Progress in Research and Application of Graphene Aerogel—A Bibliometric Analysis
Abstract
1. Introduction
2. Bibliometric Analysis
2.1. Collection of Data
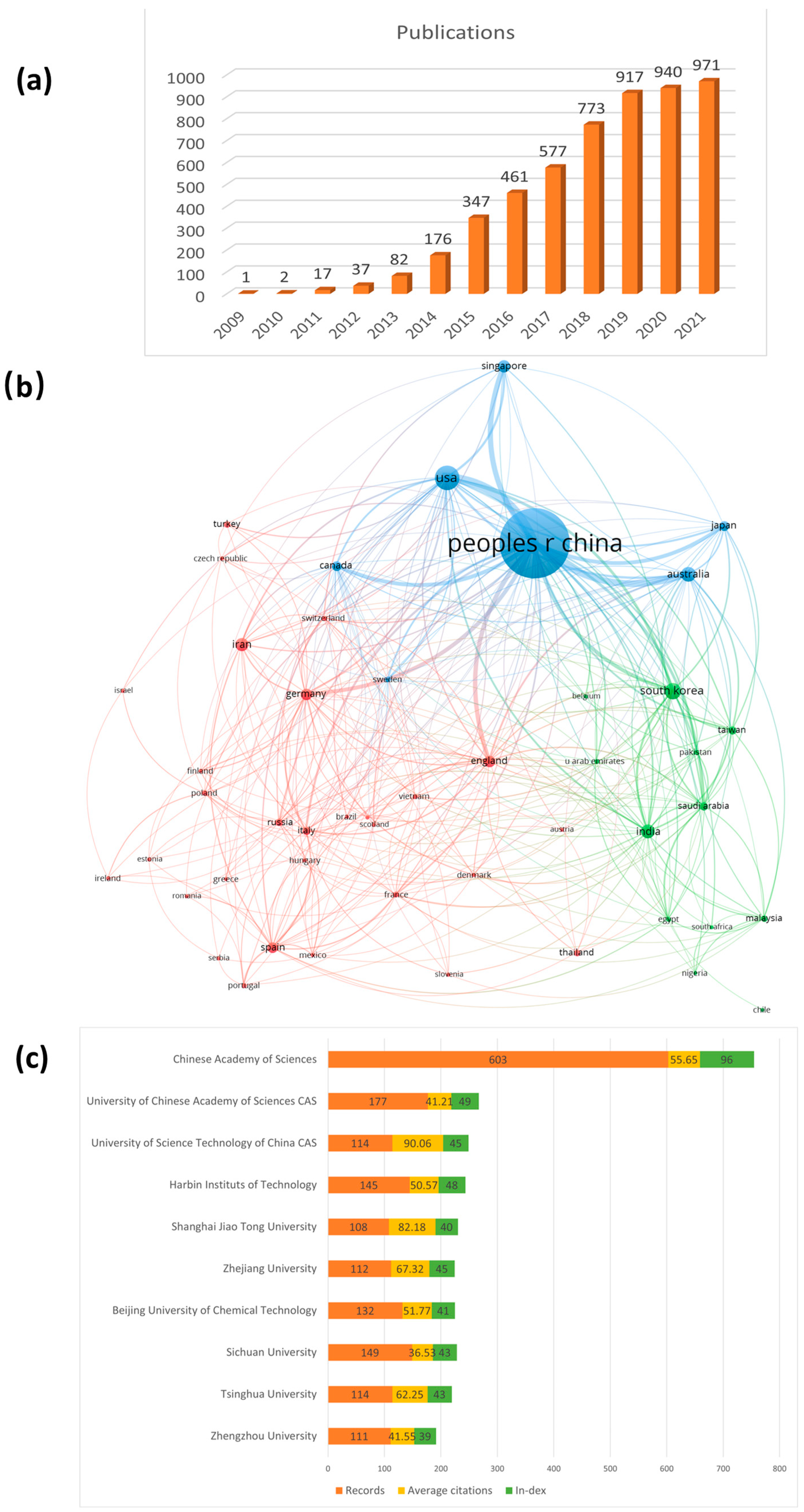
2.2. Annual Publications and Countries/Institutes Contributing to GA research
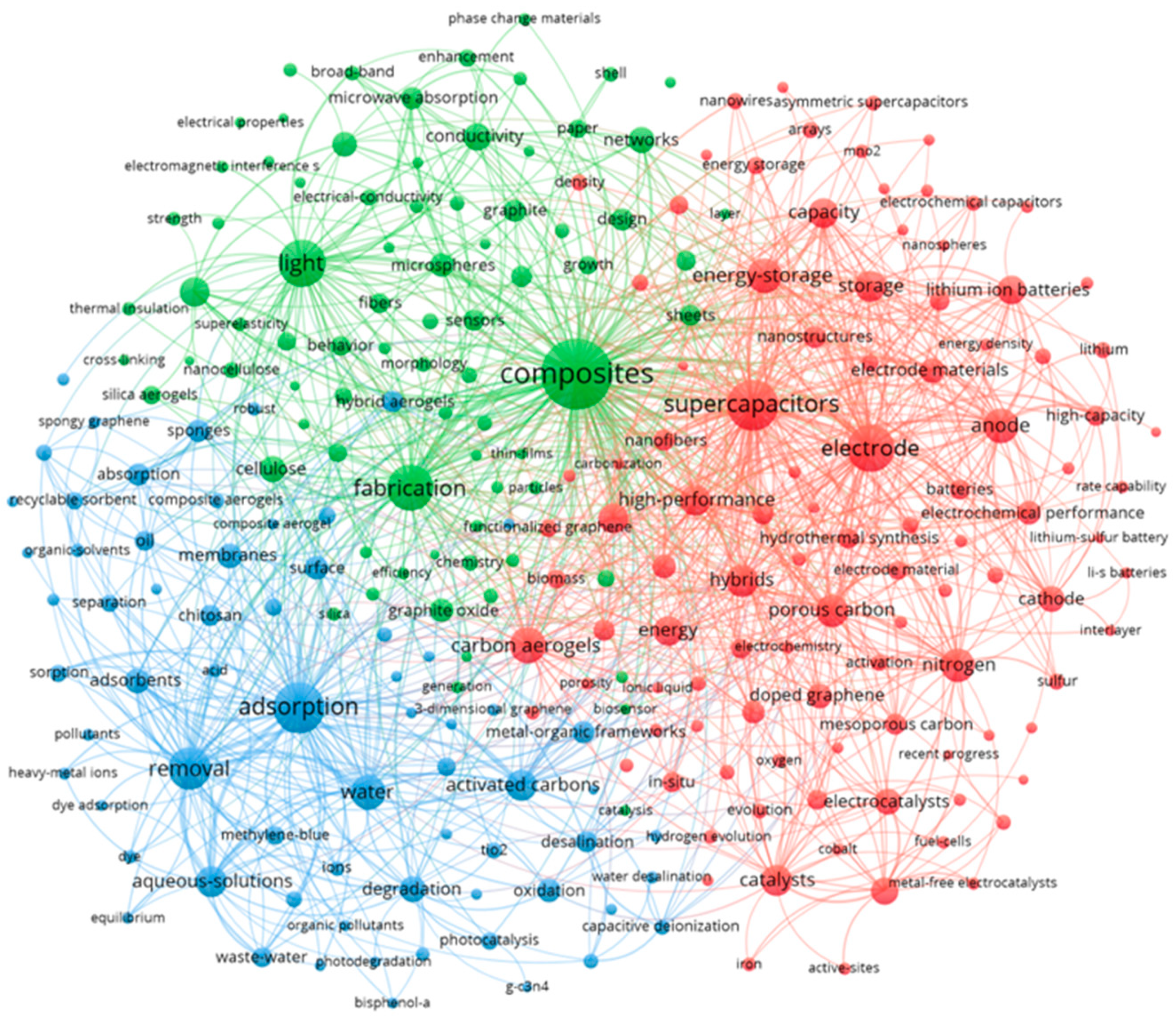
2.3. Research Hotspots
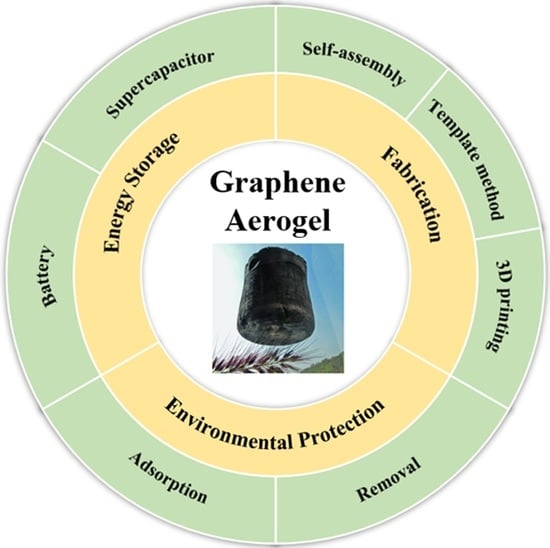
3. Fabrication of Graphene Aerogels
3.1. Self-Assembly Method for GA Fabrication
3.1.1. In-Situ Assembly Method
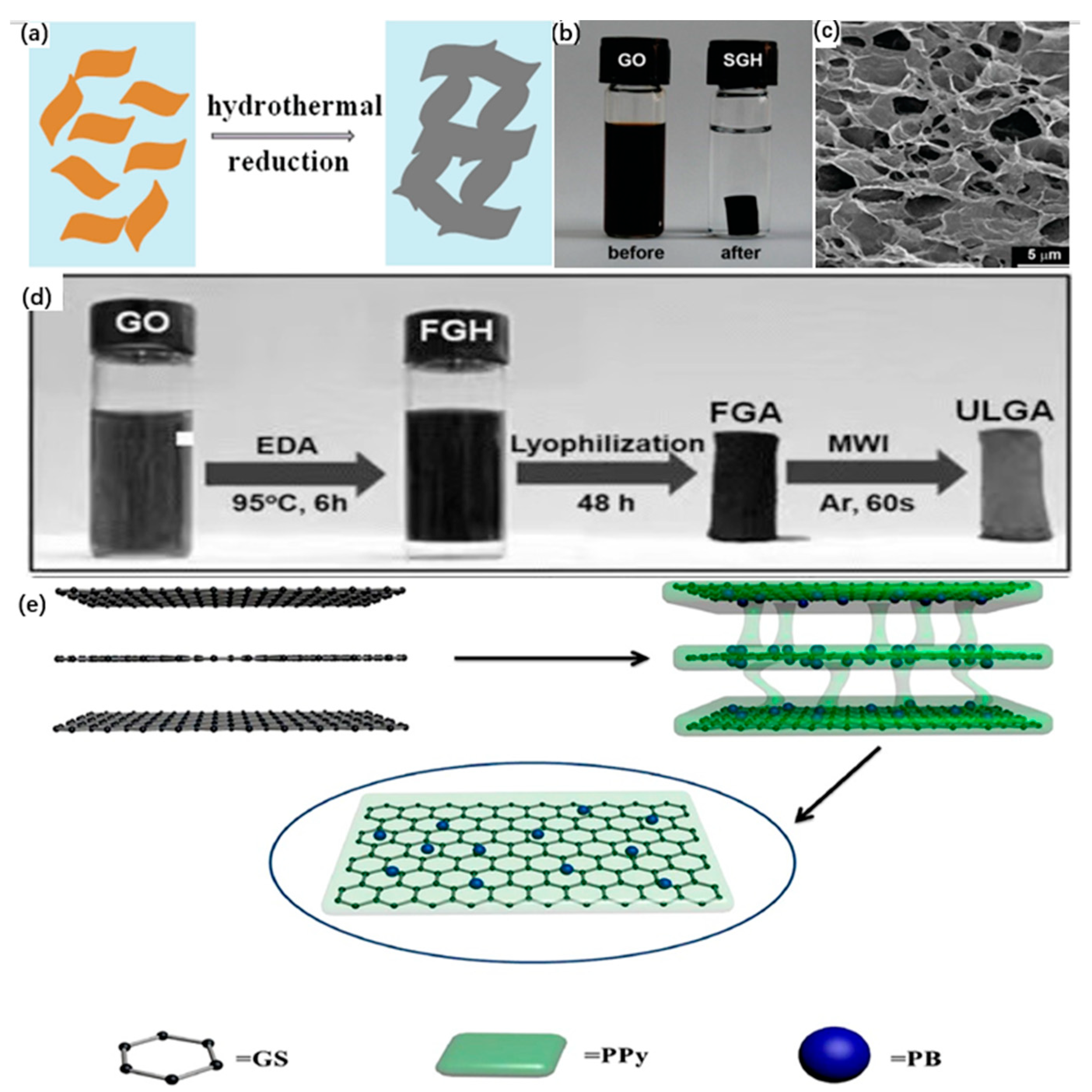
3.1.2. Cross-Linker-Induced Self-Assembly Method
3.2. Template Method for GA Fabrication
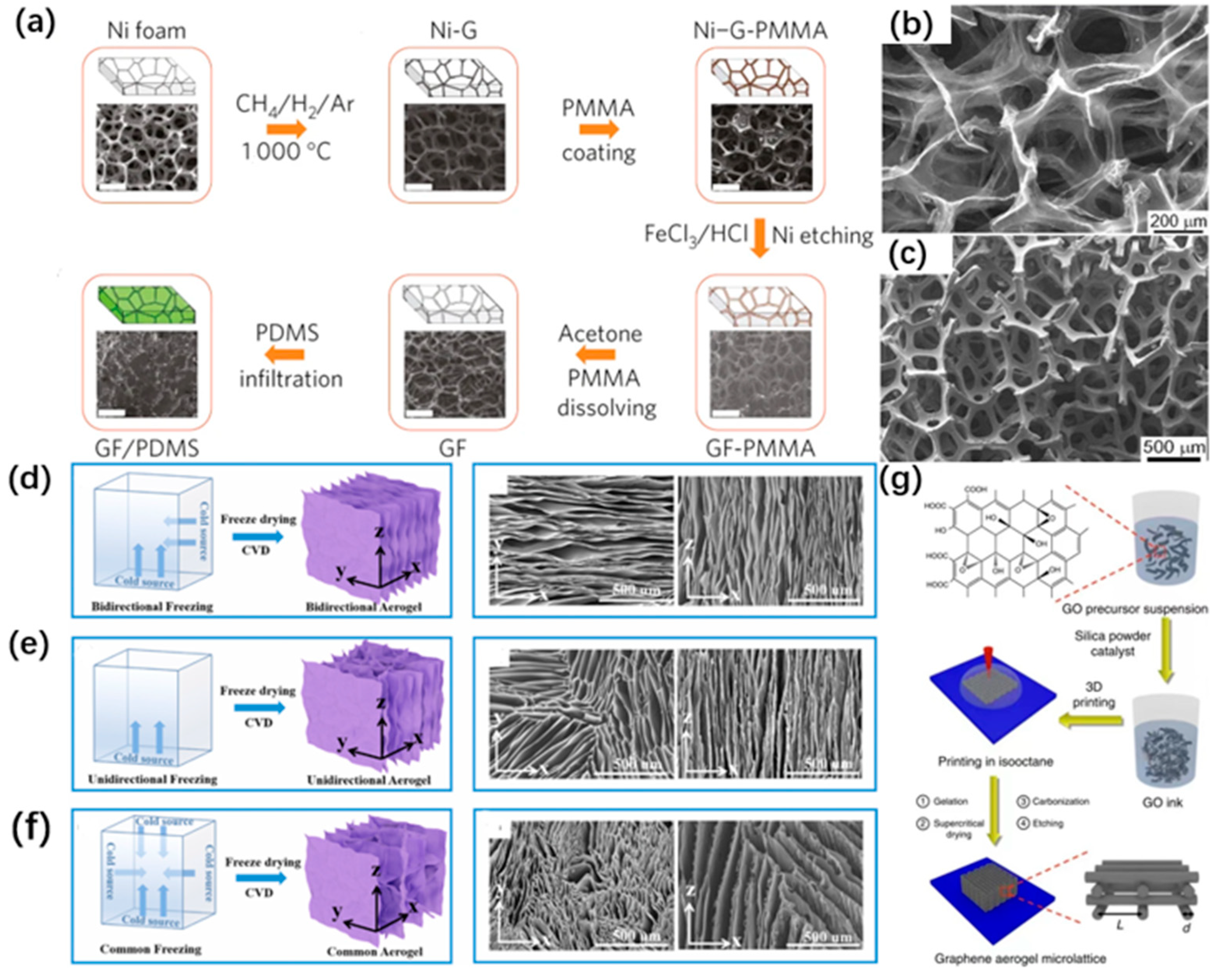
3.2.1. Template-Assisted Chemical Vapor Deposition
3.2.2. Ice Template Method
3.3. 3D Printing Methods
4. GA in the Field of Energy Storage
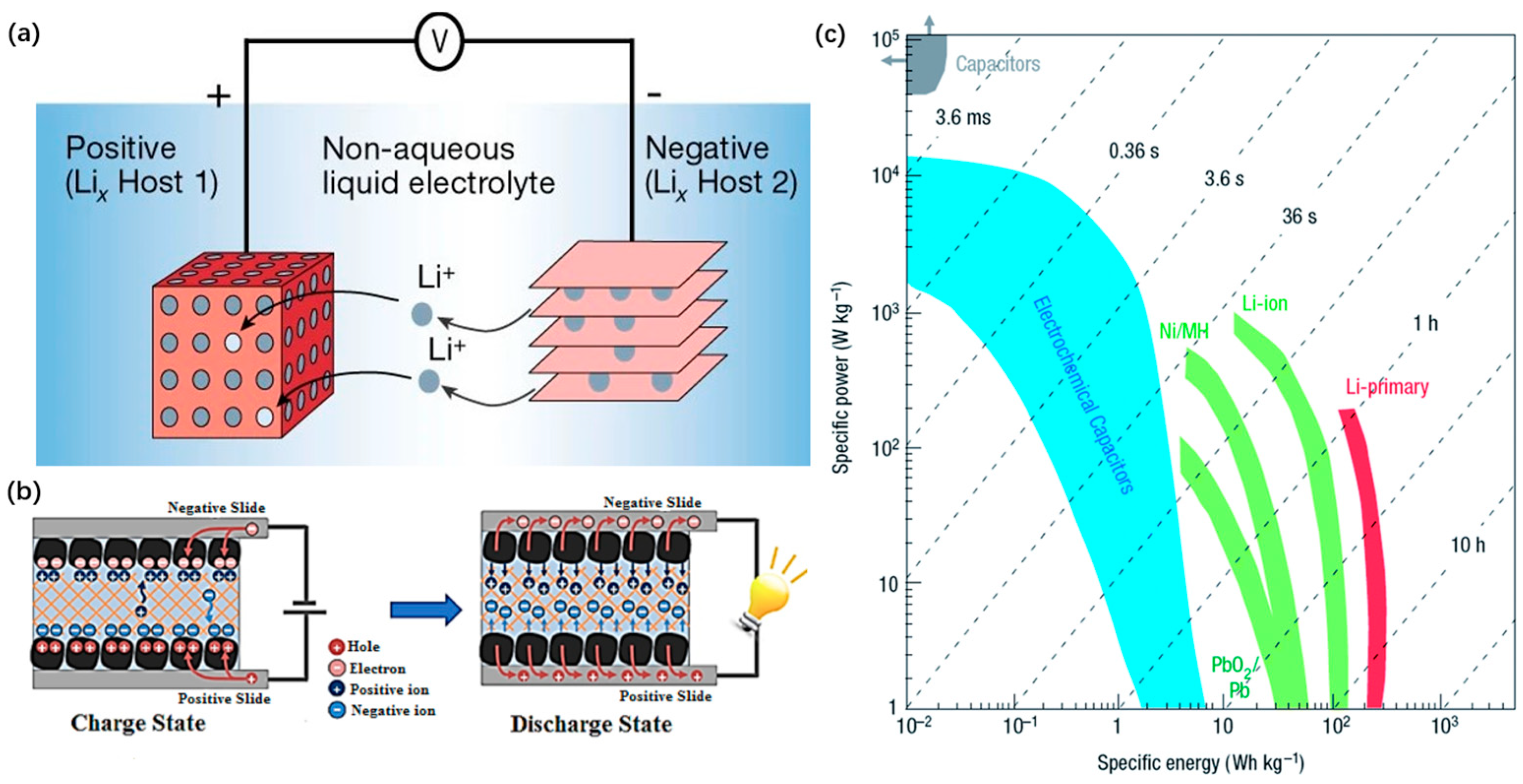
4.1. Lithium Battery Electrode
4.1.1. The LiBs Anode
- Cathode:
- Anode:
4.1.2. The Li-S Battery Electrodes
- Cathode:
- Anode:
4.1.3. The Li-O2 Battery Electrode
- Cathode:
- Anode:
4.2. Supercapacitor Electrodes
4.2.1. Electrochemical Double Layer Capacitor Electrodes
4.2.2. Pseudocapacitor Electrodes
5. Application of GA in the Environmental Protection Field
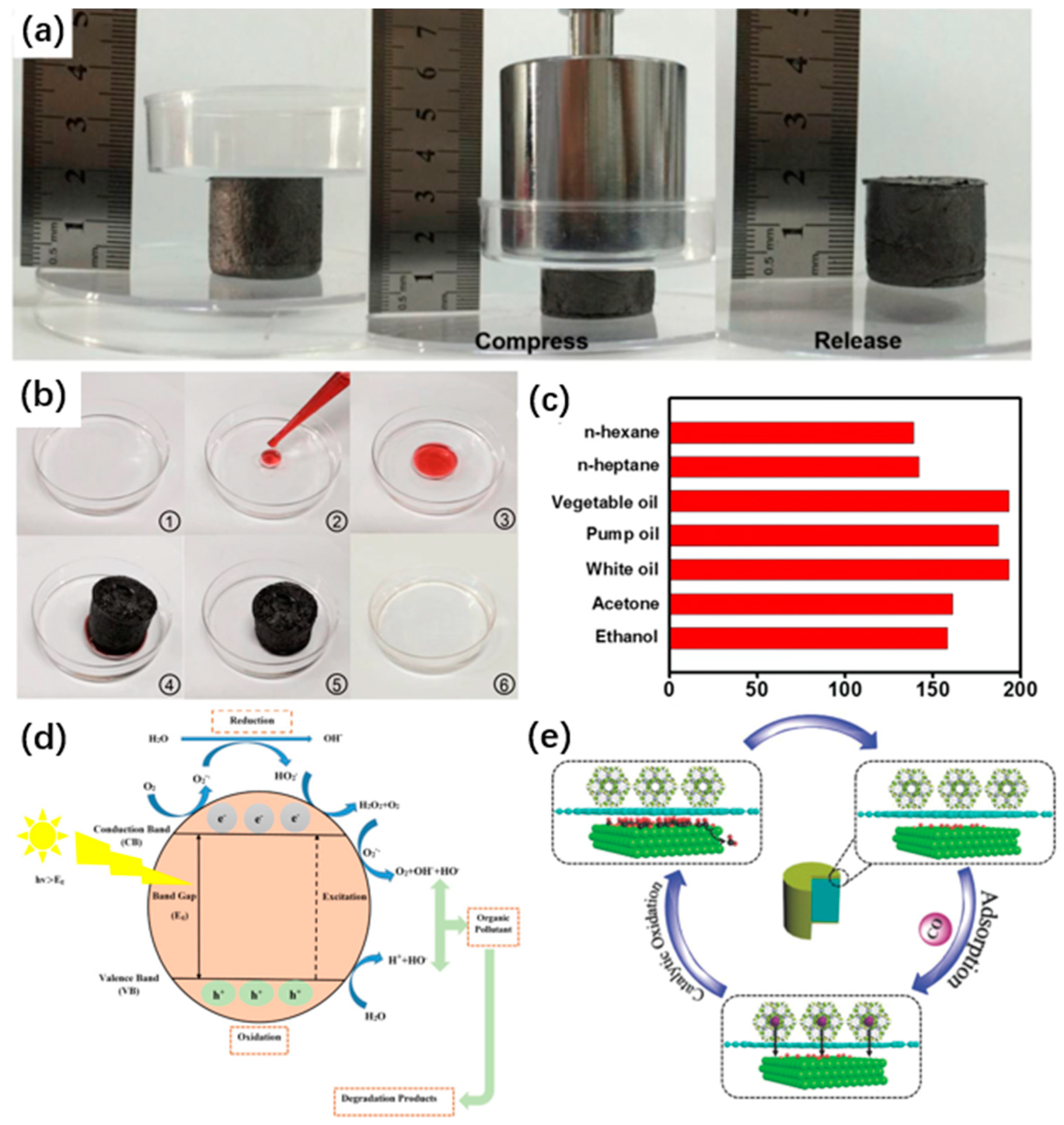
5.1. Adsorption
5.1.1. Adsorption of Wastewater Pollutants
5.1.2. Adsorption of Gases
5.2. Removal
6. Conclusions and Outlook
- (1)
- We firstly discuss the self-assembly method, the template method, and the 3D printing preparation of GA, respectively, and analyze the advantages and limitations of different preparation methods. We found that the assembly method as a commonly used preparation method has the advantages of easy operation and low cost, and is expected to be produced in large quantities on a factory scale. However, the molded GA surface often contains many defects and collapses, which can seriously impair performance. The GA obtained by template method and 3D printing has better results in terms of material structure and uniformity of properties as well as stability in long-term use. However, the relatively high cost of these two methods and the tedious preparation process makes them difficult to meet in industrial scale manufacturing at present.
- (2)
- GA with its excellent conductivity and large specific surface area is used as an electrode for batteries and supercapacitors to store energy. In lithium battery systems, GA is usually used as an electrode material alone or in combination with other electrode materials. Its good electrical conductivity and large specific surface area can effectively improve the transfer efficiency of lithium ions during charging and discharging to achieve rapid charging and discharging. In supercapacitors, the porous structure provided by GA can increase the electrode capacity as well as provide electrochemically active sites for electron attachment after doping with heteroatoms to achieve high energy density. The large specific surface area and high electrical conductivity possessed by GA are its advantages, and it has a huge advantage in the development of energy storage.
- (3)
- In the field of environmental protection, the hydrophobic properties of the oxygen-containing functional groups on the surface of GA combined with the porous structure enable effective adsorption of organic substances, and its good resilience can effectively achieve multiple cycles of adsorption-desorption under the action of mechanical pressure. Meanwhile the large surface area provides loading for photocatalysts to improve the efficiency of catalytic degradation of pollutants, which will play a very significant role in promoting the improvement of environmental problems. The development and progress of GA is closely related to the development of these three major research fields. As a visualization method, the bibliometric analysis presents objective and comprehensive content. The summary of the key sections will be presented to the reader more clearly and quickly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Dong, Q.C.; Xiao, M.; Chu, Z.Y.; Li, G.C.; Zhang, Y. Recent Progress of Toxic Gas Sensors Based on 3D Graphene Frameworks. Sensors 2021, 21, 3386. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutierrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.B.; Wan, W.B.; Gogotsi, Y.; Qiu, J.S. Ultralight and Highly Compressible Graphene Aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef]
- He, Y.Q.; Liu, Y.; Wu, T.; Ma, J.K.; Wang, X.R.; Gong, Q.J.; Kong, W.N.; Xing, F.B.; Liu, Y.; Gao, J.P. An environmentally friendly method for the fabrication of reduced graphene oxide foam with a super oil absorption capacity. J. Hazard. Mater. 2013, 260, 796–805. [Google Scholar] [CrossRef]
- Chen, Z.P.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Pei, S.F.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Garcia-Tunon, E.; Barg, S.; Franco, J.; Bell, R.; Eslava, S.; D’Elia, E.; Maher, R.C.; Guitian, F.; Saiz, E. Printing in Three Dimensions with Graphene. Adv. Mater. 2015, 27, 1688–1693. [Google Scholar] [CrossRef]
- Zhang, X.T.; Sui, Z.Y.; Xu, B.; Yue, S.F.; Luo, Y.J.; Zhan, W.C.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Fu, G.T.; Yan, X.X.; Chen, Y.F.; Xu, L.; Sun, D.M.; Lee, J.M.; Tang, Y.W. Boosting Bifunctional Oxygen Electrocatalysis with 3D Graphene Aerogel-Supported Ni/MnO Particles. Adv. Mater. 2018, 30, 1704609. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Y.; Meng, Y.N.; Xiao, P.W.; Zhao, Z.Q.; Wei, Z.X.; Han, B.H. Nitrogen-Doped Graphene Aerogels as Efficient Supercapacitor Electrodes and Gas Adsorbents. ACS Appl. Mater. Interfaces 2015, 7, 1431–1438. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.H.; Tian, J.; Li, H.D.; Sang, Y.H.; Yu, G.W.; Cai, H.Q.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Tsang, A.C.H.; Zhang, J.T.; Hui, K.N.; Hui, K.S.; Huang, H.B. Recent Development and Applications of Advanced Materials via Direct Ink Writing. Adv. Mater. Technol. 2022, 7, 31. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liang, H.W.; Chen, L.F.; Hu, B.C.; Yu, S.H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Peng, H.R.; Zhang, H.Z.; Kang, J.Z.; Zhu, C.; Delgado, G.; Byrne, D.; Faulkner, S.; Freyman, M.; Lu, X.H.; et al. Printing Porous Carbon Aerogels for Low Temperature Supercapacitors. Nano Lett. 2021, 21, 3731–3737. [Google Scholar] [CrossRef]
- Yang, Q.X.; Lu, R.; Ren, S.S.; Chen, C.T.; Chen, Z.J.; Yang, X.Y. Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Tian, Y.; Du, C.; Yong, S.H.; Zhou, X.; Zhou, C.; Yang, S.Y. Catalysis-involved 3D N-doped graphene aerogel achieves a superior solar water purification rate and efficiency. Chem. Eng. J. 2023, 453, 139793. [Google Scholar] [CrossRef]
- An, B.X.; Ma, Y.; Li, W.B.; Su, M.; Li, F.Y.; Song, Y.L. Three-dimensional multi-recognition flexible wearable sensor via graphene aerogel printing. Chem. Commun. 2016, 52, 10948–10951. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.Y.; Tang, W.Y.; Xia, L.J.; Fu, Z.; Zhou, S.J.; Zhang, J.J.; Zhang, C.H.; Li, L.; Ji, H.; Xu, W.L. Flexible and weavable 3D porous graphene/PPy/lignocellulose-based versatile fibrous wearables for thermal management and strain sensing. Chem. Eng. J. 2023, 452, 139338. [Google Scholar] [CrossRef]
- Sun, Z.X.; Fang, S.Y.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, I.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Zhi, D.D.; Li, T.; Li, J.Z.; Ren, H.S.; Meng, F.B. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Pt. B-Eng. 2021, 211, 108642. [Google Scholar] [CrossRef]
- Sun, Y.R.; Yang, M.X.; Yu, F.; Chen, J.H.; Ma, J. Synthesis of Graphene Aerogel Adsorbents and Their Applications in Water Treatment. Prog. Chem. 2015, 27, 1133–1146. [Google Scholar] [CrossRef]
- Raagulan, K.; Kim, B.M.; Chai, K.Y. Recent Advancement of Electromagnetic Interference (EMI) Shielding of Two Dimensional (2D) MXene and Graphene Aerogel Composites. Nanomaterials 2020, 10, 702. [Google Scholar] [CrossRef]
- Wang, J.; Ellsworth, M.W. Graphene Aerogels. In Proceedings of the 1st International Symposium on Emerging Materials for Post-CMOS Applications Held at the 215th Meeting of the Electrochemical-Society, San Francisco, CA, USA, 25–29 May 2009; pp. 241–247. [Google Scholar]
- Chen, W.F.; Yan, L.F. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Sheng, K.X.; Xu, Y.X.; Li, C.; Shi, G.Q. High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide. New Carbon Mater. 2011, 26, 9–15. [Google Scholar] [CrossRef]
- Gao, H.C.; Xiao, F.; Ching, C.B.; Duan, H.W. High-Performance Asymmetric Supercapacitor Based on Graphene Hydrogel and Nanostructured MnO2. ACS Appl. Mater. Interfaces 2012, 4, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.W.; Luo, H.J.; Geng, J.J.; Chen, J.D. Facile one-pot preparation of nitrogen-doped ultra-light graphene oxide aerogel and its prominent adsorption performance of Cr(VI). Chem. Eng. J. 2018, 338, 62–71. [Google Scholar] [CrossRef]
- Shu, D.; Feng, F.; Han, H.L.; Ma, Z.F. Prominent adsorption performance of amino-functionalized ultra-light graphene aerogel for methyl orange and amaranth. Chem. Eng. J. 2017, 324, 1–9. [Google Scholar] [CrossRef]
- Goldstein, A.P.; Mickelson, W.; Machness, A.; Lee, G.; Worsley, M.A.; Woo, L.; Zettl, A. Simultaneous Sheet Cross-Linking and Deoxygenation in the Graphene Oxide Sol-Gel Transition. J. Phys. Chem. C 2014, 118, 28855–28860. [Google Scholar] [CrossRef]
- Tong, X.; Jia, W.J.; Li, Y.M.; Yao, T.J.; Wu, J.; Yang, M. One-step preparation of reduced graphene oxide/Prussian blue/polypyrrole aerogel and their enhanced photo-Fenton performance. J. Taiwan Inst. Chem. Eng. 2019, 102, 92–98. [Google Scholar] [CrossRef]
- Wu, J.; Tao, K.; Zhang, J.; Guo, Y.Y.; Miao, J.M.; Norford, L.K. Chemically functionalized 3D graphene hydrogel for high performance gas sensing. J. Mater. Chem. A 2016, 4, 8130–8140. [Google Scholar] [CrossRef]
- Wan, W.C.; Zhang, F.; Yu, S.; Zhang, R.Y.; Zhou, Y. Hydrothermal formation of graphene aerogel for oil sorption: The role of reducing agent, reaction time and temperature. New J. Chem. 2016, 40, 3040–3046. [Google Scholar] [CrossRef]
- Li, J.H.; Li, J.Y.; Meng, H.; Xie, S.Y.; Zhang, B.W.; Li, L.F.; Ma, H.J.; Zhang, J.Y.; Yu, M. Ultra-light, compressible and fire-resistant graphene aerogel as a highly efficient and recyclable absorbent for organic liquids. J. Mater. Chem. A 2014, 2, 2934–2941. [Google Scholar] [CrossRef]
- Lim, M.B.; Hu, M.; Manandhar, S.; Sakshaug, A.; Strong, A.; Riley, L.; Pauzauskie, P.J. Ultrafast sol-gel synthesis of graphene aerogel materials. Carbon 2015, 95, 616–624. [Google Scholar] [CrossRef]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.L.; Yu, Z.L.; Xu, X.W.; Zheng, Y.R.; Yu, S.H. Scalable Template Synthesis of Resorcinol-Formaldehyde/Graphene Oxide Composite Aerogels with Tunable Densities and Mechanical Properties. Angew. Chem.-Int. Edit. 2015, 54, 2397–2401. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Olson, T.Y.; Lee, J.R.I.; Willey, T.M.; Nielsen, M.H.; Roberts, S.K.; Pauzauskie, P.J.; Biener, J.; Satcher, J.H.; Baumann, T.F. High Surface Area, sp(2)-Cross-Linked Three-Dimensional Graphene Monoliths. J. Phys. Chem. Lett. 2011, 2, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Xu, C.; Ma, C.Q.; Ren, W.C.; Cheng, H.M. Lightweight and Flexible Graphene Foam Composites for High-Performance Electromagnetic Interference Shielding. Adv. Mater. 2013, 25, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Wang, X.W.; Wang, L.H.; Song, H.; Zhang, H.; Huang, W.; Chen, P. 3D Graphene Foam as a Monolithic and Macroporous Carbon Electrode for Electrochemical Sensing. ACS Appl. Mater. Interfaces 2012, 4, 3129–3133. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Shi, Y.M.; Shi, W.H.; Lu, G.; Huang, X.; Yan, Q.Y.; Zhang, Q.C.; Zhang, H. Preparation of Novel 3D Graphene Networks for Supercapacitor Applications. Small 2011, 7, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Pettes, M.T.; Ji, H.X.; Ruoff, R.S.; Shi, L. Thermal Transport in Three-Dimensional Foam Architectures of Few-Layer Graphene and Ultrathin Graphite. Nano Lett. 2012, 12, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Yang, G.; Park, M.J.; Kwak, J.S.; Baik, K.H.; Kim, D.; Kim, J. Three-dimensional graphene foam-based transparent conductive electrodes in GaN-based blue light-emitting diodes. Appl. Phys. Lett. 2013, 102, 161902. [Google Scholar] [CrossRef]
- Ma, Y.X.; Yu, M.; Liu, J.H.; Li, X.J.; Li, S.M. Ultralight Interconnected Graphene-Amorphous Carbon Hierarchical Foam with Mechanical Resiliency for High Sensitivity and Durable Strain Sensors. ACS Appl. Mater. Interfaces 2017, 9, 27127–27134. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.L.; Cheng, J.B.; Zhang, A.N.; Wang, Y.Z.; Zhao, H.B. Fully bio-based, low fire-hazard and superelastic aerogel without hazardous cross-linkers for excellent thermal insulation and oil clean-up absorption. J. Hazard. Mater. 2021, 403, 123977. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.Y.J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef]
- Deville, S. The lure of ice-templating: Recent trends and opportunities for porous materials. Scr. Mater. 2018, 147, 119–124. [Google Scholar] [CrossRef]
- Xi, X.T.; Luo, X.Q.; Xia, Y.; Yi, L.F.; Wang, Y.; Song, D.Y.; Song, Y.J.; Wu, J.R.; Zhao, L.J. Ice Crystal Growth Mechanism and Structure-activity Relationships of Graphene Oxide/Poly(vinyl alcohol) Aerogels. Chin. J. Polym. Sci. 2022, 40, 772–780. [Google Scholar] [CrossRef]
- Zhao, N.F.; Li, M.; Gong, H.X.; Bai, H. Controlling ice formation on gradient wettability surface for high-performance bioinspired materials. Sci. Adv. 2020, 6, eabb4712. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.F.; Hanaor, D.A.H.; Shen, X.D.; Gurlo, A. Freeze Casting: From Low-Dimensional Building Blocks to Aligned Porous Structures—A Review of Novel Materials, Methods, and Applications. Adv. Mater. 2020, 32, 1907176. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Qin, J.D.; Shi, G.; Sun, C.; Ingram, M.; Qian, S.S.; Lu, J.; Zhang, S.Q.; Zhong, Y.L. A focus review on 3D printing of wearable energy storage devices. Carbon Energy 2022, 4, 1242–1261. [Google Scholar] [CrossRef]
- Feng, J.Z.; Su, B.L.; Xia, H.S.; Zhao, S.Y.; Gao, C.; Wang, L.K.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Xu, Z.; Huang, T.Q.; Liu, Y.J.; Guo, F.; Xi, J.B.; Gao, W.W.; Gao, C. Direct 3D Printing of Ultralight Graphene Oxide Aerogel Microlattices. Adv. Funct. Mater. 2018, 28, 1707024. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhang, F.; Medarametla, S.P.; Li, H.; Zhou, C.; Lin, D. 3D Printing of Graphene Aerogels. Small 2016, 12, 1702–1708. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Li, P.G.; Gao, C. Strong, Conductive, Lightweight, Neat Graphene Aerogel Fibers with Aligned Pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem.-Int. Edit. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.A.; He, Z.S.; Guo, J.F.; Pei, S.E.; Shao, H.B.; Wang, J.M. Self-Assembled Three-Dimensional Graphene Aerogel with an Interconnected Porous Structure for Lithium-Ion Batteries. ChemElectroChem 2019, 6, 2698–2706. [Google Scholar] [CrossRef]
- Ren, L.; Hui, K.N.; Hui, K.S.; Liu, Y.D.; Qi, X.; Zhong, J.X.; Du, Y.; Yang, J.P. 3D hierarchical porous graphene aerogel with tunable meso-pores on graphene nanosheets for high-performance energy storage. Sci Rep 2015, 5, 14229. [Google Scholar] [CrossRef]
- Shan, H.; Xiong, D.B.; Li, X.F.; Sun, Y.P.; Yan, B.; Li, D.J.; Lawes, S.; Cui, Y.H.; Sun, X.L. Tailored lithium storage performance of graphene aerogel anodes with controlled surface defects for lithium-ion batteries. Appl. Surf. Sci. 2016, 364, 651–659. [Google Scholar] [CrossRef]
- Ye, J.C.; Charnvanichborikarn, S.; Worsley, M.A.; Kucheyev, S.O.; Wood, B.C.; Wang, Y.M. Enhanced electrochemical performance of ion-beam-treated 3D graphene aerogels for lithium ion batteries. Carbon 2015, 85, 269–278. [Google Scholar] [CrossRef]
- Li, X.F.; Geng, D.S.; Zhang, Y.; Meng, X.B.; Li, R.Y.; Sun, X.L. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Shan, H.; Li, X.F.; Cui, Y.H.; Xiong, D.B.; Yan, B.; Li, D.J.; Lushington, A.; Sun, X.L. Sulfur/Nitrogen Dual-doped Porous Graphene Aerogels Enhancing Anode Performance of Lithium Ion Batteries. Electrochim. Acta 2016, 205, 188–197. [Google Scholar] [CrossRef]
- Zhang, C.J.; Chai, F.L.; Fu, L.; Hu, P.; Pang, S.P.; Cui, G.L. Lithium storage in a highly conductive Cu3Ge boosted Ge/graphene aerogel. J. Mater. Chem. A 2015, 3, 22552–22556. [Google Scholar] [CrossRef]
- Qin, J.; He, C.N.; Zhao, N.Q.; Wang, Z.Y.; Shi, C.S.; Liu, E.Z.; Li, J.J. Graphene Networks Anchored with Sn@Graphene as Lithium Ion Battery Anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.L.; Zhou, X.F.; Deng, W.; Liu, Z.P. A compressible and hierarchical porous graphene/Co composite aerogel for lithium-ion batteries with high gravimetric/volumetric capacity. J. Mater. Chem. A 2016, 4, 6021–6028. [Google Scholar] [CrossRef]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Jiang, T.C.; Bu, F.X.; Feng, X.X.; Shakir, I.; Hao, G.L.; Xu, Y.X. Porous Fe2O3 Nanoframeworks Encapsulated within Three-Dimensional Graphene as High-Performance Flexible Anode for Lithium-Ion Battery. ACS Nano 2017, 11, 5140–5147. [Google Scholar] [CrossRef]
- Shao, J.X.; Feng, J.H.; Zhou, H.; Yuan, A.H. Graphene aerogel encapsulated Fe-Co oxide nanocubes derived from Prussian blue analogue as integrated anode with enhanced Li-ion storage properties. Appl. Surf. Sci. 2019, 471, 745–752. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tao, H.C.; Ma, H.; Du, S.L.; Li, T.; Zhang, Y.K.; Li, J.H.; Yang, X.L. Three-dimensional MoO2@few-layered MoS2 covered by S-doped graphene aerogel for enhanced lithium ion storage. Electrochim. Acta 2018, 283, 619–627. [Google Scholar] [CrossRef]
- Meng, J.K.; Cao, Y.; Suo, Y.; Liu, Y.S.; Zhang, J.M.; Zheng, X.C. Facile Fabrication of 3D SiO2@Graphene Aerogel Composites as Anode Material for Lithium Ion Batteries. Electrochim. Acta 2015, 176, 1001–1009. [Google Scholar] [CrossRef]
- Entwistle, J.E.; Booth, S.G.; Keeble, D.S.; Ayub, F.; Yan, M.; Corr, S.A.; Cumming, D.J.; Patwardhan, S.V. Insights into the Electrochemical Reduction Products and Processes in Silica Anodes for Next-Generation Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2001826. [Google Scholar] [CrossRef]
- Hu, X.Z.; Jin, Y.; Zhu, B.; Tan, Y.L.; Zhang, S.; Zong, L.Q.; Lu, Z.D.; Zhu, J. Free-Standing Graphene-Encapsulated Silicon Nanoparticle Aerogel as an Anode for Lithium Ion Batteries. ChemNanoMat 2016, 2, 671–674. [Google Scholar] [CrossRef]
- Chang, P.; Liu, X.X.; Zhao, Q.J.; Huang, Y.Q.; Huang, Y.H.; Hu, X.L. Constructing Three-Dimensional Honeycombed Graphene/Silicon Skeletons for High-Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 31879–31886. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.Z.; Chung, S.H.; Zu, C.X.; Su, Y.S. Rechargeable Lithium-Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O-2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.P.; Zhao, S.Y.; Sun, Z.H.; Wang, W.; Cheng, H.M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Hu, G.J.; Xu, C.; Sun, Z.H.; Wang, S.G.; Cheng, H.M.; Li, F.; Ren, W.C. 3D Graphene-Foam-Reduced-Graphene-Oxide Hybrid Nested Hierarchical Networks for High-Performance Li-S Batteries. Adv. Mater. 2016, 28, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.D.; Zhao, X.J.; Wu, Z.S.; Dong, Y.F.; Lu, P.F.; Chen, J.; Ren, W.C.; Cheng, H.M.; Bao, X.H. Free-standing integrated cathode derived from 3D graphene/carbon nanotube aerogels serving as binder-free sulfur host and interlayer for ultrahigh volumetric-energy-density lithium-sulfur batteries. Nano Energy 2019, 60, 743–751. [Google Scholar] [CrossRef]
- Zhou, G.M.; Paek, E.; Hwang, G.S.; Manthiram, A. High-Performance Lithium-Sulfur Batteries with a Self-Supported, 3D Li2S-Doped Graphene Aerogel Cathodes. Adv. Energy Mater. 2016, 6, 1501355. [Google Scholar] [CrossRef]
- Luo, L.; Chung, S.H.; Manthiram, A. A three-dimensional self-assembled SnS2-nano-dots@graphene hybrid aerogel as an efficient polysulfide reservoir for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 7659–7667. [Google Scholar] [CrossRef]
- Liu, M.M.; Zhang, C.C.; Su, J.M.; Chen, X.; Ma, T.Y.; Huang, T.; Yu, A.S. Propelling Polysulfide Conversion by Defect-Rich MoS2 Nanosheets for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 20788–20795. [Google Scholar] [CrossRef]
- Ding, M.; Huang, S.Z.; Wang, Y.; Hu, J.P.; Pam, M.E.; Fan, S.; Shi, Y.M.; Ge, Q.; Yang, H.Y. Promoting polysulfide conversion by catalytic ternary Fe3O4/carbon/graphene composites with ordered microchannels for ultrahigh-rate lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 25078–25087. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wu, Y.F.; Kan, S.T.; Bu, M.M.; Liu, Y.; Yang, L.S.; Yang, Y.H.; Liu, H.T. A combination of MnO2-decorated graphene aerogel modified separator and I/N codoped graphene aerogel sulfur host to synergistically promote Li-S battery performance. Electrochim. Acta 2020, 348, 136173. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, X.X.; Li, M.L.; Zheng, L.J.; Guan, D.H.; Huang, X.L.; Xu, J.J.; Yu, J.H. Porous Materials Applied in Nonaqueous Li-O(2)Batteries: Status and Perspectives. Adv. Mater. 2020, 32, 2002559. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Chen, C.G.; Su, J.M.; Kuang, P.; Zhang, C.C.; Yao, Y.; Huang, T.; Yu, A.S. Three-dimensional MoSx (1 < x < 2) nanosheets decorated graphene aerogel for lithium-oxygen batteries. J. Mater. Chem. A 2016, 4, 10986–10991. [Google Scholar] [CrossRef]
- Zhao, C.T.; Yu, C.; Li, S.F.; Guo, W.; Zhao, Y.; Dong, Q.; Lin, X.T.; Song, Z.X.; Tan, X.Y.; Wang, C.H.; et al. Ultrahigh-Capacity and Long-Life Lithium-Metal Batteries Enabled by Engineering Carbon Nanofiber-Stabilized Graphene Aerogel Film Host. Small 2018, 14, 1803310. [Google Scholar] [CrossRef]
- Kim, C.H.J.; Varanasi, C.V.; Liu, J. Synergy of polypyrrole and carbon x-aerogel in lithium-oxygen batteries. Nanoscale 2018, 10, 3753–3758. [Google Scholar] [CrossRef]
- Zhao, C.T.; Yu, C.; Liu, S.H.; Yang, J.; Fan, X.M.; Huang, H.W.; Qiu, J.S. 3D Porous N-Doped Graphene Frameworks Made of Interconnected Nanocages for Ultrahigh-Rate and Long-Life Li-O-2 Batteries. Adv. Funct. Mater. 2015, 25, 6913–6920. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, L.; Zhang, J.J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Kotz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Lee, S.Y.; Wei, T.; Li, J.; Fan, Z.J. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Wu, Z.S.; Sun, Y.; Tan, Y.Z.; Yang, S.B.; Feng, X.L.; Mullen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Su, F.Y.; Xie, L.F.; Li, X.M.; Liu, Z.; Kong, Q.Q.; Guo, X.H.; Zhang, Y.; Wan, L.; Li, K.X.; et al. Self-Assembled 3D Graphene-Based Aerogel with Co3O4 Nanoparticles as High-Performance Asymmetric Supercapacitor Electrode. ChemSusChem 2015, 8, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, Y.; Zhang, J.; Zhang, H.J.; Liu, J.H. Preparation, Structures and Properties of Three-Dimensional Graphene-Based Materials. Prog. Chem. 2019, 31, 667–680. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.Y.; Qian, F.; Han, T.Y.J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors Based on Three-Dimensional Hierarchical Graphene Aerogels with Periodic Macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shi, Y.Q.; Gao, W.; Sun, Z.; Liu, T.X. Graphene-Carbon Nanotube Aerogel with a Scroll-Interconnected-Sheet Structure as an Advanced Framework for a High-Performance Asymmetric Supercapacitor Electrode. ACS Appl. Nano Mater. 2018, 1, 4435–4441. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.S.; Pan, H.; Jiang, J.Z.; Yang, X.W.; Xu, Z.X.; Tian, H.; Han, S.; Wu, D.Q. Nitrogen-Doped Porous Carbon/Graphene Aerogel with Much Enhanced Capacitive Behaviors. Electrochim. Acta 2016, 215, 100–107. [Google Scholar] [CrossRef]
- Chen, W.; Gui, D.Y.; Liu, J.H. Nickel oxide/graphene aerogel nanocomposite as a supercapacitor electrode material with extremely wide working potential window. Electrochim. Acta 2016, 222, 1424–1429. [Google Scholar] [CrossRef]
- Tian, W.W.; Cheng, D.K.; Wang, S.; Xiong, C.X.; Yang, Q.L. Phytic acid modified manganese dioxide/graphene composite aerogel as high-performance electrode materials for supercapacitors. Appl. Surf. Sci. 2019, 495, 143589. [Google Scholar] [CrossRef]
- Zheng, C.L.; Zhang, J.L.; Zhang, Q.; You, B.; Chen, G.L. Three dimensional Ni foam-supported graphene oxide for binder-free pseudocapacitor. Electrochim. Acta 2015, 152, 216–221. [Google Scholar] [CrossRef]
- Chang, J.H.; Hung, Y.H.; Luo, X.F.; Huang, C.H.; Jung, S.M.; Chang, J.K.; Kong, J.; Su, C.Y. The hierarchical porosity of a three-dimensional graphene electrode for binder-free and high performance supercapacitors. RSC Adv. 2016, 6, 8384–8394. [Google Scholar] [CrossRef]
- Su, X.L.; Fu, L.; Cheng, M.Y.; Yang, J.H.; Guan, X.X.; Zheng, X.C. 3D nitrogen-doped graphene aerogel nanomesh: Facile synthesis and electrochemical properties as the electrode materials for supercapacitors. Appl. Surf. Sci. 2017, 426, 924–932. [Google Scholar] [CrossRef]
- Xu, P.; Gan, Q.M.; Ma, L.; Li, Z.Y.; Zhang, H.; Xiao, H.; Liang, X.; Zhang, T.F.; Tian, X.H.; Liu, C.H. A high surface area N-doped holey graphene aerogel with low charge transfer resistance as high performance electrode of non-flammable thermostable supercapacitors. Carbon 2019, 149, 452–461. [Google Scholar] [CrossRef]
- Wu, Z.S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.L.; Mullen, K. Three-Dimensional Nitrogen and Boron Co-doped Graphene for High-Performance All-Solid-State Supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wan, H.C.; Zhu, W.K.; Duan, T.; Yao, W.T. Naturally Dried, Double Nitrogen-Doped 3D Graphene Aerogels Modified by Plant Extracts for Multifunctional Applications. ACS Sustain. Chem. Eng. 2018, 6, 1172–1181. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Z.E.; Sun, L.; Lu, Z.W.; Zhuo, K.L. Nitrogen and sulfur co-doped porous graphene aerogel as an efficient electrode material for high performance supercapacitor in ionic liquid electrolyte. J. Power Sources 2018, 390, 215–223. [Google Scholar] [CrossRef]
- Zou, X.X.; Wu, D.P.; Mu, Y.F.; Xing, L.Y.; Zhang, W.C.; Gao, Z.Y.; Xu, F.; Jiang, K. Boron and nitrogen Co-doped holey graphene aerogels with rich B-N motifs for flexible supercapacitors. Carbon 2020, 159, 94–101. [Google Scholar] [CrossRef]
- Hou, J.H.; Cao, C.B.; Idrees, F.; Ma, X.L. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef]
- Yao, B.; Chandrasekaran, S.; Zhang, J.; Xiao, W.; Qian, F.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Efficient 3D Printed Pseudocapacitive Electrodes with Ultrahigh MnO2 Loading. Joule 2019, 3, 459–470. [Google Scholar] [CrossRef]
- Barua, A.; Mehra, P.; Paul, A. Understanding Integrated Graphene-MOF Nanostructures as Binder- and Additive-Free High-Performance Supercapacitors at Commercial Scale Mass Loading. ACS Appl. Energ. Mater. 2021, 4, 14249–14259. [Google Scholar] [CrossRef]
- Jiang, J.X.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem. Eng. J. 2019, 358, 1539–1551. [Google Scholar] [CrossRef]
- Guo, Q.L.; Chen, S.; Liu, Z.X.; Yan, J.C.; Liu, H.E. Preparation and performance evaluation of graphene/hydroxypropyl methylcellulose composite aerogel for high viscosity oil adsorption. J. Environ. Chem. Eng. 2022, 10, 108312. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, M.L.; Li, X.F.; Wang, C.J.; Gui, C.X.; Yu, Z.Z. Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids. Carbon 2016, 100, 456–464. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Meng, Q.H.; Zhang, X.T.; Ma, R.; Cao, B. Green synthesis of carbon nanotube-graphene hybrid aerogels and their use as versatile agents for water purification. J. Mater. Chem. 2012, 22, 8767–8771. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.D.; Ge, S.Z.; Jiang, C.W.; Guo, T.Y.; Peng, T.P.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil-water separation. Surf. Coat. Technol. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Sun, A.Q.; Hou, X.A.; Hu, X.G. Super-performance photothermal conversion of 3D macrostructure graphene-CuFeSe2 aerogel contributes to durable and fast clean-up of highly viscous crude oil in seawater. Nano Energy 2020, 70, 104511. [Google Scholar] [CrossRef]
- Qu, J.F.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Engineering 3D Ru/Graphene Aerogel Using Metal-Organic Frameworks: Capture and Highly Efficient Catalytic CO Oxidation at Room Temperature. Small 2018, 14, 1800343. [Google Scholar] [CrossRef]
- Liu, J.T.; Ge, X.; Ye, X.X.; Wang, G.Z.; Zhang, H.M.; Zhou, H.J.; Zhang, Y.X.; Zhao, H.J. 3D graphene/delta-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. [Google Scholar] [CrossRef]
- Lu, M.X.; Deng, Y.J.; Luo, Y.; Lv, J.P.; Li, T.B.; Xu, J.; Chen, S.W.; Wang, J.Y. Graphene Aerogel-Metal-Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef]
- Pan, L.H.; Wang, Z.Q.; Yang, Q.; Huang, R.Y. Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, L.; Tao, Y.X.; Yao, C.; Xue, H.G.; Kong, Y. Electrosorption of Lead Ions by Nitrogen-Doped Graphene Aerogels via One-Pot Hydrothermal Route. Ind. Eng. Chem. Res. 2016, 55, 1912–1920. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Niu, Q.Y.; Gu, X.T.; Yang, N.; Zhao, G.H. Recent progress on carbon nanomaterials for the electrochemical detection and removal of environmental pollutants. Nanoscale 2019, 11, 11992–12014. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.K.; Chen, M.Q.; Hu, M.F.; Tian, M.; Zhang, Y.Y.; Long, D.H. Probing the room-temperature oxidative desulfurization activity of three-dimensional alkaline graphene aerogel. Appl. Catal. B-Environ. 2020, 262, 118266. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Xia, D.; Li, H.; Mannering, J.; Huang, P.; Zheng, X.R.; Kulak, A.; Baker, D.; Iruretagoyena, D.; Menzel, R. Electrically Heatable Graphene Aerogels as Nanoparticle Supports in Adsorptive Desulfurization and High-Pressure CO2 Capture. Adv. Funct. Mater. 2020, 30, 2002788. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.D.; Ding, Z.M.; Zhang, L.; Fu, Y.K.; Wang, J.S.; Li, Y.; Han, S.M. Remarkable hydrogen storage properties and mechanisms of the shell-core MgH2@carbon aerogel microspheres. Int. J. Hydrogen Energy 2018, 43, 3731–3740. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, S.K.; Zhang, L.M.; You, T.T.; Xu, F. Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution. Materials 2016, 9, 582. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Mesoporous TiO2 Nanocrystals Grown in Situ on Graphene Aerogels for High Photocatalysis and Lithium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Q.; Ji, S.D.; Wang, M.Q.; Dou, J.J.; Yang, Z.; Qiu, H.W.; Kou, S.; Ji, Y.Q.; Wang, H. Fabrication of porous TiO2-RGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes. J. Alloys Compd. 2020, 819, 153033. [Google Scholar] [CrossRef]
- Dong, S.Y.; Cui, Y.R.; Wang, Y.F.; Li, Y.K.; Hu, L.M.; Sun, J.Y.; Sun, J.H. Designing three-dimensional acicular sheaf shaped BiVO4/reduced graphene oxide composites for efficient sunlight-driven photocatalytic degradation of dye wastewater. Chem. Eng. J. 2014, 249, 102–110. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jiang, Y.Q.; Muthukaruppan, S.; Balasubramanian, R. Effect of boron doping level on the photocatalytic activity of graphene aerogels. Carbon 2018, 128, 237–248. [Google Scholar] [CrossRef]
- Chen, Y.X.; Wang, P.L.; Liang, Y.; Zhao, M.J.; Jiang, Y.Y.; Wang, G.T.; Zou, P.; Zeng, J.; Zhang, Y.S.; Wang, Y. Fabrication of a three-dimensional porous Z-scheme silver/silver bromide/graphitic carbon nitride@nitrogen-doped graphene aerogel with enhanced visible-light photocatalytic and antibacterial activities. J. Colloid Interface Sci. 2019, 536, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Chen, Y.X.; Chen, B.J.; Zhang, Y.S.; Lin, L.; Han, X.W.; Zou, P.; Wang, G.T.; Zeng, J.; Zhao, M.J. Fabrication of a three-dimensional visible-light-driven Ag-AgBr/TiO2/graphene aerogel composite for enhanced photocatalytic destruction of organic dyes and bacteria. New J. Chem. 2019, 43, 5088–5098. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.W.; Fan, M.G.; Tong, P.P.; Sun, J.Y.; Dong, S.Y.; Sun, J.H. Ultra-light and compressible 3D BiOCl/RGO aerogel with enriched synergistic effect of adsorption and photocatalytic degradation of oxytetracycline. J. Mater. Res. Technol.-JMRT 2019, 8, 4577–4587. [Google Scholar] [CrossRef]
- Feng, L.; Wei, P.; Song, Q.; Zhang, J.X.; Fu, Q.A.; Jia, X.H.; Yang, J.; Shao, D.; Li, Y.; Wang, S.Z.; et al. Superelastic, Highly Conductive, Superhydrophobic, and Powerful Electromagnetic Shielding Hybrid Aerogels Built from Orthogonal Graphene and Boron Nitride Nanoribbons. ACS Nano 2022, 16, 17049–17061. [Google Scholar] [CrossRef]
- Zou, X.; Chen, Y.Y.; Zheng, Z.Y.; Sun, M.Y.; Song, X.F.; Lin, P.R.; Tao, J.; Zhao, P. The sensitive monitoring of living cell-secreted dopamine based on the electrochemical biosensor modified with nitrogen-doped graphene aerogel/Co3O4 nanoparticles. Microchem. J. 2022, 183, 107957. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Sun, X.X.; Yang, S.; Wang, D.; Liang, L.; Wang, S.S.; Ning, Y.H.; Yin, W.L.; Li, Y.B. Multifunctional elastic rGO hybrid aerogels for microwave absorption, infrared stealth and heat insulation. Chem. Eng. J. 2023, 452, 139376. [Google Scholar] [CrossRef]
- Liang, L.Y.; Li, Q.M.; Yan, X.; Feng, Y.Z.; Wang, Y.M.; Zhang, H.B.; Zhou, X.P.; Liu, C.T.; Shen, C.Y.; Xie, X.L. Multifunctional Magnetic Ti3C2Tx MXene/Graphene Aerogel with Superior Electromagnetic Wave Absorption Performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.B.; Wen, L.; Yang, H.S.; Zhao, J.Y.; He, C.J.; Hu, Z.K.; Peng, L.; Hou, C.J.; Huo, D.Q. Catalytic Hairpin Assembly-Driven Ratiometric Dual-SignalElectrochemical Biosensor for Ultrasensitive Detection of MicroRNABased on the Ratios of Fe-MOFs and MB-GA-UiO-66-NH2. Anal. Chem. 2022, 94, 5846–5855. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, B.; Zhang, W.; Liu, Y.; Zhu, S.; Gu, Z.; Zhang, H. Progress in Research and Application of Graphene Aerogel—A Bibliometric Analysis. Materials 2023, 16, 272. https://doi.org/10.3390/ma16010272
Chai B, Zhang W, Liu Y, Zhu S, Gu Z, Zhang H. Progress in Research and Application of Graphene Aerogel—A Bibliometric Analysis. Materials. 2023; 16(1):272. https://doi.org/10.3390/ma16010272
Chicago/Turabian StyleChai, Bowen, Wanlin Zhang, Yuanyuan Liu, Shuang Zhu, Zhanjun Gu, and Hao Zhang. 2023. "Progress in Research and Application of Graphene Aerogel—A Bibliometric Analysis" Materials 16, no. 1: 272. https://doi.org/10.3390/ma16010272
APA StyleChai, B., Zhang, W., Liu, Y., Zhu, S., Gu, Z., & Zhang, H. (2023). Progress in Research and Application of Graphene Aerogel—A Bibliometric Analysis. Materials, 16(1), 272. https://doi.org/10.3390/ma16010272