Effect of Rice Husk-Based Silica on the Friction Properties of High Density Polyethylene Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of SiO2 from Rice Husk
2.3. Preparation of HDPE-SiO2 Composites
2.4. Preparation of Test Specimens
2.5. Characterization
3. Results and Discussion
3.1. Characterization of Fillers
3.2. Photography of Pure HDPE and HDPE Composites
3.3. Mechanical Properties of Pure HDPE and HDPE Composites
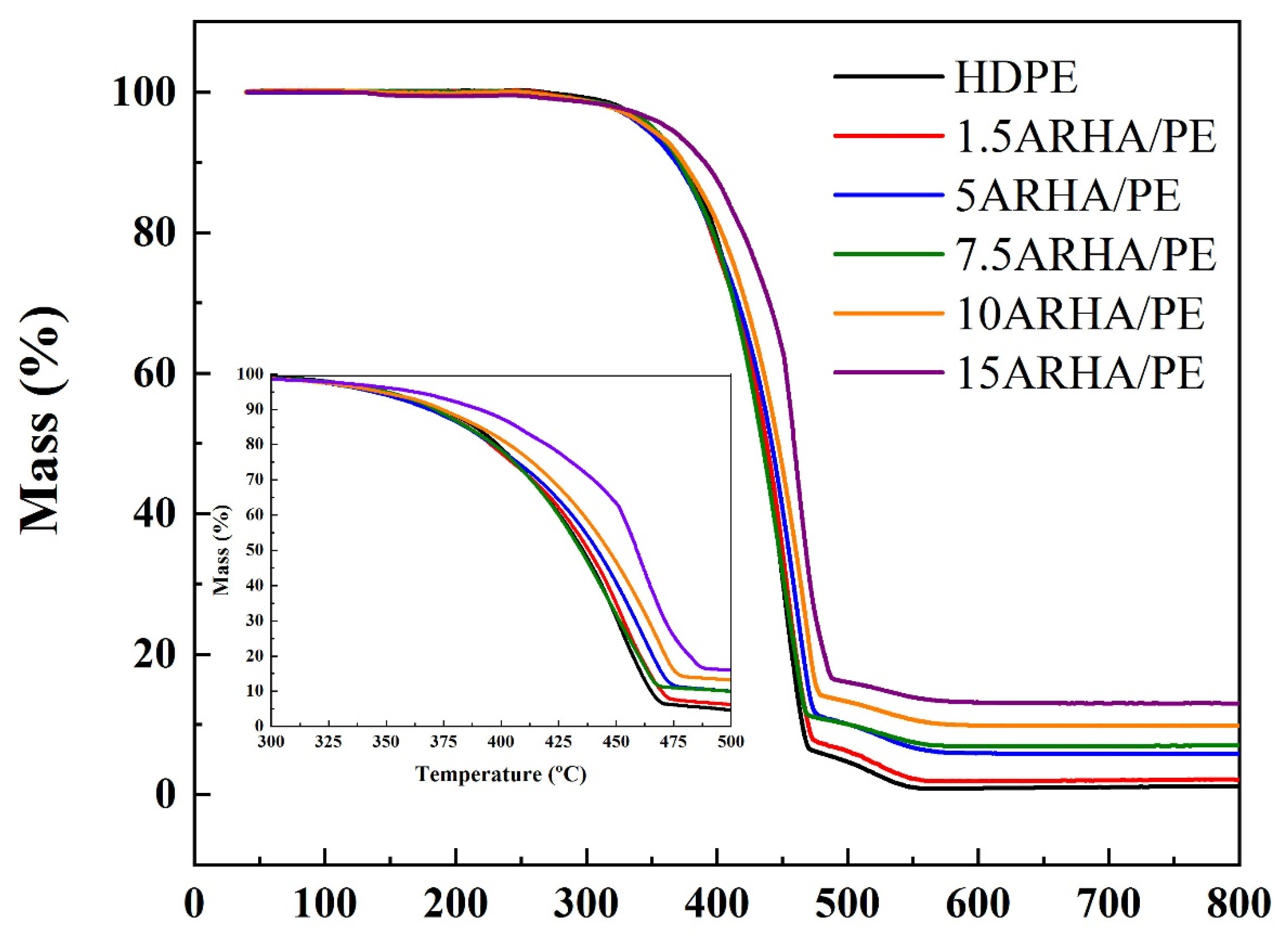
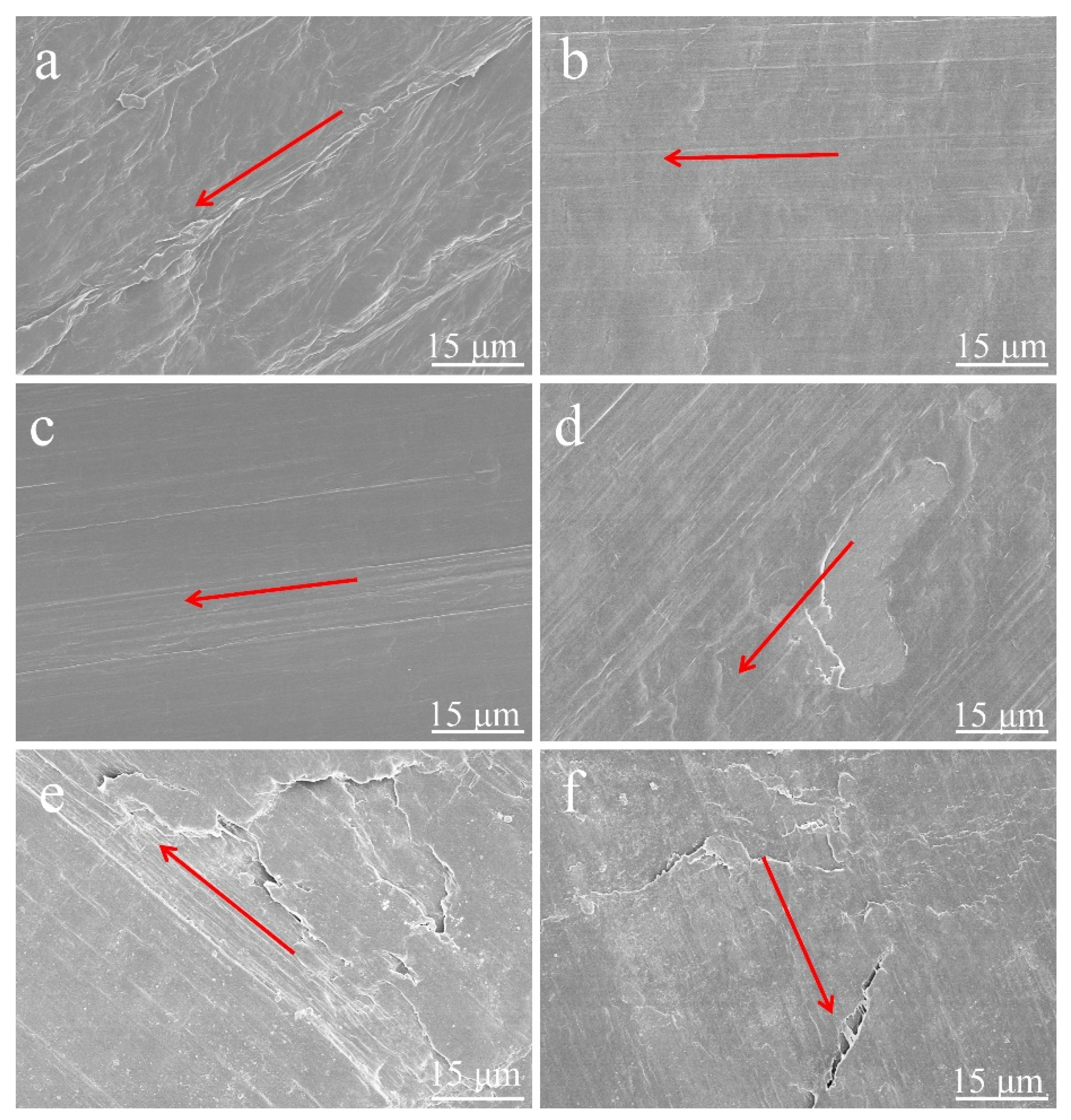
3.4. Friction Properties of Pure HDPE and HDPE Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Gao, G.; Jiang, D.; Yin, Z. Enhancement of the Water-Lubricated Tribological Properties of Hybrid PTFE/Nomex Fabric Laminate Composite via Epoxy Resin and Graphite Filler. Materials 2021, 15, 62. [Google Scholar] [CrossRef]
- Friedrich, K.; Zhang, Z.; Schlarb, A.K. Effects of various fillers on the sliding wear of polymer composites. Compos. Sci. Technol. 2005, 65, 2329–2343. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Q.; Shen, W. The friction and wear properties of nanometre SiO2 filled polyetheretherketone. Tribol. Int. 1997, 30, 193–197. [Google Scholar] [CrossRef]
- Chuang, W.; Geng-Sheng, J.; Lei, P.; Bao-Lin, Z.; Ke-Zhi, L.; Jun-Long, W. Influences of surface modification of nano-silica by silane coupling agents on the thermal and frictional properties of cyanate ester resin. Results Phys. 2018, 9, 886–896. [Google Scholar] [CrossRef]
- Aysa-Martínez, Y.; Anoro-López, S.; Cano, M.; Julve, D.; Pérez, J.; Coronas, J. Synthesis of amorphous magnesium silicates with different SiO2:MgO molar ratios at laboratory and pilot plant scales. Microporous Mesoporous Mater. 2021, 317, 110946. [Google Scholar] [CrossRef]
- Wang, Z.; Smith, A.T.; Wang, W.; Sun, L. Versatile Nanostructures from Rice Husk Biomass for Energy Applications. Angew. Chem. Int. Ed. 2018, 57, 13722–13734. [Google Scholar] [CrossRef]
- Stefani, P.M.; Cyras, V.; Barchi, A.T.; Vazquez, A. Mechanical properties and thermal stability of rice husk ash filled epoxy foams. J. Appl. Polym. Sci. 2006, 99, 2957–2965. [Google Scholar] [CrossRef]
- Sintharm, P.; Phisalaphong, M. Green Natural Rubber Composites Reinforced with Black/White Rice Husk Ashes: Effects of Reinforcing Agent on Film’s Mechanical and Dielectric Properties. Polymers 2021, 13, 882. [Google Scholar] [CrossRef]
- Cheng, Q.; Xu, C.; Huang, W.; Ma, C.; Fan, G.; Yan, J.; Jian, Z.; Zhang, Y.; Song, G. Preparing high purity white carbon black from rice husk. Food Sci. Nutr. 2019, 8, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Krishnarao, R.; Godkhindi, M. Distribution of silica in rice husks and its effect on the formation of silicon carbide. Ceram. Int. 1992, 18, 243–249. [Google Scholar] [CrossRef]
- Patel, M.; Karera, A.; Prasanna, P. Effect of thermal and chemical treatments on carbon and silica contents in rice husk. J. Mater. Sci. 1987, 22, 2457–2464. [Google Scholar] [CrossRef]
- Conradt, R.; Pimkhaokham, P.; Leela-Adisorn, U. Nano-structured silica from rice husk. J. Non-Cryst. Solids 1992, 145, 75–79. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, P.; Upadhyay, S. Thermal degradation of rice husk: Effect of pre-treatment on kinetic and thermodynamic parameters. Fuel 2020, 268, 117164. [Google Scholar] [CrossRef]
- Marcotullio, G.; Krisanti, E.; Giuntoli, J.; de Jong, W. Selective production of hemicellulose-derived carbohydrates from wheat straw using dilute HCl or FeCl3 solutions under mild conditions. X-ray and thermo-gravimetric analysis of the solid residues. Bioresour. Technol. 2011, 102, 5917–5923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Li, J.; Huang, Y.; Alsaedi, A.; Hayat, T.; Xu, X.; Wang, X. Rice husks as a sustainable silica source for hierarchical flower-like metal silicate architectures assembled into ultrathin nanosheets for adsorption and catalysis. J. Hazard. Mater. 2017, 321, 92–102. [Google Scholar] [CrossRef]
- Olugbemide, A.D.; Lajide, L.; Adebayo, A.; Owolabi, B.J. Enhanced Biogas Production from Rice Husk through Solid-State Chemical Pretreatments. Waste Biomass Valorization 2019, 11, 2397–2407. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Pirogovskaya, P.D.; Panasenko, A.Y.; Kovekhova, A.V.; Zemnukhova, L.A. The acid-base properties of amorphous silica from straw and rice husk. J. Chem. Plant Raw Mater. 2021, 327–335. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Comparative study of various pretreatment reagents on rice husk and structural changes assessment of the optimized pretreated rice husk. Bioresour. Technol. 2013, 135, 116–119. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bhattacharya, S.; Narang, H.K. Abaca fibre reinforced polymer composites: A review. J. Mater. Sci. 2020, 56, 4569–4587. [Google Scholar] [CrossRef]
- Ayswarya, E.; Francis, K.V.; Renju, V.; Thachil, E.T. Rice husk ash—A valuable reinforcement for high density polyethylene. Mater. Des. 2012, 41, 1–7. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, B.; Quan, H.; Yam, R.C.; Yuen, K.K.R.; Li, R.K. Flame retardancy of rice husk-filled high-density polyethylene ecocomposites. Compos. Sci. Technol. 2009, 69, 2675–2681. [Google Scholar] [CrossRef]
- Sulaiman, M.; Iqbal, T.; Yasin, S.; Mahmood, H.; Shakeel, A. Study of Nano-Mechanical Performance of Pretreated Natural Fiber in LDPE Composite for Packaging Applications. Materials 2020, 13, 4977. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lo, T.Y.; Memon, S.A. Microstructure and reactivity of rich husk ash. Constr. Build. Mater. 2012, 29, 541–547. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, X.-F.; Sun, R. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.; González, L. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Wang, W.; Martin, J.C.; Zhang, N.; Ma, C.; Han, A.; Sun, L. Harvesting silica nanoparticles from rice husks. J. Nanopart. Res. 2011, 13, 6981–6990. [Google Scholar] [CrossRef]
- Ma, C.; Wu, L.; Dirican, M.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. Carbon black-based porous sub-micron carbon fibers for flexible supercapacitors. Appl. Surf. Sci. 2020, 537, 147914. [Google Scholar] [CrossRef]
- de Carvalho, F.P.; Felisberti, M.I.; Oviedo, M.A.S.; Vargas, M.D.; Farah, M.; Ferreira, M.P.F. Rice husk/poly(propylene-co-ethylene) composites: Effect of different coupling agents on mechanical, thermal, and morphological properties. J. Appl. Polym. Sci. 2011, 123, 3337–3344. [Google Scholar] [CrossRef]
- Zhang, G.; Schlarb, A.; Tria, S.; Elkedim, O. Tensile and tribological behaviors of PEEK/nano-SiO2 composites compounded using a ball milling technique. Compos. Sci. Technol. 2008, 68, 3073–3080. [Google Scholar] [CrossRef]
- Dong, C.; Yuan, C.; Bai, X.; Tian, Y. A novel approach to reduce deformation behaviors of HDPE polymer during friction. Appl. Surf. Sci. 2020, 503. [Google Scholar] [CrossRef]
- Namdeo, R.; Tiwari, S.; Manepatil, S.; Chand, N. Experimental Analysis of Dry Sliding Friction and Wear Behavior of OMMT Nanoclay Added EVA/HDPE/MA-g-PE Compatibilized Polymer Blends with Parametric Study of Influencing Factors. J. Test. Eval. 2018, 48, 1122–1137. [Google Scholar] [CrossRef]
- Zhang, E.; Gao, F.; Fu, R.; Lu, Y.; Han, X.; Su, L. Tribological Behavior of Phenolic Resin-Based Friction Composites Filled with Graphite. Materials 2021, 14, 742. [Google Scholar] [CrossRef] [PubMed]
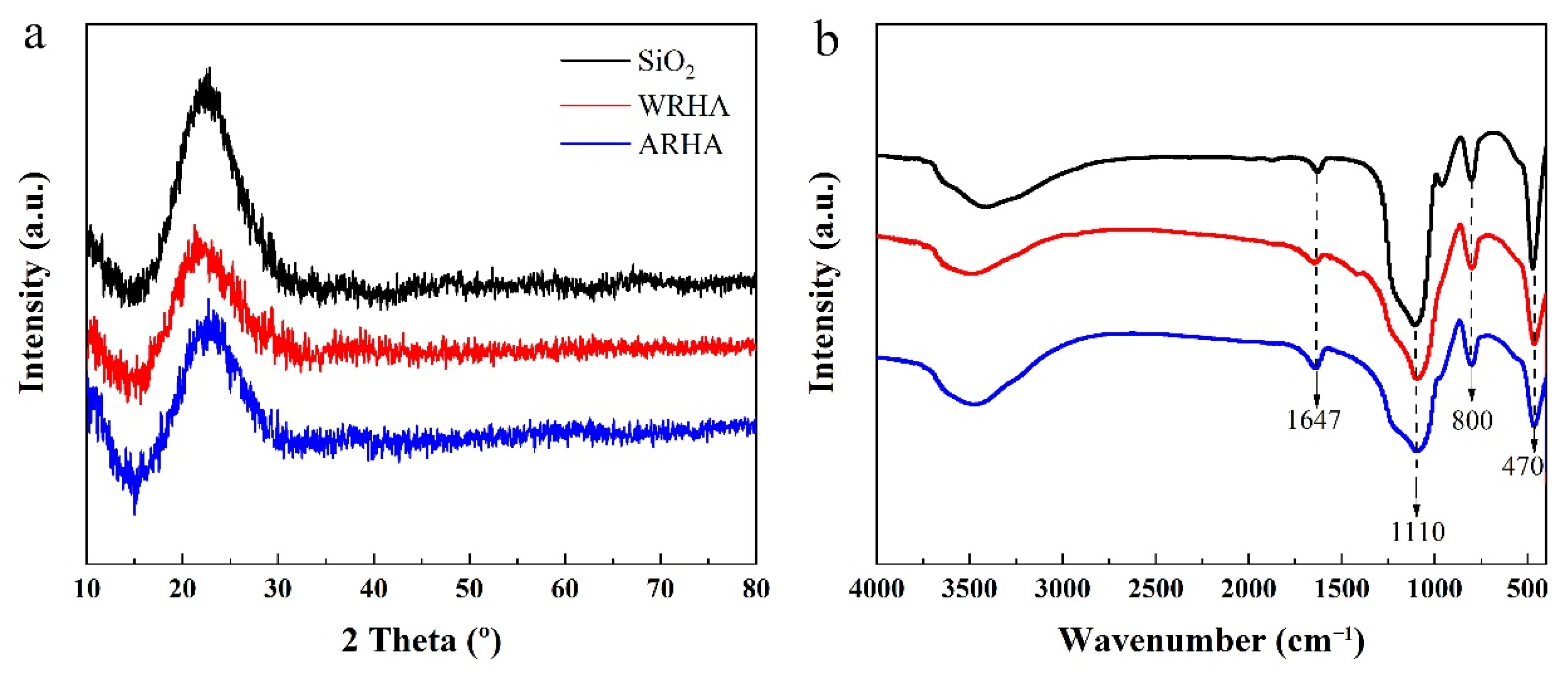

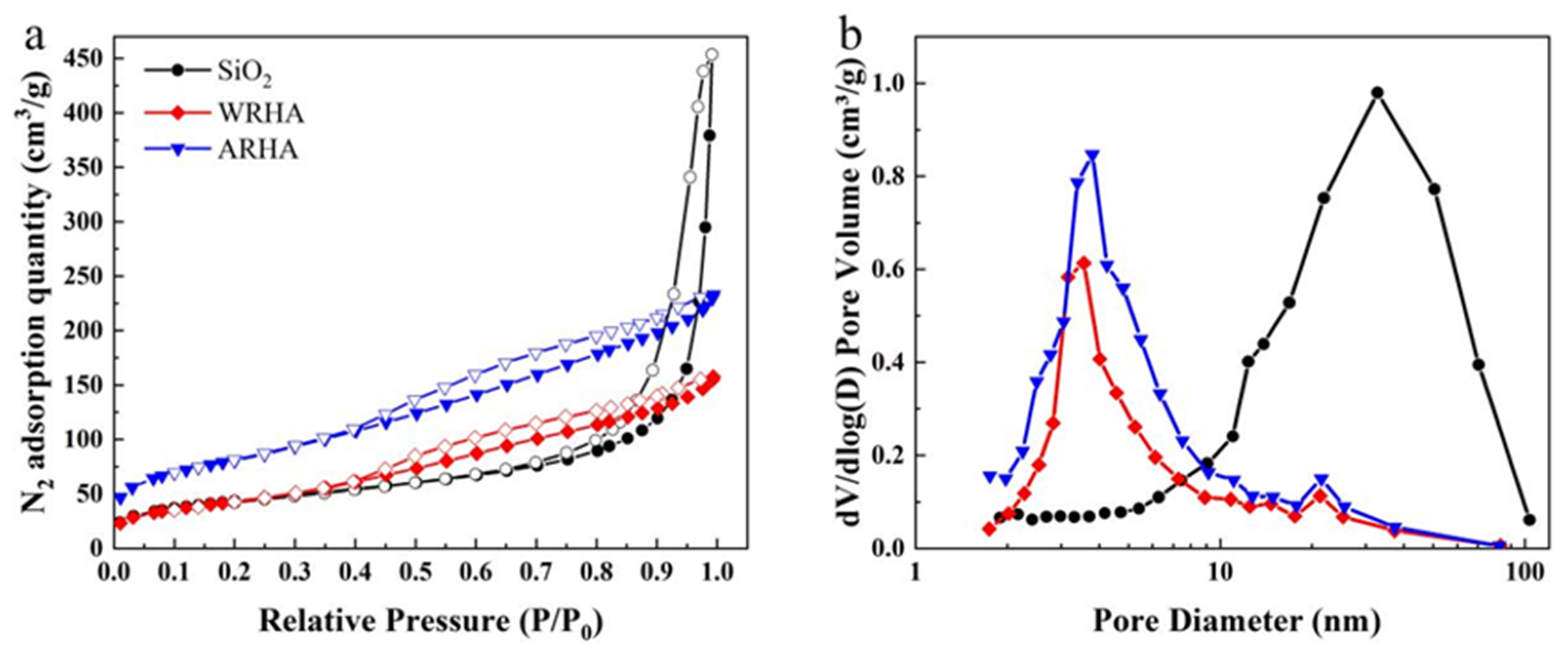
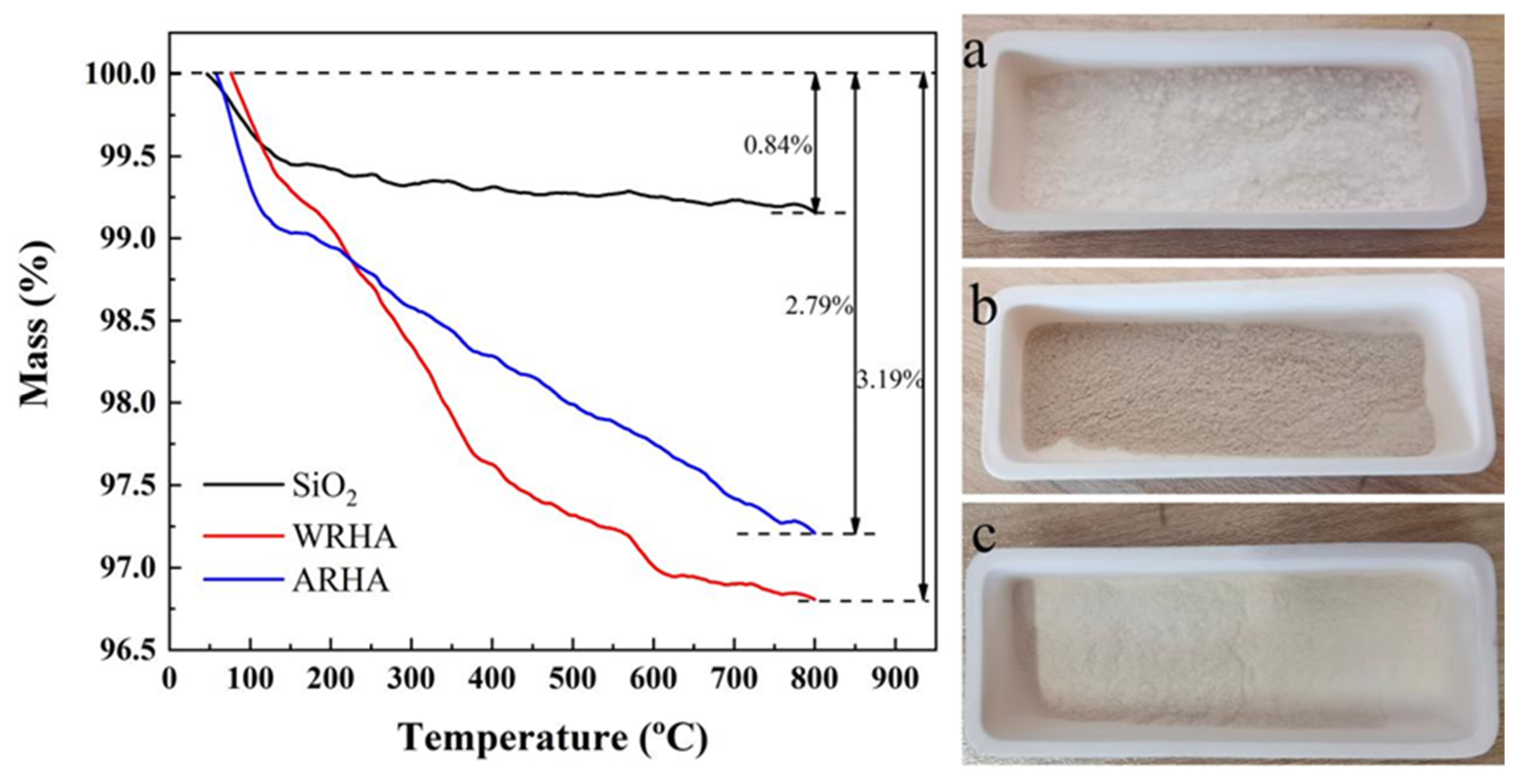
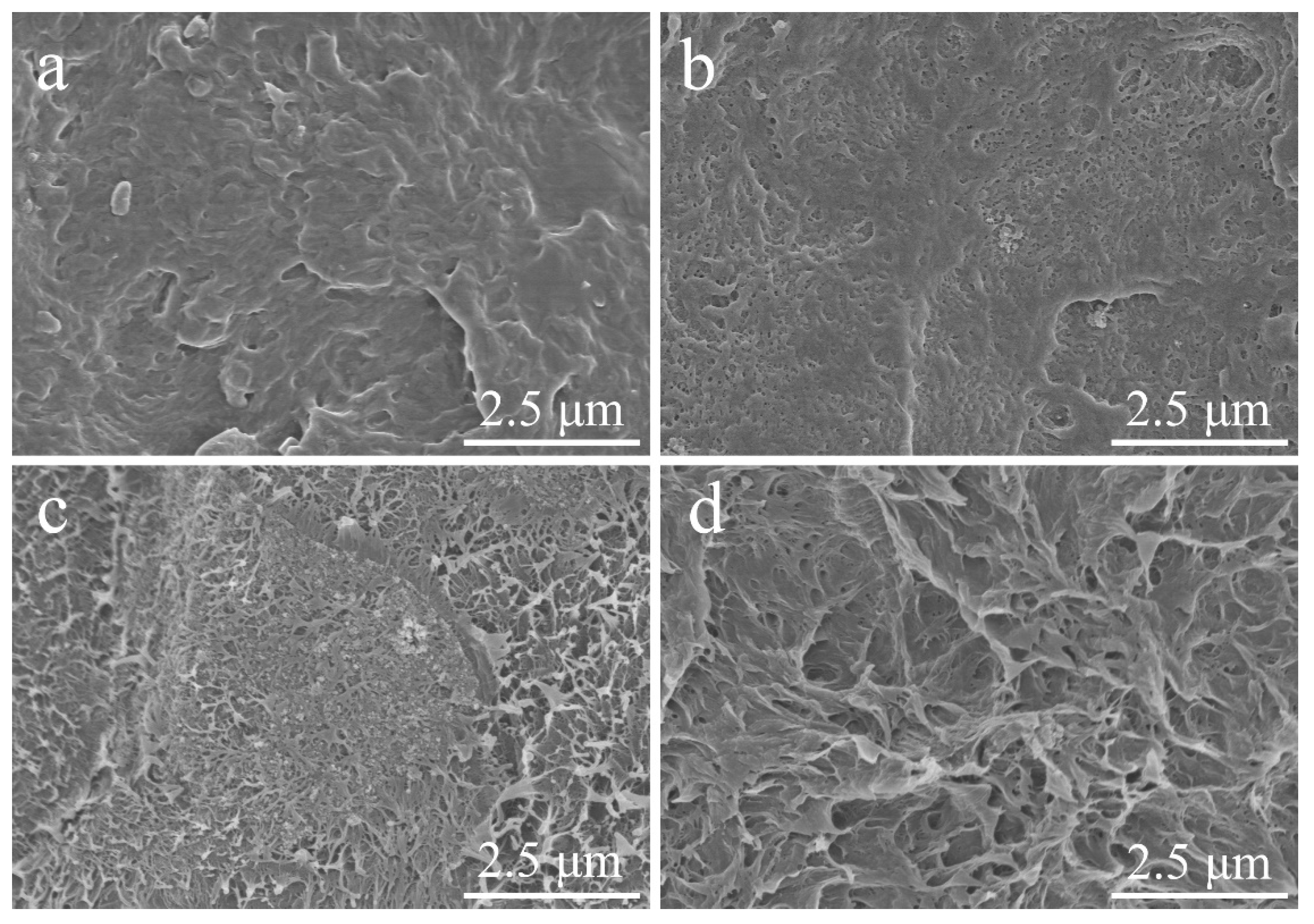



| Samples | Pre-Purification | Temperature (°C) | Air Flow |
|---|---|---|---|
| WRHA | Distilled water | 550 | 180 L/h |
| ARHA | 3 wt.% HCl | 550 | 180 L/h |
| Samples | Composition |
|---|---|
| XSiO2/PE | X g SiO2 + (100 − X) g HDPE |
| XWRHA/PE | X g WRHA + (100 − X) g HDPE |
| XARHA/PE | X g ARHA + (100 − X) g HDPE |
| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| SiO2 | 155.78 | 0.70 | 18.02 |
| WRHA | 158.80 | 0.24 | 6.14 |
| ARHA | 289.91 | 0.36 | 4.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Qian, M.; Wang, X.; Zhang, W.; Zhang, X.; Wang, X.; Zhu, Y. Effect of Rice Husk-Based Silica on the Friction Properties of High Density Polyethylene Composites. Materials 2022, 15, 3191. https://doi.org/10.3390/ma15093191
Shi Y, Qian M, Wang X, Zhang W, Zhang X, Wang X, Zhu Y. Effect of Rice Husk-Based Silica on the Friction Properties of High Density Polyethylene Composites. Materials. 2022; 15(9):3191. https://doi.org/10.3390/ma15093191
Chicago/Turabian StyleShi, Yafei, Miaomiao Qian, Xinru Wang, Wanjia Zhang, Xuewei Zhang, Xiaofeng Wang, and Yanchao Zhu. 2022. "Effect of Rice Husk-Based Silica on the Friction Properties of High Density Polyethylene Composites" Materials 15, no. 9: 3191. https://doi.org/10.3390/ma15093191
APA StyleShi, Y., Qian, M., Wang, X., Zhang, W., Zhang, X., Wang, X., & Zhu, Y. (2022). Effect of Rice Husk-Based Silica on the Friction Properties of High Density Polyethylene Composites. Materials, 15(9), 3191. https://doi.org/10.3390/ma15093191






