Influence of Al/Ti Ratio and Ta Concentration on the As-Cast Microstructure, Phase Composition, and Phase Transformation Temperatures of Lost-Wax Ni-Based Superalloy Castings
Abstract
:1. Introduction
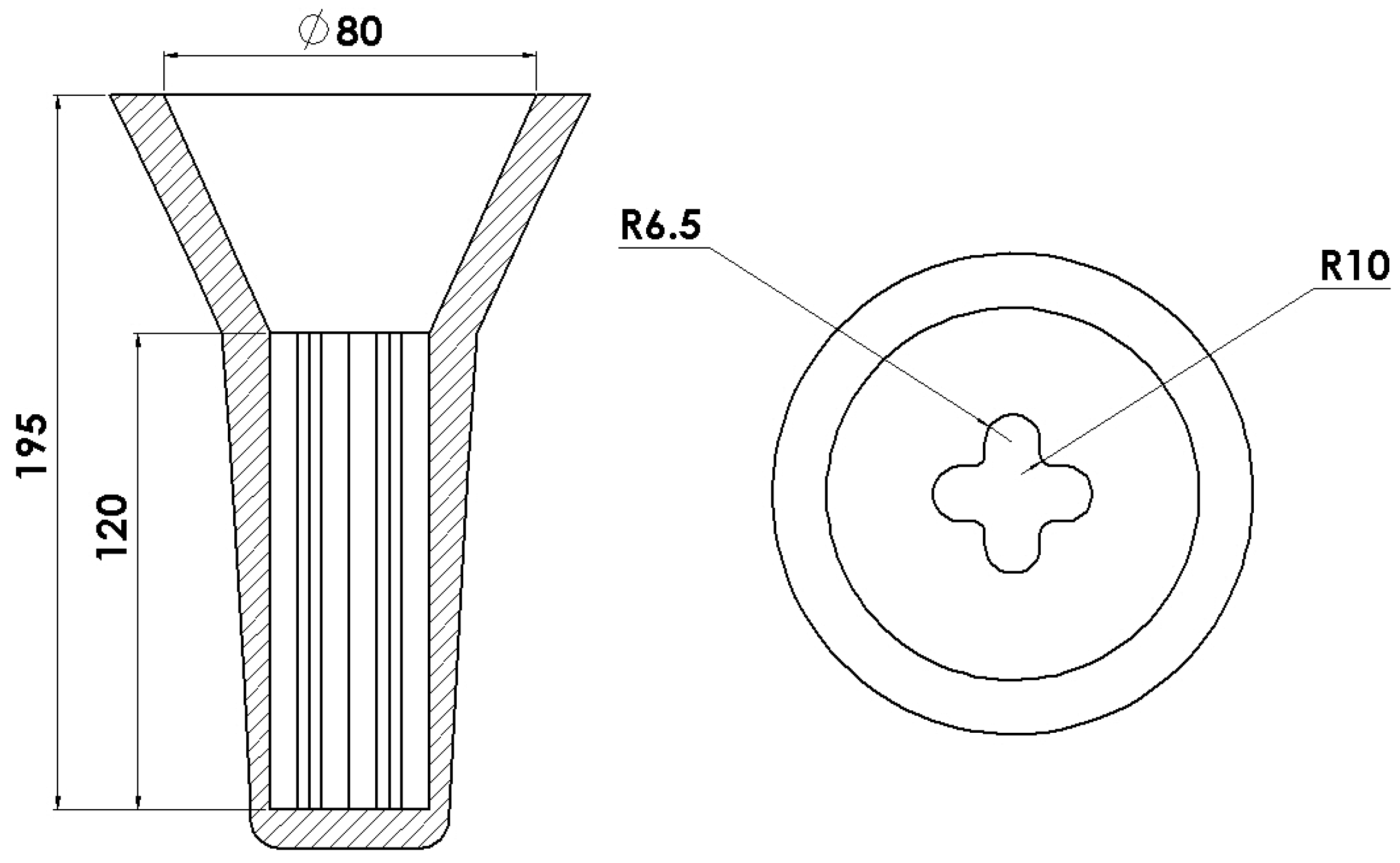
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
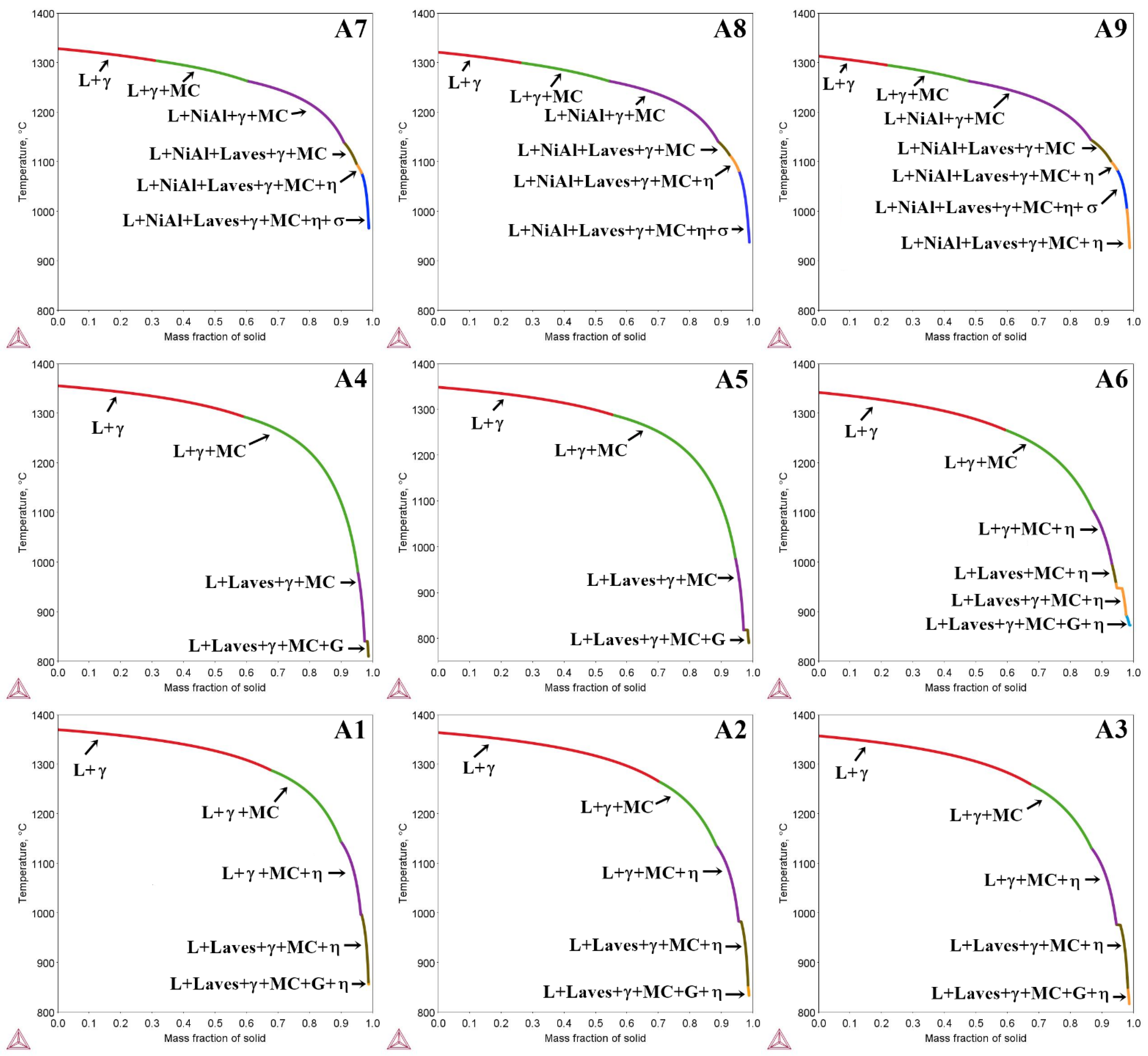
3.1. Solidification Simulation via Thermo-Calc
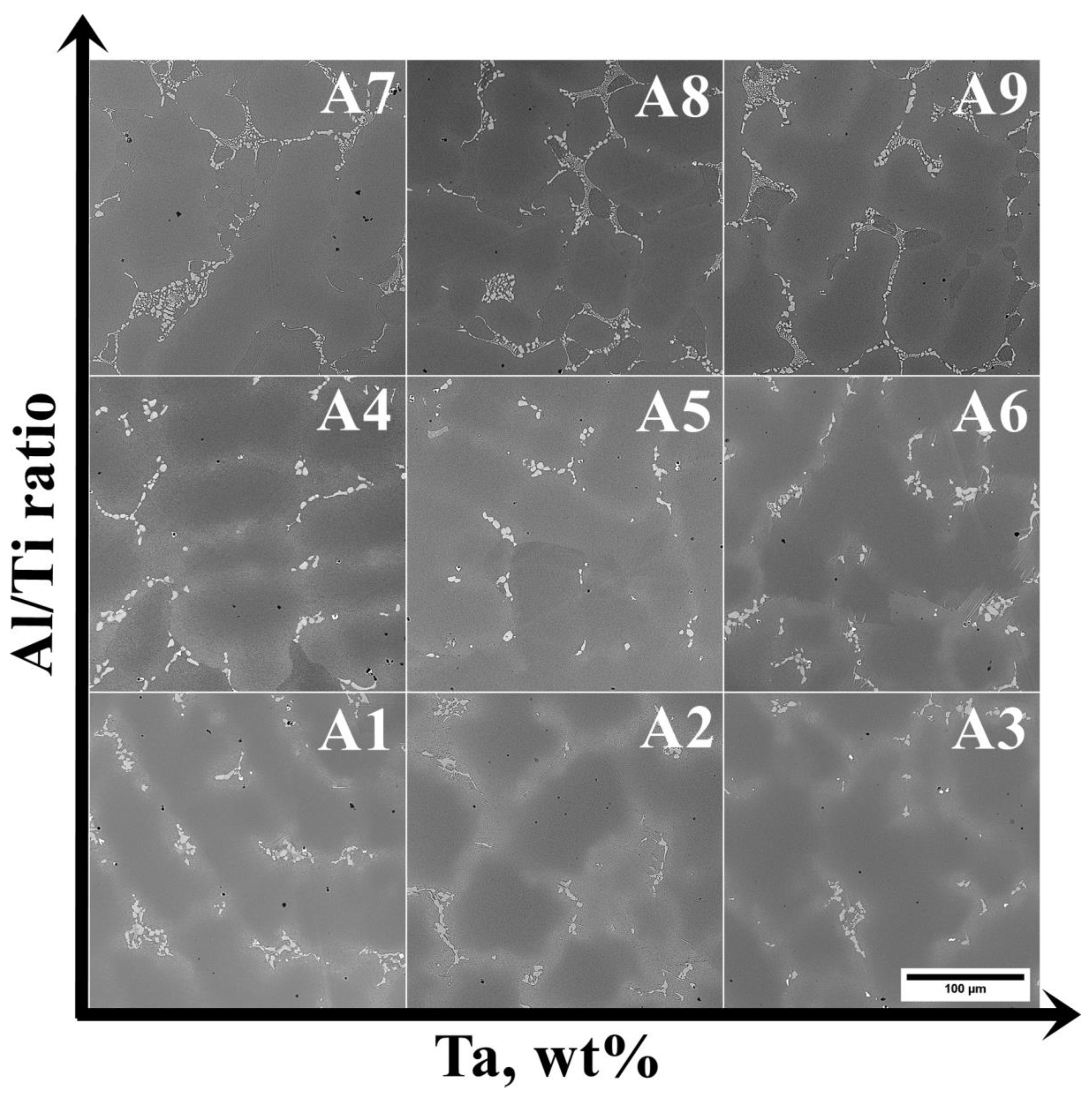
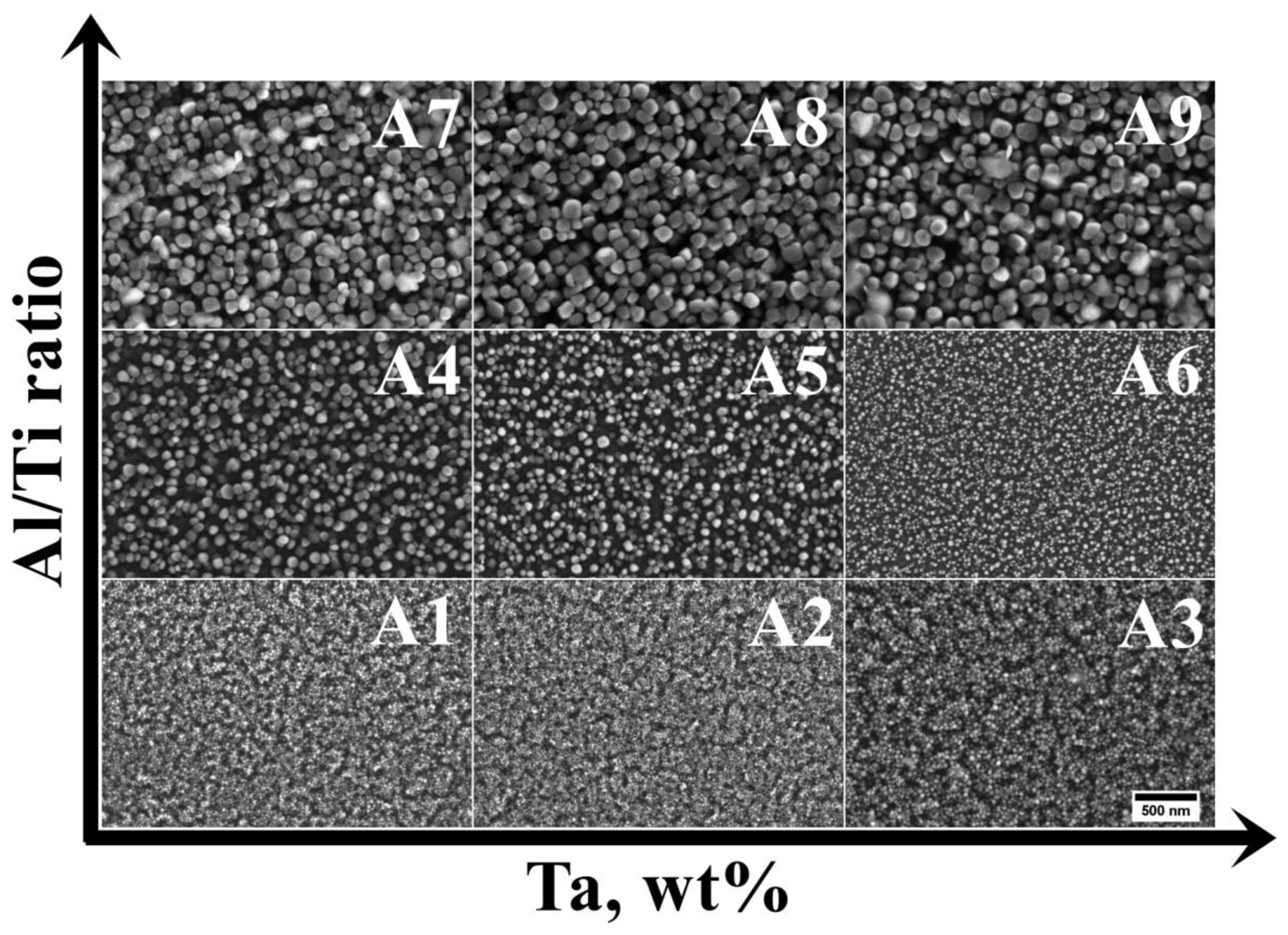
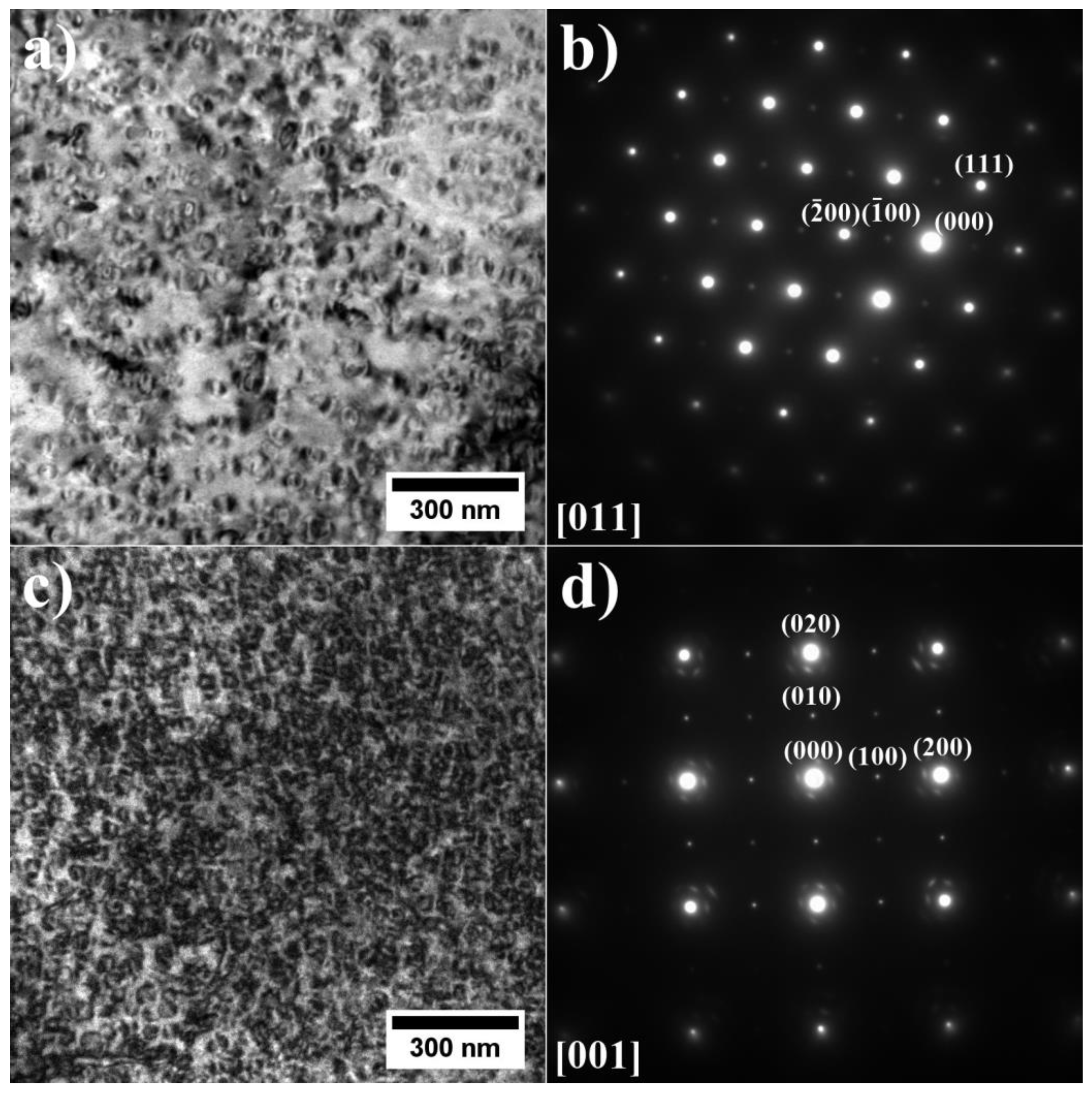
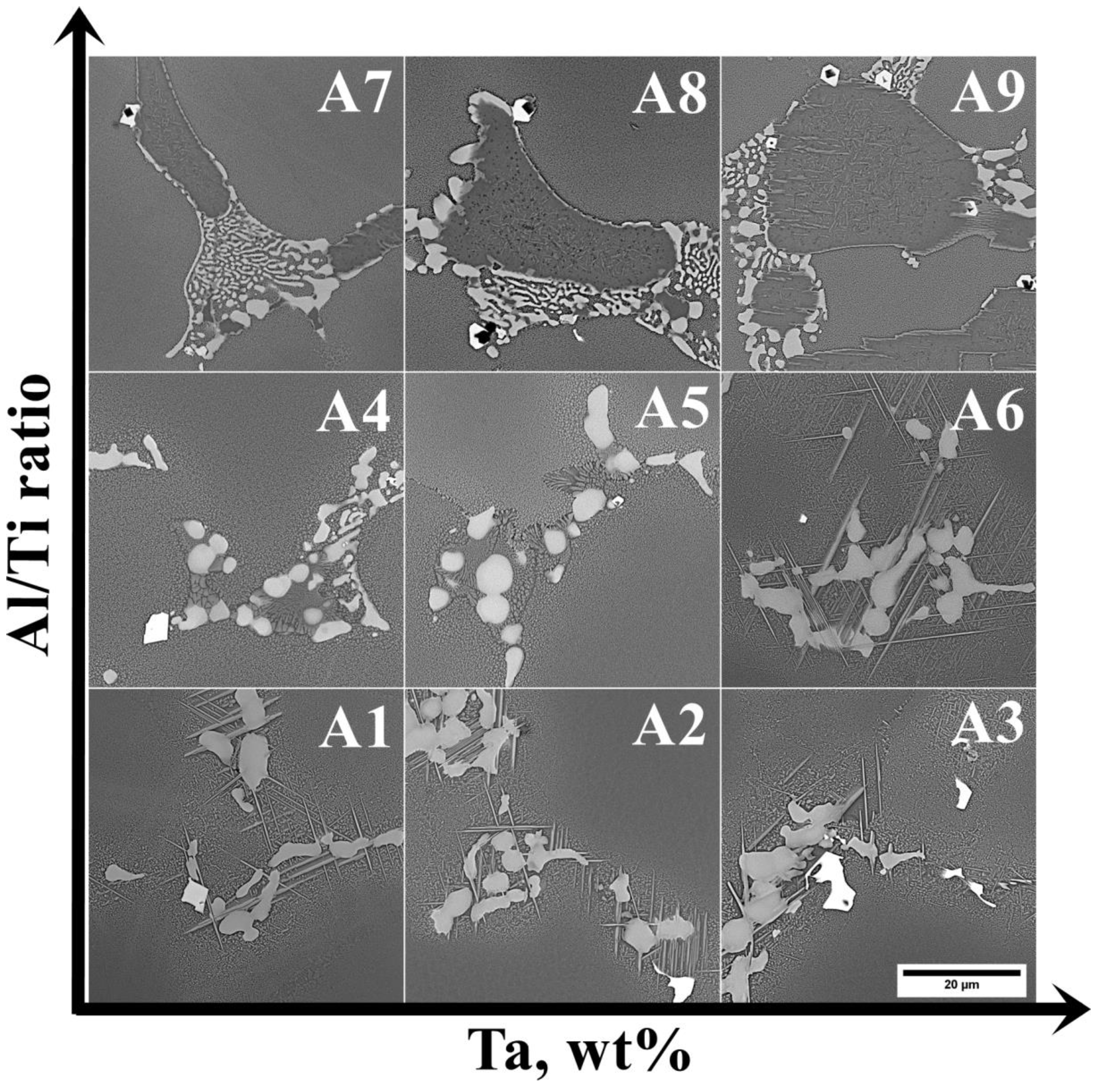
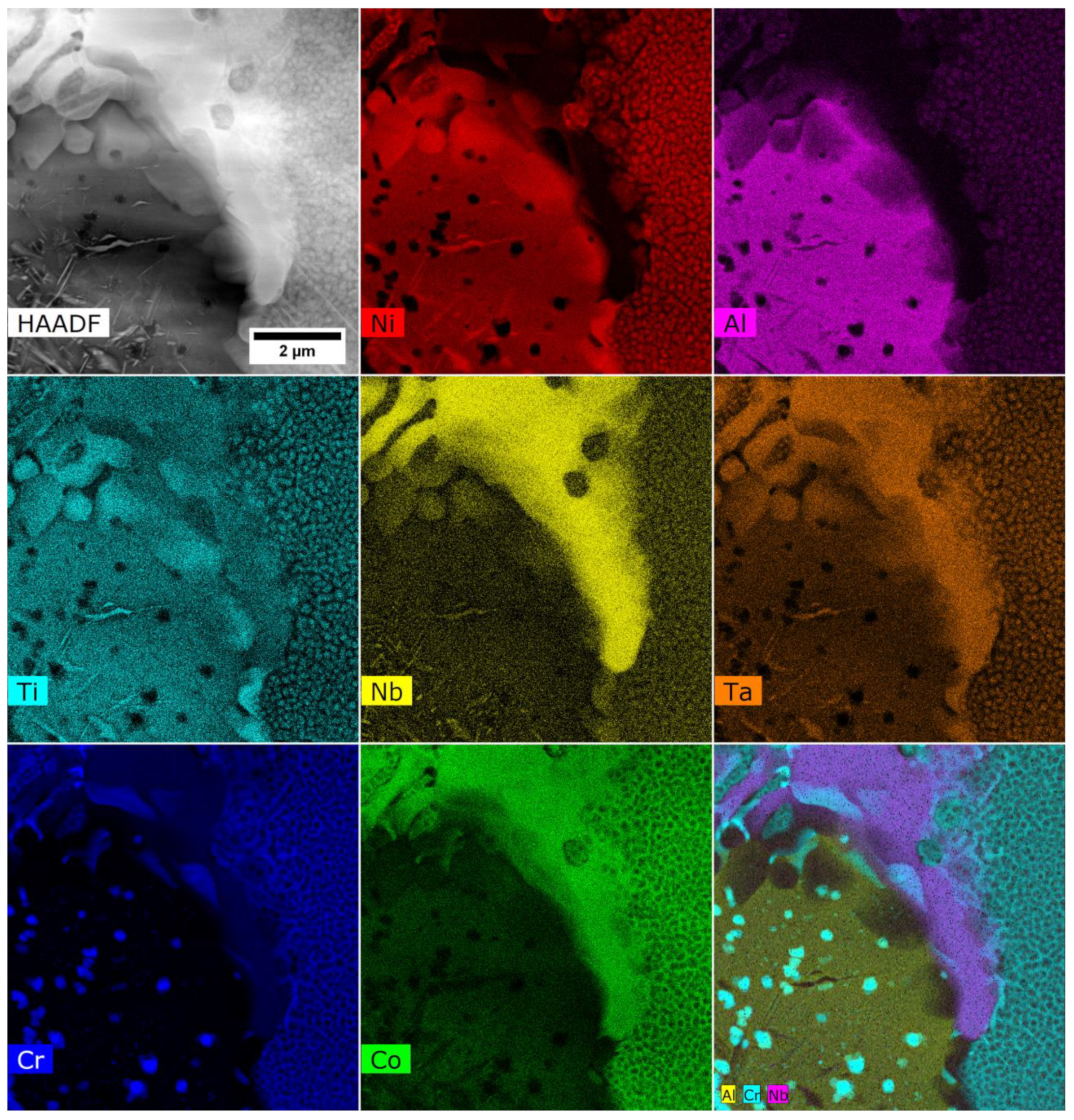
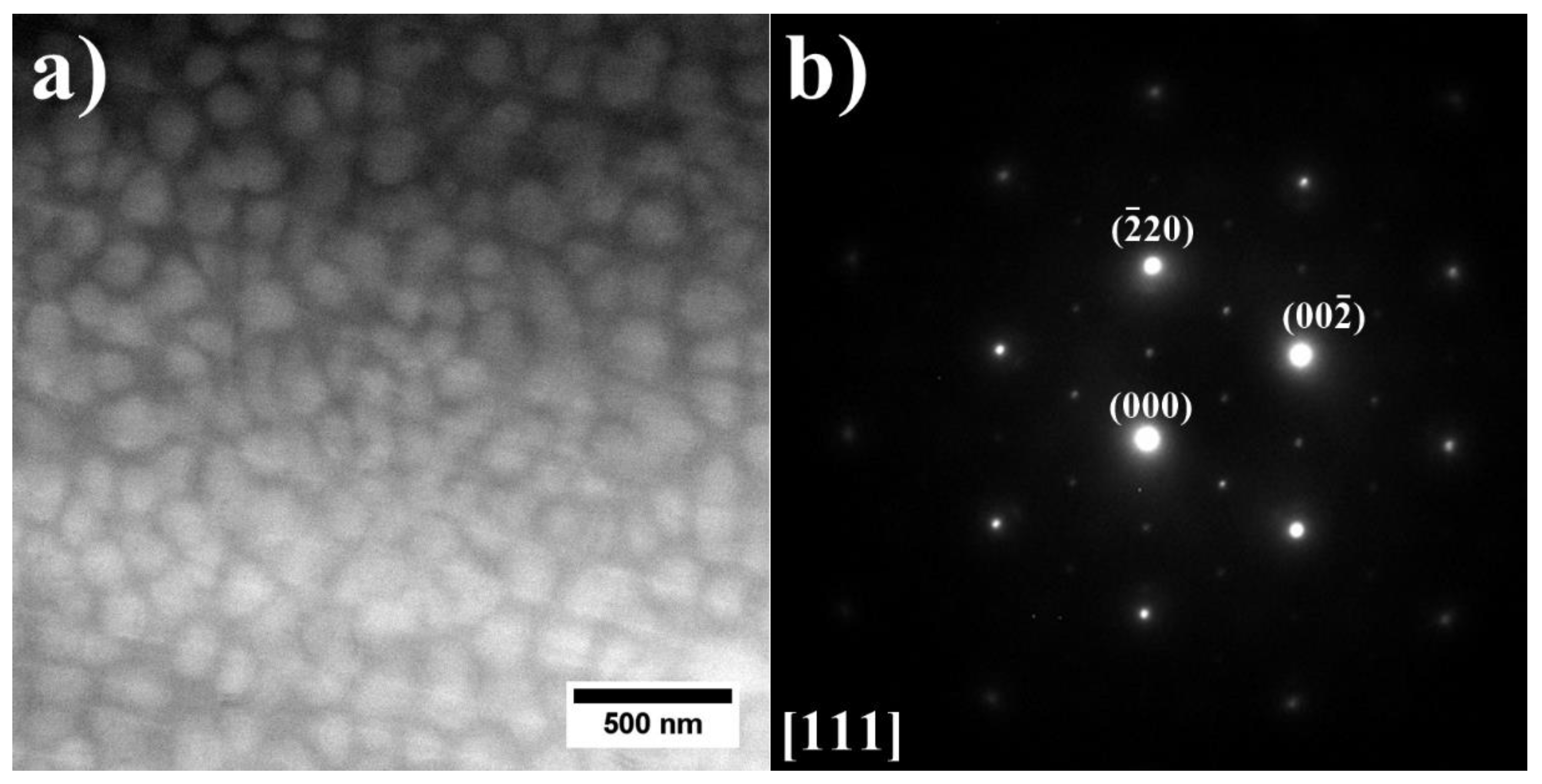
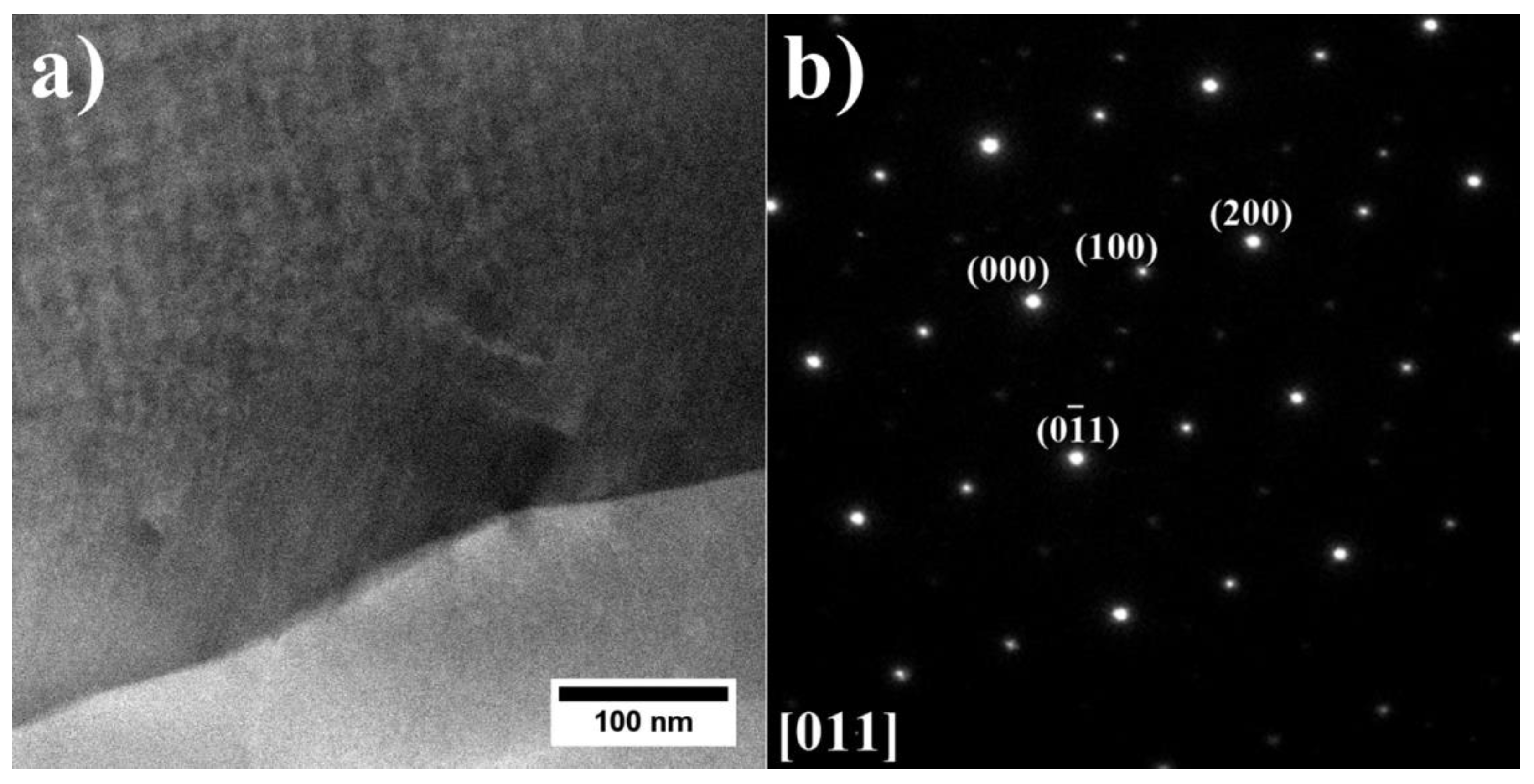
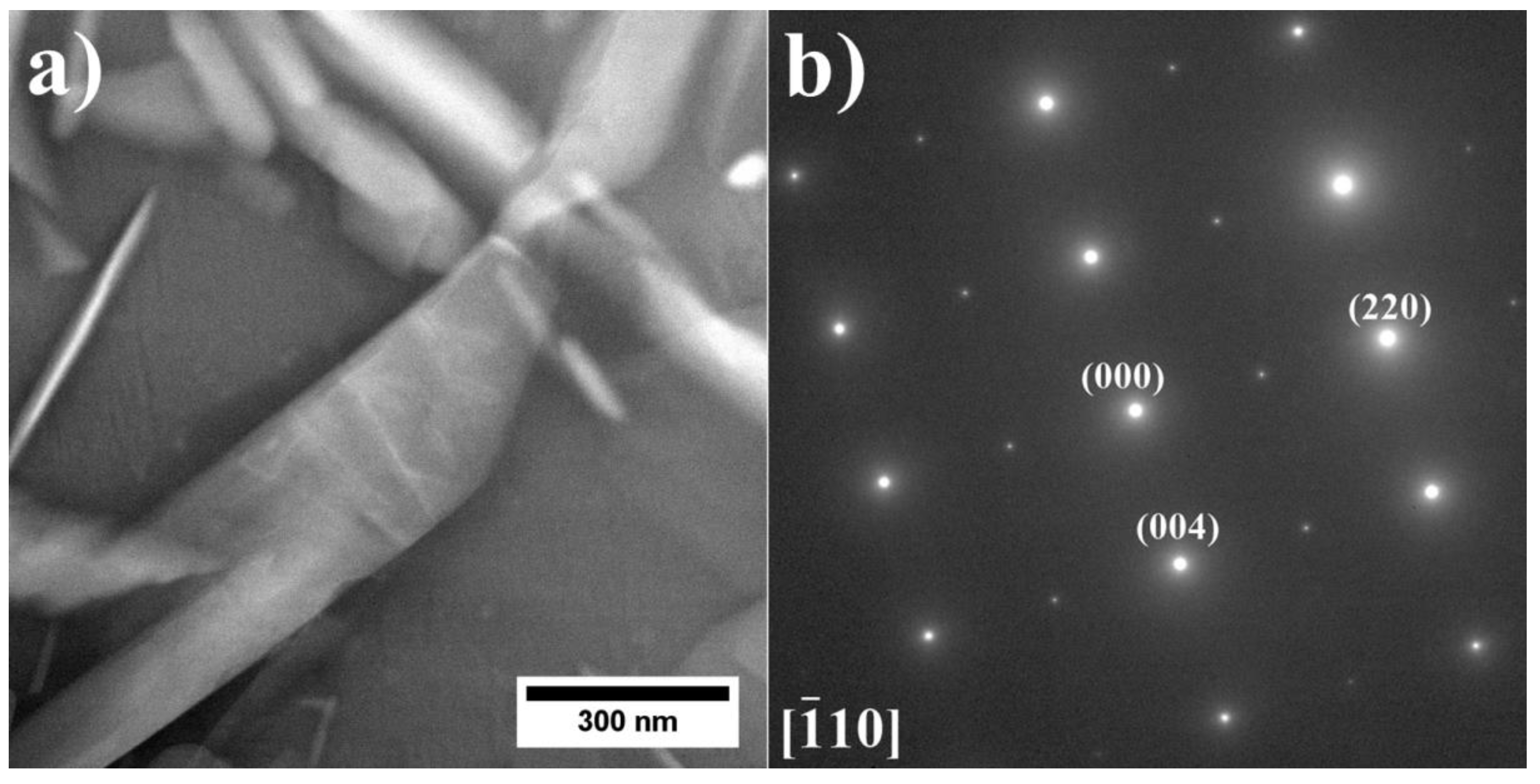
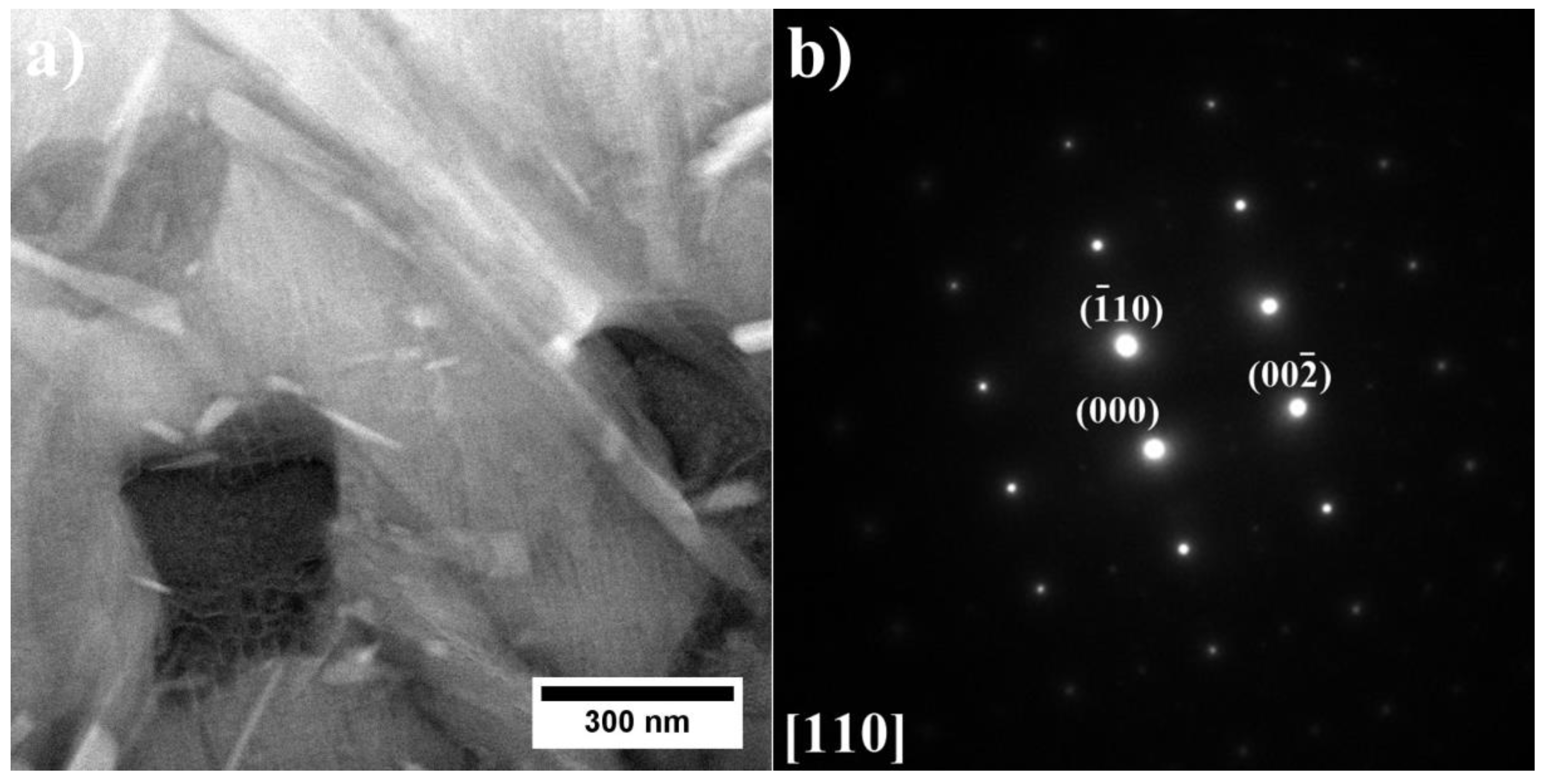
3.2. Analysis of the Microstructure of the A1–A9 Superalloys in As-Cast Condition
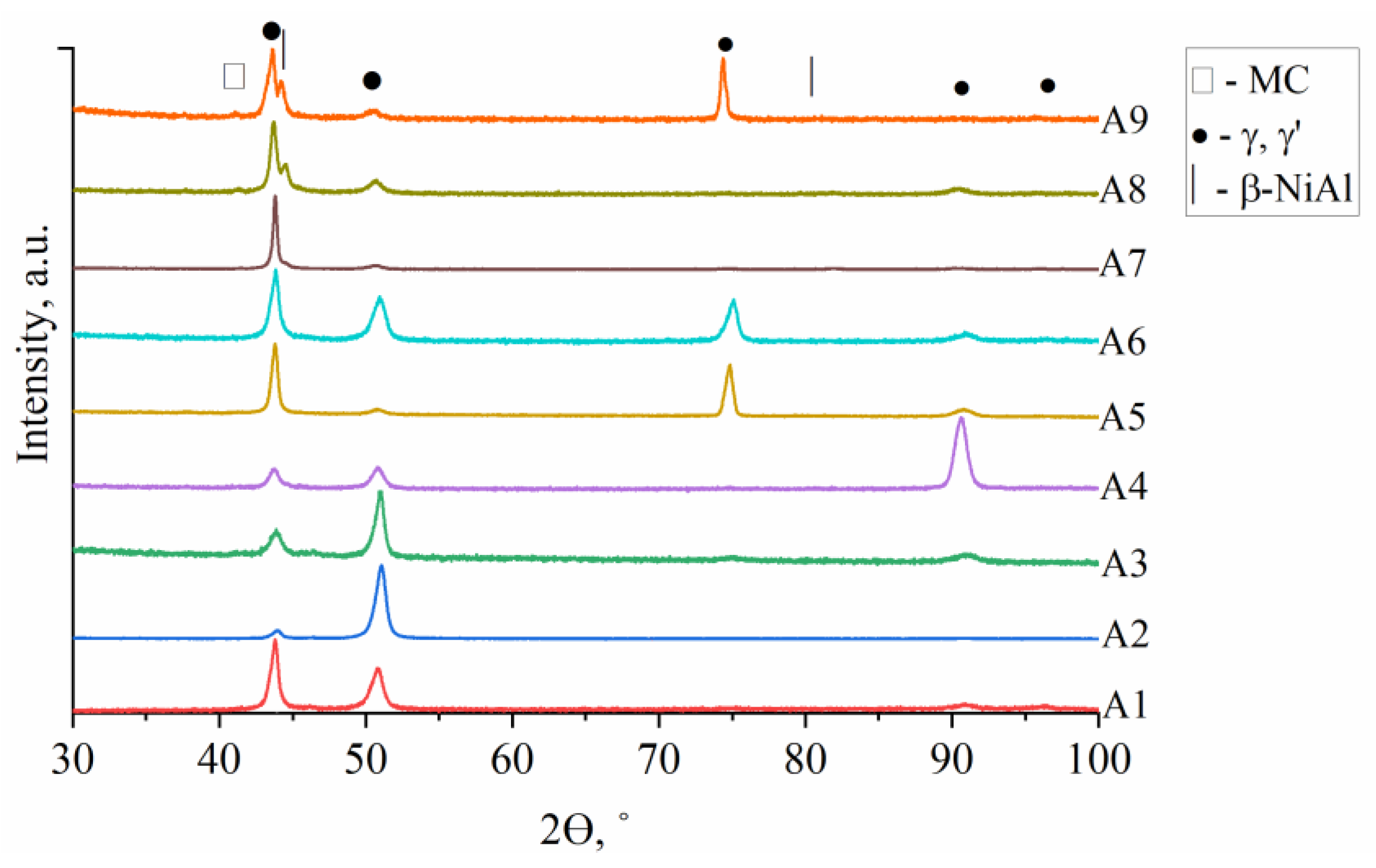
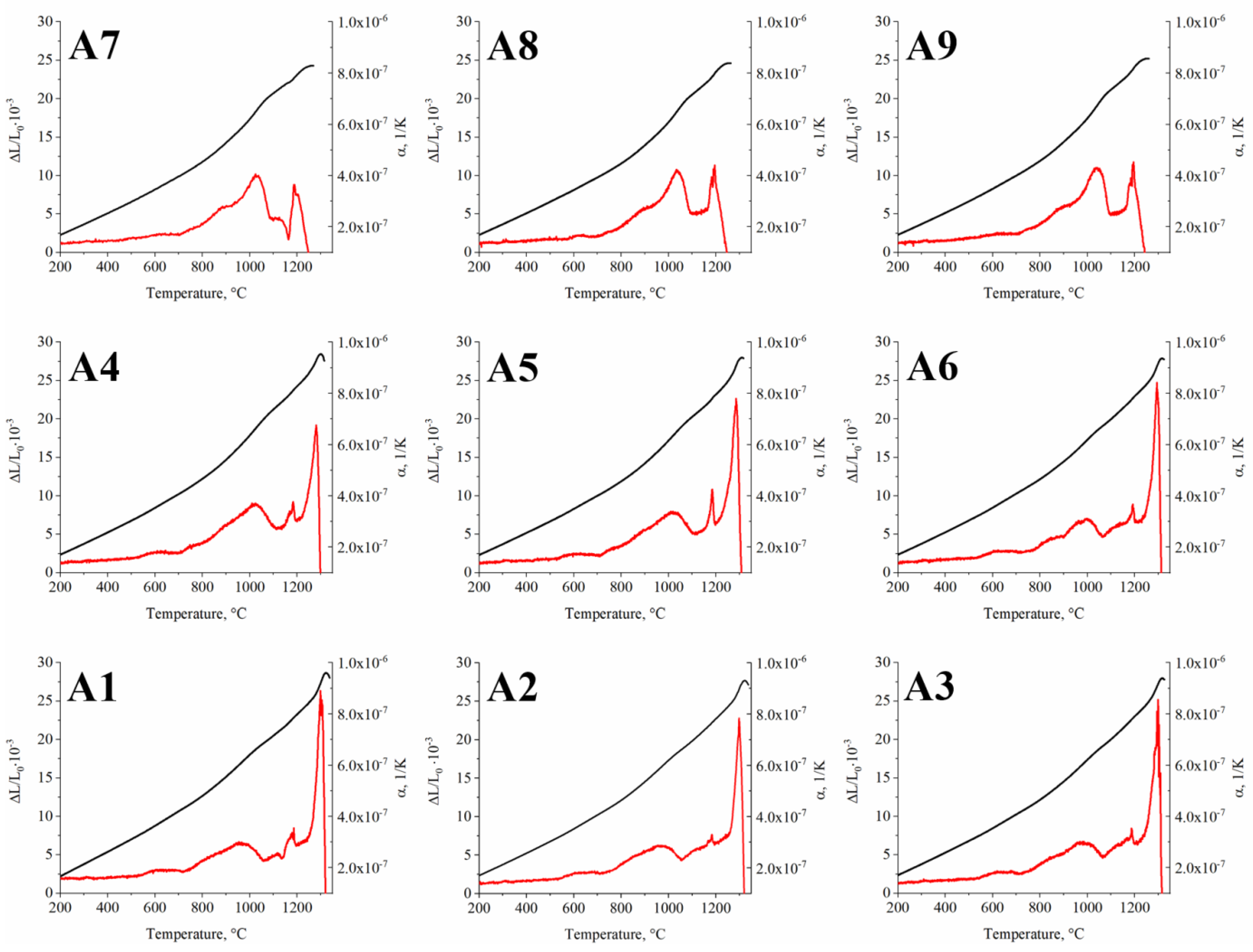
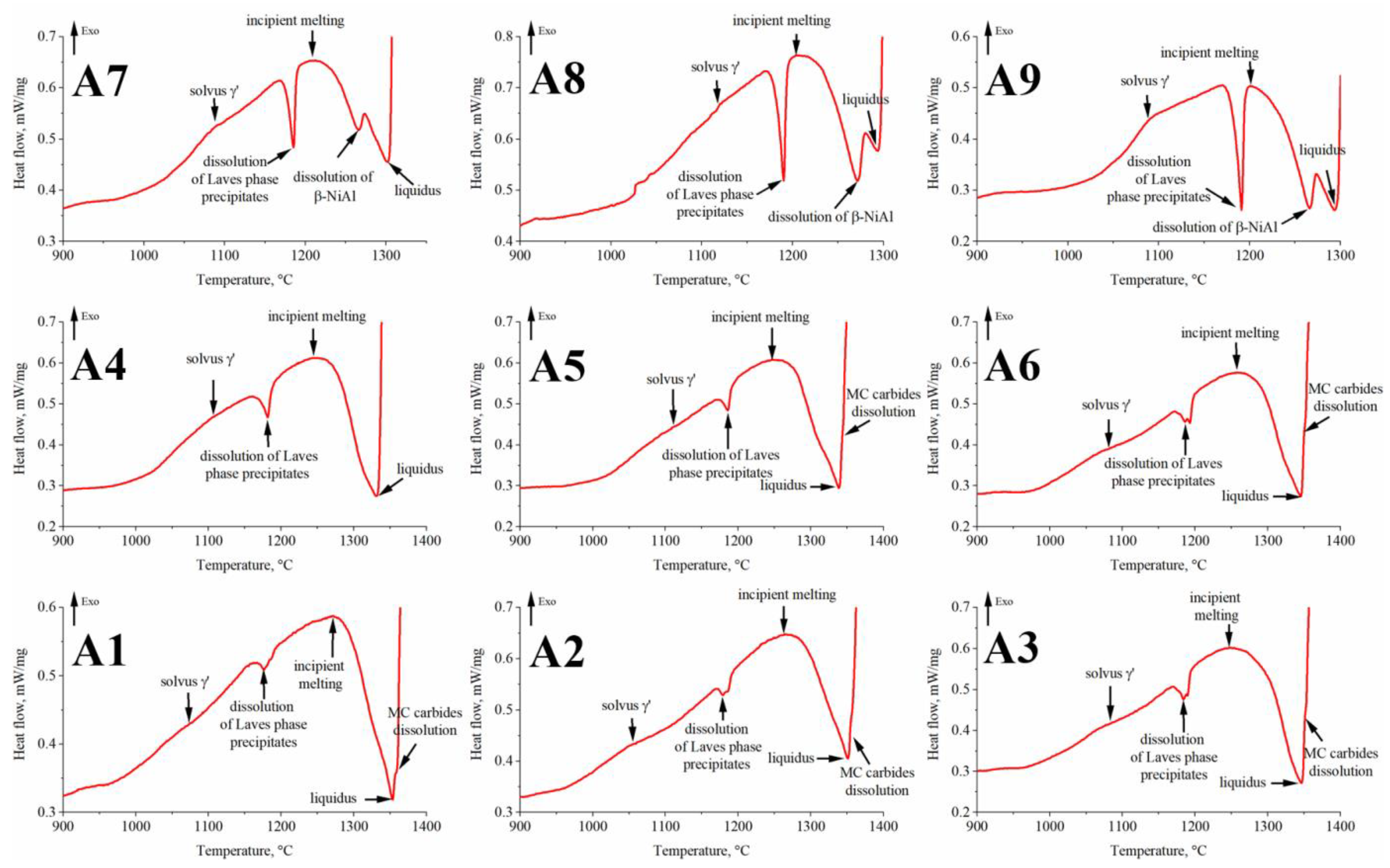
3.3. Analysis of the A1–A9 Superalloys Phase Transformation Temperatures during Heating
4. Conclusions
- -
- The Scheil solidification simulation showed the formation of the γ, γ′, MC carbides, and Laves phase in all castings. Additionally, β-NiAl was predicted in superalloys with high Al/Ti ratios. G phase precipitates could form in the final stage of solidification in superalloys with low and medium Al/Ti ratios.
- -
- For all castings, the XRD spectra revealed peaks corresponding to γ matrix, γ′ precipitates, and MC. The β-NiAl phase is present in castings characterized by high Al/Ti ratios.
- -
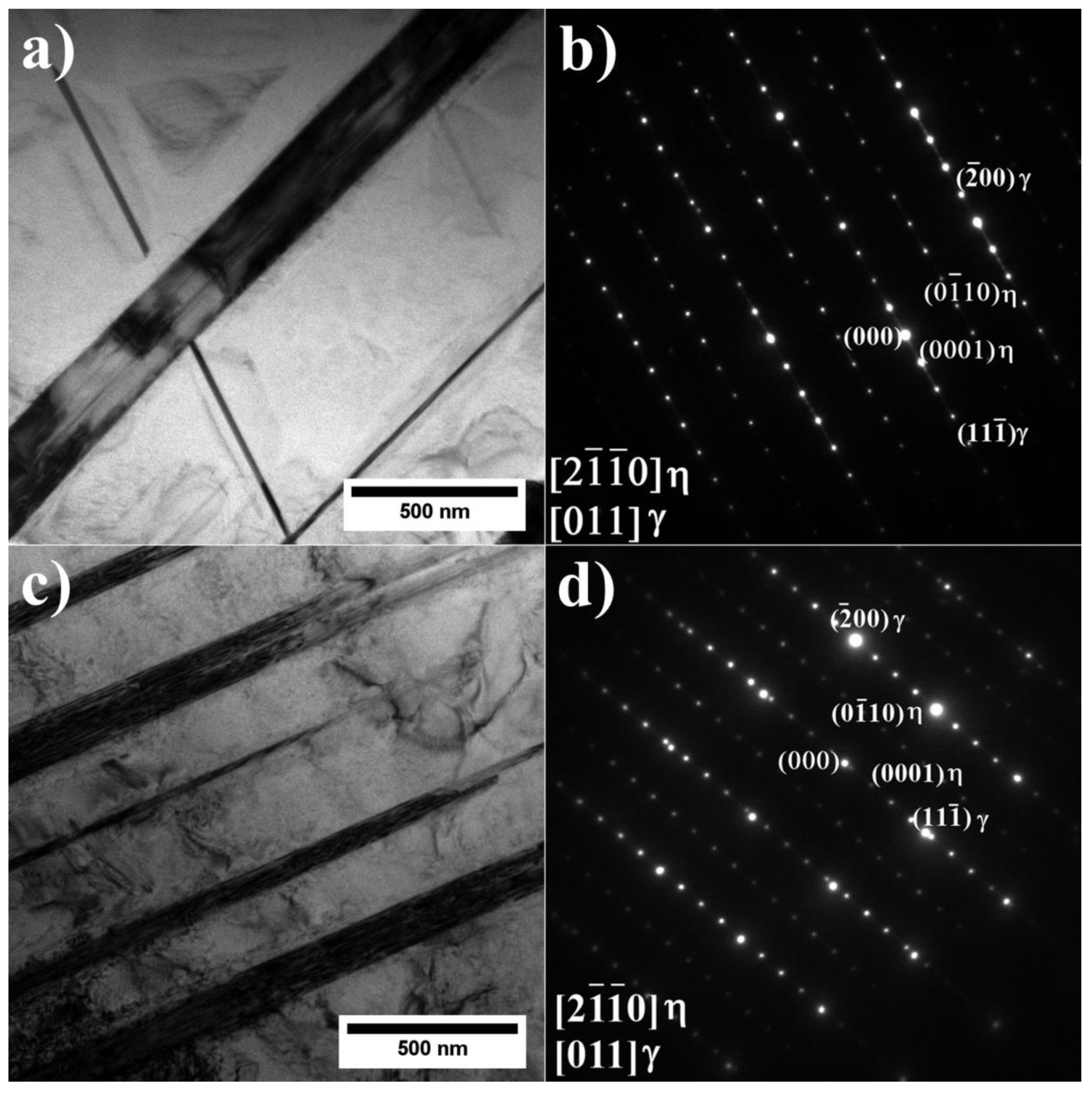
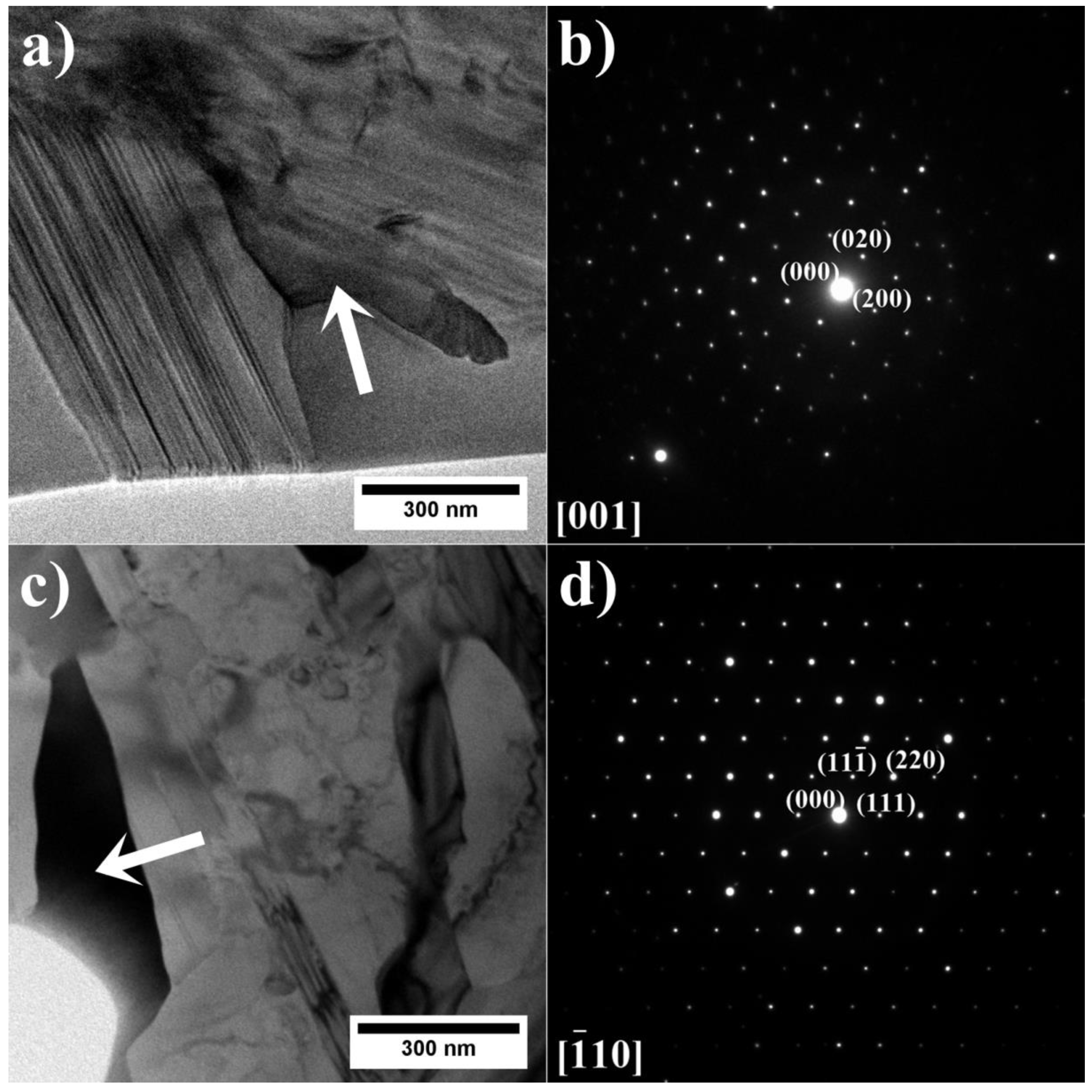
- As verified by SEM and TEM, the microstructure of alloys A1–A9 consists of a γ matrix, γ′ phase, (Nb, Ti, Ta)C carbides, and Laves phase precipitates. The alloys with low and medium Al/Ti ratios, except variants A4 and A5, were additionally strengthened by plate-like η phase precipitates.
- -
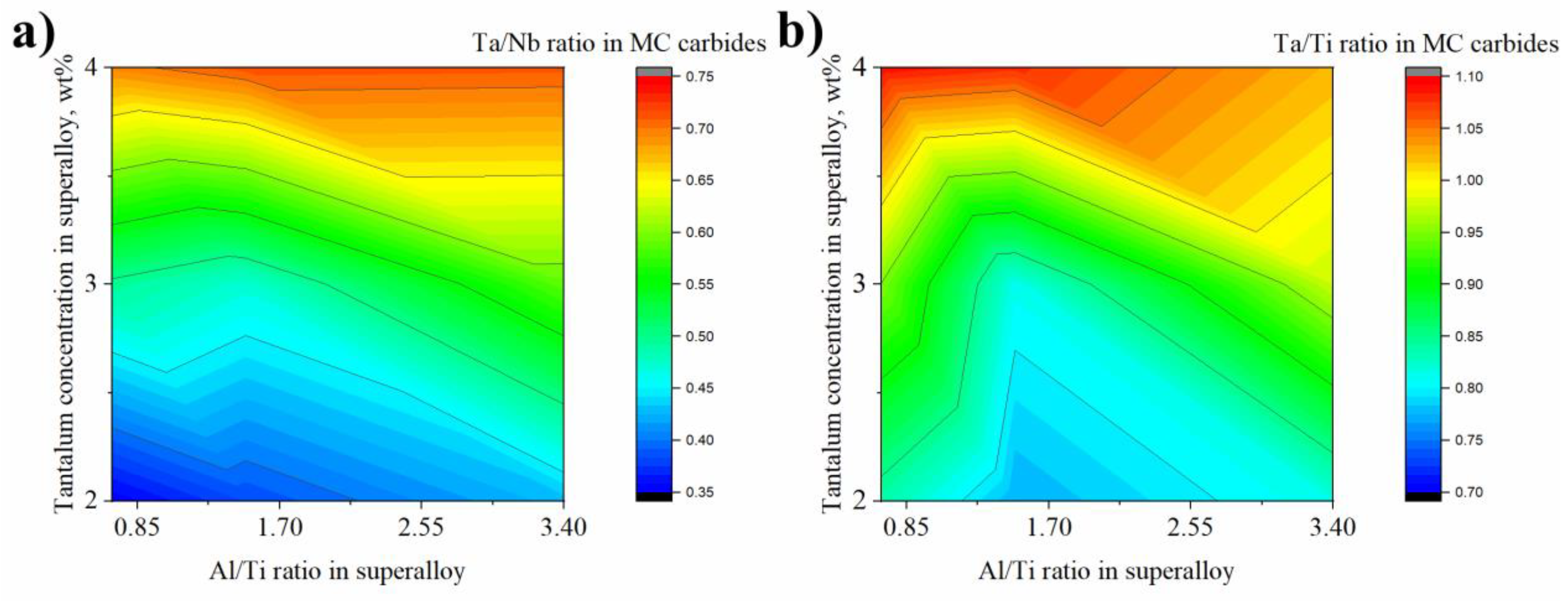
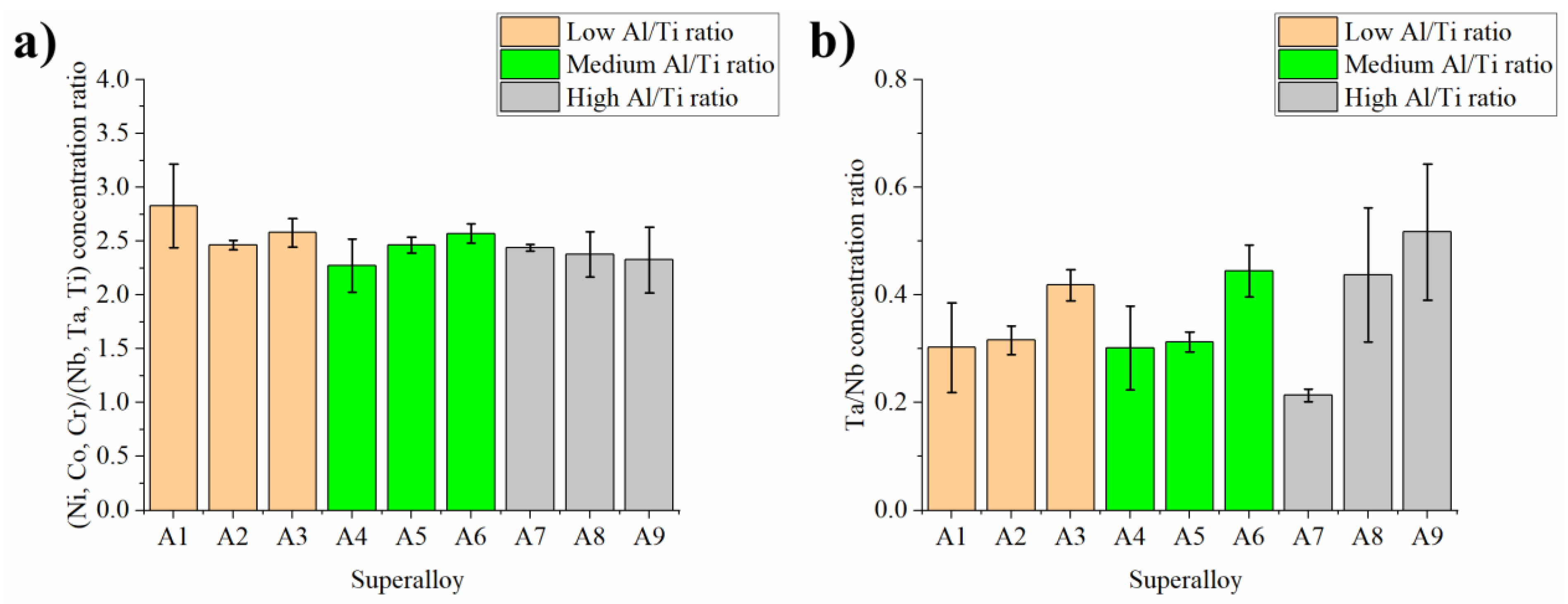
- It was shown via SEM-EDX analysis that the initial Ta concentration in the superalloy has a strong influence on the MC carbides’ composition, as it can partially replace Nb and Ti. The Ta/Nb and Ta/Ti concentration relationship in carbides increases with Ta content in the superalloy.
- -
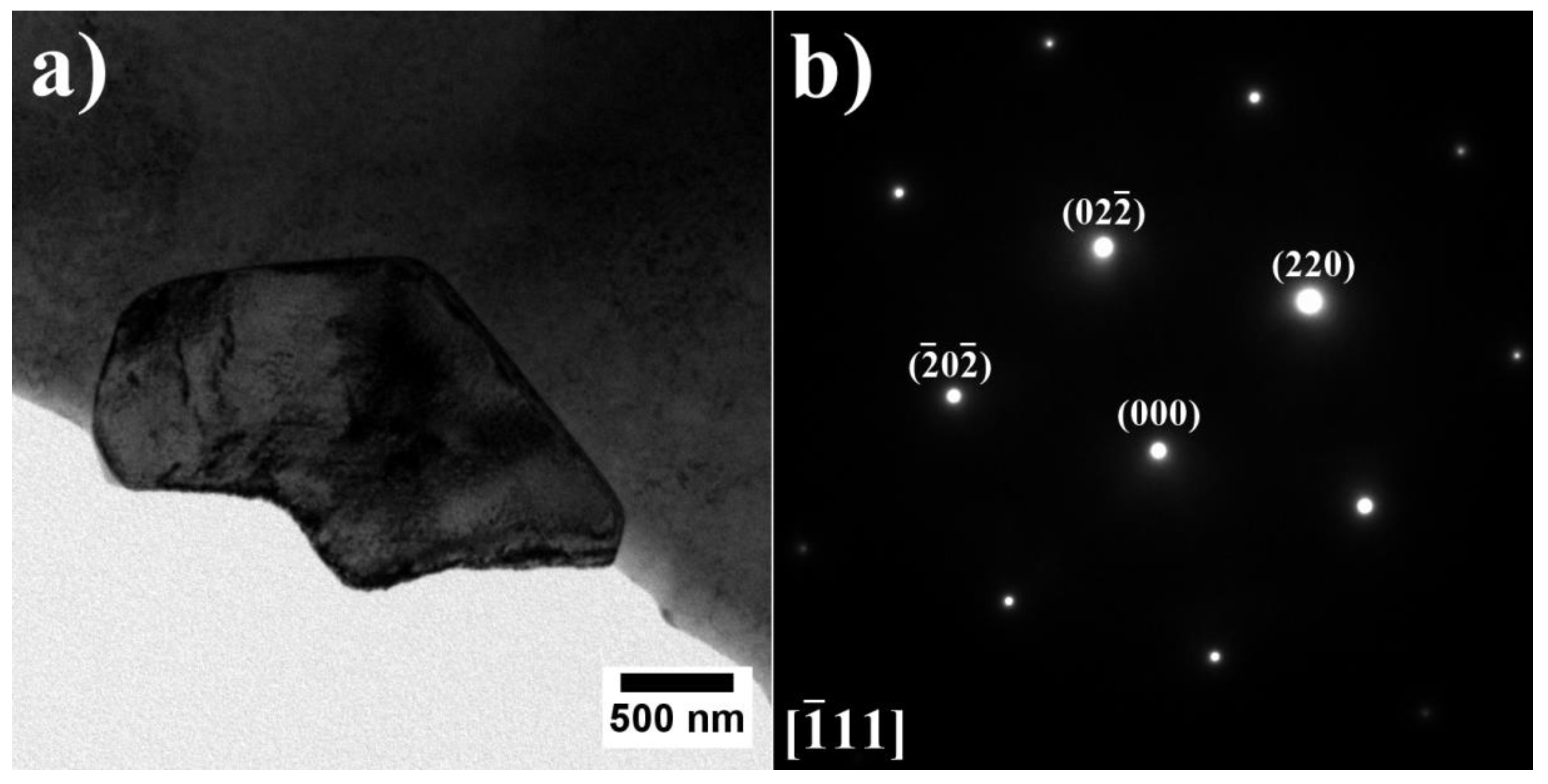
- The high initial Al/Ti concentration ratio in the A7–A9 superalloys led to the formation of the β-NiAl phase, strengthened locally by η phase and α-Cr precipitates.
- -
- On the DIL curves of all alloys, dilatation effects originating from γ′ precipitates are observed. In the A7–A9 superalloys, the effect from Laves phase dissolution was registered right before sample melting.
- -
- Based on the DSC curves, the γ′ solvus temperature in alloys with low and medium Al/Ti ratios was in the range of 1055–1084 °C and 1080–1111 °C, respectively. The liquidus temperature decreased with the increase in Al/Ti ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, R.C. The Superalloys Fundaments and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Di Gianfrancesco, A. The fossil fuel power plants technology. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Woodhead Publishing: Shaston, UK; Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Grudzień, M.; Tuz, L.; Pańcikiewicz, K.; Zielińska-Lipiec, A. Microstructure and properties of a repair weld in a nickel based superalloy gas turbine component. Adv. Mater. Sci. 2017, 17, 55–63. [Google Scholar] [CrossRef] [Green Version]
- deBarbadillo, J.J. Inconel 740H. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Woodhead Publishing: Shaston, UK; Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Baker, B.A. A new alloy designed for superaheater tubing in coal-fired ultra supercritical boilers. In Proceedings of the International Symposium on Superalloys 718, 625, 706 and Derivatives, TMS (TheMinerals, Metals & Materials Society), Pittsburgh, PA, USA, 2–5 October 2005; pp. 601–611. [Google Scholar]
- de Barbadillo, J.J.; Baker, B.; Xie, X. Superalloys for advanced energy applications: Inconel alloy 740H—A case study on international government-industry-university collaboration. In Proceedings of the 13th International Symposium on Superalloys, TMS (The Minerals, Metals & Materials Society), Pittsburgh, PA, USA, 11–15 September 2016; pp. 217–226. [Google Scholar]
- Xie, X.; Chi, C.; Yu, H.; Yu, Q.; Dong, J.; Zhao, S. Structure stability study on fossil power plant advanced heat-resistant steels and alloys in China. In Proceedings of the Advances in Materials Technology for Fossil Power Plants, Santa Fe, NM, USA, 31 August–3 September 2010; pp. 30–52. [Google Scholar]
- Xie, X.; Zhao, S.; Dong, J.; Smith, G.; Patel, S. An investigation of structure stability and its improvement on new developed Ni-Cr-Co-Mo-Nb-Ti-Al superalloy. Mater. Sci. Forum 2005, 475, 613–618. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, X.; Smith, G.; Patel, S. Microstructural stability and mechanical properties of a new nickel-based superalloy. Mater. Sci. Eng. A 2003, 355, 96–105. [Google Scholar] [CrossRef]
- Shin, G.S.; Yun, J.Y.; Park, M.C.; Kim, S.J. Effect of Mo on the thermal stability of γ′ precipitate in Inconel 740 alloy. Mater. Charact. 2014, 95, 180–186. [Google Scholar] [CrossRef]
- Abbasi, M.; Kim, D.-I.; Shim, J.H.; Jung, W.-S. Effects of alloyed aluminium and titanium on the oxidation behavior of Inconel 740 superalloy. J. All. Comp. 2016, 658, 210–221. [Google Scholar] [CrossRef]
- Ennis, P. Nickel-Base Alloys for Advanced Power Plant Components. In Coal Power Plant Materials and Life Assessment; Woodhead Publishing: Shaston, UK; Cambridge, UK, 2014; pp. 147–167. [Google Scholar] [CrossRef]
- Rozman, K.; Detrois, M.; Jablonski, P. Mechanical performance of various Inconel 740/740H alloy compositions for use in A-USC castings. In Proceedings of the 9th International Symposium on Superalloy 718 & Derivatives: Energy, Aerospace, and Industrial Applications, Pittsburgh, PA, USA, 3–6 June 2018; pp. 611–627. [Google Scholar] [CrossRef]
- Uhl, W.; Pirowski, Z.; Jaśkowiec, K. Trials to manufacture and test castings from Inconel alloy. Arch. Foundry Eng. 2010, 10, 73–78. [Google Scholar]
- Detrois, M.; Rozman, K.; Jablonski, P.; Hawk, J. An alternative casting technique to improve the creep resistance of cast Inconel alloy 740H. Metall. Mater. Trans. 2020, 51, 3819–3831. [Google Scholar] [CrossRef]
- Detrois, M.; Rozman, K.A.; Jablonski, P.D.; Hawk, J.A. Thermal processing design of cast Inconel Alloy 740H for improved mechanical performance. In Proceedings of the 9th International Symposium on Superalloy 718 & Derivatives: Energy, Aerospace, and Industrial Applications, Pittsburgh, PA, USA, 3–6 June 2018; pp. 829–846. [Google Scholar] [CrossRef]
- Janowski, G.M.; Heckel, R.W.; Pletka, B.J. The effects of tantalum on the microstructure of two polycrystalline nickel-base superalloys: B1900+Hf and MAR-M247. Metall. Trans. A 1986, 17, 1891–1905. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Rutkowski, B.; Grudzień-Rakoczy, M.; Cygan, R.; Ratuszek, W.; Zielińska-Lipiec, A. Analysis of γ′ precipitates, carbides and nano-borides in heat-treated Ni-based superalloy using SEM, STEM-EDX, and HRSTEM. Materials 2020, 13, 4452. [Google Scholar] [CrossRef]
- Patel, S.J.; Smith, G.D. The role of niobium in wrought superalloys. In Proceedings of the International Symposium Niobium 2001, Orlando, FL, USA, 2–5 December 2001; pp. 1081–1108. [Google Scholar]
- Szczotok, A.; Chmiela, B. Effect of heat treatment on chemical segregation in CMSX-4 nickel-base superalloy. J. Mater. Eng. Perform. 2014, 23, 2739–2747. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.A.; Richards, N.L. On incipient melting during high temperature heat treatment of cast Inconel 738 superalloy. J. Mater. Sci. 2004, 39, 7401–7404. [Google Scholar] [CrossRef]
- Xinxu, L.; Chonglin, J.; Yong, Z.; Shaomin, L.; Zhouhua, J. Segregation and homogenization for a new nickel-based superalloy. Vacuum 2020, 177, 109379. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Y.; Diao, A.; Li, Y.; Wang, X.; Zhou, Y.; Li, J.; Sun, X. Effect of solution heat treatment on creep properties of a nickel-based single crystal superalloy. J. Mater. Res. Tech. 2021, 15, 4702–4713. [Google Scholar] [CrossRef]
- Grudzień-Rakoczy, M.; Rakoczy, Ł.; Cygan, R.; Kromka, F.; Pirowski, Z.; Milkovič, O. Fabrication and characterization of the newly developed superalloys based on Inconel 740. Materials 2020, 13, 2362. [Google Scholar] [CrossRef]
- Walter, C.; Hallstedt, B.; Warnken, N. Simulation of the solidification of CMSX-4. Mater. Sci. Eng. A 2005, 397, 385–390. [Google Scholar] [CrossRef]
- Sims, C.T.; Stoloff, N.S.; Hagel, W.C. Superalloys II. High-temperature materials for aerospace and industrial power; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Baeslack, W.A., III; West, S.L.; Kelly, T.J. Weld cracking in Ta-modified cast Inconel 718. Scri. Metall. 1988, 22, 729–734. [Google Scholar] [CrossRef]
- Komura, Y.; Tokunaga, K. Structural studies of stacking variants in Mg-base Friauf-Laves phases. Acta Cryst. Sect. B Struct. Cryst. Cryst. Chem. 1980, 36, 1548–1554. [Google Scholar] [CrossRef]
- Cieslak, M.J.; Knorovsky, A.; Headley, T.J.; Romig Jr, A.D. The solidification metallurgy of Alloy 718 and other Nb-containing superalloys. In Superalloy 718—Metallurgy and Applications; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 1989; pp. 59–68. [Google Scholar]
- Radhakrishna, C.; Prasad Rao, K. The formation and control of Laves phase in superalloy 718 welds. J. Mater. Sci. 1997, 32, 1977–1984. [Google Scholar] [CrossRef]
- Kruk, A.; Cempura, G.; Lech, S.; Wusatowska-Sarnek, A.; Czyrska-Filemonowicz, A. Application of analytical electron microscopy and tomographic techniques for metrology and 3d imaging of microstructural elements in Allvac®718Plus™. In Proceedings of the 9th international symposium on Superalloy 718 and derivatives: Energy, aerospace and industrial applications, Pittsburgh, PA, USA, 3–6 June 2018; pp. 1035–1050. [Google Scholar] [CrossRef]
- Laves, F.; Wallbaum, H.J. Die kristallstruktur von Ni3Ti und Si2Ti. Zeitsch. für Kristall. Cryst. Mater. 1939, 101, 78–93. [Google Scholar] [CrossRef]
- Bouse, G.K. Eta (η) and Platelet Phases in Investment Cast Superalloys. In Proceedings of the 8th International Symposium Superalloys 1996, Pittsburgh, PA, USA, 22–26 September 1996; pp. 163–172. [Google Scholar]
- Seo, S.M.; Kim, I.S.; Lee, J.H.; Jo, C.Y.; Miyahara, H.; Ogi, K. Eta phase and boride formation in directionally solidified Ni-base superalloy IN792+Hf. Metall. Mater. Trans. A 2007, 38, 883–893. [Google Scholar] [CrossRef]
- Willemin, P.; Durrand-Charre, M. Phase equilibria in multicomponent alloy systems. In Proceedings of the 6th International Symposium Superalloys 1988, Pittsburgh, PA, USA, 18–22 September 1988; pp. 723–732. [Google Scholar]
- Kruk, A.; Cempura, G.; Lech, S.; Czyrska-Filemonowic, A. STEM-EDX and FIB-SEM tomography of Allvac 718Plus superalloy. Arch. Metall. Mater. 2016, 61, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.G.; Liu, F.; Zhang, A.-W.; Han, D.-W.; Yao, X.-Y.; Zhang, W.-W.; Sun, W.-R. Element Segregation and Solidification Behavior of a Nb, Ti, Al Co-Strengthened Superalloy ЭК151. Act. Metall. Sin. 2019, 32, 1298–1308. [Google Scholar] [CrossRef] [Green Version]
- Beattie, H.J.; Versnyder, F.L. A new complex phase in a high-temperature alloy. Nature 1956, 178, 208–209. [Google Scholar] [CrossRef]
- Powell, D.J.; Pilkington, R.; Miller, D.A. The precipitation characteristics of 20%Cr/25% Ni-Nb stabilised stainless steel. Acta Metall. 1988, 36, 713–724. [Google Scholar] [CrossRef]
- Xie, X.; Wu, Y.; Chi, C.; Zhang, M. Superalloys for Advanced Ultra-Super-Critical Fossil Power Plant Application. In Superalloys; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2015; pp. 51–76. [Google Scholar] [CrossRef] [Green Version]
- Ecob, R.C.; Lobb, R.C.; Kohler, V.L. The formation of G-phase in 20/25 Nb stainless steel AGR fuel cladding alloy and its effect on creep properties. J. Mater. Sci. 1987, 22, 2867–2880. [Google Scholar] [CrossRef]
- Brager, A. An X-ray examination of titanium nitride III. Investigation by the powder method. Acta Physicoch. URSS 1939, 11, 617–632. [Google Scholar]
- Sobczak, N.; Pirowski, Z.; Purgert, R.; Uhl, W.; Jaśkowiec, K.; Boroń, Ł.; Pysz, S.; Sobczak, J. Castability of Haynes 282 alloy. In Proceedings of the Advanced Ultrasupercritical Coal-fired Power Plants, Vienna, Austria, 12–20 September 2012; pp. 1–24. [Google Scholar]
- Cockcroft, S.L.; Degawa, T.; Mitchell, A.; Tripp, D.W.; Schmalz, A. Inclusion precipitation in superalloys. In Proceedings of the 7th International Symposium Superalloys 1988, Pittsburgh, PA, USA, 20–24 September 1992; pp. 577–586. [Google Scholar]
- Miracle, D.B. Overview No. 104 The physical and mechanical properties of NiAl. Acta Metall. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.G.; Li, J.G.; Meng, J.; Duan, T.F.; Yang, Y.H.; Liu, J.L.; Liu, J.D.; Pei, W.L.; Zhou, Y.Z.; et al. The formation and evolution of NiAl phase in a fourth generation nickel-based single crystal superalloy. J. All. Comp. 2020, 848, 156584. [Google Scholar] [CrossRef]
- Cao, K.; Yang, W.; Zhang, J.; Liu, C.; Qu, P.; Su, H.; Zhang, J.; Liu, L. Solidification characteristics and as-cast microstructure of a Ru-containing nickel-based single crystal superalloy. J. Mater. Res. Tech. 2021, 11, 474–486. [Google Scholar] [CrossRef]
- Tan, X.; Tan, X.; Liu, J.; Jin, T. Precipitation of β-NiAl/Laves eutectics in a Ru-containing single crystal Ni-based superalloy. Met. Mater. Inter. 2015, 21, 222–226. [Google Scholar] [CrossRef]
- Lopez-Galilea, I.; Zenk, C.; Neumeier, S.; Huth, S.; Theisen, W.; Göken, M. The thermal stability of intermetallic compounds in an as-cast SX Co-base superalloy. Adv. Eng. Mater. 2015, 17, 741–747. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Y.; Yang, S.; Sun, W.; Guo, S.; Sun, X.; Hu, Z. As-cast microstructure and solidification behavior of a high Al- and Nb containing superalloy. J. Mater. Sci. 2010, 45, 3448–3456. [Google Scholar] [CrossRef]
- Yuan, L.; Xiong, J.; Zhao, W.; Shi, J.; Li, J. Transient liquid phase bonding of Ni3Al based superalloy using Mn-Ni-Cr filler. J. Mater. Res. Tech. 2021, 11, 1583–1593. [Google Scholar] [CrossRef]
- Pathare, V.; Michal, G.M.; Vedula, K. Analysis of NiAlTa precipitates in β-NiAl + 2 at.% Ta alloy. Scri. Metall. 1987, 21, 283–288. [Google Scholar] [CrossRef]
- Cotton, J.D.; Noebe, R.D.; Kaufman, M.J. The effects of chromium on NiAl intermetallic alloys: Part II. Slip systems. Intermetallics 1993, 1, 117–126. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures 1; Interscience Publishers: New York, NY, USA, 1963. [Google Scholar]
- Tian, W.H.; Han, C.S.; Nemoto, M. Precipitation of α-Cr in B2-ordered NiAl. Intermetallics 1999, 7, 59–67. [Google Scholar] [CrossRef]
- Pyczak, F.; Devrient, B.; Mughrabi, H. The effects of different alloying elements on the thermal expansion coefficients, lattice constants and misfit of nickel-based superalloys investigated by X-ray diffraction. In Proceedings of the 10th International Symposium Superalloys, Pittsburgh, PA, USA, 19–23 September 2004; pp. 827–836. [Google Scholar]
- Rakoczy, Ł.; Milkovič, O.; Rutkowski, B.; Cygan, R.; Grudzień-Rakoczy, M.; Kromka, F.; Zielińska-Lipiec, A. Characterization of γ′ precipitates in cast Ni-based superalloy and their behaviour at high-homologous temperatures studied by TEM and in Situ XRD. Materials 2020, 13, 2397. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Grudzień-Rakoczy, M.; Hanning, F.; Cempura, G.; Cygan, R.; Andersson, J.; Zielińska-Lipiec, A. Investigation of the γ′ precipitates dissolution in a Ni-based superalloy during stress-free short-term annealing at high homologous temperatures. Metall. Mater. Trans. A 2021, 52, 4767–4784. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Cempura, G.; Kruk, A.; Czyrska-Filemonowicz, A.; Zielińska-Lipiec, A. Evolution of γ′ morphology and γ/γ′ lattice parameter misfit in a nickel-based superalloy during non-equilibrium cooling. Int. J. Mater. Res. 2019, 110, 66–69. [Google Scholar] [CrossRef]
- Grosdidier, T.; Hazotte, A.; Simon, A. Precipitation and dissolution processes in γ/γ′ single crystal nickel-based superalloys. Mater. Sci. Eng. A 1998, 256, 183–196. [Google Scholar] [CrossRef]
- Trexler, M.D.; Church, B.C.; Sanders, T.H., Jr. Determination of the Ni3(Ti, Al) dissolution boundary in a directionally solidified superalloy. Scri. Mater. 2006, 55, 561–564. [Google Scholar] [CrossRef]
- Sponseller, D.L. Differential thermal analysis of nickel-base superalloys. In Proceedings of the 8th International Symposium Superalloys 1996, Pittsburgh, PA, USA, 22–26 September 1996; pp. 259–270. [Google Scholar]
- Liu, L.; Zhen, B.L.; Banerji, A.; Reif, W.; Sommer, F. Effect of melt homogenization temperature on the cast structures of IN 738 LC superalloy. Scri. Metall. Mater. 1994, 30, 593–598. [Google Scholar] [CrossRef]
- Zlá, S.; Dobrovská, J.; Smetana, B.; Žaludová, M.; Vodárek, V.; Konečná, K. Differential thermal analysis and phase analysis of nickel based superalloy IN 738LC. In Proceedings of the 19th International Conference on Metallurgy and Materials, Rožnov pod Radhoštěm, Roznov pod Radhostem, Czech Republic, 18–20 May 2010; pp. 790–795. [Google Scholar]

| Ta Concentration | Al/Ti Concentration Ratio | ||
|---|---|---|---|
| Low: 0.7 (±0.10) | Medium: 1.5 (±0.15) | High: 3.4 (±0.20) | |
| Low: 2.0 (±0.10) | A1 | A4 | A7 |
| Medium: 3.0 (±0.15) | A2 | A5 | A8 |
| High: 4.0 (±0.20) | A3 | A6 | A9 |
| Alloy | Cr | Co | Al | Ti | Nb | Fe | Mo | Mn | Si | C | Ta | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 22.34 | 19.96 | 1.30 | 1.72 | 1.80 | 0.62 | 0.43 | 0.30 | 0.59 | 0.03 | 2.00 | Balance |
| A2 | 22.46 | 19.91 | 1.28 | 1.78 | 1.92 | 0.61 | 0.43 | 0.30 | 0.60 | 0.02 | 3.00 | |
| A3 | 22.41 | 19.91 | 1.31 | 1.68 | 1.88 | 0.62 | 0.44 | 0.30 | 0.61 | 0.02 | 4.00 | |
| A4 | 22.84 | 19.71 | 2.80 | 1.82 | 1.92 | 0.63 | 0.42 | 0.29 | 0.59 | 0.03 | 2.00 | |
| A5 | 22.74 | 19.69 | 2.82 | 1.76 | 1.80 | 0.64 | 0.43 | 0.30 | 0.60 | 0.03 | 3.00 | |
| A6 | 22.28 | 19.85 | 2.75 | 1.83 | 1.94 | 0.56 | 0.43 | 0.22 | 0.56 | 0.02 | 4.00 | |
| A7 | 21.76 | 18.85 | 6.06 | 1.81 | 1.88 | 0.63 | 0.42 | 0.29 | 0.60 | 0.03 | 2.00 | |
| A8 | 21.20 | 19.05 | 6.34 | 1.76 | 1.82 | 0.61 | 0.40 | 0.28 | 0.56 | 0.03 | 3.00 | |
| A9 | 21.43 | 18.65 | 6.12 | 1.84 | 1.86 | 0.62 | 0.42 | 0.29 | 0.60 | 0.03 | 4.00 |
| Parameter/Casting | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 |
|---|---|---|---|---|---|---|---|---|---|
| Mean size of the γ′ precipitates, nm | 26 (±3) | 31 (±4) | 46 (±6) | 99 (±10) | 76 (±9) | 41 (±4) | 110 (±16) | 112 (±25) | 134 (±18) |
| Circularity, ξ | 0.96 (±0.04) | 0.93 (±0.03) | 0.93 (±0.02) | 0.90 (±0.02) | 0.91 (±0.04) | 0.94 (±0.02) | 0.89 (±0.02) | 0.88 (±0.03) | 0.87 (±0.03) |
| Superalloy | Mean Size, μm | Standard Deviation, μm |
|---|---|---|
| A1 | 3.1 | 1.2 |
| A2 | 3.8 | 1.2 |
| A3 | 3.1 | 1.6 |
| A4 | 3.5 | 1.5 |
| A5 | 3.0 | 0.7 |
| A6 | 2.7 | 0.7 |
| A7 | 4.5 | 1.3 |
| A8 | 2.6 | 0.9 |
| A9 | 3.1 | 0.9 |
| Alloy | γ′ solvus | Dissolution of Laves Phase | Incipient Melting (Solidus) | β-NiAl Dissolution | Liquidus | MC Carbides Dissolution |
|---|---|---|---|---|---|---|
| A1 | 1072 | 1176 | 1224 | not registered | 1353 | 1357 |
| A2 | 1055 | 1179 | 1263 | 1351 | 1354 | |
| A3 | 1084 | 1184 | 1247 | 1346 | 1350 | |
| A4 | 1106 | 1182 | 1245 | 1330 | not registered | |
| A5 | 1111 | 1185 | 1250 | 1339 | 1343 | |
| A6 | 1080 | 1193 | 1258 | 1345 | 1350 | |
| A7 | 1089 | 1185 | 1211 | 1267 | 1302 | not registered |
| A8 | 1117 | 1189 | 1208 | 1272 | 1293 | |
| A9 | 1087 | 1190 | 1201 | 1266 | 1292 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudzień-Rakoczy, M.; Rakoczy, Ł.; Cygan, R.; Chrzan, K.; Milkovič, O.; Pirowski, Z. Influence of Al/Ti Ratio and Ta Concentration on the As-Cast Microstructure, Phase Composition, and Phase Transformation Temperatures of Lost-Wax Ni-Based Superalloy Castings. Materials 2022, 15, 3296. https://doi.org/10.3390/ma15093296
Grudzień-Rakoczy M, Rakoczy Ł, Cygan R, Chrzan K, Milkovič O, Pirowski Z. Influence of Al/Ti Ratio and Ta Concentration on the As-Cast Microstructure, Phase Composition, and Phase Transformation Temperatures of Lost-Wax Ni-Based Superalloy Castings. Materials. 2022; 15(9):3296. https://doi.org/10.3390/ma15093296
Chicago/Turabian StyleGrudzień-Rakoczy, Małgorzata, Łukasz Rakoczy, Rafał Cygan, Konrad Chrzan, Ondrej Milkovič, and Zenon Pirowski. 2022. "Influence of Al/Ti Ratio and Ta Concentration on the As-Cast Microstructure, Phase Composition, and Phase Transformation Temperatures of Lost-Wax Ni-Based Superalloy Castings" Materials 15, no. 9: 3296. https://doi.org/10.3390/ma15093296
APA StyleGrudzień-Rakoczy, M., Rakoczy, Ł., Cygan, R., Chrzan, K., Milkovič, O., & Pirowski, Z. (2022). Influence of Al/Ti Ratio and Ta Concentration on the As-Cast Microstructure, Phase Composition, and Phase Transformation Temperatures of Lost-Wax Ni-Based Superalloy Castings. Materials, 15(9), 3296. https://doi.org/10.3390/ma15093296







