Structure-Controlled Porous Cordierite Ceramics with High Solid Content Prepared by Pickering Emulsion Technique Using Sucrose as a Porogen
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Preparation
2.2. Characterization
3. Results and Discussion
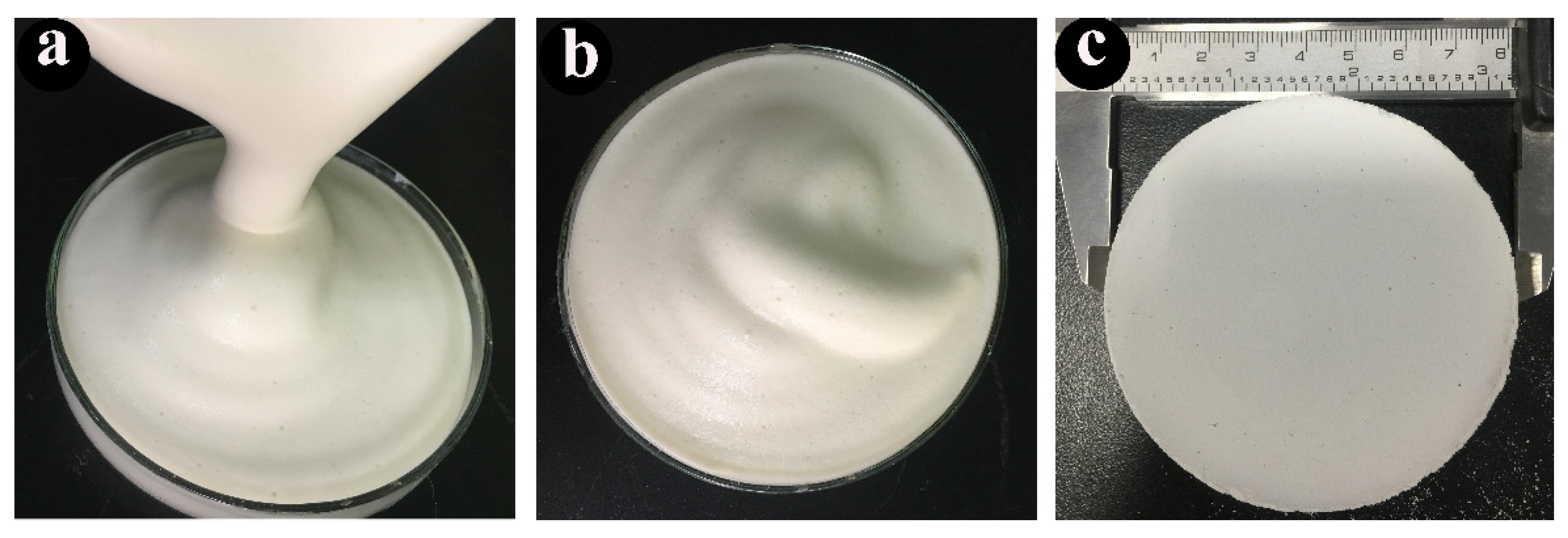
3.1. Properties of PCCs Emulsions
3.2. Effects of Oil Contents on the Morphology of PCCs
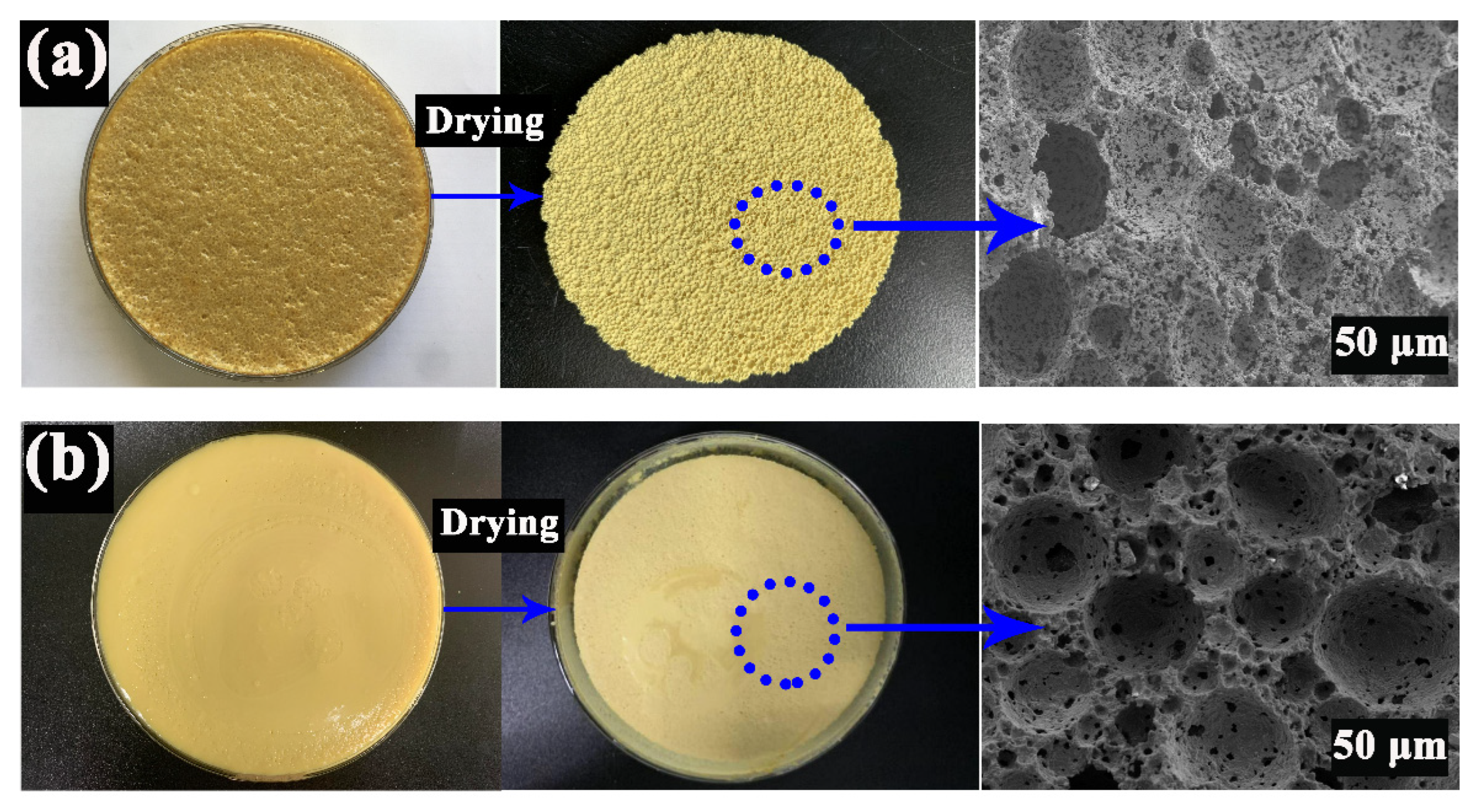
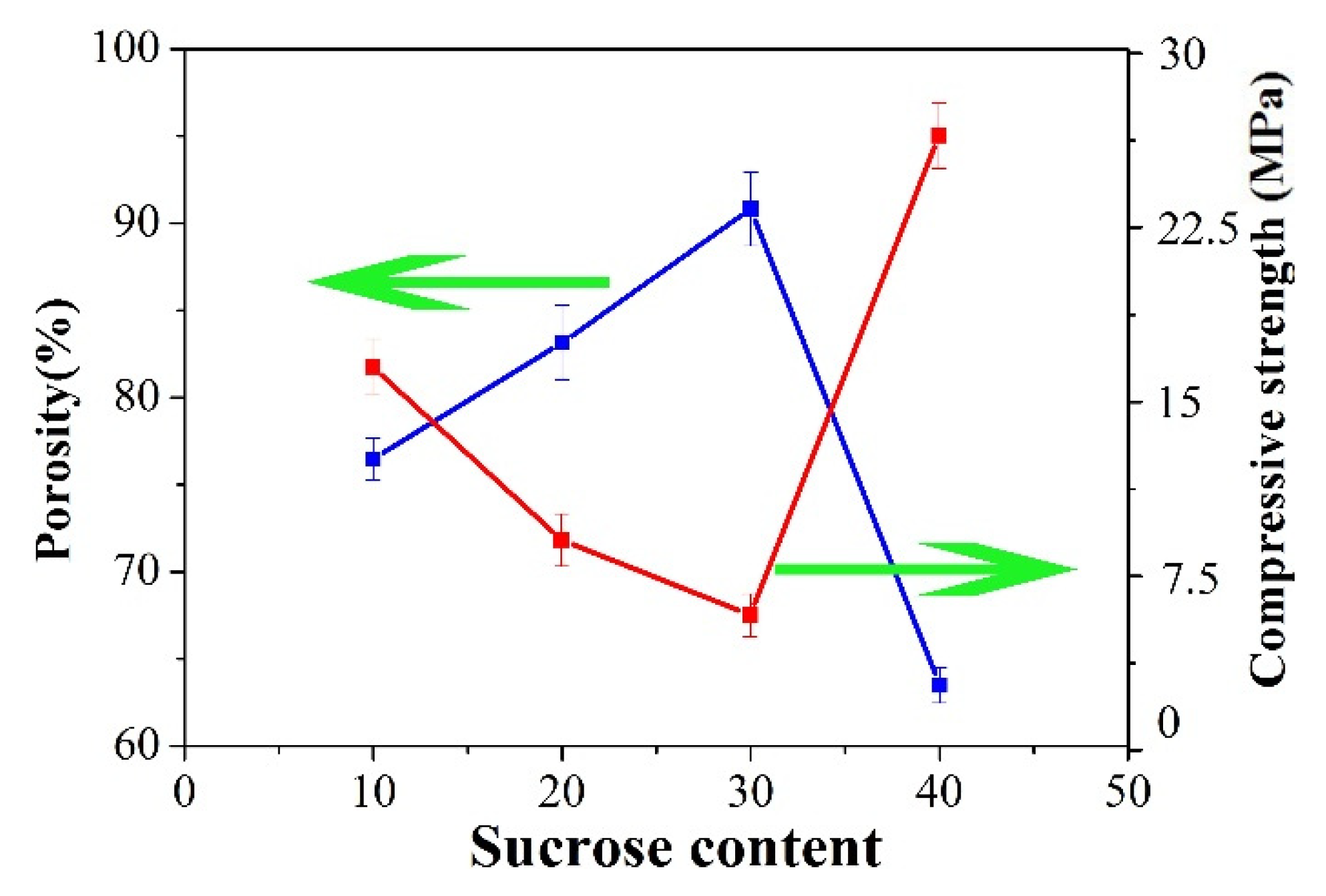
3.3. Effects of Sucrose Content on the Morphology and Property of PCCs
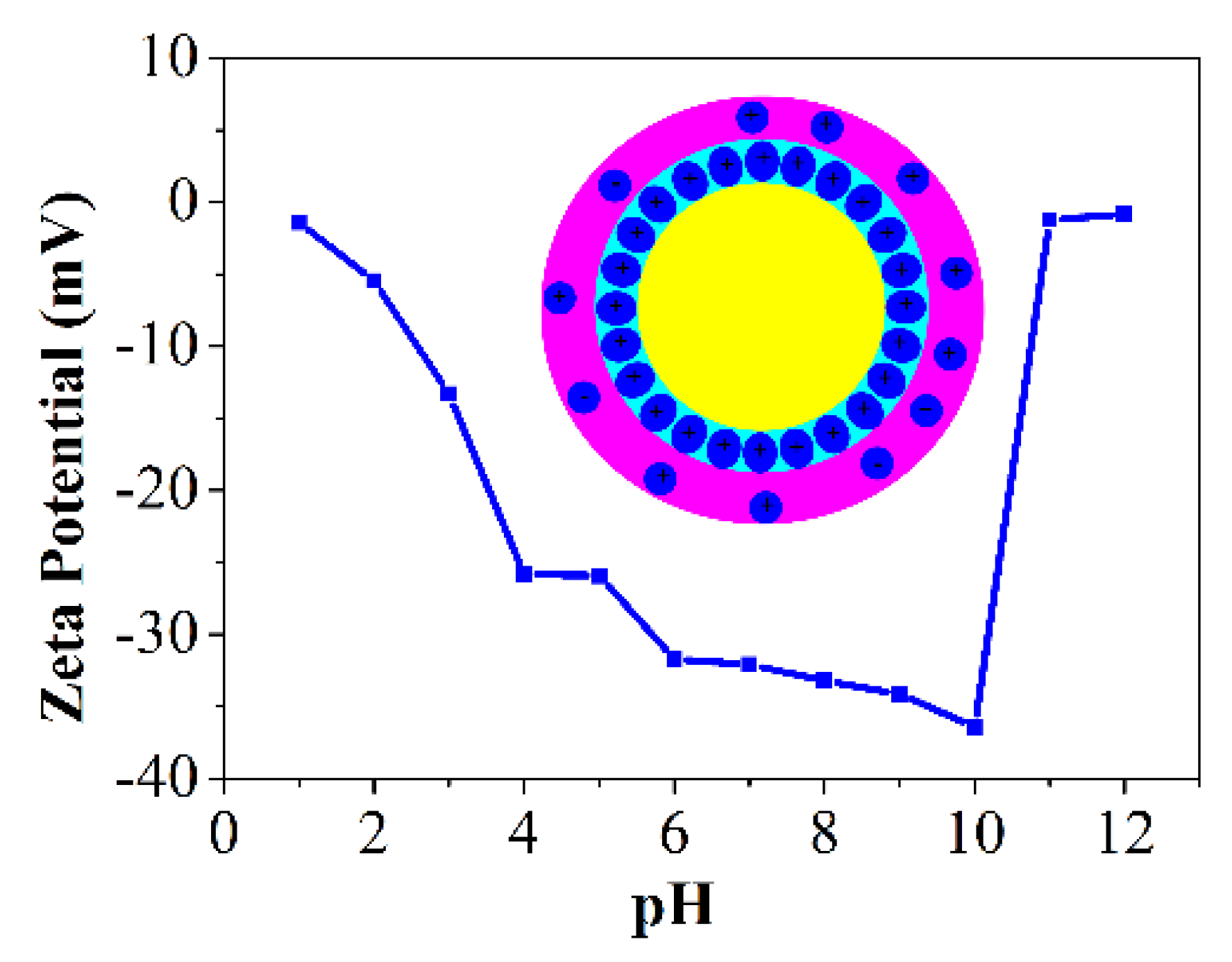
3.4. Effects of Sucrose on the Stability of PCCs Emulsions
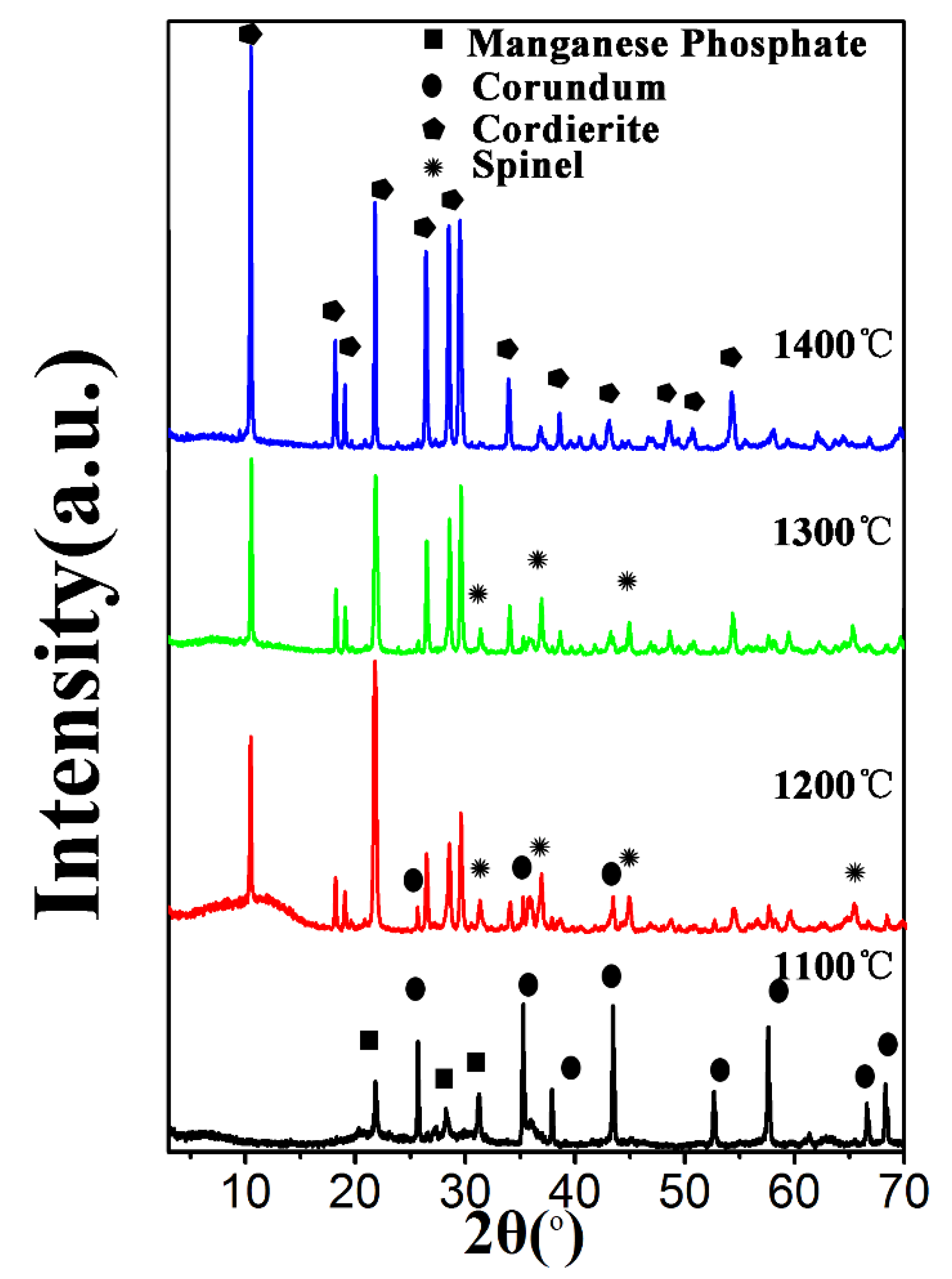
3.5. Effects of Temperature on the Morphology of PCCs with 30% Sucrose Content
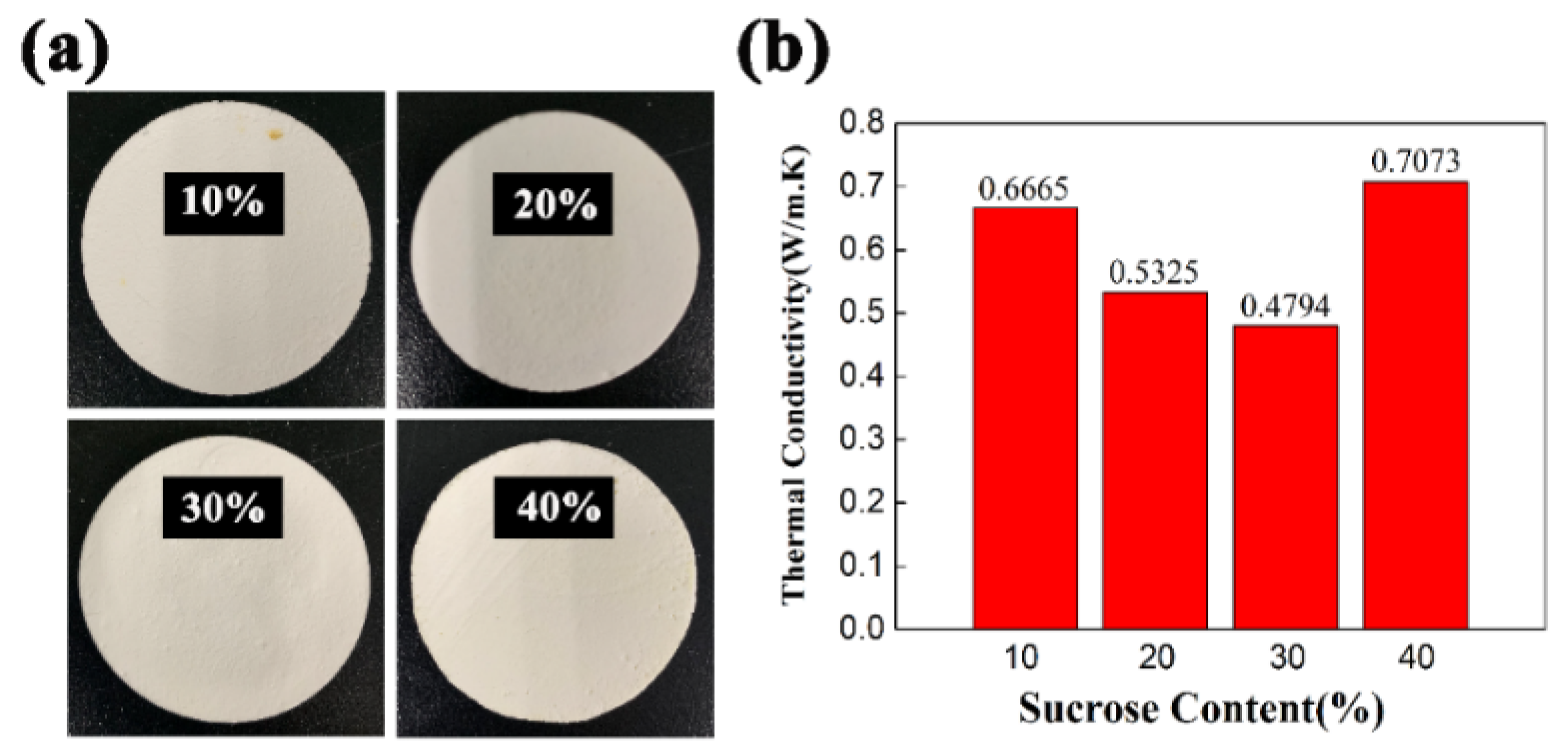
3.6. Effects of Sucrose Content on the Thermal Conductivity of PCCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benhammou, A.; El Hafiane, Y.; Abourriche, A.; Abouliatim, Y.; Nibou, L.; Yaacoubi, A.; Tessier-Doyen, N.; Smith, A.; Tanouti, B. Effects of oil shale addition and sintering cycle on the microstructure and mechanical properties of porous cordierite-ceramic. Ceram. Int. 2014, 40, 8937–8944. [Google Scholar] [CrossRef]
- Baitalik, S.; Kayal, N. Processing and properties of cordierite-silica bonded porous SiC ceramics. Ceram. Int. 2017, 43, 14683–14692. [Google Scholar] [CrossRef]
- Fuji, M.; Shiroki, Y.; Menchavez, R.L.; Takegami, H.; Takahashi, M.; Suzuki, H.; Izuhara, S.; Yokoyama, T. Fabrication of cordierite filter by in-situ solidification for high temperature dust collection. Powder Technol. 2007, 172, 57–62. [Google Scholar] [CrossRef]
- Richardson, J.T.; Peng, Y.; Remue, D. Properties of ceramic foam catalyst supports: Pressure drop. Appl. Catal. A Gen. 2000, 204, 19–32. [Google Scholar] [CrossRef]
- Uhlhorn, R.J.R.; Keizer, K.; Burggraaf, A.J. Gas transport and separation with ceramic membranes. Part I. Multilayer diffusion and capillary condensation. J. Membr. Sci. 1992, 66, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Julbe, A.; Farrusseng, D.; Guizard, C. Porous ceramic membranes for catalytic reactors—Overview and new ideas. J. Membr. Sci. 2001, 181, 3–20. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, W.; Xu, N. Design and Fabrication of Ceramic Catalytic Membrane Reactors for Green Chemical Engineering Applications. Engineering 2018, 4, 848–860. [Google Scholar] [CrossRef]
- Ohji, T.; Fukushima, M. Macro-porous ceramics: Processing and properties. Int. Mater. Rev. 2012, 57, 115–131. [Google Scholar] [CrossRef]
- Hasmaliza, M.; Yu Min, L.; Norfadhilah, I. Porous cordierite synthesized using corn starch. Adv. Mater. Res. 2014, 858, 137–140. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Wu, L. Porous cordierite ceramics prepared by foam-gelcasting technique: Phase evolution and properties. J. Alloys Compd. 2019, 791, 690–699. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, J. Approaching extremely low thermal conductivity by crystal structure engineering in Mg2Al4Si5O18. J. Mater. Res. 2015, 30, 3729–3739. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.-Y.; Wang, Y.-L.; Cui, S.-P.; Nie, Z.-R.; Li, Q.-Y.; Wei, Q. Preparation and desalination performance of porous planar cordierite membranes using industrial solid waste as main silica source. Ceram. Int. 2019, 45, 5932–5940. [Google Scholar] [CrossRef]
- Wang, S.; Lu, H.; Hou, Z. Sol–emulsion–gel synthesis of cordierite ceramics for high-frequency multilayer chip inductors. Ceram. Int. 2013, 39, 991–997. [Google Scholar] [CrossRef]
- Zhou, J.-E.; Dong, Y.; Hampshire, S.; Meng, G. Utilization of sepiolite in the synthesis of porous cordierite ceramics. Appl. Clay Sci. 2011, 52, 328–332. [Google Scholar] [CrossRef]
- Sandoval, M.L.; Talou, M.H.; Tomba Martinez, A.G.; Camerucci, M.A.; Gregorová, E.; Pabst, W. Porous cordierite-based ceramics processed by starch consolidation casting—Microstructure and high-temperature mechanical behavior. Ceram. Int. 2018, 44, 3893–3903. [Google Scholar] [CrossRef]
- Pia, G.; Casnedi, L.; Sanna, U. Porosity and pore size distribution influence on thermal conductivity of yttria-stabilized zirconia: Experimental findings and model predictions. Ceram. Int. 2016, 42, 5802–5809. [Google Scholar] [CrossRef]
- Qiu, L.; Zou, H.; Tang, D.; Wen, D.; Feng, Y.; Zhang, X. Inhomogeneity in pore size appreciably lowering thermal conductivity for porous thermal insulators. Appl. Therm. Eng. 2018, 130, 1004–1011. [Google Scholar] [CrossRef]
- Pelissari, P.I.B.G.B.; Angélico, R.A.; Salvini, V.R.; Vivaldini, D.O.; Pandolfelli, V.C. Analysis and modeling of the pore size effect on the thermal conductivity of alumina foams for high temperature applications. Ceram. Int. 2017, 43, 13356–13363. [Google Scholar] [CrossRef]
- Pia, G.; Sanna, U. An intermingled fractal units model to evaluate pore size distribution influence on thermal conductivity values in porous materials. Appl. Therm. Eng. 2014, 65, 330–336. [Google Scholar] [CrossRef]
- Jin, X.; Dong, L.; Xu, H.; Liu, L.; Li, N.; Zhang, X.; Han, J. Effects of porosity and pore size on mechanical and thermal properties as well as thermal shock fracture resistance of porous ZrB2–SiC ceramics. Ceram. Int. 2016, 42, 9051–9057. [Google Scholar] [CrossRef]
- Han, L.; Li, F.; Deng, X.; Wang, J.; Zhang, H.; Zhang, S. Foam-gelcasting preparation, microstructure and thermal insulation performance of porous diatomite ceramics with hierarchical pore structures. J. Eur. Ceram. Soc. 2017, 37, 2717–2725. [Google Scholar] [CrossRef]
- Pia, G. High porous yttria-stabilized zirconia with aligned pore channels: Morphology directionality influence on heat transfer. Ceram. Int. 2016, 42, 11674–11681. [Google Scholar] [CrossRef]
- Pia, G.; Casnedi, L.; Sanna, U. Porous ceramic materials by pore-forming agent method: An intermingled fractal units analysis and procedure to predict thermal conductivity. Ceram. Int. 2015, 41, 6350–6357. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, W.; Han, B.; Li, Y.; Yan, W.; Xu, N.; Li, N.; Wei, J. Preparation and characterization of eco-friendly and low-cost mullite-corundum foamed ceramics with low thermal conductivity. Ceram. Int. 2019, 45, 13203–13209. [Google Scholar] [CrossRef]
- Han, Y.; Li, C.; Bian, C.; Li, S.; Wang, C.-A. Porous anorthite ceramics with ultra-low thermal conductivity. J. Eur. Ceram. Soc. 2013, 33, 2573–2578. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.; Liu, X.; Huang, Z.; Lee, S.-H. Aqueous gelcasting and pressureless sintering of zirconium diboride foams. Ceram. Int. 2014, 40, 6325–6330. [Google Scholar] [CrossRef]
- Li, C.; Bian, C.; Han, Y.; Wang, C.-A.; An, L. Mullite whisker reinforced porous anorthite ceramics with low thermal conductivity and high strength. J. Eur. Ceram. Soc. 2016, 36, 761–765. [Google Scholar] [CrossRef]
- Zou, S.; Yang, Y.; Liu, H.; Wang, C. Synergistic stabilization and tunable structures of Pickering high internal phase emulsions by nanoparticles and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1–9. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Ge, H.B.; Wang, G.; Yuan, B.; Dong, B.B.; Li, H.X. Fabrication and microstructure of porous SiC ceramics using suspension emulsions as pore-forming agents. Ceram. Int. 2014, 40, 11705–11711. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.H.; Liu, L.J.; Zhang, H.Y. Large gas permeability nickel/alumina substrates with hierarchical pore structure for solid oxide fuel cells derived from particle-stabilized emulsions. J. Power Sources 2015, 285, 400–405. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wang, X.; Liu, L.; Wang, C. Highly permeable macroporous cordierite ceramics with controlled microstructure produced by particle-stabilized emulsions with a reactive thermal treatment. J. Eur. Ceram. Soc. 2017, 37, 3203–3211. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.-h.; Xie, Y.-m.; Zhang, H.-y. Three-dimensional fully interconnected highly porous hydroxyapatite scaffolds derived from particle-stabilized emulsions. Ceram. Int. 2016, 42, 5455–5460. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Deng, Y.; Guan, W.; Wang, X.; Qian, T. Preparation of paraffin/porous TiO2 foams with enhanced thermal conductivity as PCM, by covering the TiO2 surface with a carbon layer. Appl. Energy 2016, 171, 37–45. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, X.; Ma, Y.; Guan, W.; Qian, T. Macrostructure-controlled titania ceramics derived from particle-stabilized emulsions: Preparation and photocatalysis performance. Mater. Chem. Phys. 2016, 182, 402–408. [Google Scholar] [CrossRef]
- Luan, X.Z.; Li, J.H.; Wang, Z.Y.; Feng, W.W.; Huang, K.Y.; Liu, S. Hierarchically cell-window structured porous cordierite prepared by particle-stabilized emulsions using potato starch as a modifier. J. Eur. Ceram. Soc. 2021, 41, 4369–4380. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Feng, W.; Wang, X.; Nian, H. Design and Preparation of the Phase Change Materials Paraffin/Porous Al2O3@Graphite Foams with Enhanced Heat Storage Capacity and Thermal Conductivity. ACS Sustain. Chem. Eng. 2017, 5, 7594–7603. [Google Scholar] [CrossRef]
- Innocentini, M.D.; Rodrigues, V.P.; Romano, R.C.; Pileggi, R.G.; Silva, G.M.; Coury, J.R. Permeability optimization and performance evaluation of hot aerosol filters made using foam incorporated alumina suspension. J. Hazard. Mater. 2009, 162, 212–221. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, X.; Li, J.; Feng, W.; Liu, R.; Liu, S.; Wang, Z. Structure-Controlled Porous Cordierite Ceramics with High Solid Content Prepared by Pickering Emulsion Technique Using Sucrose as a Porogen. Materials 2022, 15, 3410. https://doi.org/10.3390/ma15093410
Luan X, Li J, Feng W, Liu R, Liu S, Wang Z. Structure-Controlled Porous Cordierite Ceramics with High Solid Content Prepared by Pickering Emulsion Technique Using Sucrose as a Porogen. Materials. 2022; 15(9):3410. https://doi.org/10.3390/ma15093410
Chicago/Turabian StyleLuan, Xuezhu, Jinhong Li, Wuwei Feng, Rui Liu, Shuo Liu, and Ziyao Wang. 2022. "Structure-Controlled Porous Cordierite Ceramics with High Solid Content Prepared by Pickering Emulsion Technique Using Sucrose as a Porogen" Materials 15, no. 9: 3410. https://doi.org/10.3390/ma15093410
APA StyleLuan, X., Li, J., Feng, W., Liu, R., Liu, S., & Wang, Z. (2022). Structure-Controlled Porous Cordierite Ceramics with High Solid Content Prepared by Pickering Emulsion Technique Using Sucrose as a Porogen. Materials, 15(9), 3410. https://doi.org/10.3390/ma15093410





