Exploration of Zero-Valent Iron Stabilized Calcium–Silicate–Alginate Beads’ Catalytic Activity and Stability for Perchlorate Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Fabrication of Alginate–Silicate Hybrid Polymer
2.3. Characterization of nZVI-ASB
2.4. Fabrication of nZVI Loaded Alginate–Silicate Polymer Bead (nZVI-ASB)
2.5. Perchlorate Degradation Using nZVI-ASB
3. Results and Discussion
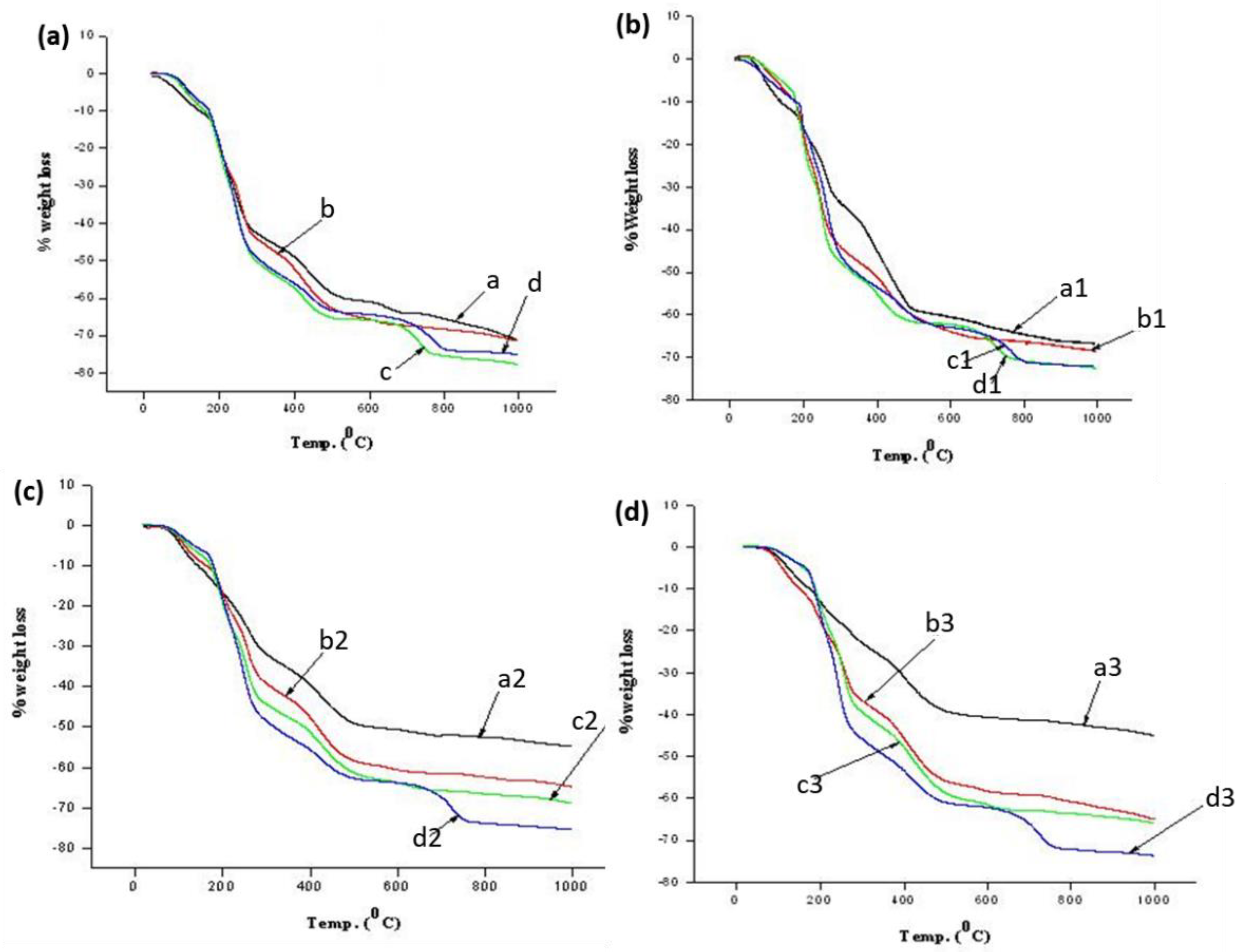
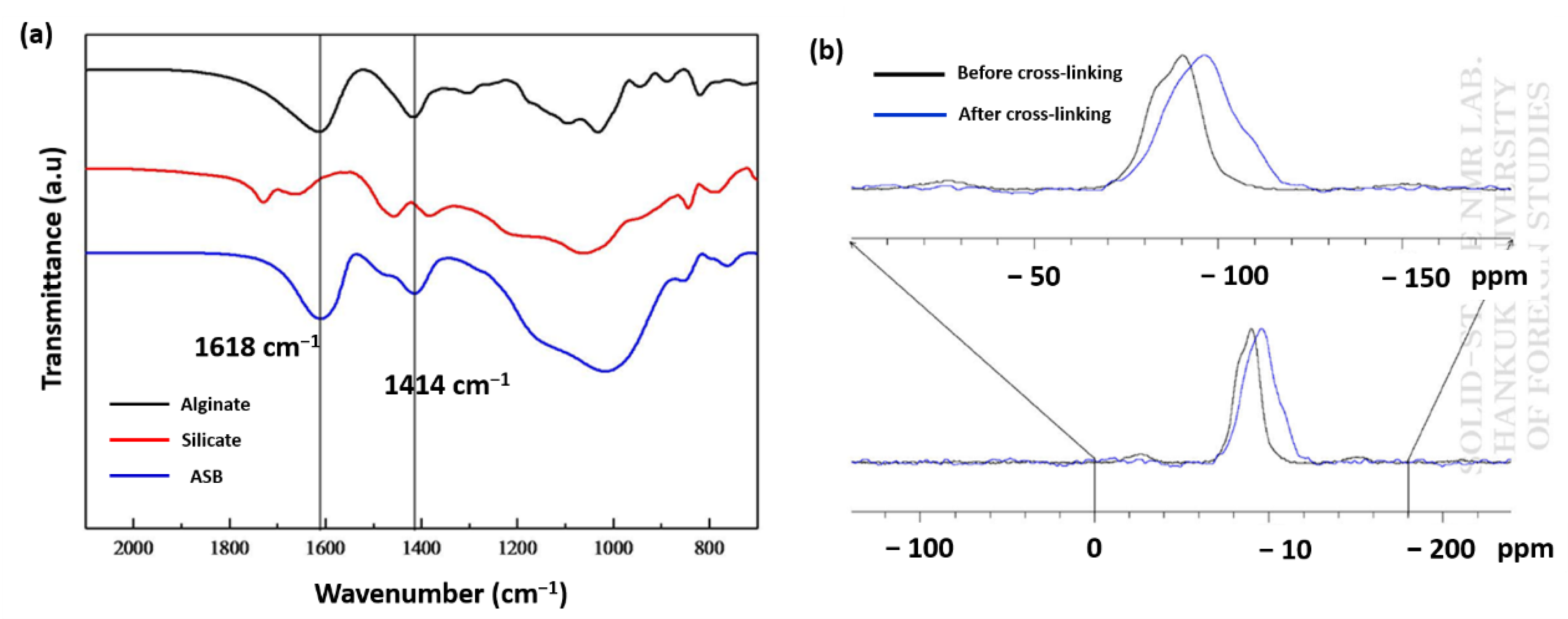
3.1. Characterization of Alginate/Silicate Hybrid Polymer
3.2. Investigating the Decomposition Conditions of Perchloric Acid Using nZVI-ASB
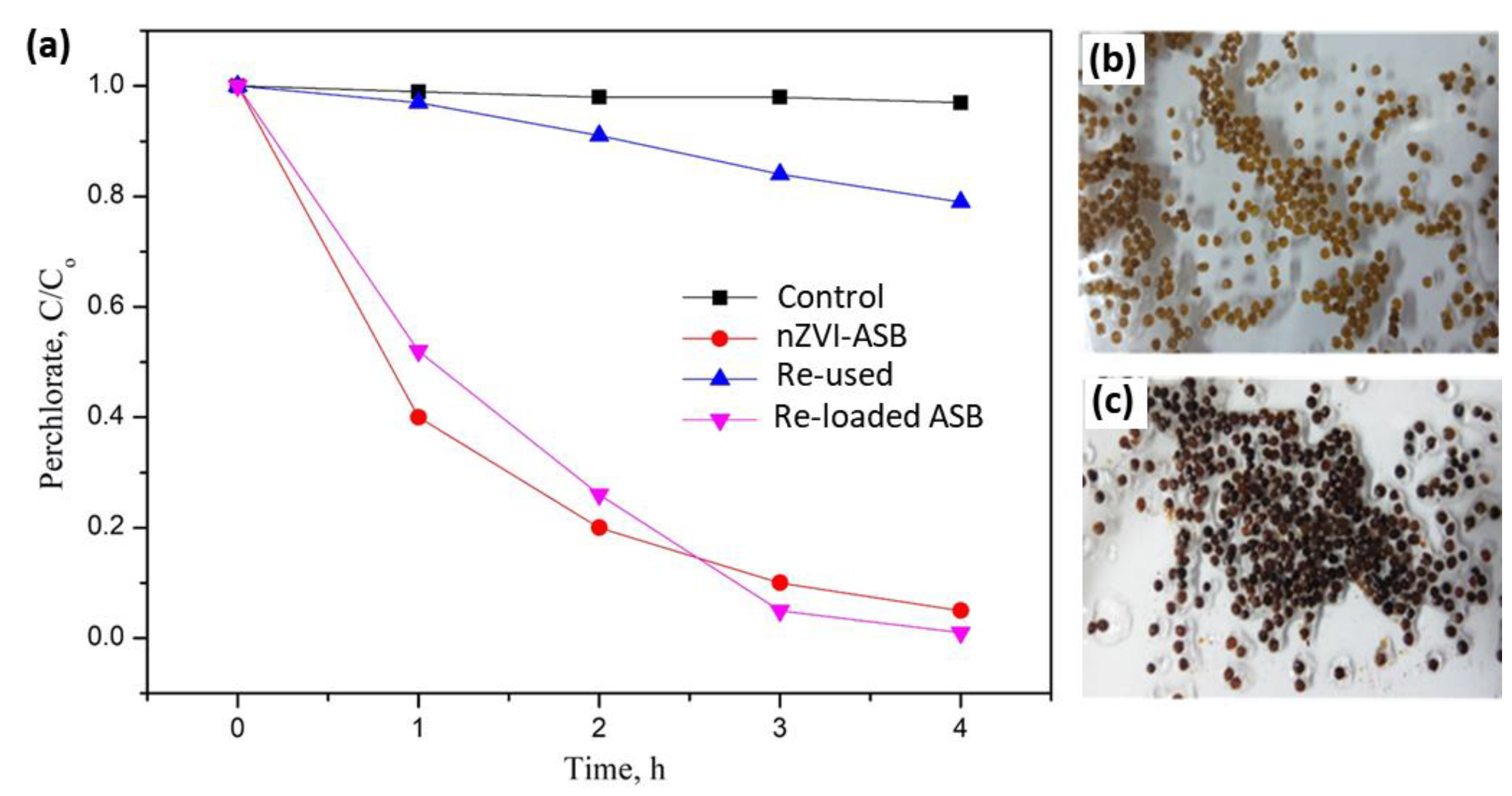
3.3. Stability/Reusable Capabilities of nZVI-ASB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcon, L.; Oliveras, J.; Puntes, V.F. In Situ Nanoremediation of Soils and Groundwaters from the Nanoparticle’s Standpoint: A Review. Sci. Total Environ. 2021, 791, 148324. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, A.; Das, D.; Prabhakar, S.; Kumar, M.M.; Anbalagan, K.; Rajesh, M. Adsorption of Perchlorate from Water Using Quaternary Ammonium-Functionalized Chitosan Beads. Environ. Prog. Sustain. Energy 2020, 39, e13325. [Google Scholar] [CrossRef]
- Xie, Y.; Ren, L.; Zhu, X.; Gou, X.; Chen, S. Physical and Chemical Treatments for Removal of Perchlorate from Water–A Review. Process Saf. Environ. Prot. 2018, 116, 180–198. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Chen, H.; Zhang, M.; Cao, X. Biochar-Supported Zerovalent Iron for Removal of Various Contaminants from Aqueous Solutions. Bioresour. Technol. 2014, 152, 538–542. [Google Scholar] [CrossRef]
- Joo, T.; Lee, J.-C.; Paeng, K.-J. Reduction of Perchlorate in Aqueous Solution Using Zero Valece Iron Stabilized with Alginate Bead. Anal. Sci. Technol. 2010, 23, 560–565. [Google Scholar] [CrossRef]
- Rangan, S.M.; Mouti, A.; LaPat-Polasko, L.; Lowry, G.V.; Krajmalnik-Brown, R.; Delgado, A.G. Synergistic Zerovalent Iron (Fe0) and Microbiological Trichloroethene and Perchlorate Reductions Are Determined by the Concentration and Speciation of Fe. Environ. Sci. Technol. 2020, 54, 14422–14431. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, Y.; Ni, B.-J. Zero Valent Iron Significantly Enhances Methane Production from Waste Activated Sludge by Improving Biochemical Methane Potential Rather Than Hydrolysis Rate. Sci. Rep. 2015, 5, 8263. [Google Scholar] [CrossRef] [Green Version]
- Coradin, T.; Livage, J. Mesoporous Alginate/Silica Biocomposites for Enzyme Immobilisation. Comptes Rendus Chim. 2003, 6, 147–152. [Google Scholar] [CrossRef]
- Coradin, T.; Allouche, J.; Boissière, M.; Livage, J. Sol-Gel Biopolymer / Silica Nanocomposites in Biotechnology. Curr. Nanosci. 2006, 2, 219–230. [Google Scholar] [CrossRef]
- Sakai, S.; Ono, T.; Ijima, H.; Kawakami, K. In Vitro and in Vivo Evaluation of Alginate/Sol–Gel Synthesized Aminopropyl-Silicate/Alginate Membrane for Bioartificial Pancreas. Biomaterials 2002, 23, 4177–4183. [Google Scholar] [CrossRef]
- Sakai, S.; Ono, T.; Ijima, H.; Kawakami, K. MIN6 Cells-Enclosing Aminopropyl-Silicate Membrane Templated by Alginate Gels Differences in Guluronic Acid Content. Int. J. Pharm. 2004, 270, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Boninsegna, S.; Dal Toso, R.; Dal Monte, R.; Carturan, G. Alginate Microspheres Loaded with Animal Cells and Coated by a Siliceous Layer. J. Sol-Gel Sci. Technol. 2003, 26, 1151–1157. [Google Scholar] [CrossRef]
- Liu, X.; Qian, L.; Shu, T.; Tong, Z. Rheology Characterization of Sol–Gel Transition in Aqueous Alginate Solutions Induced by Calcium Cations through in Situ Release. Polymer 2003, 44, 407–412. [Google Scholar] [CrossRef]
- Desimone, M.F.; Alvarez, G.S.; Foglia, M.L.; Diaz, L.E. Development of Sol-Gel Hybrid Materials for Whole Cell Immobilization. Recent Pat. Biothechnology 2009, 3, 55–60. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, S.; Jiang, Z.; Yuan, W.; Wang, T. Diffusion of Nicotinamide Adenine Dinuncleotide in Calcium Alginate Hydrogel Beads Doped with Carbon and Silica Nanotubes. J. Chem. Eng. Data 2005, 50, 1319–1323. [Google Scholar] [CrossRef]
- Blandino, A.; Macías, M.; Cantero, D. Immobilization of Glucose Oxidase within Calcium Alginate Gel Capsules. Process Biochem. 2001, 36, 601–606. [Google Scholar] [CrossRef]
- Blandino, A.; Macías, M.; Cantero, D. Glucose Oxidase Release from Calcium Alginate Gel Capsules. Enzyme Microb. Technol. 2000, 27, 319–324. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Saxena, S. Dynamic Release of Riboflavin from a Starch-Based Semi IPN via Partial Enzymatic Degradation: Part II. React. Funct. Polym. 2004, 61, 115–129. [Google Scholar] [CrossRef]
- Vandenberg, G.W.; Drolet, C.; Scott, S.L.; de la Noüe, J. Factors Affecting Protein Release from Alginate–Chitosan Coacervate Microcapsules during Production and Gastric/Intestinal Simulation. J. Control. Release 2001, 77, 297–307. [Google Scholar] [CrossRef]
- Taqieddin, E.; Amiji, M. Enzyme Immobilization in Novel Alginate–Chitosan Core-Shell Microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar] [CrossRef]
- Cellesi, F.; Tirelli, N.; Hubbell, J.A. Towards a Fully-Synthetic Substitute of Alginate: Development of a New Process Using Thermal Gelation and Chemical Cross-Linking. Biomaterials 2004, 25, 5115–5124. [Google Scholar] [CrossRef] [PubMed]
- Betigeri, S.S.; Neau, S.H. Immobilization of Lipase Using Hydrophilic Polymers in the Form of Hydrogel Beads. Biomaterials 2002, 23, 3627–3636. [Google Scholar] [CrossRef]
- Coradin, T.; Nassif, N.; Livage, J. Silica–Alginate Composites for Microencapsulation. Appl. Microbiol. Biotechnol. 2003, 61, 429–434. [Google Scholar] [CrossRef]
- Meng, X. An Overview of Molecular Layer Deposition for Organic and Organic–Inorganic Hybrid Materials: Mechanisms, Growth Characteristics, and Promising Applications. J. Mater. Chem. A 2017, 5, 18326–18378. [Google Scholar] [CrossRef]
- Kusuktham, B.; Prasertgul, J.; Srinun, P. Morphology and Property of Calcium Silicate Encapsulated with Alginate Beads. Silicon 2014, 6, 191–197. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent Bio-Applications of Sol–Gel Materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, J.; Shen, J. Construction of Multilayer Coating onto Poly-(Dl-Lactide) to Promote Cytocompatibility. Biomaterials 2004, 25, 109–117. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, Z.; Lu, Y.; Wu, H.; Yuan, W.-K. Preparation and Catalytic Properties of Novel Alginate−Silica−Dehydrogenase Hybrid Biocomposite Beads. Ind. Eng. Chem. Res. 2006, 45, 511–517. [Google Scholar] [CrossRef]
- Xu, S.-W.; Lu, Y.; Li, J.; Zhang, Y.-F.; Jiang, Z.-Y. Preparation of Novel Silica-Coated Alginate Gel Beads for Efficient Encapsulation of Yeast Alcohol Dehydrogenase. J. Biomater. Sci. Polym. Ed. 2007, 18, 71–80. [Google Scholar] [CrossRef]
- Wong, T.W.; Chan, L.W.; Kho, S.B.; Sia Heng, P.W. Design of Controlled-Release Solid Dosage Forms of Alginate and Chitosan Using Microwave. J. Control. Release 2002, 84, 99–114. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, D.; Zhang, Z.; Zhang, R. Effectively Modified Surface Microstructure and Properties of Carbon Fiber via In-Situ Growth of Nano-SiO2. ECS J. Solid State Sci. Technol. 2021, 10, 091011. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M.; Dong, W.; Le, Z.; Zhang, D.; Henderson, I.M. A 29Si, 1H, and 13C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Park, A.A. Si Solid State MAS NMR Study on Leaching Behaviors and Chemical Stability of Different Mg-Silicate Structures for CO2 Sequestration. Chem. Eng. J. 2020, 396, 125204. [Google Scholar] [CrossRef]
- Donadelli, J.A.; Carlos, L.; Arques, A.; García Einschlag, F.S. Kinetic and Mechanistic Analysis of Azo Dyes Decolorization by ZVI-Assisted Fenton Systems: PH-Dependent Shift in the Contributions of Reductive and Oxidative Transformation Pathways. Appl. Catal. B Environ. 2018, 231, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Zhao, D.; Pan, G. Rapid and Complete Destruction of Perchlorate in Water and Ion-Exchange Brine Using Stabilized Zero-Valent Iron Nanoparticles. Water Res. 2007, 41, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yi, Y.; Qin, Y.; Wang, L.; Liu, G.; Wu, Y.; Diao, Z.; Zhou, T.; Xu, M. Perchlorate Degradation in Aqueous Solution Using Chitosan-Stabilized Zero-Valent Iron Nanoparticles. Sep. Purif. Technol. 2016, 171, 164–173. [Google Scholar] [CrossRef]

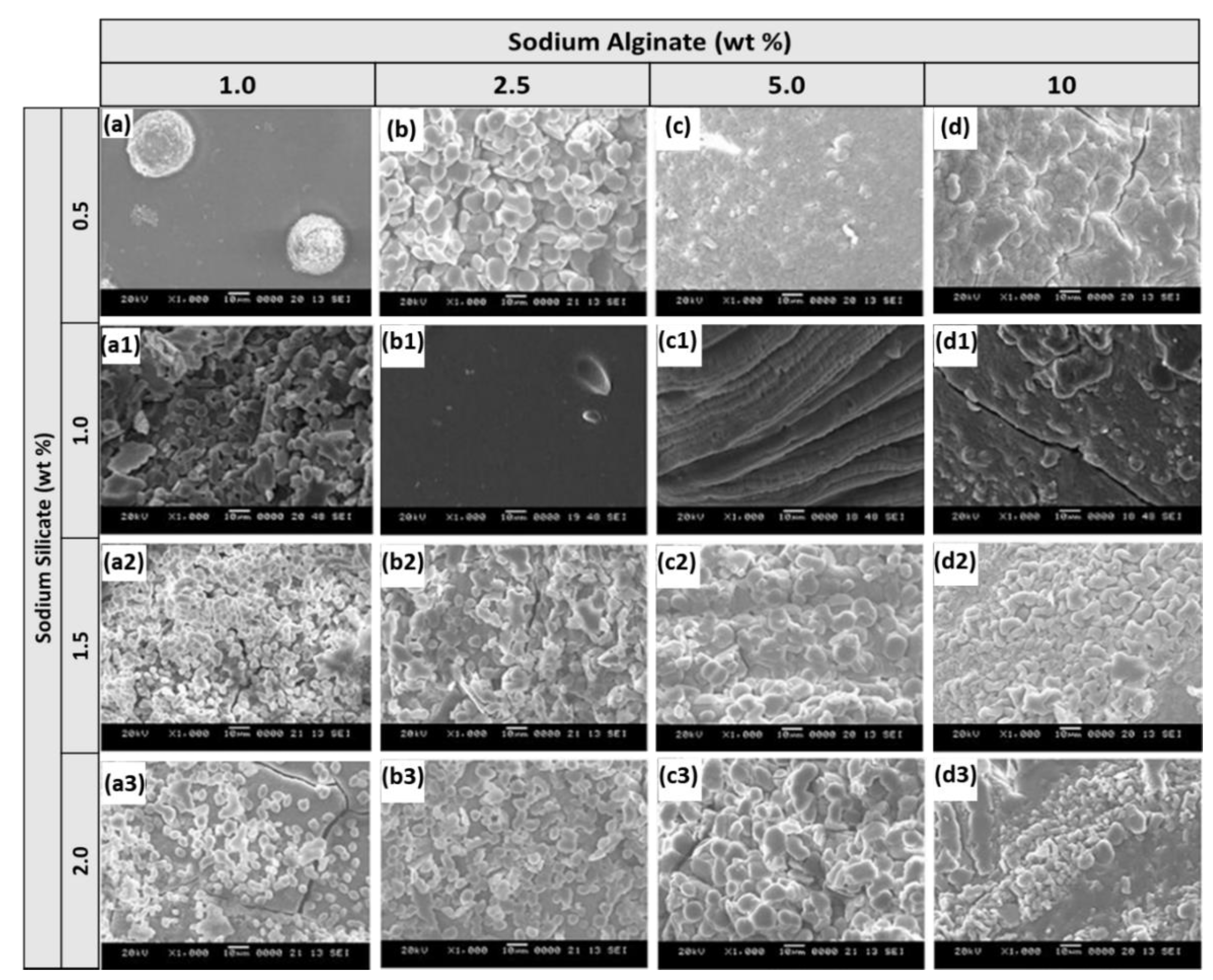

| Sample Name | Sodium Alginate (wt%) | Sodium Silicate (wt%) | Calcium Chloride (wt%) | wt% loss after Air Drying |
|---|---|---|---|---|
| a | 1 | 0.5 | 1 | 98.05 |
| b | 2.5 | 0.5 | 1 | 97.45 |
| c | 5 | 0.5 | 1 | 95.86 |
| d | 10 | 0.5 | 1 | 94.12 |
| a1 | 1 | 1 | 1 | 97.16 |
| b1 | 2.5 | 1 | 1 | 97.28 |
| c1 | 5 | 1 | 1 | 95.33 |
| d1 | 10 | 1 | 1 | 94.09 |
| a2 | 1 | 1.5 | 1 | 97.78 |
| b2 | 2.5 | 1.5 | 1 | 96.76 |
| c2 | 5 | 1.5 | 1 | 95.57 |
| d2 | 10 | 1.5 | 1 | 90.40 |
| a3 | 1 | 2 | 1 | 97.70 |
| b3 | 2.5 | 2 | 1 | 96.76 |
| c3 | 5 | 2 | 1 | 95.57 |
| d3 | 10 | 2 | 1 | 90.40 |
| Sample Name | % Weight Loss of ASB at Different Temperature (°C). | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 | 1000 | |
| a | 5.82 | 19.25 | 42.64 | 49.01 | 58.67 | 60.94 | 64.01 | 65.59 | 67.88 | 71.25 |
| b | 3.27 | 19.55 | 44.08 | 52.19 | 62.67 | 65.63 | 67.31 | 68.32 | 69.40 | 71.32 |
| c | 3.47 | 20.58 | 50.20 | 57.33 | 65.23 | 65.87 | 68.55 | 75.41 | 76.39 | 77.62 |
| d | 2.44 | 18.49 | 49.18 | 56.09 | 63.28 | 64.37 | 66.41 | 73.69 | 74.35 | 74.99 |
| a1 | 5.59 | 16.28 | 33.57 | 45.08 | 58.84 | 60.49 | 62.71 | 64.66 | 66.15 | 66.69 |
| b1 | 2.77 | 17.30 | 44.01 | 51.08 | 60.28 | 63.94 | 65.68 | 66.32 | 67.35 | 68.52 |
| c1 | 2.21 | 19.20 | 47.55 | 55.12 | 61.59 | 62.28 | 65.51 | 70.81 | 71.65 | 72.54 |
| d1 | 4.38 | 16.09 | 45.92 | 53.55 | 60.48 | 62.95 | 64.87 | 70.99 | 71.73 | 72.05 |
| a2 | 4.25 | 16.81 | 31.95 | 39.66 | 49.07 | 50.62 | 52.02 | 52.51 | 53.58 | 54.76 |
| b2 | 3.12 | 16.88 | 39.23 | 47.83 | 58.41 | 60.64 | 61.52 | 62.38 | 63.33 | 64.81 |
| c2 | 2.71 | 19.39 | 44.39 | 51.45 | 61.38 | 63.80 | 65.74 | 66.52 | 67.30 | 68.82 |
| d2 | 2.13 | 18.31 | 48.55 | 55.76 | 62.73 | 63.78 | 67.62 | 73.81 | 74.55 | 75.35 |
| a3 | 2.34 | 12.89 | 22.89 | 31.11 | 39.20 | 40.61 | 41.35 | 42.14 | 43.39 | 45.01 |
| b3 | 3.55 | 17.50 | 36.66 | 45.76 | 55.91 | 58.36 | 59.17 | 60.78 | 62.73 | 65.04 |
| c3 | 0.91 | 13.67 | 39.44 | 47.89 | 58.84 | 61.59 | 62.88 | 63.64 | 64.56 | 65.85 |
| d3 | 1.02 | 15.70 | 45.81 | 53.44 | 61.04 | 62.20 | 66.28 | 72.18 | 72.85 | 73.71 |
| Sample Name | Total Intrusion Volume (mL/g) | Porosity (%) | Bulk Density (g/mL) | Apparent Density (g/mL) |
|---|---|---|---|---|
| a | 0.0525 | 11.9591 | 2.2780 | 2.5874 |
| b | 0.0336 | 5.0924 | 1.5150 | 1.5963 |
| c | 0.0153 | 3.0963 | 2.0252 | 2.0899 |
| d | 0.0177 | 3.4383 | 1.9417 | 2.0109 |
| a1 | 0.0903 | 0.3361 | 0.0372 | 0.0374 |
| b1 | 0.0352 | 0.1481 | 0.0421 | 0.0421 |
| c1 | 0.0306 | 0.1560 | 0.0510 | 0.0511 |
| d1 | 0.0243 | 0.1695 | 0.0698 | 0.0700 |
| a2 | 0.1081 | 18.0628 | 1.6707 | 2.0391 |
| b2 | 0.0598 | 10.4051 | 1.7400 | 1.9421 |
| c2 | 0.0294 | 5.0000 | 1.7023 | 1.7919 |
| d2 | 0.0265 | 5.5343 | 2.0871 | 2.2093 |
| a3 | 0.0499 | 8.9412 | 1.7935 | 1.9696 |
| b3 | 0.0454 | 8.0920 | 1.7812 | 1.9380 |
| c3 | 0.0326 | 5.6307 | 1.7264 | 1.8295 |
| d3 | 0.0299 | 5.5616 | 1.8621 | 1.9718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.-K.; Narendra Kumar, A.V.; Jeon, B.-H.; Kim, E.Y.; Yum, T.; Paeng, K.-J. Exploration of Zero-Valent Iron Stabilized Calcium–Silicate–Alginate Beads’ Catalytic Activity and Stability for Perchlorate Degradation. Materials 2022, 15, 3340. https://doi.org/10.3390/ma15093340
Jung Y-K, Narendra Kumar AV, Jeon B-H, Kim EY, Yum T, Paeng K-J. Exploration of Zero-Valent Iron Stabilized Calcium–Silicate–Alginate Beads’ Catalytic Activity and Stability for Perchlorate Degradation. Materials. 2022; 15(9):3340. https://doi.org/10.3390/ma15093340
Chicago/Turabian StyleJung, Yu-Kyung, Alam Venugopal Narendra Kumar, Byong-Hun Jeon, Eun Young Kim, Taewoo Yum, and Ki-Jung Paeng. 2022. "Exploration of Zero-Valent Iron Stabilized Calcium–Silicate–Alginate Beads’ Catalytic Activity and Stability for Perchlorate Degradation" Materials 15, no. 9: 3340. https://doi.org/10.3390/ma15093340
APA StyleJung, Y.-K., Narendra Kumar, A. V., Jeon, B.-H., Kim, E. Y., Yum, T., & Paeng, K.-J. (2022). Exploration of Zero-Valent Iron Stabilized Calcium–Silicate–Alginate Beads’ Catalytic Activity and Stability for Perchlorate Degradation. Materials, 15(9), 3340. https://doi.org/10.3390/ma15093340








