercial) | Inhibition of fruit deterioration in a broad storage temperature range; reduction in chilling injury | [175] |
| CO2 absorber | Mushroom | Ca(OH)2 (chemical absorption) | Improving mushroom preservation; reducing yeast/mold growth and decay | [176] |
| CO2 absorber | Kimchi | Zeolite (physical adsorption) | Inhibition of volume expansion and pressure buildup | [177] |
| CO2 absorber | Kimchi | Ca(OH)2/zeolite (combination of physical adsorption and chemical absorption) | Solving volume expansion problems and breakage of the package without affecting the kimchi’s ripening | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Lu, C.; Tang, H.; Pouri, H.; Joulin, E.; Zhang, J. Active Food Packaging Made of Biopolymer-Based Composites. Materials 2023, 16, 279. https://doi.org/10.3390/ma16010279
Hu X, Lu C, Tang H, Pouri H, Joulin E, Zhang J. Active Food Packaging Made of Biopolymer-Based Composites. Materials. 2023; 16(1):279. https://doi.org/10.3390/ma16010279
Chicago/Turabian StyleHu, Xuanjun, Chao Lu, Howyn Tang, Hossein Pouri, Etienne Joulin, and Jin Zhang. 2023. "Active Food Packaging Made of Biopolymer-Based Composites" Materials 16, no. 1: 279. https://doi.org/10.3390/ma16010279
APA StyleHu, X., Lu, C., Tang, H., Pouri, H., Joulin, E., & Zhang, J. (2023). Active Food Packaging Made of Biopolymer-Based Composites. Materials, 16(1), 279. https://doi.org/10.3390/ma16010279







