Surface Modification of Magnesium Oxysulfate Whisker Based on SiO2@silane Coupling Agent and SiO2@polydopamine Double-Layer Structure for Reinforcing HDPE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
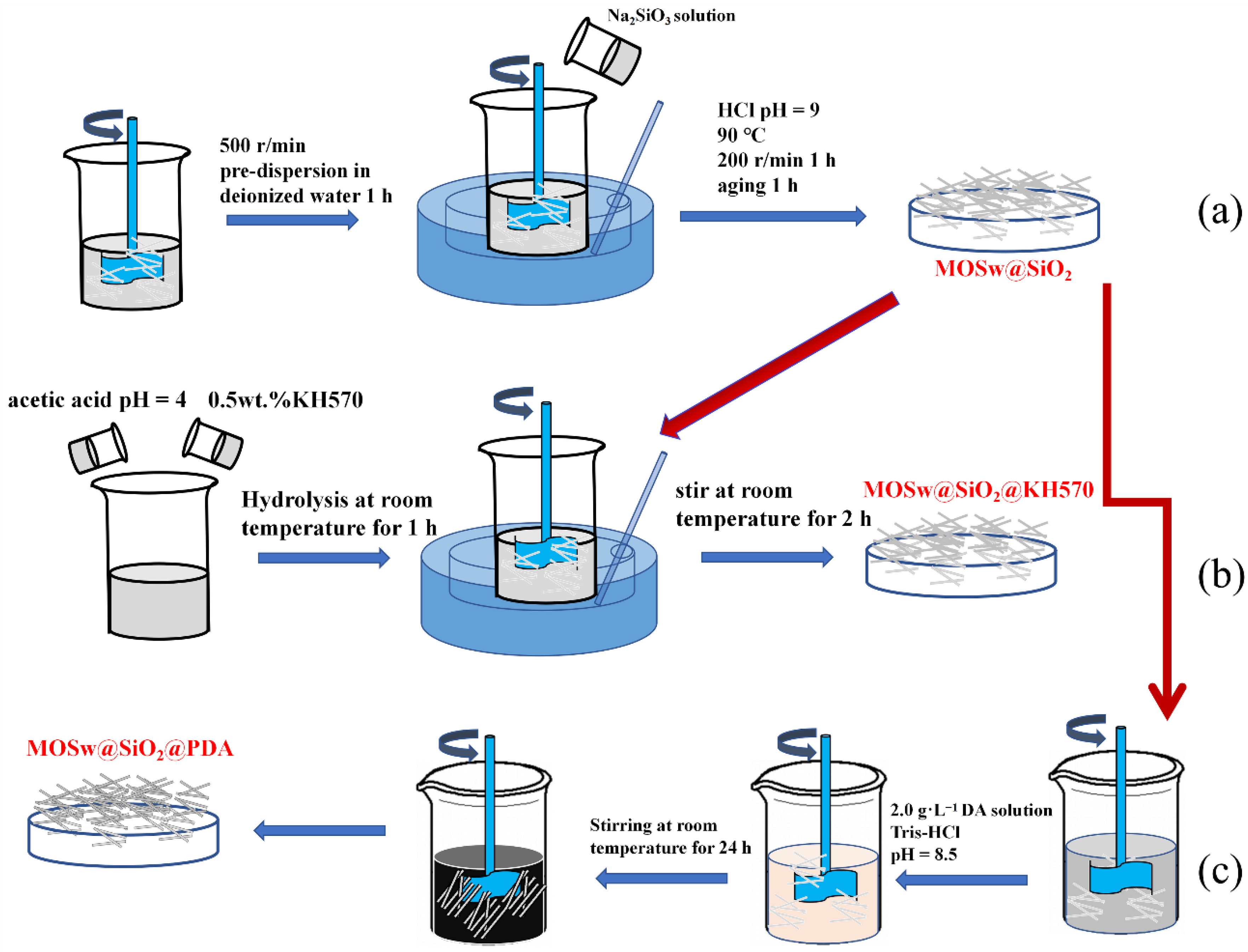
2.2. Surface Modification of MOSw
2.2.1. The Preparation of MOSw@SiO2@KH570
2.2.2. The Preparation of MOSw@SiO2@PDA
2.3. Preparation of HDPE/MOSw Composites
2.4. X-ray Photoelectron Spectroscopy (XPS) Analyses
2.5. X-ray Diffraction (XRD) Analyses
2.6. Scanning Electron Microscopy (SEM) Analyses
2.7. Dynamic Mechanical Thermal Analysis (DMTA)
2.8. Thermal Analyses
2.9. Mechanical Properties
2.10. Limit Oxygen Index (LOI) Analyses
2.11. Contact Angle Analyses
3. Results
3.1. XPS Analysis
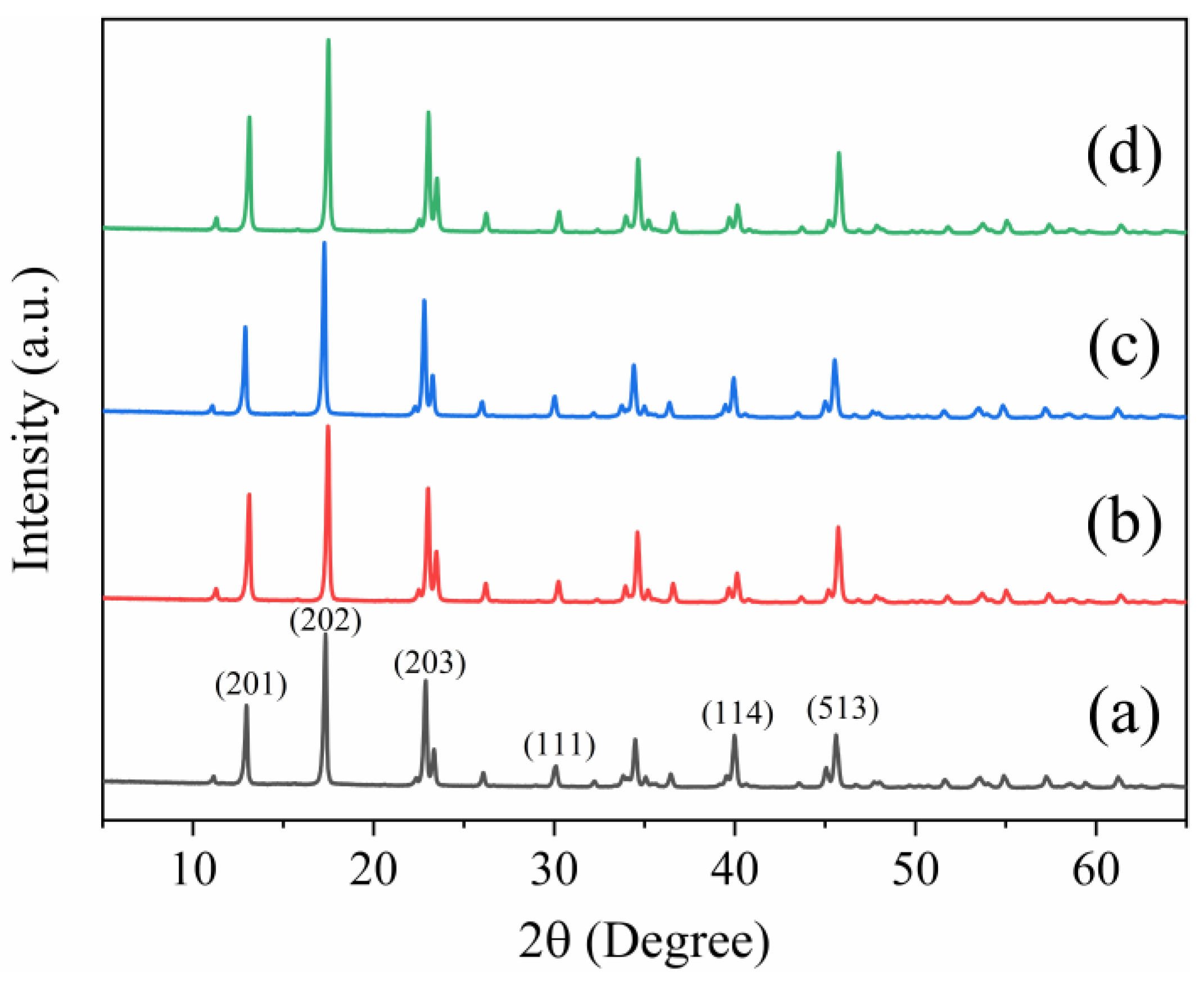
3.2. XRD Analysis
3.3. Mechanical Properties
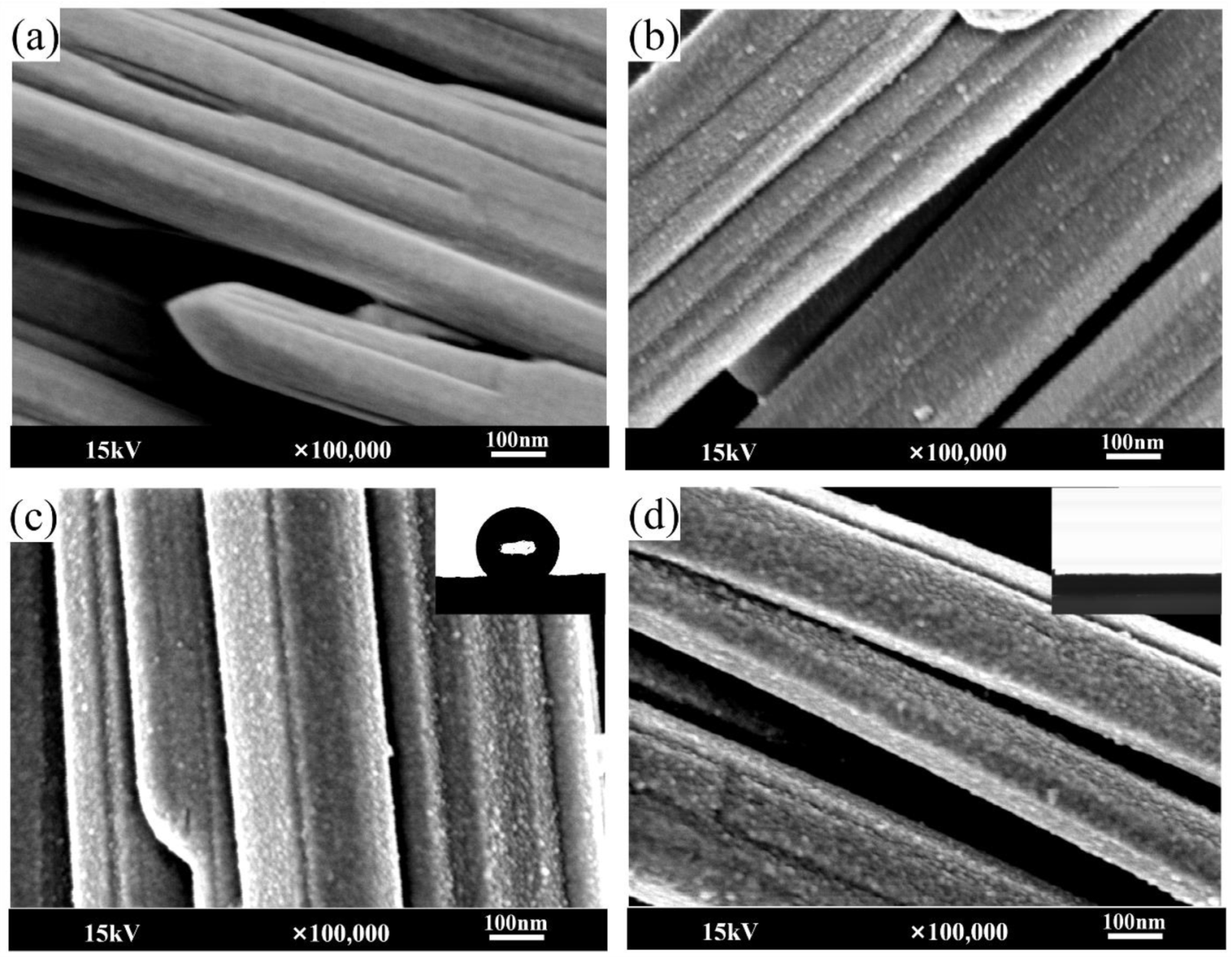
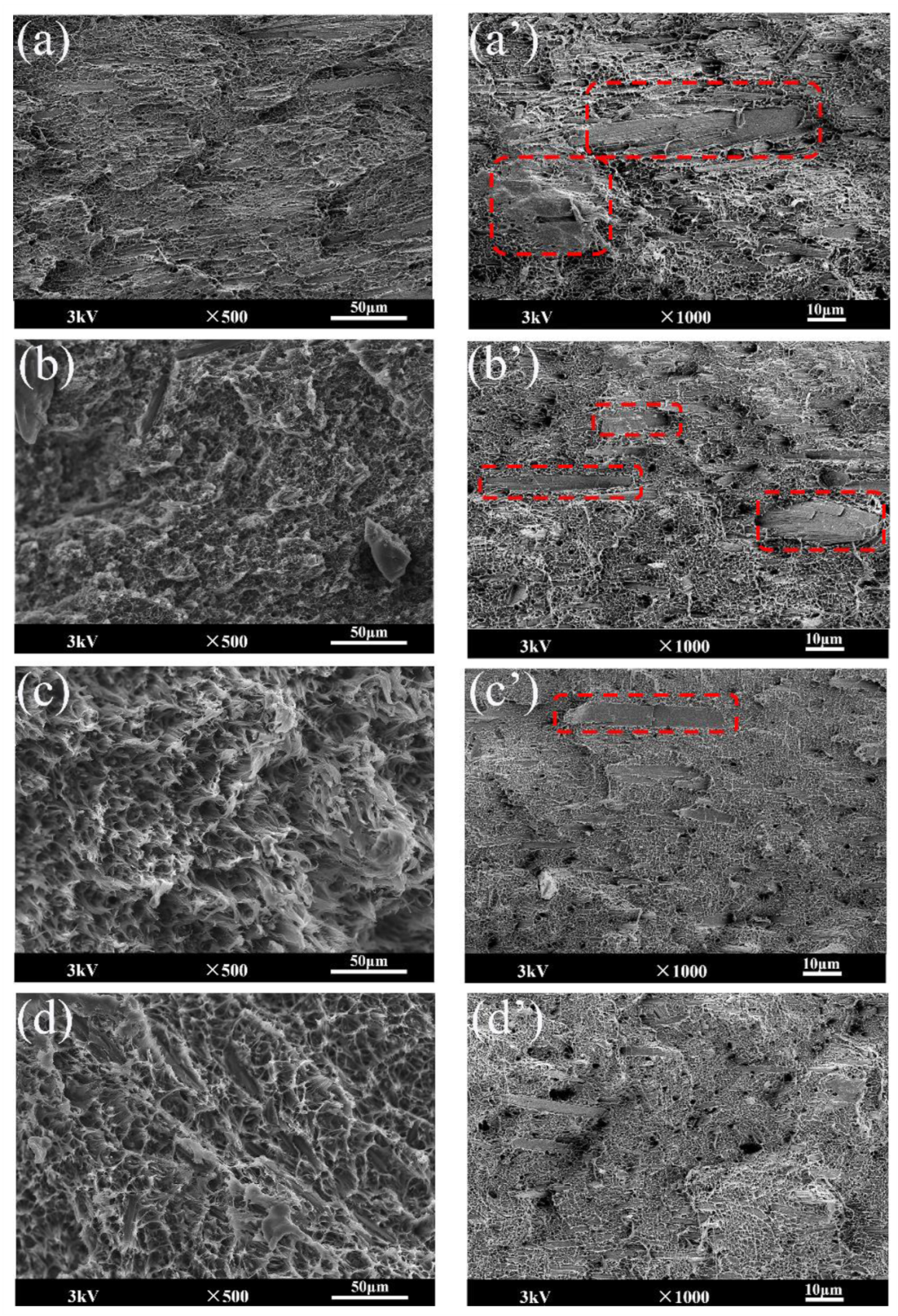
3.4. SEM Analysis
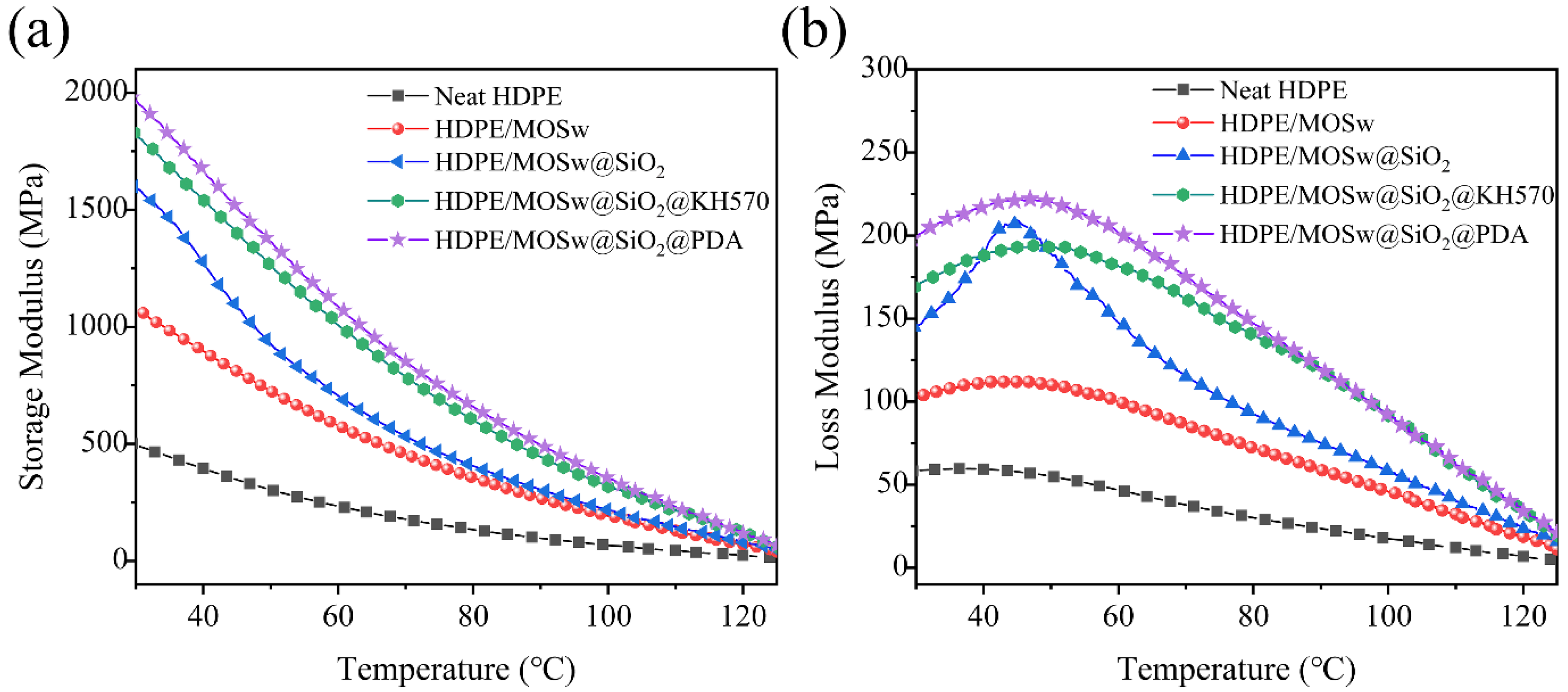
3.5. Dynamic Mechanical Thermal Analysis
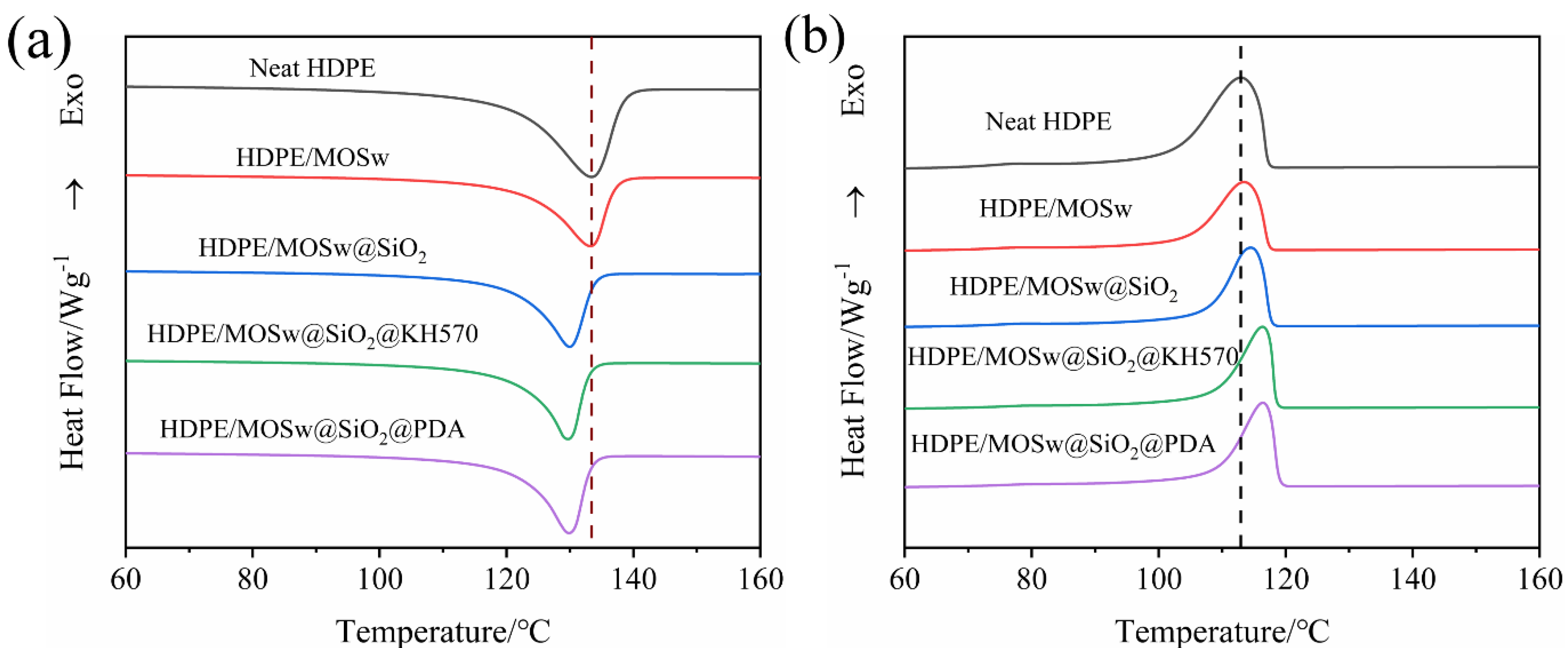
3.6. Differential Scanning Calorimeter Analysis
3.7. TG and Flame Retardant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wang, D.; Chen, S.; Zhang, Y.; Wu, Y.; Wang, N.; Chen, X.; Qin, J.; Zhang, K.; Wu, H. Effect of organic grafting expandable graphite on combustion behaviors and thermal stability of low-density polyethylene composites. Polym. Compos. 2019, 41, 719–728. [Google Scholar] [CrossRef]
- Jia, Z.; Luo, Y.; Guo, B.; Yang, B.; Du, M.; Jia, D. Reinforcing and Flame-Retardant Effects of Halloysite Nanotubes on LLDPE. Polym.-Plast. Technol. 2009, 48, 607–613. [Google Scholar] [CrossRef]
- Depeng, L.; Chixiang, L.; Xiulei, J.; Tao, L.; Ling, Z. Synergistic effects of intumescent flame retardant and nano-CaCO3 on foamability and flame-retardant property of polypropylene composites foams. J. Cell. Plast. 2018, 54, 615–631. [Google Scholar] [CrossRef]
- Chiang, W.; Hu, H.C.H. Phosphate-containing flame-retardant polymers with good compatibility to polypropylene. II. Effect of the flame-retardant polymers on polypropylene. J. Appl. Polym. Sci. 2001, 82, 2399–2403. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Li, J.; Jin, N.; Li, C.; Zhou, X.; Ma, J. A new way for lead–boron resin composite modification: SiO2 coated lead powders by a sol-gel method. RSC Adv. 2019, 9, 30752–30759. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhu, J.; Yu, J.; Wang, Y.; Hu, Z. Mussel-inspired polydopamine/polystyrene composites with 3D continuous structure and improved thermal, mechanical, and flame retarding properties. J. Appl. Polym. Sci. 2019, 136, 47740. [Google Scholar] [CrossRef]
- Lan, S.; Zhu, D.; Dang, L.; Huang, Z.; Yang, L.; Xu, Z. Surface treatment of magnesium oxysulfate whiskers through plasma polymerization. Surf. Interface Anal. 2019, 51, 1316–1324. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Yang, L.; Wang, Z.; Chen, Z.; Fan, W. Study of the Fire Performance of Magnesium Hydroxide Sulfate Hydrate Whisker Flame Retardant Polyethylene. Macromol. Mater. Eng. 2004, 289, 984–989. [Google Scholar] [CrossRef]
- Nai, X.Y.; Ye, X.S.; Cui, X.M.; Li, W. Progress of inorganic whisker research (Ⅲ): Preparation and application of hydrous magnesium oxysulfate whiskers. J. Salt Lake Res. 2005, 3, 22–28. (In Chinese) [Google Scholar]
- Domka, L.; Malicka, A.; Stachowiak, N. The effect of kaolin modification of silane coupling agents on the properties of the polyethylene composites. Pol. J. Chem. Technol. 2008, 10, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.J.; Al-Juhani, A.A.; Ul-Hamid, A.; Shawabkeh, R.; Hussein, I.A. Effect of chemical modification of oil fly ash and compatibilization on the rheological and morphological properties of low-density polyethylene composites. J. Appl. Polym. Sci. 2011, 122, 2486–2496. [Google Scholar] [CrossRef]
- Dogan, S.D.; Tayfun, U.; Dogan, M. New route for modifying cellulosic fibers with fatty acids and its application to polyethylene/jute fiber composites. J. Compos. Mater. 2016, 50, 2477–2485. [Google Scholar] [CrossRef]
- Denev, Y.G.; Denev, G.D.; Popov, A.N. Surface Modification of Phosphogypsum Used as Reinforcing Material in Polyethylene Composites. J. Elastom. Plast. 2009, 41, 119–132. [Google Scholar] [CrossRef]
- Chimeni, D.Y.; Toupe, J.L.; Dubois, C.; Rodrigue, D. Effect of hemp surface modification on the morphological and tensile properties of linear medium density polyethylene (LMDPE) composites. Compos. Interface 2016, 23, 405–421. [Google Scholar] [CrossRef]
- Li, Q.; Gao, X.; Cheng, W.; Han, G. Effect of Modified Red Pottery Clay on the Moisture Absorption Behavior and Weatherability of Polyethylene-Based Wood-Plastic Composites. Materials 2017, 10, 111. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Lai, W.; Wang, C. Effects of Surface Modification on the Mechanical Properties of Flax/β-Polypropylene Composites. Materials 2016, 9, 314. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Han, G.; Li, Q.; Gao, X.; Cheng, W. High-Temperature Hot Air/Silane Coupling Modification of Wood Fiber and Its Effect on Properties of Wood Fiber/HDPE Composites. Materials 2017, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kim, Y.C.; Park, J.; Kim, Y.; Kim, S.; Kim, K.J.; Suhr, J.; Lee, Y.; Lee, S.H.; Kim, D.; et al. Mechanical properties and flame retardancy of surface-modified magnesium oxysulfate (5Mg(OH)2·MgSO4·3H2O) whisker for polypropylene composites. J. Mater. 2018, 4, 149–156. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Liu, X.; Zhu, D.; Dong, Y.; Li, W. Crystallization, mechanical, thermal, and rheological properties of polypropylene composites reinforced by magnesium oxysulfate whisker. Chin. J. Polym. Sci. 2017, 35, 659–671. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Dong, Y.; Li, W. Functional group effect on flame retardancy, thermal, and mechanical properties of organophosphorus-based magnesium oxysulfate whiskers as a flame retardant in polypropylene. RSC Adv. 2017, 7, 21655–21665. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qiu, T. Natural rubber composites reinforced with basic magnesium oxysulfate whiskers: Processing and ultraviolet resistance/flame retardant properties. Polym. Test. 2020, 81, 106271. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Zhu, D.; Xu, N.; Dong, Y.; Li, W. Effects of different compatilizers on mechanical, crystallization, and thermal properties of polypropylene/magnesium oxysulfate whisker composites. J. Adhes. Sci. Technol. 2017, 31, 1839–1857. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wan, C.; Zhang, Y.; Li, R.; Liu, G. Thermal stability, flame retardancy and rheological behavior of ABS filled with magnesium hydroxide sulfate hydrate whisker. Polym. Bull. 2007, 58, 747–755. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Cao, C.; Li, H.; Xiao, L.; Qian, Q.; Huang, B.; Chen, Q. Simultaneously enhanced mechanical properties and thermal properties of ultrahigh-molecular-weight polyethylene with polydopamine-coated α-alumina platelets. Polym. Int. 2019, 68, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Nai, X.; Lan, S.; Bian, S.; Liu, X.; Li, W. Surface modification of magnesium hydroxide sulfate hydrate whiskers using a silane coupling agent by dry process. Appl. Surf. Sci. 2016, 390, 25–30. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The improved performance of a Mg-rich epoxy coating on AZ91D magnesium alloy by silane pretreatment. Corros. Sci. 2012, 60, 165–172. [Google Scholar] [CrossRef]
- Wang, K.; Liu, M.; Song, C.; Shen, L.; Chen, P.; Xu, S. Surface-conductive UHMWPE fibres via in situ reduction and deposition of graphene oxide. Mater. Des. 2018, 148, 167–176. [Google Scholar] [CrossRef]
- Tang, Z.; Zhu, C.; Fan, F.; Qing, B.; Shao, F.; Lei, F.; Deng, X. Green synthesis of the excellent magnesium oxysulfate whiskers under controlled reaction conditions. Mater. Chem. Phys. 2017, 195, 143–148. [Google Scholar] [CrossRef]
- Kang, K.; Lee, D. Synthesis of magnesium oxysulfate whiskers using triethanolamine as a morphology control agent. J. Ind. Eng. Chem. 2014, 20, 2580–2583. [Google Scholar] [CrossRef]
- Savas, L.A.; Tayfun, U.; Dogan, M. The use of polyethylene copolymers as compatibilizers in carbon fiber reinforced high density polyethylene composites. Compos. Part B Eng. 2016, 99, 188–195. [Google Scholar] [CrossRef]
- Cha, J.H.; Choi, H.; Jung, Y.; Choi, S.; An, G.S. Novel synthesis of core-shell structured Fe3O4@SiO2 nanoparticles via sodium silicate. Ceram. Int. 2020, 46, 14384–14390. [Google Scholar] [CrossRef]
- Chen, P.; Wei, B.; Zhu, X.; Gao, D.; Gao, Y.; Cheng, J.; Liu, Y. Fabrication and characterization of highly hydrophobic rutile TiO2-based coatings for self-cleaning. Ceram. Int. 2019, 45, 6111–6118. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. A facile method for fabricating robust cellulose nanocrystal/SiO2 superhydrophobic coatings. J. Colloid Interface Sci. 2019, 536, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, J.; Yang, K.; Guo, J.; Wang, W.; Pan, R.; Wu, G. Simultaneous enhancements of mechanical properties and hydrophilic properties of polypropylene via nano-silicon dioxide modified by polydopamine. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Zhang, N.; Wang, X.; Wei, Q.; Zhang, Y. Impact of titanate coupling agent on properties of high density polyethylene composite filled with coal gangue. Surf. Interface Anal. 2020, 52, 645–655. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Q.; Han, G.; Yao, F.; Kojima, Y.; Suzuki, S. Compatibilizing and toughening bamboo flour-filled HDPE composites: Mechanical properties and morphologies. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1891–1900. [Google Scholar] [CrossRef]
- Gao, C.; Ren, X.; Zhang, J.; Wang, B.; Liu, Y.; Wu, Y. Effect of magnesium hydroxide sulfate hydrate whisker on non-isothermal crystallization kinetics of poly(butylene succinate). Thermochim. Acta 2018, 663, 9–18. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Effects of maleated polypropylene on the morphology, thermal and mechanical properties of short carbon fiber reinforced polypropylene composites. Mater. Des. 2011, 32, 4069–4073. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, Z.; Yang, J.; Yang, B.; Lu, H. Preparation of Thermoplastic Polyester Elastomer/Cerium Carbonate Hydroxide Composites Containing Aluminum Phosphinate with Improved Flame-Retardant and Mechanical Properties. Ind. Eng. Chem. Res. 2015, 54, 11048–11055. [Google Scholar] [CrossRef]
- Oyama, H.T.; Sekikawa, M.; Shida, S. Effect of the interface structure on the morphology and the mechanical, thermal, and flammability properties of polypropylene/poly(phenylene ether)/magnesium hydroxide composites. Polym. Degrad. Stabil. 2012, 97, 755–765. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Xiao, J.; Wang, Z.; Chen, Z.; Fan, W. Magnesium hydroxide sulfate hydrate whisker flame retardant polyethylene/montmorillonite nanocomposites. J. Mater. Sci. 2006, 41, 363–367. [Google Scholar] [CrossRef]
- Cho, J.H.; Vasagar, V.; Shanmuganathan, K.; Jones, A.R.; Nazarenko, S.; Ellison, C.J. Bioinspired Catecholic Flame Retardant Nanocoating for Flexible Polyurethane Foams. Chem. Mater. 2015, 27, 6784–6790. [Google Scholar] [CrossRef]



| MOSw | MOSw@SiO2 | MOSw@SiO2@KH570 | MOSw@SiO2@PDA | |
|---|---|---|---|---|
| ∆Si | ─ | + 4.91% | + 5.98% | + 2.89% |
| ∆N | ─ | ─ | ─ | + 4.03% |
| ∆C | ─ | ─ | + 2.18% | + 7.66% |
| Samples | Tc (°C) | Tp (°C) | Tm (°C) | ∆H (J/g) | Xc (%) |
|---|---|---|---|---|---|
| Neat HDPE | 117.2 | 112.9 | 133.4 | 200.4 | 72.3 |
| HDPE/MOSw | 117.4 | 113.4 | 133.2 | 135.8 | 70.1 |
| HDPE/MOSw@SiO2 | 117.7 | 114.5 | 130.0 | 129.3 | 66.7 |
| HDPE/MOSw@SiO2@KH570 HDPE/MOSw@SiO2@PDA | 118.5 119.0 | 116.3 116.4 | 129.7 129.9 | 126.7 127.3 | 65.3 65.6 |
| Sample | Tonset (°C) | Tmax (°C) | Residue (%) | LOI (%) |
|---|---|---|---|---|
| HDPE | 420 | 462 | - | 19.4 |
| HDPE/MOSw | 353 | 436 | 21.8 | 22.5 |
| HDPE/MOSw@SiO2 | 341 | 467 | 22.0 | 22.6 |
| HDPE/MOSw@SiO2@KH570 | 329 | 468 | 24.3 | 22.9 |
| HDPE/MOSw@SiO2@PDA | 355 | 478 | 22.7 | 23.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhang, Y.; Jiang, G. Surface Modification of Magnesium Oxysulfate Whisker Based on SiO2@silane Coupling Agent and SiO2@polydopamine Double-Layer Structure for Reinforcing HDPE. Materials 2022, 15, 3272. https://doi.org/10.3390/ma15093272
Zhou X, Zhang Y, Jiang G. Surface Modification of Magnesium Oxysulfate Whisker Based on SiO2@silane Coupling Agent and SiO2@polydopamine Double-Layer Structure for Reinforcing HDPE. Materials. 2022; 15(9):3272. https://doi.org/10.3390/ma15093272
Chicago/Turabian StyleZhou, Xiaochen, Yao Zhang, and Guodong Jiang. 2022. "Surface Modification of Magnesium Oxysulfate Whisker Based on SiO2@silane Coupling Agent and SiO2@polydopamine Double-Layer Structure for Reinforcing HDPE" Materials 15, no. 9: 3272. https://doi.org/10.3390/ma15093272
APA StyleZhou, X., Zhang, Y., & Jiang, G. (2022). Surface Modification of Magnesium Oxysulfate Whisker Based on SiO2@silane Coupling Agent and SiO2@polydopamine Double-Layer Structure for Reinforcing HDPE. Materials, 15(9), 3272. https://doi.org/10.3390/ma15093272





