Flammability of Polymer Compositions Filled with Wheat Bran
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Research Programme and Methodology
3. Results
3.1. Horizontal Flammability Tests
3.2. Vertical Flammability Tests
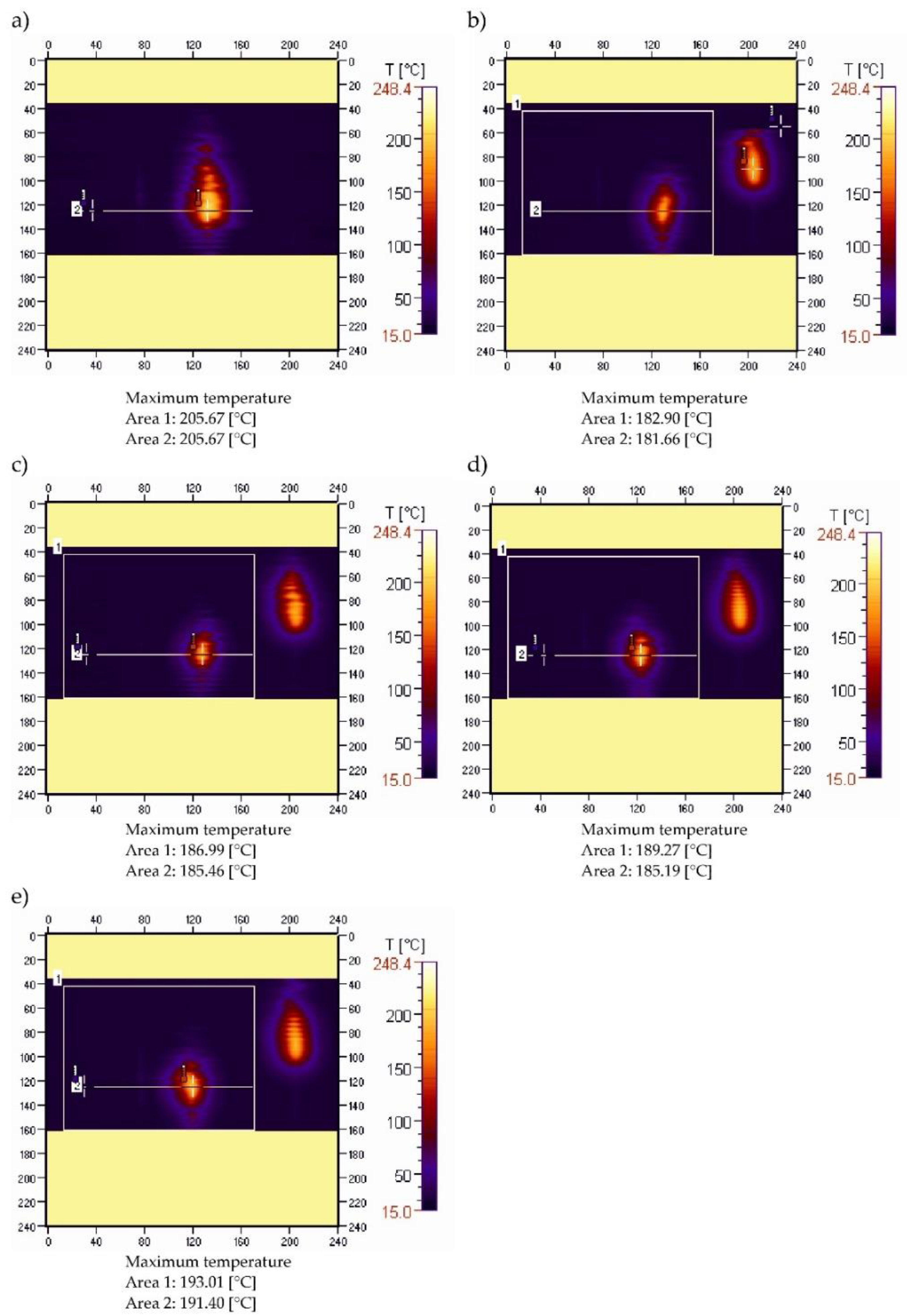
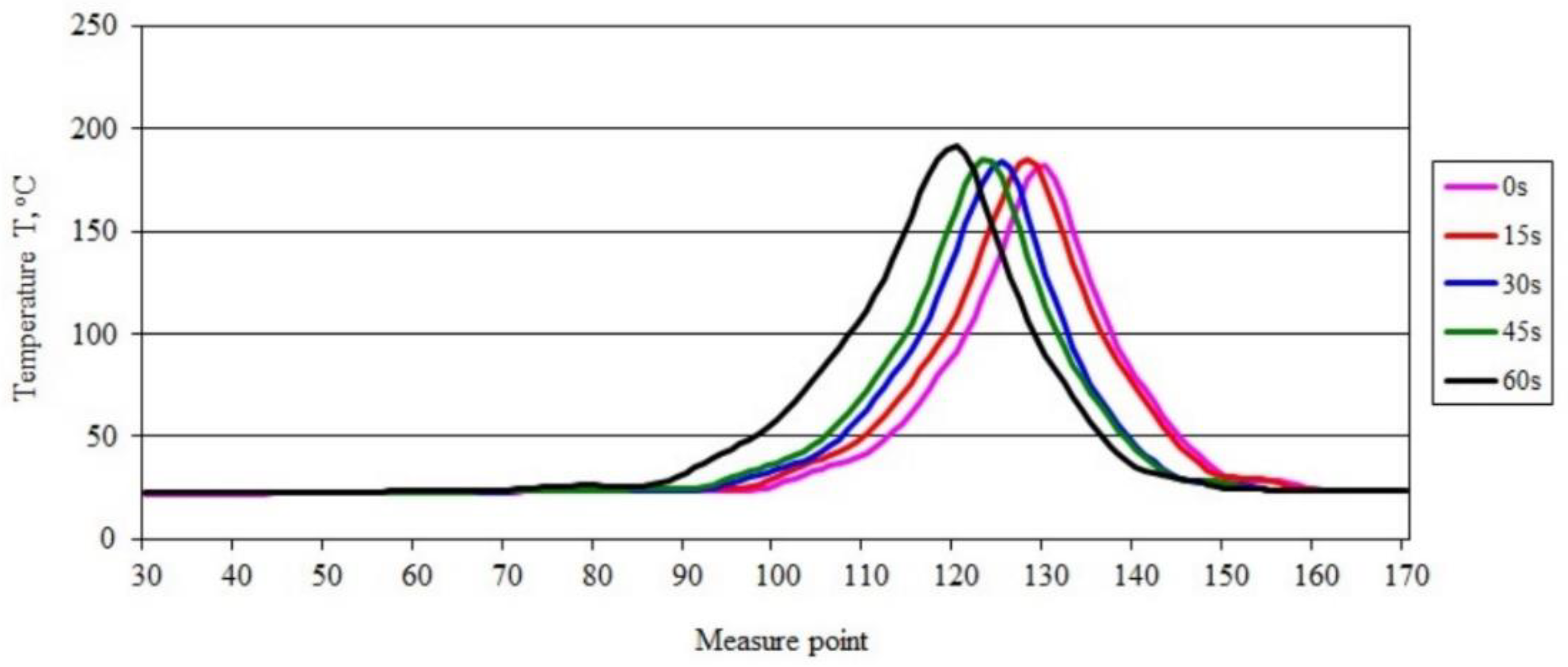
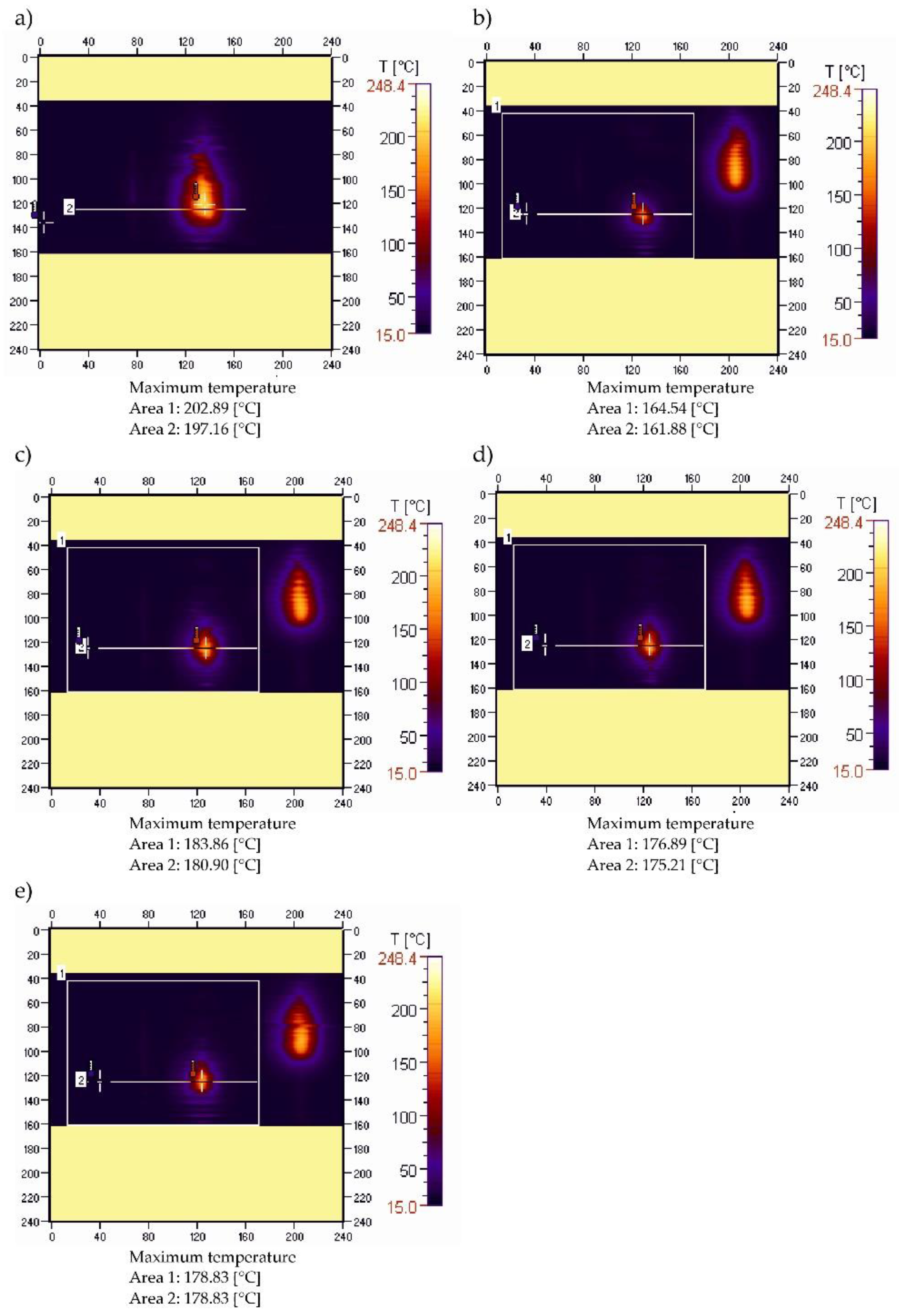
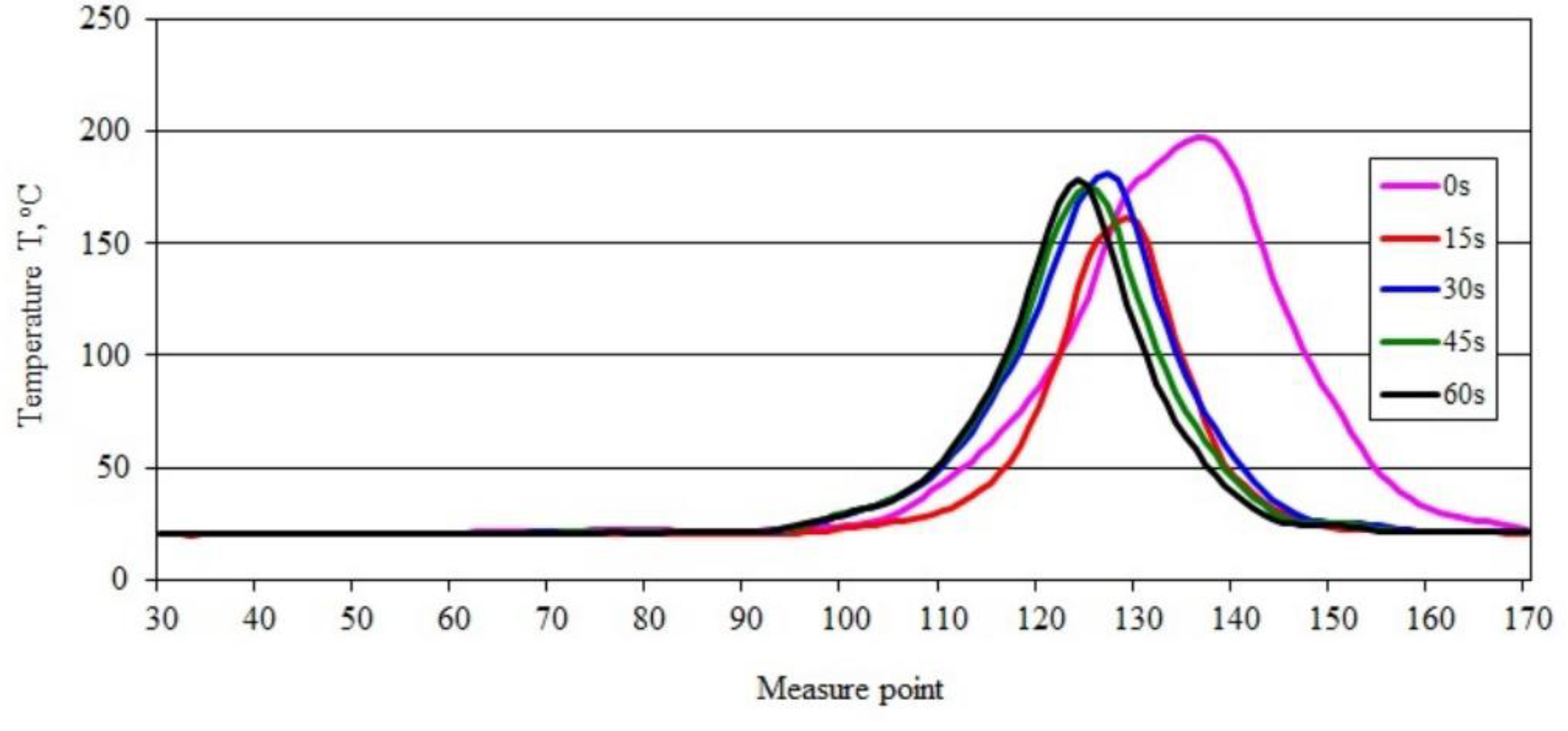
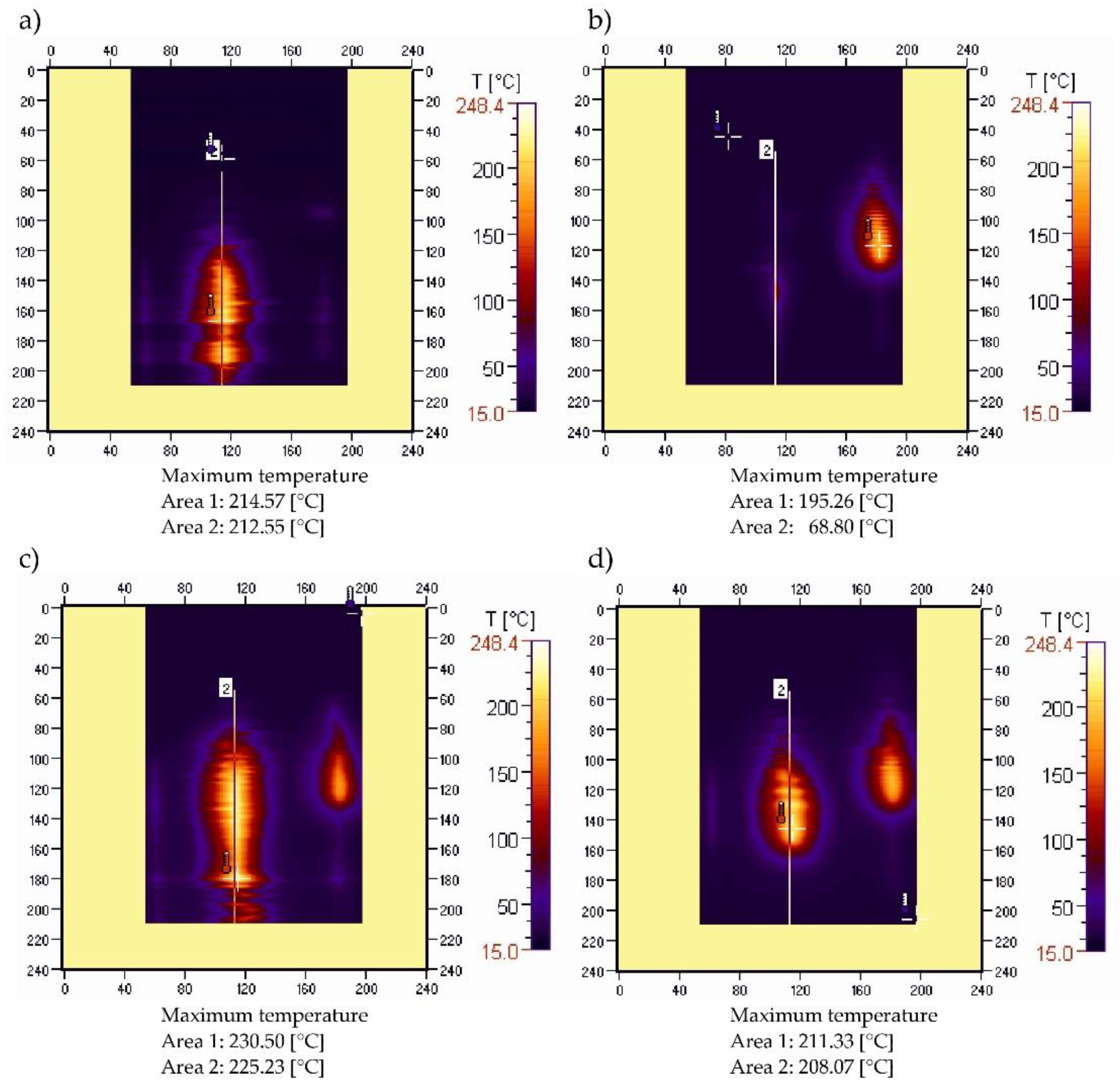
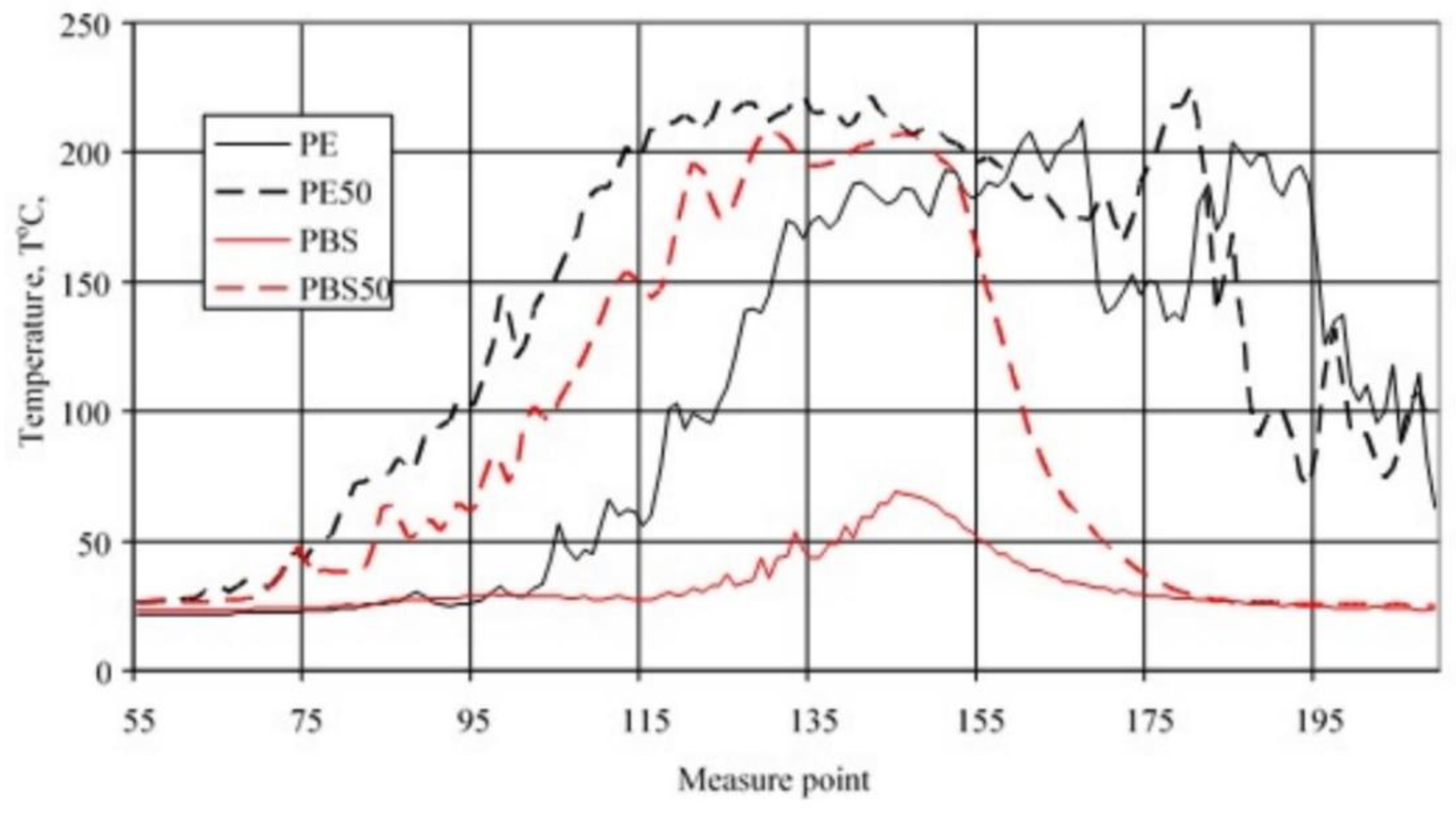
3.3. Sample Temperature during Horizontal Combustion
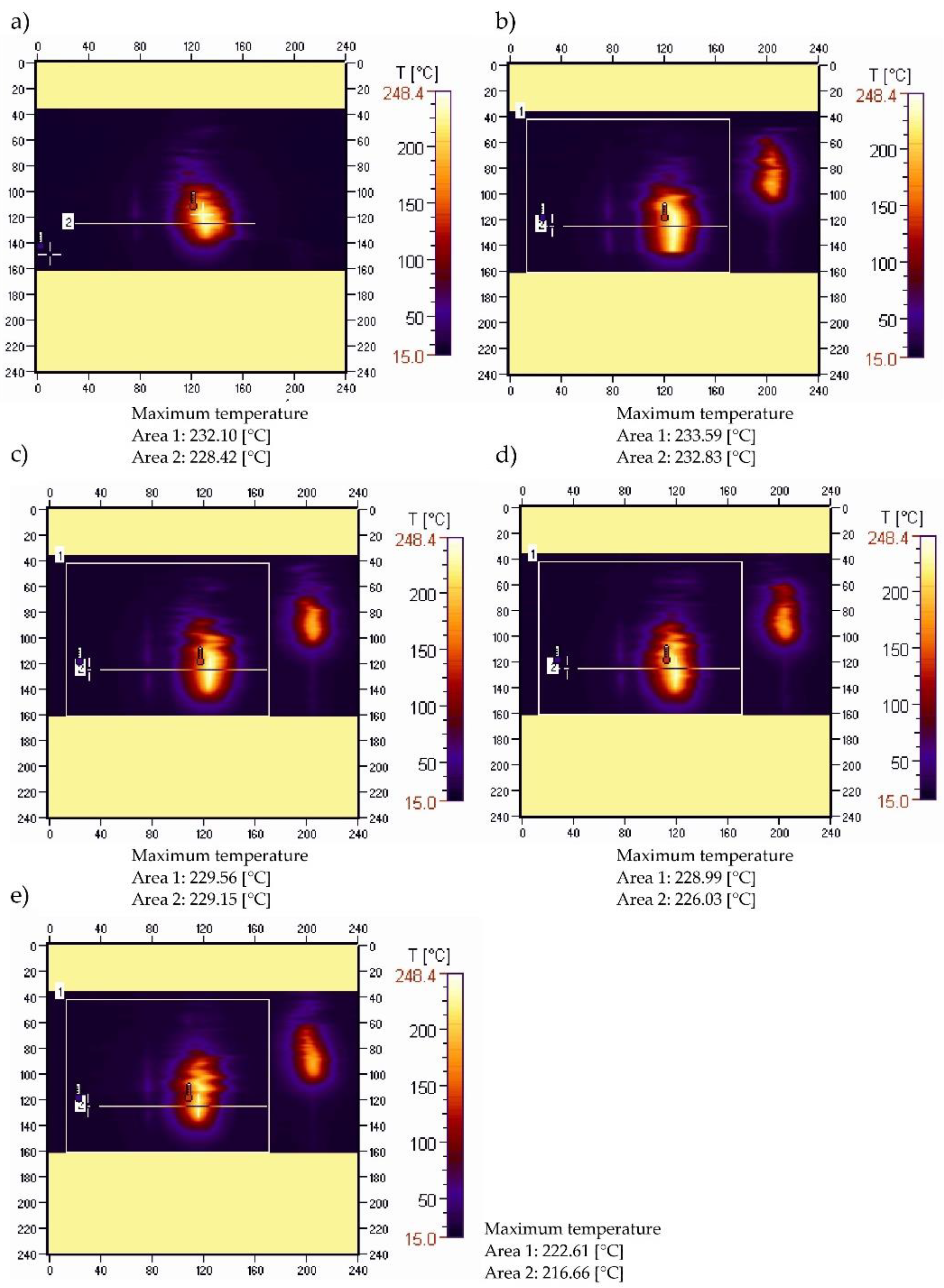
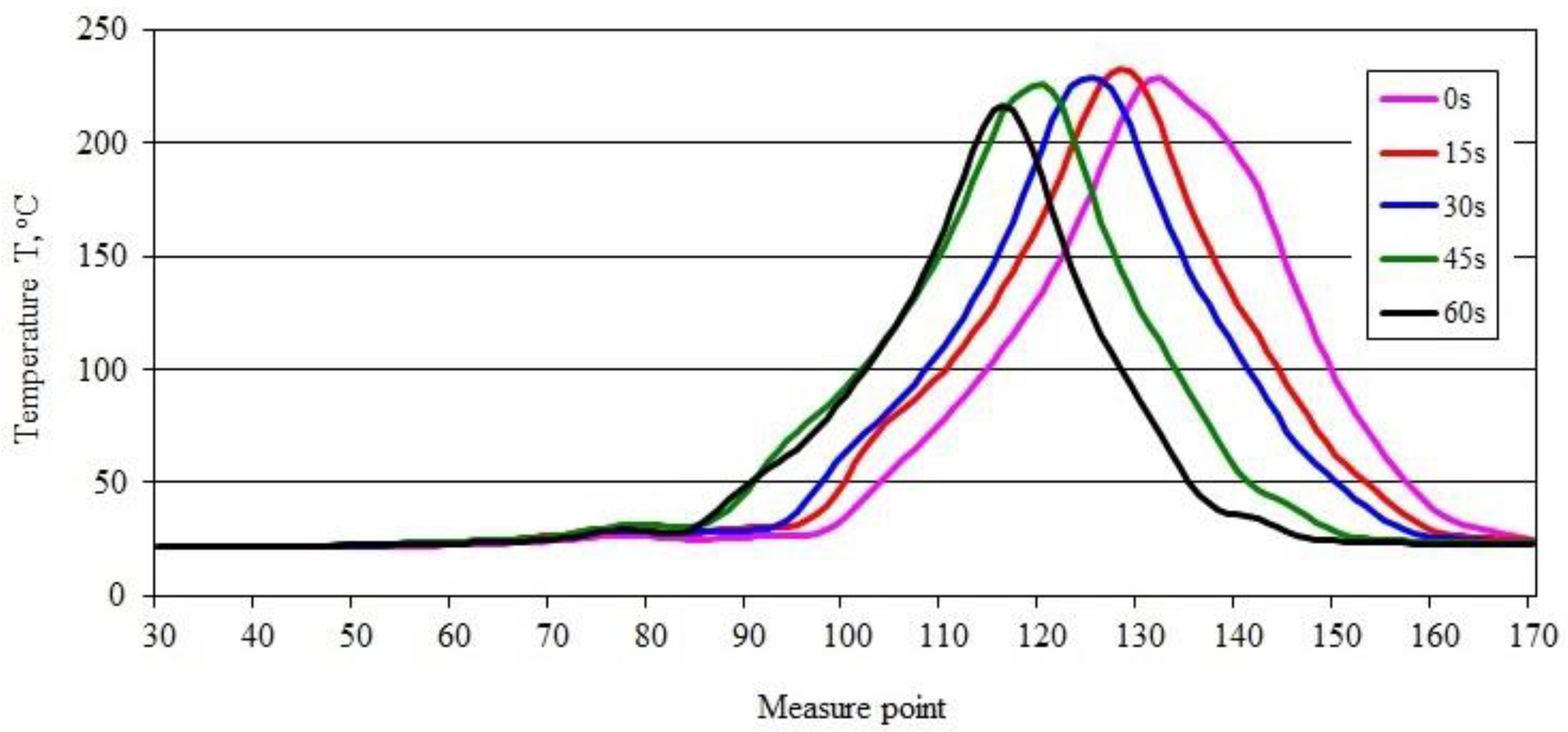
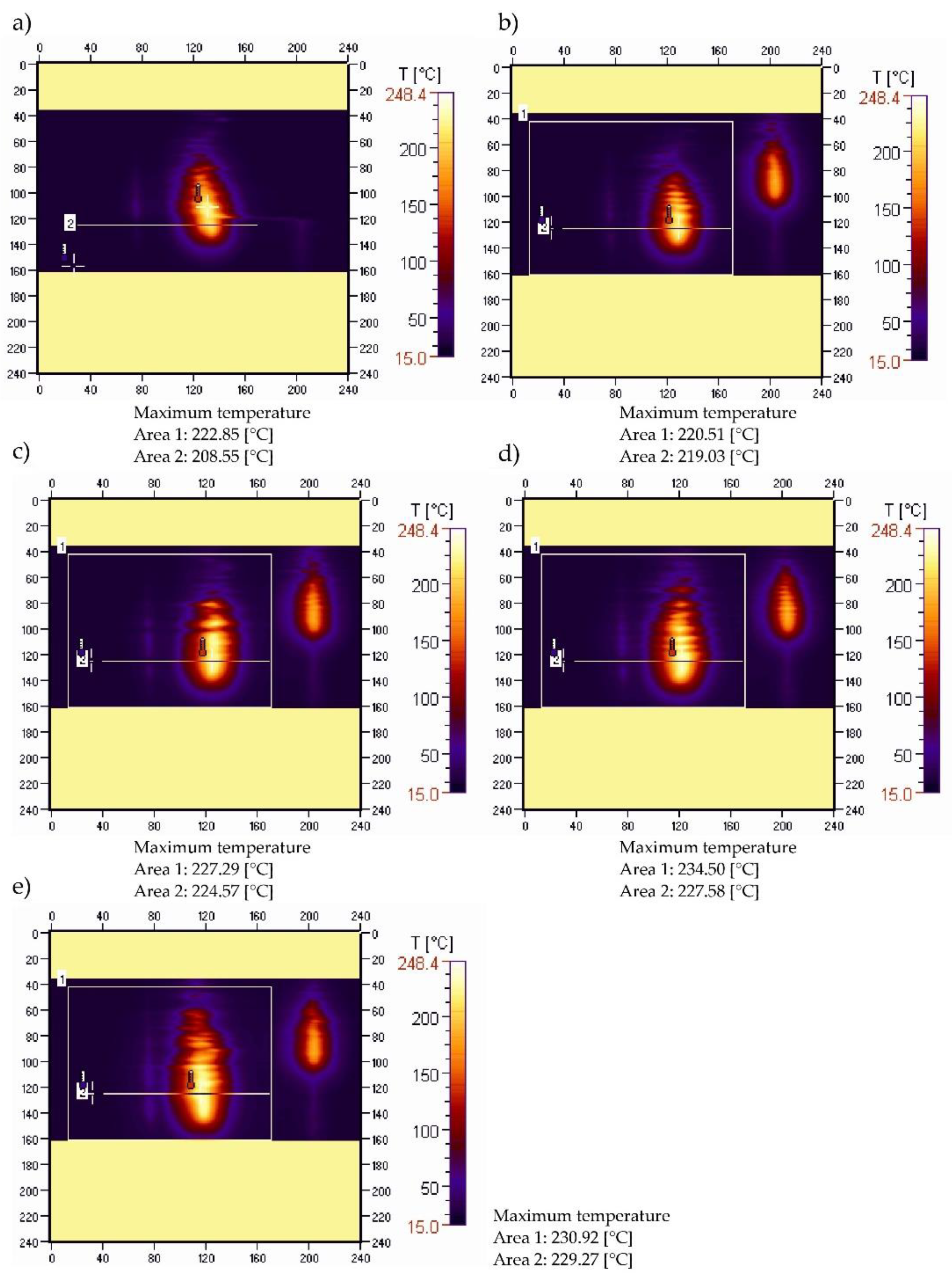
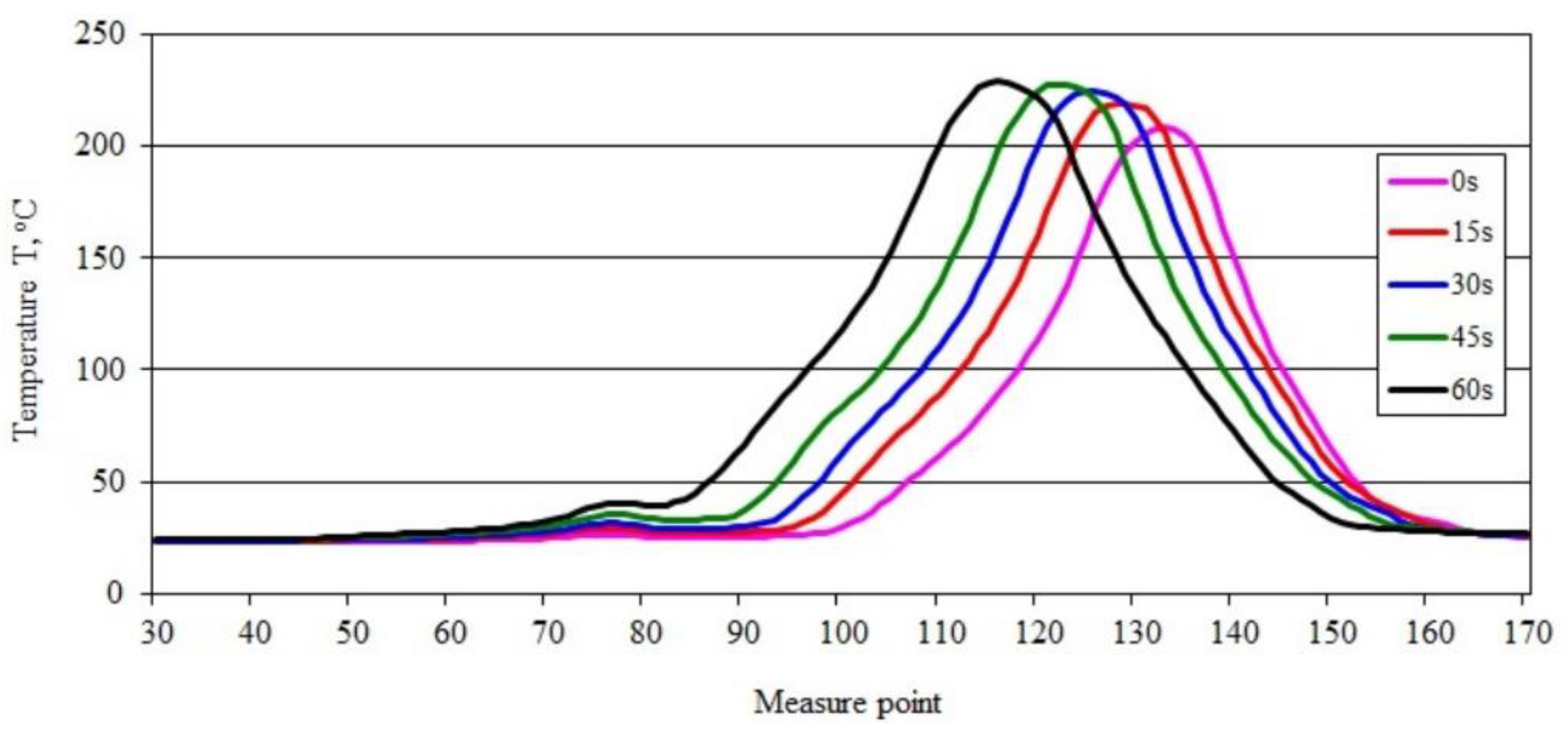
3.4. Sample Temperature during Vertical Combustion
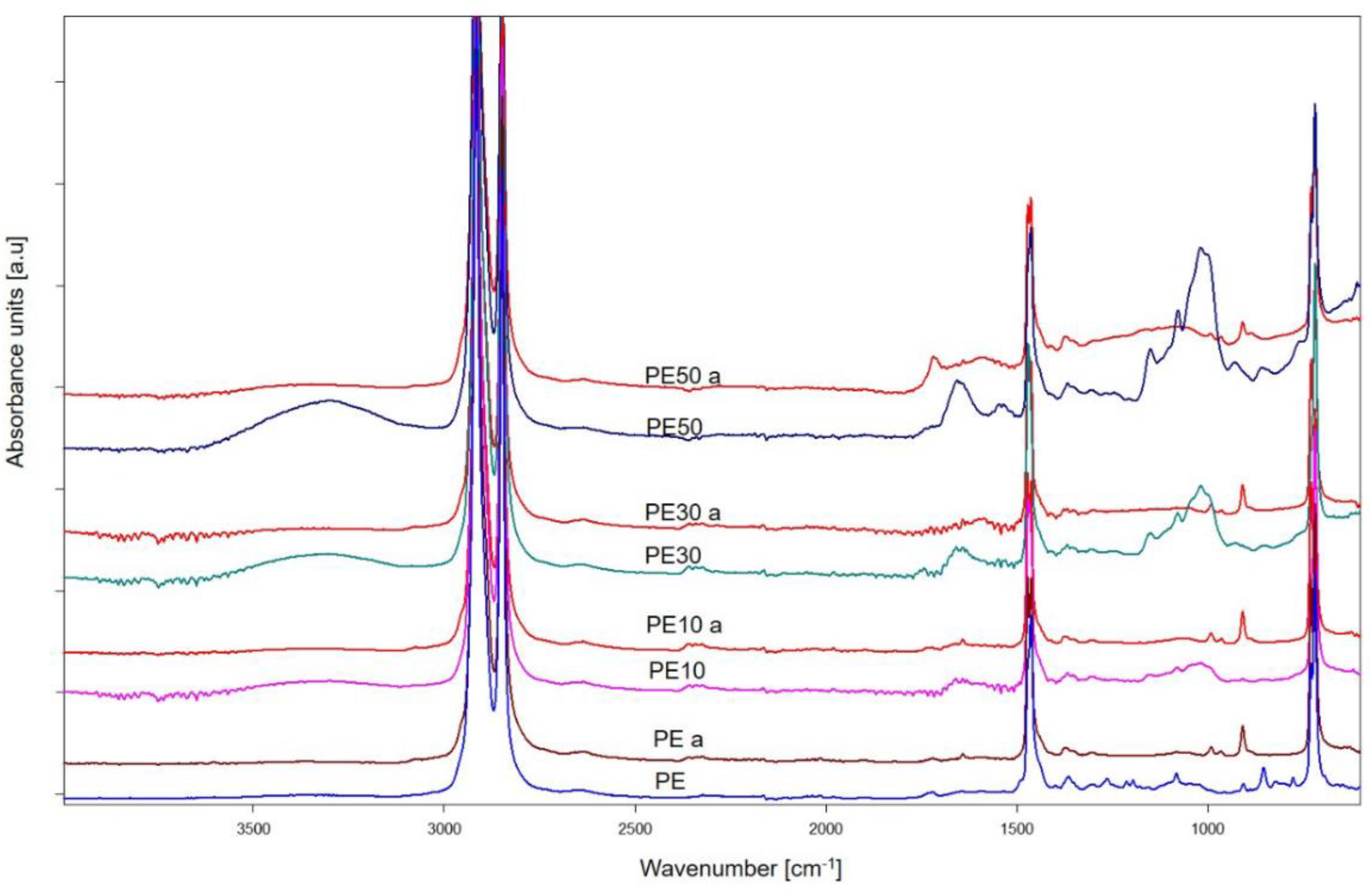
3.5. Chemical Structure of Sample Residues after Flammability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muniyadi, M.; Yit Siew Ng, T.; Munusamy, Y.; Xian Ooi, Z. Mimusops elengi seed Shell powder as a new bio-filler for polypropylene-based bio-composites. Bioresources 2018, 13, 272–289. [Google Scholar] [CrossRef]
- Bekele, L.D.; Zhang, W.; Liu, Y.; Duns, G.J.; Yu, C.; Jin, X.; Li, X.; Jia, Q.; Chen, J. Preparation and characterization of lemongrass fiber (Cymbopogon species) for reinforcing application in thermoplastic composites. Bioresources 2017, 12, 5664–5681. [Google Scholar] [CrossRef]
- Schirp, A.; Mannheim, M.; Plinke, B. Influence of refiner fibre quality and fibre modification treatment on properties of injection-moulded beech wood-plastic composites. Compos. Part A 2014, 61, 245–257. [Google Scholar] [CrossRef]
- Haque, M.; Hasan, M.; Islam, S.; Ali, E. Physico-mechanical properties of chemically treated palm and coir fiber reinforced polypropylene. Bioresour. Technol. 2009, 100, 4903–4906. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.U.; Kumarappa, S.; Gaitonde, V.N. Mechanical an physical characterization of agricultural waste reinforced polymer composites. J. Mater. Environ. Sci. 2012, 3, 907–916. [Google Scholar]
- Ashori, A.; Nourbakhsh, A. Bio-based composites from waste agricultural residues. Waste Manag. 2010, 2, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhuang, J.; Fu, Y.; Wang, Z.; Li, Q. Enhanced mechanical strength of polypropylene-based lignocellulosic-plastic composites by cellulose fibers. Bioresources 2019, 14, 1668–1678. [Google Scholar]
- Fabiyi, J.S.; McDonald, A.G. Effect of wood species on property and weathering performance of wood plastic composites. Compos. Part A 2010, 41, 1434–1440. [Google Scholar] [CrossRef]
- Hattotuwa, G.; Premalal, B.; Ismail, H.; Bahrain, A. Comparison of the mechanical properties of rice husk powder filled polypropylene composites with talc filled polyethylene composites. Polym. Test. 2002, 21, 833–839. [Google Scholar]
- Rahmat, W.; Tin Sin, L.; Rahmat, A.R.; Isa, N.M.; Salleh, M.S.N.; Mokhtar, M. Comparison of rice husk-filled polyethylene composite and natural wood under weathering effects. J. Compos. Mater. 2010, 45, 1403–1410. [Google Scholar] [CrossRef]
- George, J.; Kumar, R.; Jayaprahash, C.; Ramakrishna, A.; Sabapathy, S.N.; Bawa, A. Rice bran-filled biodegradable low-density polyethylene films: Development and characterization for packaging applications. J. Appl. Polym. Sci. 2006, 105, 4514–4522. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Majewski, Ł.; Gaspar Cunha, A. Evaluation of suitability of wheat bran as a natural filler in polymer processing. Bioresources 2018, 13, 7037–7052. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Efficiency of twin-screw extrusion of biodegradable poly(butylene succin-ate)-wheat bran blend. Materials 2021, 14, 424. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Injection Moulded Sustainable Biocomposites from Poly(butylene succinate) and Wheat Bran. Materials 2021, 14, 7049. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Artificial Ageing, Chemical Resistance, and Biodegradation of Biocomposites from Poly(Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7580. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Jachowicz, T.; Sąsiadek, M. Experimental Determination of Coefficients for the Renner Model of the Thermodynamic Equation of State of the Poly(butylene succinate) and Wheat Bran Biocomposites. Materials 2021, 14, 5293. [Google Scholar] [CrossRef]
- Tufan, M.; Ayrilmis, N. Potential use of halzenut husk in recycled high-density polyethylene composites. Bioresources 2016, 11, 7476–7489. [Google Scholar] [CrossRef]
- Sarsari, N.A.; Pourmousa, S.; Tajdini, A. Physical and mechanical properties of walnut shell flour-filled thermoplastic starch composites. Bioresources 2016, 11, 6968–6983. [Google Scholar]
- Obasi, H.C. Peanut husk filled polyethylene composites: Effect of filler content and compatibilizer on properties. J. Polym. 2015, 2015, 189289. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Properties of coconut shell powder filled polylactic acid ecocomposites: Effect of maleic acid. Polym. Eng. Sci. 2013, 53, 1109–1116. [Google Scholar] [CrossRef]
- Essabir, H.; Achaby, M.E.; Hilali, E.M.; Bouhfid, R.; Qaiss, A.E. Morphological, structural, thermal and tensile properties of high density polyethylene composites reinforced with treated argan nut shell particles. J. Bionic Eng. 2015, 12, 129–141. [Google Scholar] [CrossRef]
- Hongsriphan, N.; Kamsantia, P.; Sillapasangloed, P.; Loychuen, S. Bio-based composite from poly(butylene succinate) and peanut shell waste adding maleinized linseed oil. IOP Conf. Ser. Mater. Sci. Eng. 2020, 773, 012046. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Husney, N.A.A.; Ismail, N.H.; Mariatti, M. Structure and performance of poly(lactic acid)/poly(butylene succinate-co-L-lactate) blend reinforced with rice husk and coconutshell filler. Polym. Polym. Compos. 2020, 29, 992–1002. [Google Scholar]
- Mirmehdi, S.M.; Zeinaly, F.; Dabbagh, F. Date palm wood flour as filler of linear low-density polyethylene. Compos. Part B 2014, 56, 137–141. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Han, S.Y.; Park, J.S.; Lee, E.A.; Park, J.Y.; Kwon, G.J.; Kim, S.J.; Lee, S.H. Influence of lignin and polymeric diphenylmethane diisocyante addition on the properties of poly(butylene succinate)/wood flour composite. Polymers 2019, 11, 1161. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Poh, B.T.; Issam, A.M.; Jawaid, M.; Ridzuan, R. Recycled polypropylene-oil pal biomass: The effect on mechanical and physical properties. J. Reinf. Plast. Compos. 2010, 29, 1117–1130. [Google Scholar] [CrossRef]
- Luz, S.M.; Tio, J.D.; Rocha, G.J.M.; Goncalves, A.R.; Del’Arco, A.P. Cellulose and cellullignin from sugarcane bagasse reinforced polypropylene composites: Effect of acetylation on mechanical and thermal properties. Compos. Part A 2008, 39, 1362–1369. [Google Scholar] [CrossRef]
- Mengeloglu, F.; Karakus, K. Thermal degradation, mechanical properties and morphology of wheat straw flour filled recycled thermoplastic composites. Sensors 2008, 8, 500–519. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Characteristics of wood-fiber plastic composites made of recycled materials. Waste Manag. 2009, 29, 1291–1295. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamuna, A.A.; Volk, J. Barley husk and coco nut shell reinforced polypropylene composites: The effect of fibre physical, chemical and surface properties. Compos. Sci. Technol. 2010, 70, 840–846. [Google Scholar] [CrossRef]
- Taha, I.; Ziegmann, G. A comparison of mechanical properties of natural fiber filled biodegradable and polyolefin polymers. J. Compos. Mater. 2006, 40, 1933–1946. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Yu, W.; Zhou, C. Isothermal crystallization kinetics of highly filled wood plastic composites: Effect of wood particles content and compatibilizer. J. Macromol. Sci. Part B 2011, 50, 2271–2289. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A review. Bioresources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Meeks, D.; Hottle, T.; Bilec, M.M.; Landis, A.E. Compostable biopolymer use in the real world: Stakeholder interviews to better understand the motivations and realities of use and disposal in US. Resour. Conserv. Recycl. 2015, 105, 134–142. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and biodegradability evaluation of composites from poly(butylene succinate) (PBS) bioplastic and biofuel co-products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutkowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and supramolecular changes in polybutylene succinate (PBS) and polybutylene succinate adipate (PBSA) copolymer during degradation In various en-virnomental conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef]
- Samujło, B. The effect of the aging process on selected properties of polypropylene modified by natural fillers. Adv. Sci. Technol. 2020, 14, 139–147. [Google Scholar] [CrossRef]
- Podkościelna, B.; Wnuczek, K.; Goliszek, M.; Klepka, T.; Dziuba, K. Flammability Tests and Investigations of Properties of Lignin-Containing Polymer Composites Based on Acrylates. Molecules 2020, 25, 5947. [Google Scholar] [CrossRef] [PubMed]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Influence of the Design Solutions of Extruder Screw Mixing Tip on Selected Properties of Wheat Bran-Polyethylene Biocomposite. Polymers 2019, 11, 2120. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Biocomposites Based on Polyethylene with a Natural Origin Filler. Materials 2020, 13, 4182. [Google Scholar] [CrossRef]
- Dowlex 2631.10EU Polyethylene Resin—Technical Data Sheet. Available online: https://www.dow.com/en-us/document-viewer.html?ramdomVar=9174234036439704424&docPath=/content/dam/dcc/documents/en-us/productdatasheet/400/400-00089061en-dowlex-2631ue-tds.pdf (accessed on 22 August 2022).
- BioPBS FZ91PB—Technical Data Sheet. Available online: https://www.pttmcc.com/file_upload/tds/TDS-FZ91PM_PB.pdf (accessed on 22 August 2022).
- Khalili, P.; Liu, X.; Zhao, Z.; Blinzler, B. Fully Biodegradable Composites: Thermal, Flammability, Moisture Absorption and Mechanical Properties of Natural Fibre-Reinforced Composites with Nano-Hydroxyapatite. Materials 2019, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Matykiewicz, D.; Barczewski, M.; Michałowski, S. Basalt powder as an eco-friendly filler for epoxy composites: Thermal and thermo-mechanical properties assessment. Compos. Part B Eng. 2019, 164, 272–279. [Google Scholar] [CrossRef]



| Experimental Design Layout | u, %wt | Matrix Material |
|---|---|---|
| PE | 0 | PE |
| PE10 | 10 | PE |
| PE30 | 30 | PE |
| PE50 | 50 | PE |
| PBS | 0 | PBS |
| PBS10 | 10 | PBS |
| PBS30 | 30 | PBS |
| PBS50 | 50 | PBS |
| Experimental Design Layout | Burning Time of Measuring Length t1, s | Linear Combustion Rate v, mm/min | Flammability Class According to Horizontal Test |
|---|---|---|---|
| PE | 157.64 | 28.55 | HB40 |
| PE10 | 128.33 | 35.07 | HB40 |
| PE30 | 98.27 | 45.79 | HB75 |
| PE50 | 141.02 | 31.91 | HB40 |
| PBS | 264.76 | 17.00 | HB40 |
| PBS10 | 203.78 | 22.08 | HB40 |
| PBS30 | 129.00 | 34.88 | HB40 |
| PBS50 | 141.67 | 31.76 | HB40 |
| Experimental Design Layout | Burning Time after First Ignition t2, s | Sample Burned Completely YES or NO | Ignition of the Cotton Batting YES or NO |
|---|---|---|---|
| PE | 78.29 | YES | YES |
| PE10 | 40.40 | YES | YES |
| PE30 | 38.44 | YES | YES |
| PE50 | 30.90 | YES | YES |
| PBS | 28.89 | NO | YES |
| PBS10 | 28.72 | NO | YES |
| PBS30 | 50.84 | YES | YES |
| PBS50 | 41.85 | YES | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasimowski, E.; Samujło, B.; Grochowicz, M.; Majewski, Ł. Flammability of Polymer Compositions Filled with Wheat Bran. Materials 2022, 15, 8955. https://doi.org/10.3390/ma15248955
Sasimowski E, Samujło B, Grochowicz M, Majewski Ł. Flammability of Polymer Compositions Filled with Wheat Bran. Materials. 2022; 15(24):8955. https://doi.org/10.3390/ma15248955
Chicago/Turabian StyleSasimowski, Emil, Bronisław Samujło, Marta Grochowicz, and Łukasz Majewski. 2022. "Flammability of Polymer Compositions Filled with Wheat Bran" Materials 15, no. 24: 8955. https://doi.org/10.3390/ma15248955
APA StyleSasimowski, E., Samujło, B., Grochowicz, M., & Majewski, Ł. (2022). Flammability of Polymer Compositions Filled with Wheat Bran. Materials, 15(24), 8955. https://doi.org/10.3390/ma15248955






