Abstract
In this study, in situ TiO2 was grown on the surface of graphene by a facile sol–gel method to form black TiO2/graphene composites with highly improved photocatalytic activity. The combination of graphene and TiO2 was beneficial to eliminate the recombination of photogenerated electron holes. The self-doping Ti3+ was introduced, accompanied by the crystallization of amorphous TiO2, during the hydrogenation process. Consequently, the narrowed bandgap caused by self-doping Ti3+ enhanced the visible light absorption and thus made the composites appear black. Both of them improved the photocatalytic performance of the synthesized black TiO2/graphene composites. The band structure of the composite was analyzed by valence band XPS, revealing the reason for the high visible light catalytic performance of the composite. The results proved that the black TiO2/graphene composites synthesized show attractive potential for applications in environmental and energy issues.
1. Introduction
Titanium dioxide (TiO2) with good photoelectric properties is generally regarded as an important photocatalyst [1,2]. Typically, TiO2 has four crystal phases, in which the anatase and rutile TiO2 are the most common and have been extensively investigated due to their excellent photoactivity [3,4]. However, their large band gap (rutile at ~3.0 eV and anatase at ~3.2 eV) severely limits the activity to the ultraviolet (UV) region of light. Typically, less than 5% of the entire solar energy is used for TiO2 photocatalysts [5,6]. Moreover, the photogenerated electron-hole pairs in TiO2 will recombine during the conduction process instead of participating in the photocatalytic reaction. This recombination of the electron hole would reduce the quantum efficiency that weakens the photocatalytic efficiency [4]. Therefore, great efforts have been made to shorten the absorption range of light and reduce the recombination of the photogenerated electron holes.
Over decades, the improvement of the photocatalytic activity of TiO2 has typically been achieved through appropriate structural design, synthesis of metal and non-metal element doping and semiconductor composite materials [7,8,9,10]. In recent years, some nanomaterials, such as carbon nanotubes, g-C3N4 and graphene with excellent electrical conductivity, have been recognized as attractive composite materials for improving the photocatalytic activity of TiO2 [11,12,13]. Notably, many reports have revealed that the excellent conductivity of graphene is conducive to the transfer of electrons. Therefore, the contact between TiO2 and graphene can significantly promote the recombination of photogenerated electron holes, thereby improving photocatalytic performance [14,15]. However, there are still some issues for the TiO2-graphene composites, such as their low visible light utilization [16,17]. In 2011, Chen et al. synthesized black TiO2 nanoparticles with a long wavelength absorption and substantial visible light photocatalytic activities by the hydrogenation method [18]. These remarkably changed properties made the black TiO2/graphene composite a promising candidate for the development of photocatalytic performance.
In this paper, in situ amorphous TiO2 was grown on the surface of graphene by a facile sol–gel method to form a series of black TiO2/graphene composites. The synthesized photocatalyst showed a narrow band gap and excellent photocatalytic performance. The influence of graphene on the photocatalytic activity of black TiO2/graphene composites has been systematically investigated by the degradation of methyl blue.
2. Materials and Methods
2.1. Synthesis of Black TiO2/Graphene Composites
Typically, the black TiO2/graphene composites were synthesized by a versatile sol–gel method, as shown in Figure 1. Tetrabutyl titanate (TBOT) (98.0%, Sinopharm Group Chemical Reagent Co., Ltd., Tianjin, China) and graphene (Strem Chemicals, Inc., Newburyport, MA, USA) were used as starting materials. Firstly, 5 mL TBOT, 250 mL C2H5OH (Eth), and various ratios of graphene were well mixed to obtain a mixture solution. The polyethylene glycol (PEG) was used as surfactant for enhancing the surface bonding between TiO2 and graphene. Then, the mixture solution was slowly dripped into a solution of 250 mL C2H5OH and 250 mL H2O while stirring. Through this process, the hydrolysis and polymerization of TBOT and H2O occurred to form a sol [19,20]. The overall reaction process is as follows,
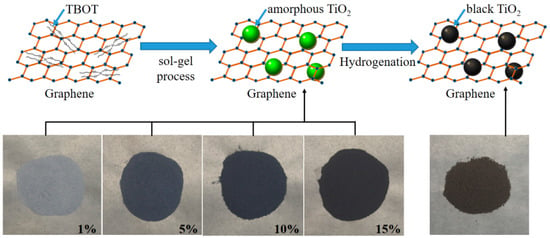
Figure 1.
Schematic diagram showing synthetic procedure of black TiO2/graphene composites.
The above reaction was held for 1 h and then precipitated for 3 h. The precipitate was centrifuged and washed 3 times with ethanol. Subsequently, the amorphous TiO2/graphene composites were obtained after drying at 80 °C for 6 h and calcining at 200 °C for 2 h in air. As shown in Figure 1, the obtained composites were gradually darkened in color with various graphene contents (1 wt%, 5 wt%, 10 wt%, 15 wt%). Finally, the amorphous TiO2/graphene composites were calcined in H2 flow at 500 °C for 2 h under atmospheric pressure. Then the black TiO2/graphene composites (denoted as BTG-1, BTG-5, BTG-10, BTG-15 corresponding to their various graphene contents of 1 wt%, 5 wt%, 10 wt%, 15 wt%, respectively) were synthesized.
2.2. Characterization
The morphology, structure and element distribution of the black TiO2/graphene composites were examined by high-resolution transmission electron microscope (HRTEM, Tecnai G2 20, Hillsboro, OR, USA). The crystal structure of composites was detected by X-ray diffraction (XRD) on Rigaku D/MAX-2400 (Tokyo, Japan). In order to confirm the chemical compositions and band status of the composites, XPS spectrum was characterized on AXIS-Ultra DLD-600 W (Manchester, UK). The state of the graphene was characterized by Raman spectroscopy with LabRAM HR800 (Piscataway, NJ, USA) using laser excitation at 532 nm. In order to examine the light absorption range of the composites, UV–Vis absorption spectra examinations were performed on a Shimadzu UV-3600 Plus (Tokyo, Japan) UV-VIS-NIR Spectrophotometer.
The photocatalytic activity of the composites was determined in the decomposition of the methyl blue (MB). The 10 mg/L MB solution was prepared to test the visible light catalytic performance of the as-synthesized composites (BTG). A sample of 10 mg BTG was added to 100 mL MB solution and stirred in the dark for 30 min to achieve adsorption/desorption equilibrium. The visible light irradiation of the photocatalysis experiment was from a 300 W halogen tungsten lamp with a cut-off filter (λ > 420 nm). The reaction solution was controlled at 20 °C with a water-cooling system. In the photocatalysis experiment, 3 mL of reaction solution was taken every 10 min, and the catalyst was removed by centrifugation (10,000 rpm). The concentrations of residual MB were analyzed by the absorption band maximum (660 nm).
3. Results and Discussion
The XRD patterns of TiO2/graphene composites before and after the hydrogenation process are shown in Figure 2A. The XRD patterns of the TiO2/graphene composites before the hydrogenation process show only some diffuse peaks, indicating that the composites before hydrogenation are amorphous. After the hydrogenation process, the peaks occur at 25.28° (101), 37.80° (004), 48.05° (200), 53.89° (105), 55.07° (211), 62.69° (204), 68.93° (116), 70.31° (220), and 75.03° (215) (Figure 2A(b)), corresponding to the diffractions of anatase TiO2 (JCPDS 21-1272) [19,21]. The results indicate that the samples after surface hydrogenation were crystallized from amorphous to anatase structure, and the average crystal size was approximately 21 nm calculated by Scherrer formula, in agreement with TEM observation.
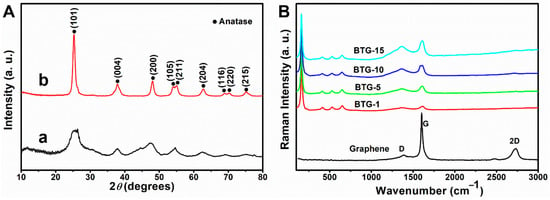
Figure 2.
(A) XRD patterns of TiO2/graphene composites (a) before and (b) after hydrogenation process. (B) Raman spectra of black TiO2/graphene composites with different graphene contents.
The structure of black TiO2/graphene composites can also be characterized by Raman spectra. The Raman spectra of the BTG with various graphene contents are shown in Figure 2B. The three bands at around 1365 cm−1 (D band), 1580 cm−1 (G band) and 2700 cm−1 (2D band) correspond to graphene [22]. For all the BTG samples, the Raman peaks occurred at around 156 cm−1 (Eg(1)), 406 cm−1 (B1g(1)), 523 cm−1 (A1g + B1g(2)), and 646 cm−1 (Eg(2)), which matched with the characteristic peaks of anatase TiO2 [23]. Compared with the characteristic peaks of anatase TiO2, the Eg(1) mode shifted from 144 cm−1 of bare bulk TiO2 to 156 cm−1 of BTG. The shift toward high frequency indicated the ultra-dispersed characteristics of the TiO2 nanoparticles and their combination with graphene, and the disappearance of the graphene 2D band in the BTG may be attributed to the composite of graphene and TiO2. From the Raman analysis, the characteristic peaks of TiO2 and graphene appeared in the spectra of BTG, indicating that the black TiO2/graphene composites were successfully synthesized.
The XPS spectra of the black TiO2/graphene composites are shown in Figure 3A. The characteristic peaks of C 1s, Ti 2p, and O 1s were present at 284.6, 457.8, and 529.7 eV, respectively. In the Ti 2p XPS spectrum (Figure 3B), the Ti 2p3/2 and Ti 2p1/2 of TiO2 were revealed at 457.8 and 463.3 eV. The Ti 2p3/2 peak of the BTG shifted from 458.6 eV to a lower binding energy corresponding to the presence of a high Ti3+ concentration [24,25,26,27]. All Ti 2p spectra were symmetrical on the low energy side, indicating that TiO2 was not doped with carbon. The curve fit of C 1s spectra of BTG is shown in Figure 3C. The peak at 284.5 eV was ascribed to the C=C/C-C bond, indicating the presence of graphene. The weak peak at 286.5 confirmed the presence of the C-O bond. In addition, there was no Ti-C peak observed in Figure 3B,C, which confirms that graphene does not exist as a dopant in BTG composites. The curve fit of O 1s spectra of BTG is shown in Figure 3D. The peak at 529.6 and 532.2 eV were ascribed to Ti-O and C-OH bonds. In Figure 3C,D, the appearance of C-O and C-OH bonds indicated the existence of a bond between carbon and oxygen atoms in BTG. This phenomenon is attributed to the oxidation of graphene since TiO2 is a well-known catalyst.
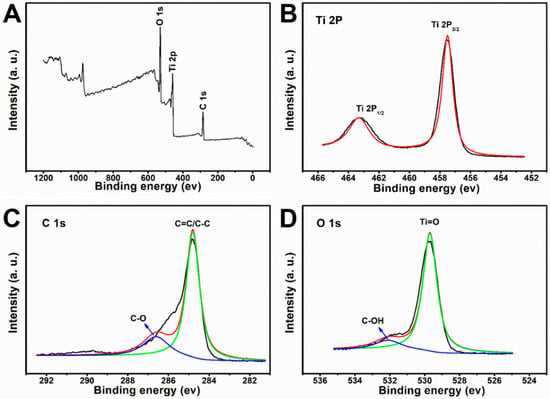
Figure 3.
XPS spectra of black TiO2/graphene composites. (A) Full survey, (B) Ti 2p spectra. (C) C 1s spectra. (D) O 1s spectra.
The selected area electron diffraction (SAED) pattern is shown in the inset of Figure 4A. The (101), (004), (200), (204) and (105) diffraction rings detected in the SAED pattern indicated that TiO2 in the composites was anatase structure. The SAED result was consistent with the XRD characterization, which indicated that the TiO2 in the composite was anatase structure with excellent photocatalytic activity. Figure 4B shows the HRTEM image of black TiO2/graphene composites. It can be seen that the size of individual TiO2 nanocrystals was approximately 15 nm in diameter. There was a disordered surface layer surrounding the TiO2 nanocrystal, as shown by the dotted red circle in Figure 4B. The thickness of the disordered layer is ~1 nm, which is consistent with the black TiO2 reported by Chen et al. [18]. The disordered layer surrounding the TiO2 nanocrystal was created by hydrogenation, which caused a significant color change and enhancement of visible light photocatalytic activity. The schematic diagram of the sample color change (from blue to black) after hydrogenation is shown in Figure 1. The inset in Figure 4B shows that the interplanar spacing was 3.58 Å, corresponding to the (101) plane of anatase TiO2. The energy dispersive X-ray (EDX) elemental mappings of Ti, C, O taken from the STEM image of Figure 4C are given in Figure 4D–F, respectively. It can be seen from the figures that the Ti and O elements were uniformly aggregated and dispersed on the C element of graphene, which was consistent with the TEM result of Figure 4A. The results further demonstrate the successful assembly of TiO2 on graphene in the black TiO2/graphene composites.
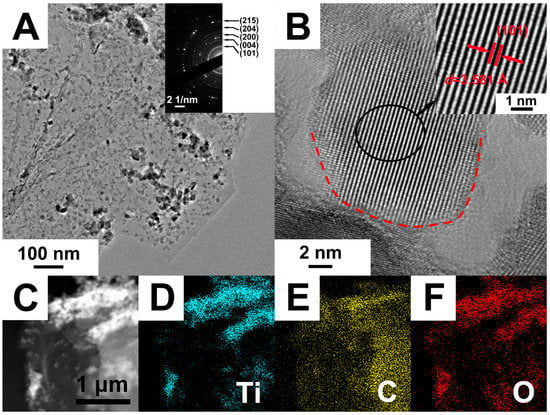
Figure 4.
(A) TEM, (B) HRTEM and (C) STEM images of black TiO2/graphene composites. Elemental mapping of (D) titanium, (E) carbon and (F) oxygen taken from the whole area of (C).
Figure 5A shows the UV–vis diffuse reflectance spectra (DRS) of the black TiO2/graphene composites. The presence of graphene significantly improved the visible light absorption of the black TiO2/graphene composites. The visible light absorption intensity of the composites was enhanced with increasing graphene content. In order to characterize the band gaps of the composites, the Kubelka–Munk function (F(R∞)·E)1/n versus the energy of light (E = hv) is shown in Figure 5B. For an indirect transition of anatase TiO2, n = 2 will give the best linear fit. As the graphene content increased from 1% to 15%, the band gaps were estimated roughly to decrease from 3.05 to 2.94 eV. It is well known that the band gap energy of anatase TiO2 is 3.2 eV. The band gap around 3 eV of the composites was lower than that of anatase TiO2, which was attributed to the self-doping of Ti3+. Moreover, the composites had enhanced light absorption in the range of visible light, which was consistent with the darker sample color with increasing graphene content. The results suggest that both graphene combination and self-doping of Ti3+ play a crucial role in the photocatalytic activity of the composites. Figure 5C illustrates the normalized MB concentration in the degradation solution as a function of visible light irradiation time. After a visible light irradiation time of 60 min, 95, 98, 99 and 96% of MB was decomposed in the presence of the BTG-1, BTG-5, BTG-10 and BTG-15, respectively. A comparative experiment without catalyst during visible light irradiation exhibited only 15% of MB decomposition. The photocatalytic process follows first-order kinetics, c = c0exp(-kt), where c0 and c are the MB concentration before and after visible light irradiation, respectively. The k value in the formula represents the photocatalytic reaction rate. Through fitting calculation, the photocatalytic reaction rates k for BTG-1, BTG-5, BTG-10 and BTG-15 were determined to be 2.88, 3.61, 3.99 and 3.23 h−1, respectively. The BTG-10 exhibited the highest photocatalytic activity. To determine the recyclability of the composites, the BTG-10 was recycled under several visible light irradiation cycles. As shown in Figure 5D, the degradation rate of MB still reached 90% after five cycles in the presence of the BTG-10, indicating that the catalyst had good stability.
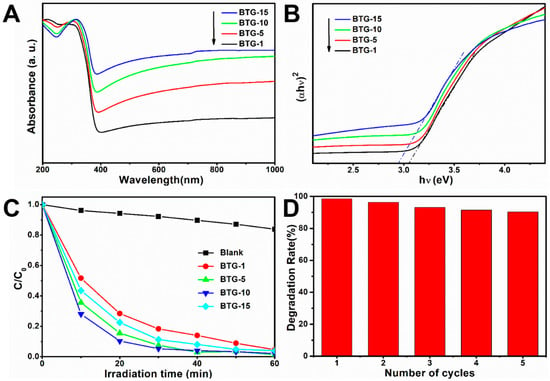
Figure 5.
(A) UV–vis diffuse reflectance spectra of black TiO2/graphene composites, (B) the Kubelka–Munk function versus the energy of light, (C) photocatalytic degradation of methylene blue (MB) under visible light (λ > 420 nm), and (D) cycle test of the samples’ degradation of MB.
Since BTG-10 had the best catalytic activity of the TiO2/graphene composites, BTG-10 was selected for valence band (VB) XPS analysis in Figure 6A. The VB of BTG-10 is located at 2.68 eV, which is lower than the commonly used TiO2 (3.0 eV). The insets in Figure 6A show the energy band diagrams. According to the energy band model, the conductance band (CB) can be calculated by CB = VB − Eg, where Eg represents the energy of the band gaps. The Eg of BTG-10 was estimated to be 3.0 eV by Figure 5B, and the CB of BTG-10 was calculated as −0.32 eV. The results suggest that the disordered surface layer surrounding the TiO2 nanocrystal introduced by hydrogenation can upshift both the VB and CB edge of the TiO2/graphene composites. According to the energy band analyses, the VB of the BTG-10 was higher than that of the O2/H2O, and the CB was lower than H+/H2, potentially suggesting that the composites have attractive potential for applications in environmental and energy issues.
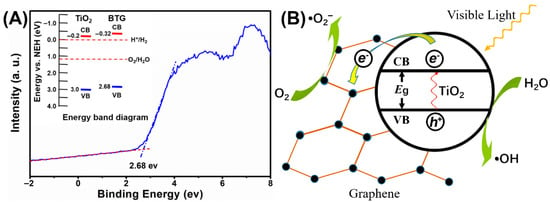
Figure 6.
(A) The valence band XPS spectra of BTG-10, and (B) the proposed photocatalytic mechanism of BTG-10.
The photocatalytic mechanism of the black TiO2/graphene composites is shown in Figure 6B. The disordered surface layer introduced by hydrogenation narrows the band gap of the composites, which improves the optical absorption properties. Consequently, the electrons in the VB can easily transit to the CB of TiO2 under visible light irradiation. It is well known that the graphene to which the nano-sized black TiO2 attached has good electrical conductivity [28]. Therefore, electrons will be transferred to graphene instead of CB, which is conducive to reducing the opportunities of electron-hole recombination and enhancing photocatalytic activity. However, the graphene itself has no photocatalytic activity, and excessive graphene will hinder the absorption of photons by TiO2. This hinder effect is the reason why BTG-10 had a higher catalytic activity than BTG-15.
4. Conclusions
A series of black TiO2/graphene composites with different graphene contents were successfully synthesized. In the sol–gel process, TiO2 was generated in situ on the surface of graphene from TBOT as a titanium source. The good conductivity of graphene is beneficial for eliminating the opportunity for photogenerated electron-hole recombination. In the hydrogenation process, self-doping Ti3+ was introduced, accompanying the crystallization of amorphous TiO2. The narrowed bandgap (2.94~3.05 eV) caused by self-doping Ti3+ enhanced the visible light absorption. Moreover, the nanostructured black TiO2-graphene composites showed enhanced visible light photocatalytic activity in methyl blue degradation. The sample with 10 wt% graphene showed the highest photocatalytic activity and good stability. The incorporation of black TiO2 caused by hydrogenation and graphene composite expanded the light absorption range and reduced the recombination of photogenerated electron holes, both of which enhanced the capacity of photodegrading organic dyes. Therefore, this work is expected to open up a new way for the synthesis of black TiO2/graphene composites, and its high photocatalytic activity proves that the black TiO2/graphene composites have attractive potential for applications in environmental and energy issues.
Author Contributions
Methodology, Z.L. (Zhaoqing Li) and Z.L. (Zhufeng Liu), writing—original draft preparation, Z.L. (Zhaoqing Li); writing—review and editing, X.Y., A.C., P.C. and L.Y.; project administration, C.Y., Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51805185), the Hubei Provincial technical innovation major program (No. 2017AAA109), the Wuhan Science and Technique Foundation (No. 2018010401011281), and Shenzhen Knowledge Innovation Program (No. 20180213102634650).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, L.; Tan, X.; Chen, X. Synthesis, properties, and applications of black titanium dioxide nanomaterials. Sci. Bull. 2017, 62, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Dette, C.; Pérezosorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, S.-W. Template-sacrificial conversion of MnCO3 microspheres to fabricate Mn-doped TiO2 visible light photocatalysts. Mater. Des. 2020, 189, 108497. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285, 13–28. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638. [Google Scholar] [CrossRef] [Green Version]
- Bouslama, M.; Amamra, M.C.; Jia, Z.; Amar, M.B.; Chhor, K.; Brinza, O.; Abderrabba, M.; Vignes, J.L.; Kanaev, A. Nanoparticulate TiO2–Al2O3 Photocatalytic Media: Effect of Particle Size and Polymorphism on Photocatalytic Activity. ACS Catal. 2012, 2, 1884–1892. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Xie, Q.; Li, J.; Qiang, T.; Shi, R. Template-free synthesis of zinc citrate yolk–shell microspheres and their transformation to ZnO yolk–shell nanospheres. J. Mater. Chem. 2012, 22, 13541–13547. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A mini-review on the synthesis and structural modification of g-C3N4-based materials, and their applications in solar energy conversion and environmental remediation. Sustain. Energy Fuels 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Huang, Q.; Tian, S.; Zeng, D.; Wang, X.; Song, W.; Li, Y.; Xiao, W.; Xie, C. Enhanced Photocatalytic Activity of Chemically Bonded TiO2/Graphene Composites Based on the Effective Interfacial Charge Transfer through the C–Ti Bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, K.H.; Choi, H.O.; Jung, H.T. Highly photoactive, visible-light-driven graphene/2D mesoporous TiO2 photocatalyst. Green Chem. 2015, 17, 3972–3978. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Tang, Y.; Feng, X. Graphene encapsulated hollow TiO2 nanospheres: Efficient synthesis and enhanced photocatalytic activity. J. Mater. Chem. A 2013, 1, 3752–3756. [Google Scholar] [CrossRef]
- Zhu, P.; Nair, A.S.; Shengjie, P.; Shengyuan, Y.; Ramakrishna, S. Correction to “Facile Fabrication of TiO2-Graphene Composite with Enhanced Photovoltaic and Photocatalytic Properties by Electrospinning”. ACS Appl. Mater. Interfaces 2012, 4, 581–585. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Wang, L.; Wang, J.; Guo, Q.; Li, J. A facile method for the structure control of TiO2 particles at low temperature. Appl. Surf. Sci. 2015, 355, 1051–1056. [Google Scholar] [CrossRef]
- Hague, D.C.; Mayo, M.J. Controlling crystallinity during processing of nanocrystalline titania. J. Am. Ceram. Soc. 1994, 77, 1957–1960. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Feng, S.; Wang, J.; Sun, Z.; Li, B.; Li, Y.; Yang, J.; Elzatahry, A.A.; Xia, Y. Sol-gel design strategy for ultradispersed TiO2 nanoparticles on graphene for high-performance lithium ion batteries. J. Am. Chem. Soc. 2013, 135, 18300–18303. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Z.; Li, J.; Qiang, W.; Wei, Z.; Tian, G.; Kai, P.; Tian, C.; Zou, J.; Fu, H. A Floating Porous Crystalline TiO2 Ceramic with Enhanced Photocatalytic Performance for Wastewater Decontamination. Eur. J. Inorg. Chem. 2013, 2013, 2411–2417. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, Y.; Zhang, J.; Wei, J.; Huang, P.; Zhang, X.; Feng, B. Preparation of carbon quantum dots/TiO2 nanotubes composites and their visible light catalytic applications. J. Mater. Chem. A 2014, 2, 18082–18086. [Google Scholar] [CrossRef]
- Zhou, G.; Shen, L.; Xing, Z.; Kou, X.; Duan, S.; Fan, L.; Meng, H.; Xu, Q.; Zhang, X.; Li, L. Ti3+ self-doped mesoporous black TiO2/graphene assemblies for unpredicted-high solar-driven photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2017, 505, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yip, C.T.; Wang, L.; Huang, H.; Zhou, L. Hydrogenated TiO2 Nanotube Arrays as High-Rate Anodes for Lithium-Ion Microbatteries. Chempluschem 2012, 77, 991–1000. [Google Scholar] [CrossRef]
- Pan, J.; Dong, Z.; Wang, B.; Jiang, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The enhancement of photocatalytic hydrogen production via Ti3+ self-doping black TiO2/g-C3N4 hollow core-shell nano-heterojunction. Appl. Catal. B 2019, 242, 92–99. [Google Scholar] [CrossRef]
- Xiong, R.; Hu, K.; Grant, A.M.; Ma, R.; Xu, W.; Lu, C.; Zhang, X.; Tsukruk, V.V. Ultrarobust Transparent Cellulose Nanocrystal-Graphene Membranes with High Electrical Conductivity. Adv. Mater. 2016, 28, 1501–1509. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).