Modulation of Structure and Optical Property of Nitrogen-Incorporated VO2 (M1) Thin Films by Polyvinyl Pyrrolidone
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.3. Ab Initio Calculations
3. Results and Discussion
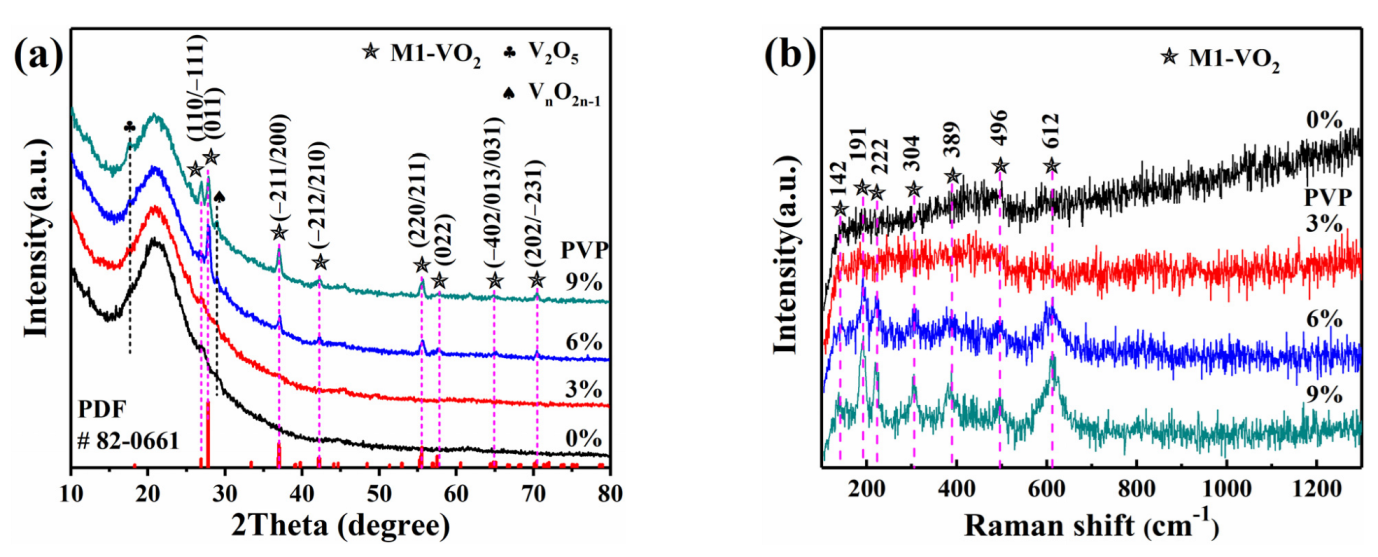
3.1. Phase Determination of VO2 (M1) Thin Films
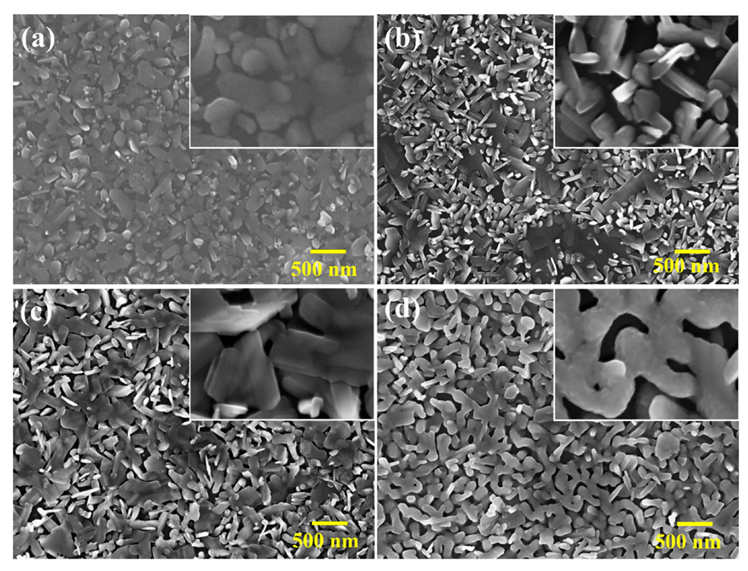
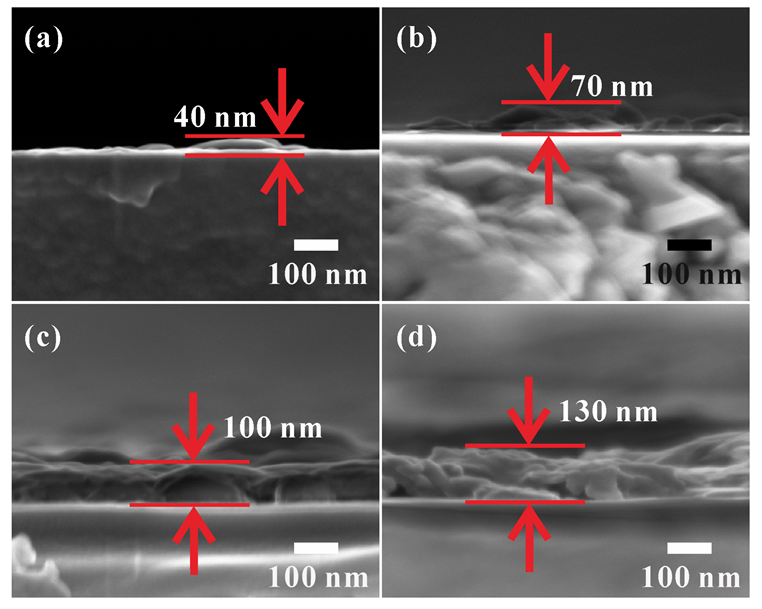
3.2. Microstructures of VO2 (M1) Thin Films
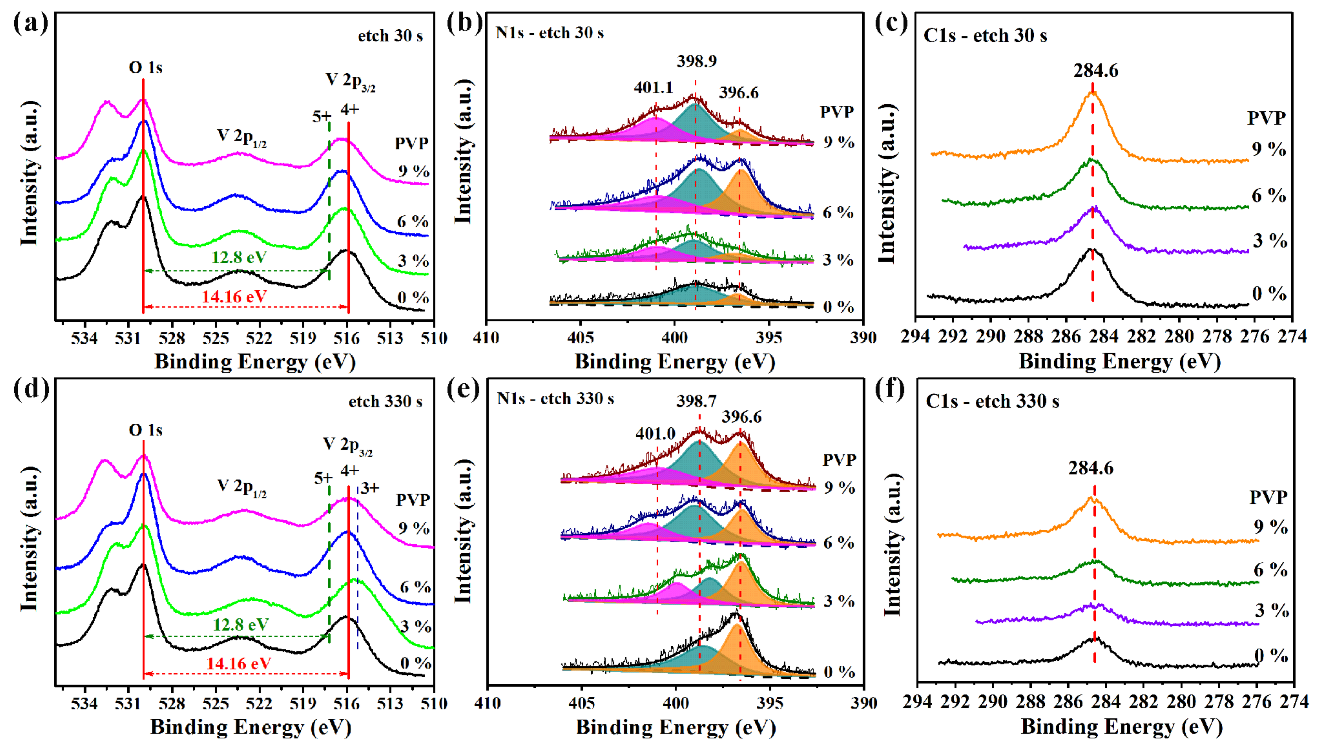
3.3. Chemical Analysis of VO2 (M1) Thin Films
3.4. Optical Properties of N-Incorporated VO2 (M1) Thin Films
3.5. Band Structure of N-Incorporated VO2 (M1)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VO2 (M1): | M1 phase VO2 |
| PVP: | polyvinyl pyrrolidone |
| Tlum: | visible integrated transmittance |
| Tsol: | solar integrated transmittance |
| ΔTsol: | solar modulation efficiency |
| ΔTNIR: | near-infrared modulation efficiency |
| ΔT2000 nm: | modulation efficiency at 2000 nm |
| τc: | critical temperature of phase transition |
| EF: | Fermi energy level |
| Eg: | bandgap energy |
| Eg1: | bandgap energy related to metal-to-insulator transition |
| Eg2: | bandgap energy related to the visible transparency |
| GIXRD: | glance incident X-ray diffraction |
| XPS: | X-ray photoemission spectroscopy |
| FESEM: | field-emission scanning electron microscopy |
| UV/Vis/NIR: | ultraviolet/visible/near-infrared light |
| DFT: | density functional theory |
| VASP: | Vienna Ab initio Simulation Package |
| Nads: | adsorbed N |
| Nsub: | substituted N |
| Nint: | interstitial N |
References
- Morin, F.J. Oxides Which Show a Metal-to-Insulator Transition at the Neel Temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Cao, X.; Chang, T.; Shao, Z.; Xu, F.; Luo, H.; Jin, P. Challenges and Opportunities toward Real Application of VO2-Based Smart Glazing. Matter 2020, 2, 862–881. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, W.; Chen, W.; Zheng, Y. Recent Progress on Vanadium Dioxide Nanostructures and Devices: Fabrication, Properties, Applications and Perspectives. Nanomaterials 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Burrell, A.K.; Mark McCleskey, T.; Jia, Q.X. Polymer assisted deposition. Chem. Commun. 2008, 11, 1271–1277. [Google Scholar] [CrossRef]
- Kang, L.; Gao, Y.; Luo, H. A novel solution process for the synthesis of VO2 thin films with excellent thermochromic properties. ACS Appl. Mater. Interfaces 2009, 1, 2211–2218. [Google Scholar] [CrossRef]
- Kang, L.T.; Gao, Y.F.; Luo, H.J. VO2 Films by Polymer-Assisted Deposition: Investigation of Thermal Decomposition of Precursor Gel and Control of Phase Transition Temperatures. Mater. Sci. Forum 2011, 687, 791–797. [Google Scholar] [CrossRef]
- Chouteau, S.; Mansouri, S.; Mohamedou, M.L.O.N.; Chaillou, J.; Suleiman, A.O.; Drogoff, B.L.; Chaker, M. Investigation of the metal-to-insulator transition of N-doped VO2(M1) thin films. Appl. Surf. Sci. 2021, 554, 149661. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, Z.; Hou, C.; Meng, X. In-Situ thermochromic mechanism of Spin-Coated VO2 film. Appl. Surf. Sci. 2021, 564, 150441. [Google Scholar] [CrossRef]
- Zong, H.; Wu, J.; Zhou, D.; Yin, Y.; Yan, L.; Li, M.; Qiao, W.; Hu, Q.; Yang, Y. Realization of high luminous transmittance and solar modulation ability of VO2 films by multistep deposition and in-situ annealing method. Surf. Interfaces 2022, 30, 101882. [Google Scholar] [CrossRef]
- Li, B.; Tian, S.; Tao, H.; Zhao, X. Tungsten doped M-phase VO2 mesoporous nanocrystals with enhanced comprehensive thermochromic properties for smart windows. Ceram. Int. 2019, 45, 4342–4350. [Google Scholar] [CrossRef]
- Chae, J.-Y.; Lee, D.; Lee, D.W.; Woo, H.-Y.; Kim, J.B.; Paik, T. Direct transfer of thermochromic tungsten-doped vanadium dioxide thin-films onto flexible polymeric substrates. Appl. Surf. Sci. 2021, 545, 148937. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, F.; Yao, J.; Zhu, T.; Xia, H.; Yang, G.; Liu, B.; Gao, Y. Facile synthesis, formation mechanism and thermochromic properties of W-doped VO2(M) nanoparticles for smart window applications. J. Mater. Chem. C 2020, 8, 13396–13404. [Google Scholar] [CrossRef]
- Li, B.; Tian, S.; Wang, Z.; Liu, B.; Gong, X.; Zhao, X. Thermochromic Ta Doped VO2 Films: Enhanced Luminous Transmittance, Significantly Depressed Phase Transition Temperature and Hysteresis Width. Appl. Surf. Sci. 2021, 568, 150959. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; You, Z.; Yu, Y.; Li, P.; Xiong, L.; He, Y. Nb-doped VO2 thin films with enhanced thermal sensing performance for uncooled infrared detection. Mater. Res. Bull. 2022, 146, 111615. [Google Scholar] [CrossRef]
- Suleiman, A.O.; Mansouri, S.; Margot, J.; Chaker, M. Tuning VO2 phase stability by a combined effect of Cr doping and oxygen pressure. Appl. Surf. Sci. 2022, 571, 151267. [Google Scholar] [CrossRef]
- Wang, N.; Duchamp, M.; Dunin-Borkowski, R.E.; Liu, S.; Zeng, X.; Cao, X.; Long, Y. Terbium-Doped VO2 Thin Films: Reduced Phase Transition Temperature and Largely Enhanced Luminous Transmittance. Langmuir 2016, 32, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chai, X.; Lv, L.; Cao, Y.; Zhang, Y.; Song, L. Preparation of porous Mo-doped VO2 films via atomic layer deposition and post annealing. Jpn. J. Appl. Phys. 2021, 60, 085501. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Z.; Wang, C.; Deng, S.; Yang, J.; Wang, X.; Li, J.; Zhao, Y.; Jin, H. Influence of the charge compensation effect on the metal–insulator transition of Mg-W co-doped VO2. Appl. Surf. Sci. 2022, 579, 151990. [Google Scholar] [CrossRef]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.G. Thermochromic undoped and Mg-doped VO2 thin films and nanoparticles: Optical properties and performance limits for energy efficient windows. J. Appl. Phys. 2014, 115, 053513. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Ren, H.; Chen, Y.; Yan, W.; Wang, C.; Li, B.; Jiang, J.; Zou, C. Gate-controlled VO2 phase transition for high-performance smart windows. Sci. Adv. 2019, 5, eaav6815. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhu, Y.; Zhao, S.; Wang, Z.; Liu, Z.; Zhu, L.; Wang, B.; Zhang, Q. Modulation of VO2 metal-insulator transition by co-doping of hydrogen and oxygen vacancy. Sol. Energy Mater. Sol. Cells 2020, 212, 110562. [Google Scholar] [CrossRef]
- Zhou, Q.; Lv, W.; Qiu, Q.; Zhou, T.; Huang, C.; Li, L. Boron doped M-phase VO2 nanoparticles with low metal-insulator phase transition temperature for smart windows. Ceram. Int. 2020, 46, 4786–4794. [Google Scholar] [CrossRef]
- Dai, L.; Chen, S.; Liu, J.; Gao, Y.; Zhou, J.; Chen, Z.; Cao, C.; Luo, H.; Kanehira, M. F-doped VO2 nanoparticles for thermochromic energy-saving foils with modified color and enhanced solar-heat shielding ability. Phys. Chem. Chem. Phys. 2013, 15, 11723–11729. [Google Scholar] [CrossRef] [PubMed]
- Liubchenko, O.; Kladko, V.; Melnik, V.; Romanyuk, B.; Gudymenko, O.; Sabov, T.; Dubikovskyi, O.; Maksimenko, Z.; Kosulya, O.; Kulbachynskyi, O. Ar-implanted vanadium dioxide thin film with the reduced phase transition temperature. Mater. Lett. 2022, 314, 131895. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Fan, L.; Liu, L.; Guo, P.; Zou, C.; Wang, J.; Qian, H.; Ibrahim, K.; Yan, W.; et al. Hole Carriers Doping Effect on the Metal–Insulator Transition of N-Incorporated Vanadium Dioxide Thin Films. J. Phys. Chem. C 2014, 118, 12837–12844. [Google Scholar] [CrossRef]
- Wan, M.; Xiong, M.; Li, N.; Liu, B.; Wang, S.; Ching, W.-Y.; Zhao, X. Observation of reduced phase transition temperature in N-doped thermochromic film of monoclinic VO2. Appl. Surf. Sci. 2017, 410, 363–372. [Google Scholar] [CrossRef]
- Wan, M.; Liu, B.; Wang, S.; Hu, L.; He, Y.; Tao, H.; Zhao, X. Optical properties and formation mechanism of M1-phase VO2 thin films annealed in a closed NH3 atmosphere. J. Alloys Compd. 2017, 706, 289–296. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Biermann, S.; Poteryaev, A.; Lichtenstein, A.I.; Georges, A. Dynamical singlets and correlation-assisted Peierls transition in VO2. Phys. Rev. Lett. 2005, 94, 026404. [Google Scholar] [CrossRef]
- Suh, J.Y.; Lopez, R.; Feldman, L.C.; Haglund, R.F. Semiconductor to metal phase transition in the nucleation and growth of VO2 nanoparticles and thin films. J. Appl. Phys. 2004, 96, 1209–1213. [Google Scholar] [CrossRef]
- Wei, J.; Ji, H.; Guo, W.; Nevidomskyy, A.H.; Natelson, D. Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams. Nat. Nanotechnol. 2012, 7, 357–362. [Google Scholar] [CrossRef]
- Liang, W.; Gao, M.; Lu, C.; Zhang, Z.; Chan, C.H.; Zhuge, L.; Dai, J.; Yang, H.; Chen, C.; Park, B.H.; et al. Enhanced Metal-Insulator Transition Performance in Scalable Vanadium Dioxide Thin Films Prepared Using a Moisture-Assisted Chemical Solution Approach. ACS Appl. Mater. Interfaces 2018, 10, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Ashok, P.; Chauhan, Y.S.; Verma, A. Effect of vanadium thickness and deposition temperature on VO2 synthesis using atmospheric pressure thermal oxidation. Thin Solid 2021, 724, 138630. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Ureña-Begara, F.; Crunteanu, A.; Raskin, J.-P. Raman and XPS characterization of vanadium oxide thin films with temperature. Appl. Surf. Sci. 2017, 403, 717–727. [Google Scholar] [CrossRef]
- Lamb, R.N.; Baxter, J.; Grunze, M.; Kong, C.W.; Unertl, W.N. An XPS study of the composition of thin polyimide films formed by vapor deposition. Langmuir 1988, 4, 249–256. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhang, M.; Yang, J.; Zhang, Z. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Myhre, K.G.; Delashmitt, J.C.; Sims, N.J.; Van Cleve, S.M.; Boll, R.A. Samarium thin films molecular plated from N,N-dimethylformamide characterized by XPS. Surf. Sci. Spectra 2018, 25, 024003. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Morone, A.; Salvi, A.M. XPS determination of oxygen-containing functional groups on carbon-fibre surfaces and the cleaning of these surfaces. Surf. Interface Anal. 1990, 15, 627–634. [Google Scholar] [CrossRef]
- Tyan, Y.-C.; Liao, J.-D.; Klauser, R.; Wu, I.-D.; Weng, C.-C. Assessment and characterization of degradation effect for the varied degrees of ultra-violet radiation onto the collagen-bonded polypropylene non-woven fabric surfaces. Biomaterials 2002, 23, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-X.; Li, G.-D.; Zhao, J.; Su, J.; Wei, X.; Wang, K.-X.; Wang, Y.-N.; Chen, J.-S. Light-Driven Preparation, Microstructure, and Visible-Light Photocatalytic Property of Porous Carbon-Doped TiO2. Int. J. Photoenergy 2012, 2012, 720183. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Catalytic Performance and Characterization of VO2+-Exchanged Titania-Pillared Clays for Selective Catalytic Reduction of Nitric Oxide with Ammonia. J. Catal. 2000, 196, 73–85. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Ed, G.G.; Walck, S.D.; Yates, J.T. Photochemical Activity of Nitrogen-Doped Rutile TiO2(110) in Visible Light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Lin, Z.; Orlov, A.; Lambert, R.M.; Payne, M.C. New insights into the origin of visible light photocatalytic activity of nitrogen-doped and oxygen-deficient anatase TiO2. J. Phys. Chem. B 2005, 109, 20948–20952. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Wang, J.; Tafen de, N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef]
- Burkhardt, W.; Christmann, T.; Franke, S.; Kriegseis, W.; Meister, D.; Meyer, B.K.; Niessner, W.; Schalch, D.; Scharmann, A. Tungsten and fluorine co-doping of VO2 films. Thin Solid Film. 2002, 402, 226–231. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Y.; Liu, X.; Chen, Z.; Dai, L.; Cao, C.; Luo, H.; Kanahira, M.; Sun, C.; Yan, L. Mg-doped VO2 nanoparticles: Hydrothermal synthesis, enhanced visible transmittance and decreased metal-insulator transition temperature. Phys. Chem. Chem. Phys. 2013, 15, 7505–7511. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Mlyuka, N.R.; Primetzhofer, D.; Hallén, A.; Possnert, G.; Niklasson, G.A.; Granqvist, C.G. Bandgap widening in thermochromic Mg-doped VO2 thin films: Quantitative data based on optical absorption. Appl. Phys. Lett. 2013, 103, 161907. [Google Scholar] [CrossRef]
- Haverkort, M.W.; Hu, Z.; Tanaka, A.; Reichelt, W.; Streltsov, S.V.; Korotin, M.A.; Anisimov, V.I.; Hsieh, H.H.; Lin, H.J.; Chen, C.T.; et al. Orbital-assisted metal-insulator transition in VO2. Phys. Rev. Lett. 2005, 95, 196404. [Google Scholar] [CrossRef] [PubMed]
- Ruzmetov, D.; Senanayake, S.D.; Narayanamurti, V.; Ramanathan, S. Correlation between metal-insulator transition characteristics and electronic structure changes in vanadium oxide thin films. Phys. Rev. B 2008, 77, 195442. [Google Scholar] [CrossRef]
- Geng, C.; Dou, S.; Zhao, J.; Ren, F.; Gu, J.; Wei, H.; Guan, H.; Liang, S.; Li, L.; Li, Y.; et al. Self-templated method to fabricate VO2 nanoparticles with ultrahigh luminous transmittance for energy-efficient thermochromic windows. Appl. Surf. Sci. 2022, 592, 153267. [Google Scholar] [CrossRef]
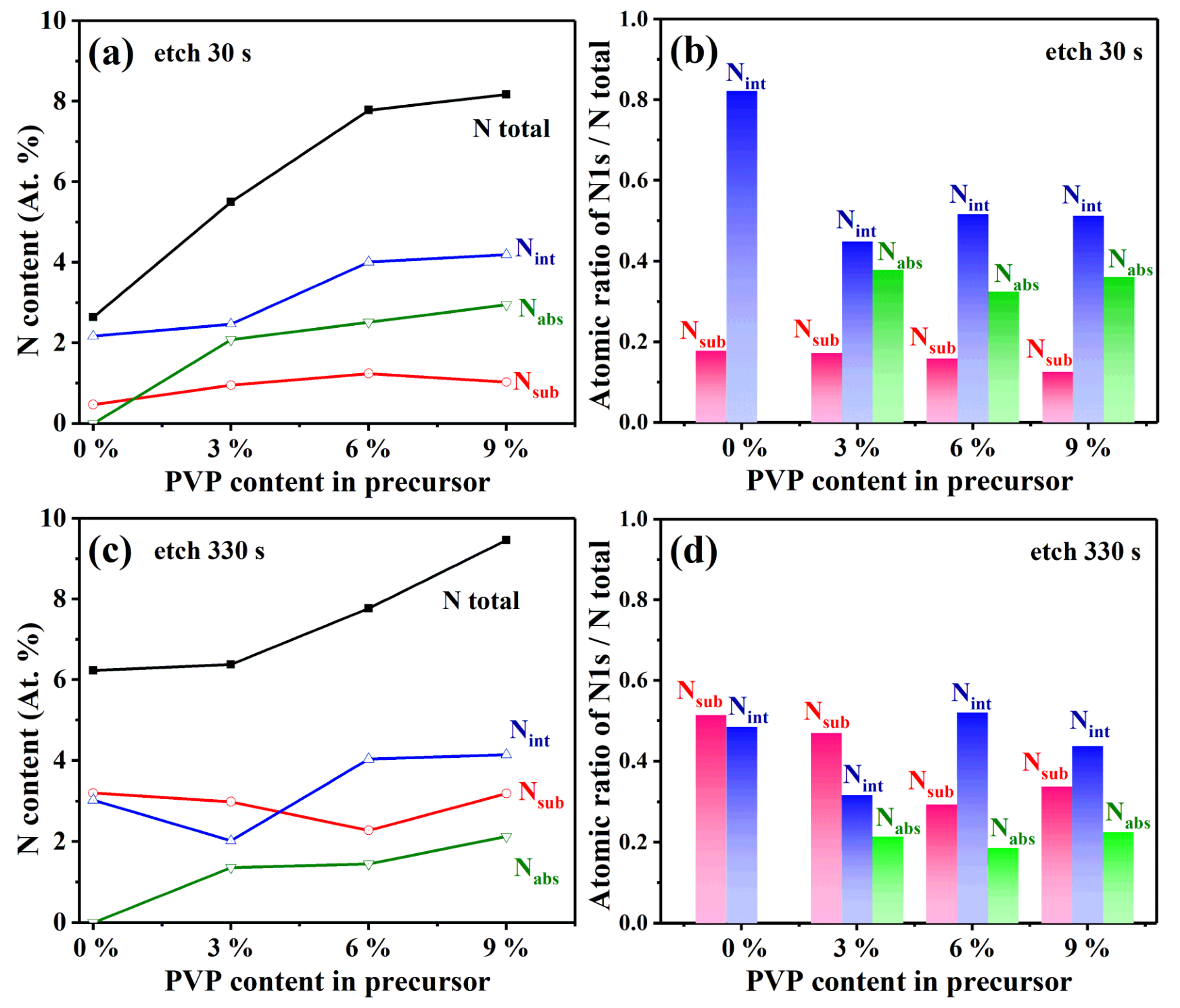
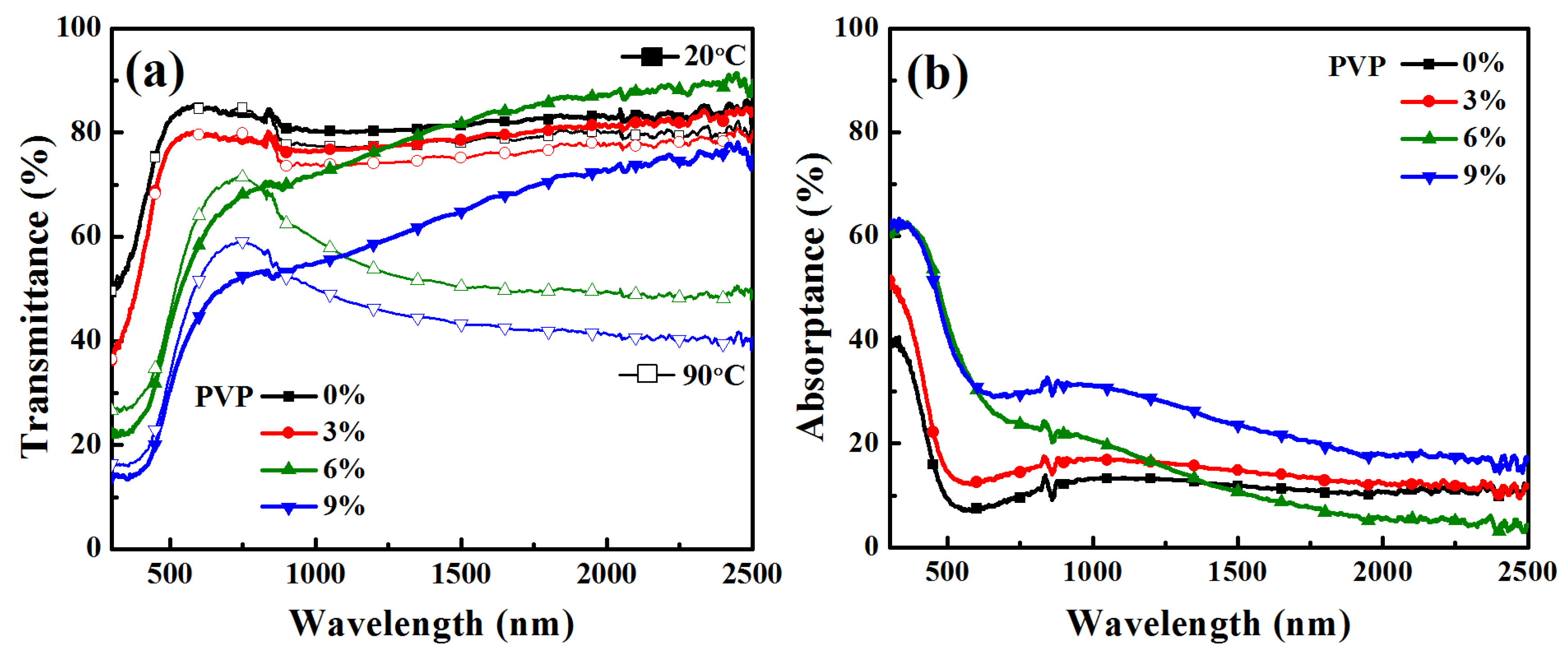

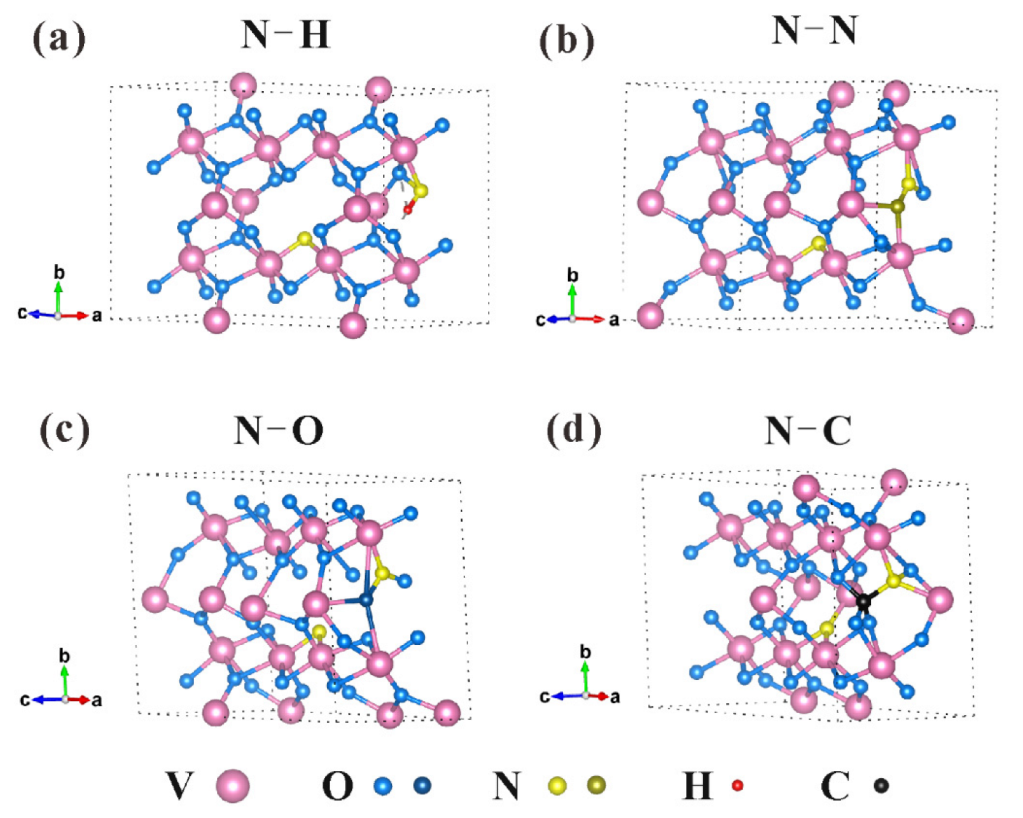
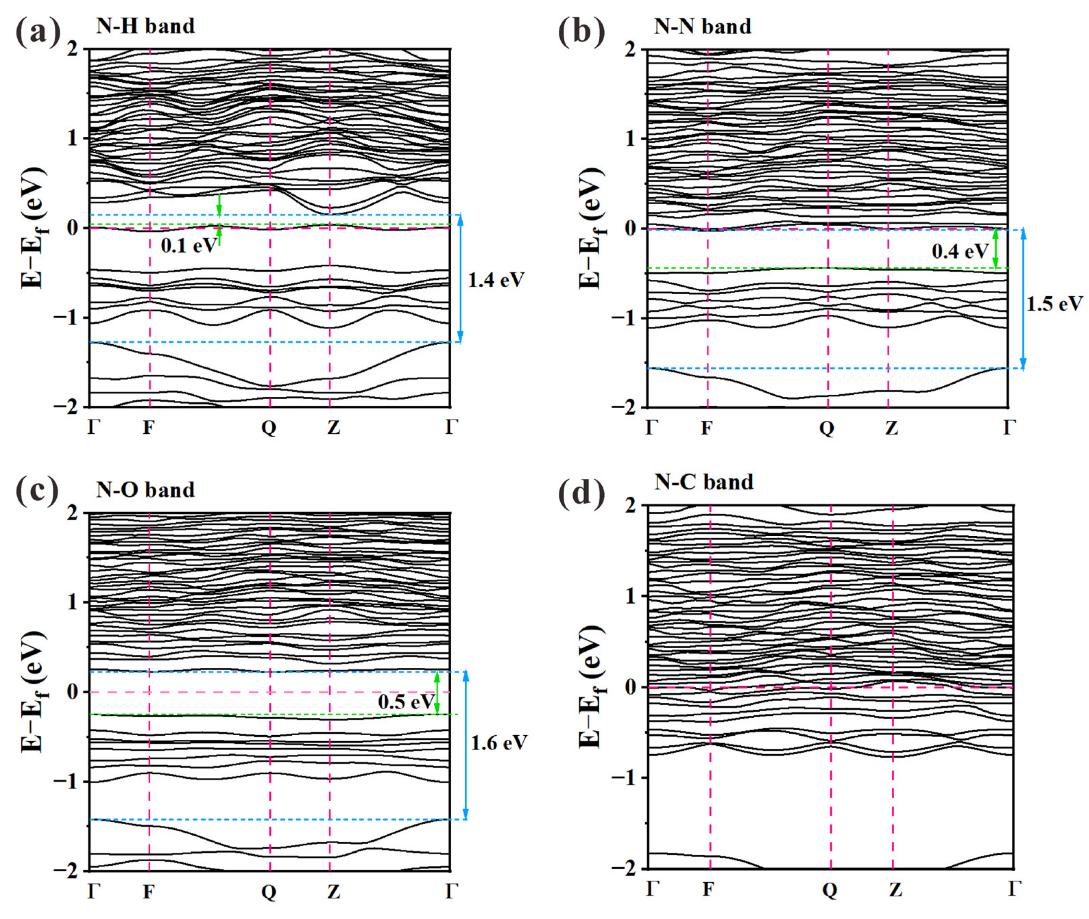
| Sample | PVP Content (wt.%) | N/(N+O) | Nsub | Nint | Nads | N/(N+O) | Nsub | Nint | Nads |
|---|---|---|---|---|---|---|---|---|---|
| Etching for 30 s | Etching for 330 s | ||||||||
| P1 | 0 | 2.64% | 0.47% | 2.17% | 0 | 6.23% | 3.20% | 3.03% | 0 |
| P2 | 3 | 5.50% | 0.95% | 2.47% | 2.08% | 6.37% | 2.99% | 2.02% | 1.36% |
| P3 | 6 | 7.78% | 1.24% | 4.01% | 2.52% | 7.77% | 2.28% | 4.04% | 1.45% |
| P4 | 9 | 8.17% | 1.03% | 4.19% | 2.95% | 9.46% | 3.19% | 4.14% | 2.13% |
| Sample | PVP Dosage (wt.%) | Film Color | λ* (nm) | Tlum (%) | ∆Tsol (%) | ∆TNIR (%) | ∆T2000 nm (%) | |
|---|---|---|---|---|---|---|---|---|
| 20 °C | 90 °C | |||||||
| P1 | 0 | Pale yellow | 426 | 84.08 | 84.03 | 0.92 | 1.02 | 2.9 |
| P2 | 3 | Pale yellow | 432 | 78.95 | 78.95 | 0.97 | 1.03 | 3.9 |
| P3 | 6 | Yellow green | 472 | 52.19 | 56.79 | 4.99 | 7.39 | 37.6 |
| P4 | 9 | Yellow green | 482 | 39.26 | 44.55 | 1.27 | 4.17 | 31.3 |
| Pure VO2 cal. | N: VO2 (N-sub-int-ads) cal. | N: VO2 exp. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N–H | N–N | N–O | N–C | P1 | P2 | P3 | P4 | ||
| Eg1 | 0.62 | 0.1 | 0.4 | 0.5 | - | - | - | 0.26 | 0.16 |
| Eg2 | 1.44 | 1.4 | 1.5 | 1.6 | - | 1.90 | 1.90 | 1.31 | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, M.; Xiong, M.; Tian, S.; Chen, X.; Li, B.; Lu, X.; Zhao, X. Modulation of Structure and Optical Property of Nitrogen-Incorporated VO2 (M1) Thin Films by Polyvinyl Pyrrolidone. Materials 2023, 16, 208. https://doi.org/10.3390/ma16010208
Wan M, Xiong M, Tian S, Chen X, Li B, Lu X, Zhao X. Modulation of Structure and Optical Property of Nitrogen-Incorporated VO2 (M1) Thin Films by Polyvinyl Pyrrolidone. Materials. 2023; 16(1):208. https://doi.org/10.3390/ma16010208
Chicago/Turabian StyleWan, Meinan, Mo Xiong, Shouqin Tian, Xingzhu Chen, Bin Li, Xuesong Lu, and Xiujian Zhao. 2023. "Modulation of Structure and Optical Property of Nitrogen-Incorporated VO2 (M1) Thin Films by Polyvinyl Pyrrolidone" Materials 16, no. 1: 208. https://doi.org/10.3390/ma16010208
APA StyleWan, M., Xiong, M., Tian, S., Chen, X., Li, B., Lu, X., & Zhao, X. (2023). Modulation of Structure and Optical Property of Nitrogen-Incorporated VO2 (M1) Thin Films by Polyvinyl Pyrrolidone. Materials, 16(1), 208. https://doi.org/10.3390/ma16010208








