Advantages of Cubosomal Formulation for Gatifloxacin Delivery in the Treatment of Bacterial Keratitis: In Vitro and In Vivo Approach Using Clinical Isolate of Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GTX-Loaded Cubosomes Formulation
2.3. Particle Size Analysis, Polydispersity Index and Zeta Potential
2.4. Transmission Electron Microscopy (TEM)
2.5. Entrapment Efficiency (EE %)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Ex Vivo Corneal Permeation Study
2.8. HPLC Assay of GTX
2.9. Determination of Minimum Inhibitory Concentration (MIC) of GTX
2.10. In Vivo Studies
2.11. Histological Examination
2.12. Statistical Analysis
3. Results and Discussion
3.1. Particle Size, Polydispersity Index and Zeta Potential
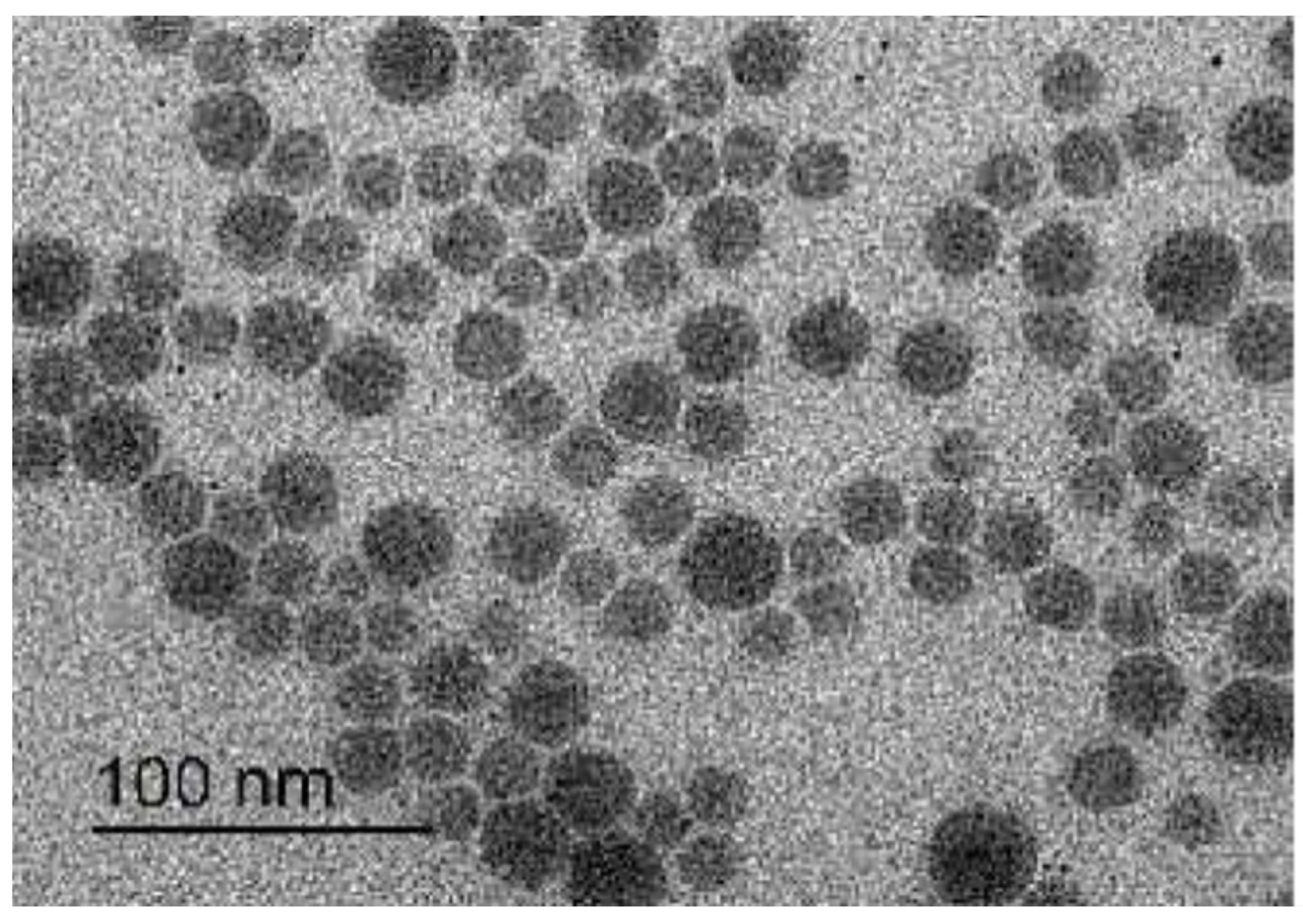
3.2. Morphology of GTX-Cubosomal Nanoparticles
3.3. Entrapment Efficiency
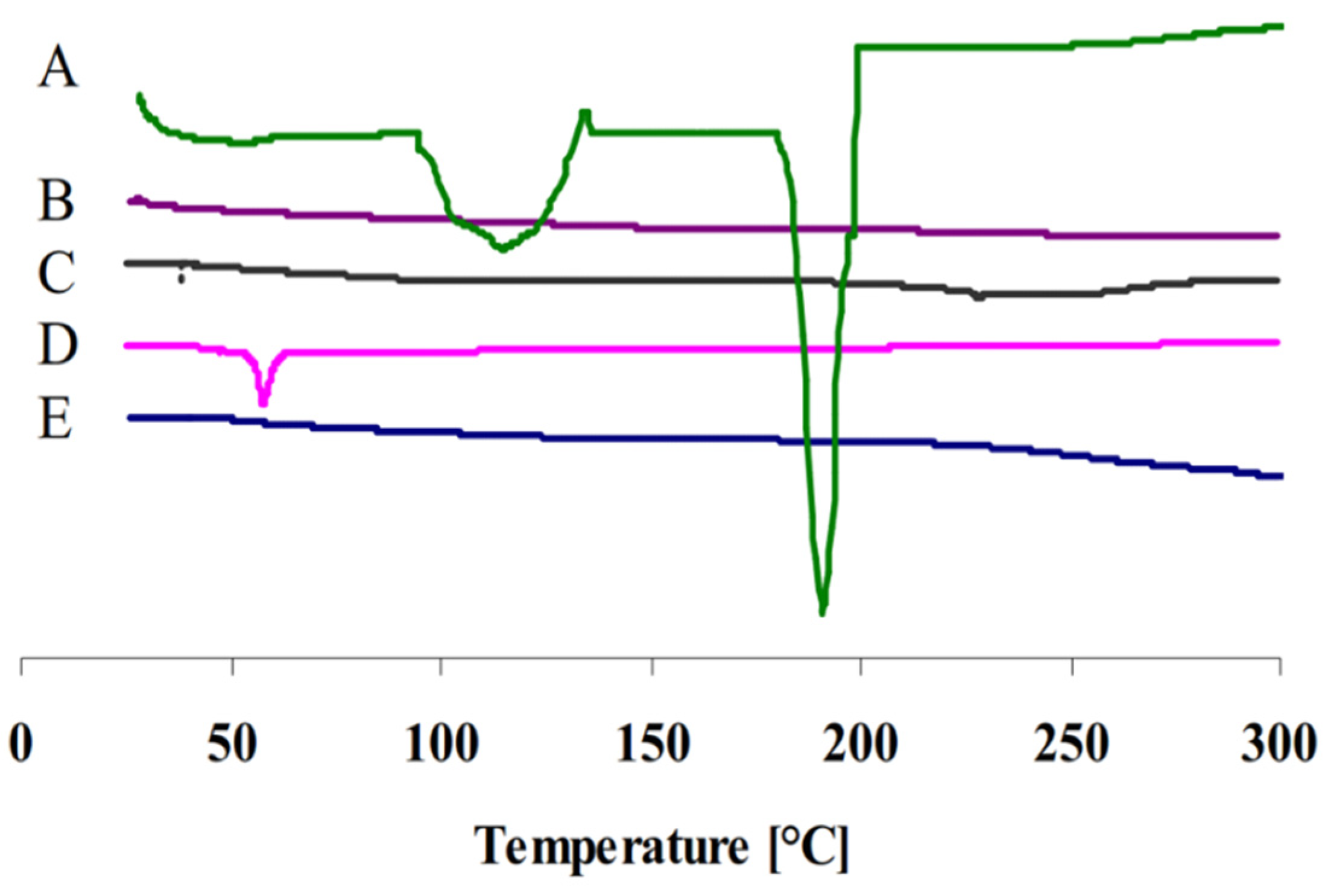
3.4. Differential Scanning Calorimetry
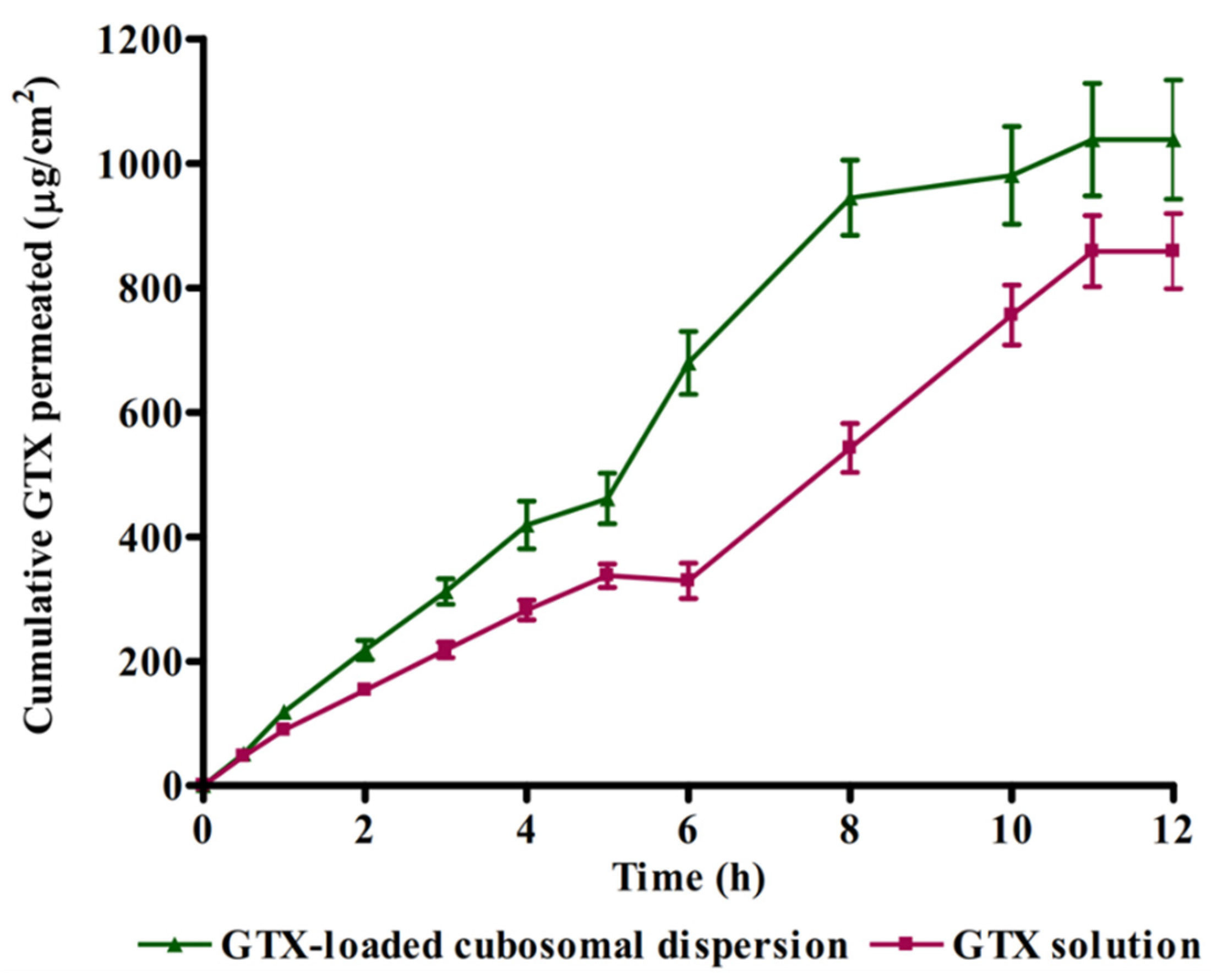
3.5. Ex Vivo Corneal Permeation
3.6. Influence of GTX-Loaded Cubosomal Nanoparticles on MIC
3.7. In Vivo Studies
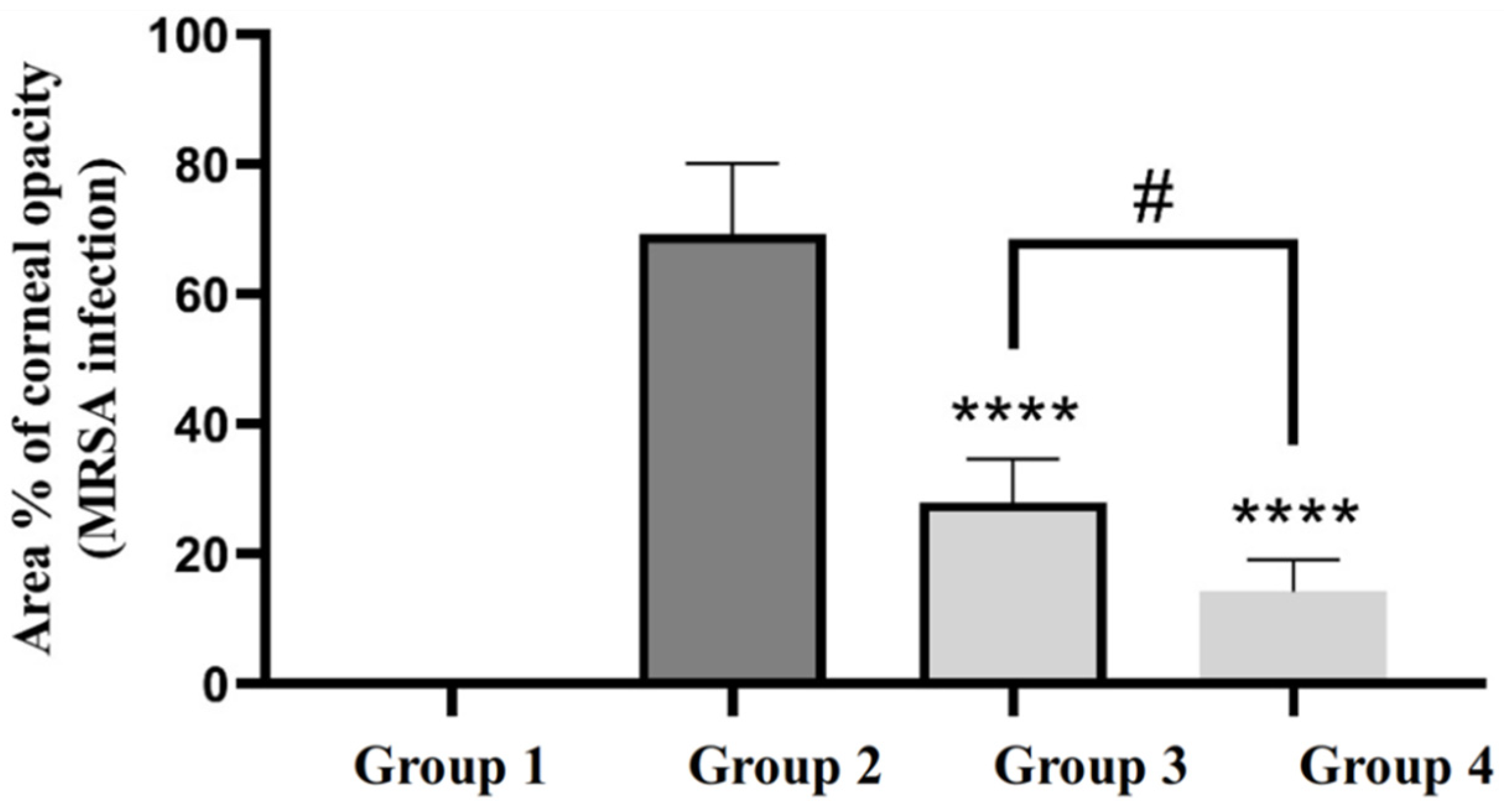
3.7.1. Efficacy of GTX-Loaded Cubosomal Nanoparticles in the Treatment of MRSA-Induced Ocular Keratitis
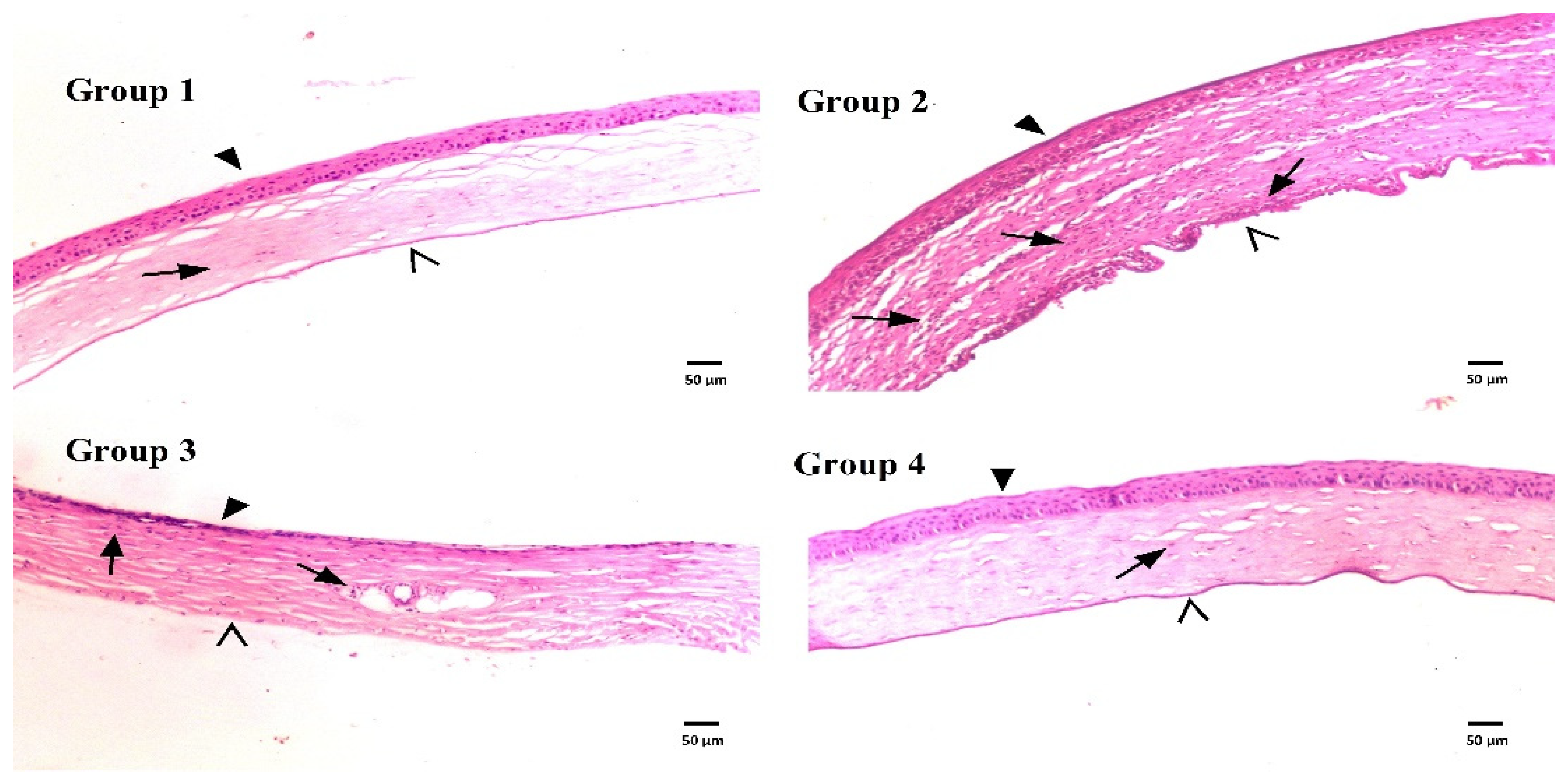
3.7.2. Histological Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, S.; Mitra, A.K. Transporters and receptors in ocular drug delivery: Opportunities and challenges. Expert Opin. Drug Deliv. 2005, 2, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Civiale, C.; Licciardi, M.; Cavallaro, G.; Giammona, G.; Mazzone, M. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int. J. Pharm. 2009, 378, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.-P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.D.; Lai, J.-Y. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics 2021, 11, 5447. [Google Scholar] [CrossRef]
- Pepic, I.; Lovric, J.; Filipovic-Grcic, J. Polymeric micelles in ocular drug delivery: Rationale, strategies and challenges. Chem. Biochem. Eng. Q. 2012, 26, 365–377. [Google Scholar]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef]
- Aksungur, P.; Demirbilek, M.; Denkbaş, E.B.; Vandervoort, J.; Ludwig, A.; Ünlü, N. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake, and kinetic studies. J. Control. Release 2011, 151, 286–294. [Google Scholar] [CrossRef]
- Katara, R.; Majumdar, D.K. Eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf. B Biointerfaces 2013, 103, 455–462. [Google Scholar] [CrossRef]
- Gupta, A.K.; Madan, S.; Majumdar, D.; Maitra, A. Ketorolac entrapped in polymeric micelles: Preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 2000, 209, 1–14. [Google Scholar] [CrossRef]
- Cavalli, R.; Gasco, M.R.; Chetoni, P.; Burgalassi, S.; Saettone, M. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int. J. Pharm. 2002, 238, 241–245. [Google Scholar] [CrossRef]
- Bose, S.; Michniak-Kohn, B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur. J. Pharm. Sci. 2013, 48, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorle, A.P.; Gattani, S.G. Design and Evaluation of Polymeric Ocular Drug Delivery System. Chem. Pharm. Bull. 2009, 57, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, C.M.; Barman Balfour, J.A.; Lamb, H.M. GTX. Drugs 1999, 58, 683–696. [Google Scholar] [CrossRef]
- Perry, C.M.; Ormrod, D.; Hust, M. GTX, a review of its use in the management of bacterial infections. Drugs 2002, 62, 169–207. [Google Scholar] [CrossRef]
- Kwatra, D.; Vadlapatla, R.K.; Vadlapudi, A.D.; Pal, D.; Mitra, A.K. Interaction of gatifloxacin with efflux transporters: A possible mechanism for drug resistance. Int. J. Pharm. 2010, 395, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Maaz, A.; Abdelwahed, W.; Tekko, I.; Trefi, S. Influence of nanoprecipitation method parameters on nanoparticles loaded with gatifloxacin for ocular drug delivery. IJASR 2015, 3, 101–112. [Google Scholar]
- Gan, L.; Han, S.; Shen, J.; Zhu, J.; Zhu, C.; Zhang, X.; Gan, Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010, 396, 179–187. [Google Scholar] [CrossRef]
- Nasr, M.; Teiama, M.; Ismail, A.; Ebada, A.; Saber, S. In vitro and in vivo evaluation of cubosomal nanoparticles as an ocular delivery system for fluconazole in treatment of keratomycosis. Drug Deliv. Transl. Res. 2020, 10, 1841–1852. [Google Scholar] [CrossRef]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2014, 5, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saber, S.; Nasr, M.; Kaddah, M.M.; Mostafa-Hedeab, G.; Cavalu, S.; Mourad, A.A.; Gaafar, A.G.A.; Zaghlool, S.S.; Saleh, S.; Hafez, M.M.; et al. Nifuroxazide-loaded cubosomes exhibit an advancement in pulmonary delivery and attenuate bleomycin-induced lung fibrosis by regulating the STAT3 and NF-κB signaling: A new challenge for unmet therapeutic needs. Biomed. Pharmacother. 2022, 148, 112731. [Google Scholar] [CrossRef] [PubMed]
- Khurana, G.; Arora, G.; Pawar, P. Ocular insert for sustained delivery of GTX sesquihydrate: Preparation and evaluations. Int. J. Pharm. Investig. 2012, 2, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalam, M.A.; Sultana, Y.; Ali, A.; Aqil, M.; Mishra, A.K.; Chuttani, K.; Aljuffali, I.A.; Alshamsan, A. Part II: Enhancement of transcorneal delivery of gatifloxacin by solid lipid nanoparticles in comparison to commercial aqueous eye drops. J. Biomed. Mater. Res. Part A 2012, 101, 1828–1836. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standardrs Institute. CLSI Document M07-A9. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard-Ninth Edition; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Ong, S.J.; Huang, Y.-C.; Tan, H.-Y.; Ma, D.H.K.; Lin, H.-C.; Yeh, L.-K.; Chen, P.Y.F.; Chen, H.-C.; Chuang, C.-C.; Chang, C.-J.; et al. Staphylococcus aureus Keratitis: A Review of Hospital Cases. PLoS ONE 2013, 8, e80119. [Google Scholar] [CrossRef]
- Banchroft, J.; Stevens, A.; Turner, D. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Arthanari, S.; Mani, G.; Jang, J.H.; Choi, J.O.; Cho, Y.H.; Lee, J.H.; Cha, S.E.; Oh, H.S.; Kwon, D.H.; Jang, H.T. Preparation and characterization of gatifloxacin-loaded alginate/poly (vinyl alcohol) electrospun nanofibers. Artif. Cells Nanomed. Biotechnol. 2014, 44, 847–852. [Google Scholar] [CrossRef]
- Sharma, R.; Kaur, G.; Kapoor, D.K. Fluconazole loaded cubosomal vesicles for topical delivery. Int. J. Drug Dev. Res. 2015, 7, 32–41. [Google Scholar]
- Cornaire, G.; Woodley, J.; Hermann, P.; Cloarec, A.; Arellano, C.; Houin, G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int. J. Pharm. 2004, 278, 119–131. [Google Scholar] [CrossRef]
- Han, S.; Shen, J.-Q.; Gan, Y.; Geng, H.-M.; Zhang, X.-X.; Zhu, C.-L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Corneal targeted Sertaconazole nitrate loaded cubosomes: Preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int. J. Pharm. 2018, 553, 386–397. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Elbanna, T.E.; Sonbol, F.I.; Gamaleldin, N.M.; El Maghraby, G.M. Optimization of niosomes for enhanced antibacterial activity and reduced bacterial resistance: In vitro and in vivo evaluation. Expert Opin. Drug Deliv. 2015, 12, 163–180. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, M.; Saber, S.; Bazeed, A.Y.; Ramadan, H.A.; Ebada, A.; Ciorba, A.L.; Cavalu, S.; Elagamy, H.I. Advantages of Cubosomal Formulation for Gatifloxacin Delivery in the Treatment of Bacterial Keratitis: In Vitro and In Vivo Approach Using Clinical Isolate of Methicillin-Resistant Staphylococcus aureus. Materials 2022, 15, 3374. https://doi.org/10.3390/ma15093374
Nasr M, Saber S, Bazeed AY, Ramadan HA, Ebada A, Ciorba AL, Cavalu S, Elagamy HI. Advantages of Cubosomal Formulation for Gatifloxacin Delivery in the Treatment of Bacterial Keratitis: In Vitro and In Vivo Approach Using Clinical Isolate of Methicillin-Resistant Staphylococcus aureus. Materials. 2022; 15(9):3374. https://doi.org/10.3390/ma15093374
Chicago/Turabian StyleNasr, Mohamed, Sameh Saber, Alaa Y. Bazeed, Heba A. Ramadan, Asmaa Ebada, Adela Laura Ciorba, Simona Cavalu, and Heba I. Elagamy. 2022. "Advantages of Cubosomal Formulation for Gatifloxacin Delivery in the Treatment of Bacterial Keratitis: In Vitro and In Vivo Approach Using Clinical Isolate of Methicillin-Resistant Staphylococcus aureus" Materials 15, no. 9: 3374. https://doi.org/10.3390/ma15093374
APA StyleNasr, M., Saber, S., Bazeed, A. Y., Ramadan, H. A., Ebada, A., Ciorba, A. L., Cavalu, S., & Elagamy, H. I. (2022). Advantages of Cubosomal Formulation for Gatifloxacin Delivery in the Treatment of Bacterial Keratitis: In Vitro and In Vivo Approach Using Clinical Isolate of Methicillin-Resistant Staphylococcus aureus. Materials, 15(9), 3374. https://doi.org/10.3390/ma15093374








