The Influence of Precipitate Morphology on the Growth of Austenite Grain in Nb-Ti-Al Microalloyed Steels
Abstract
:1. Introduction
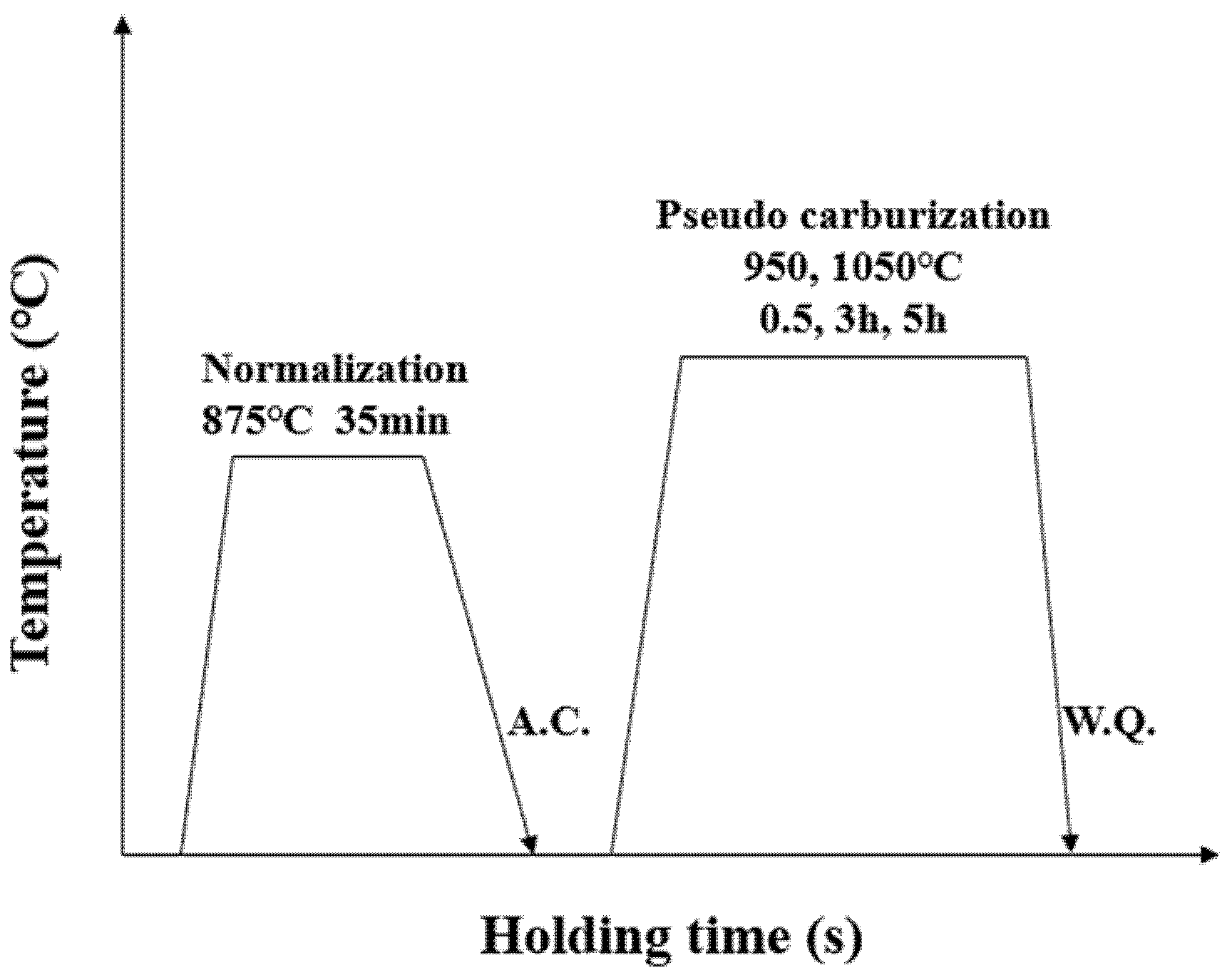
2. Experimental Procedure
3. Results and Discussion
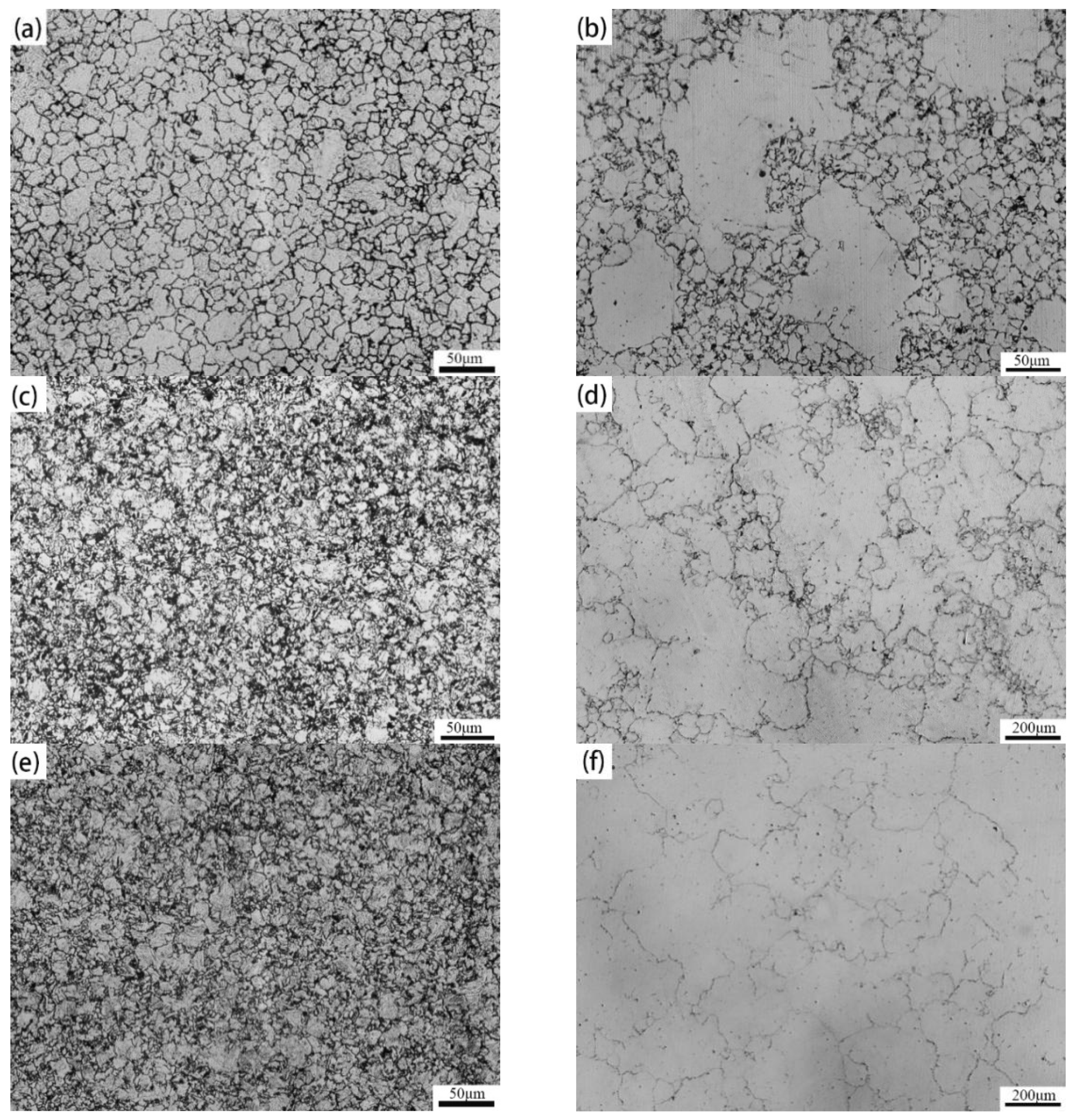
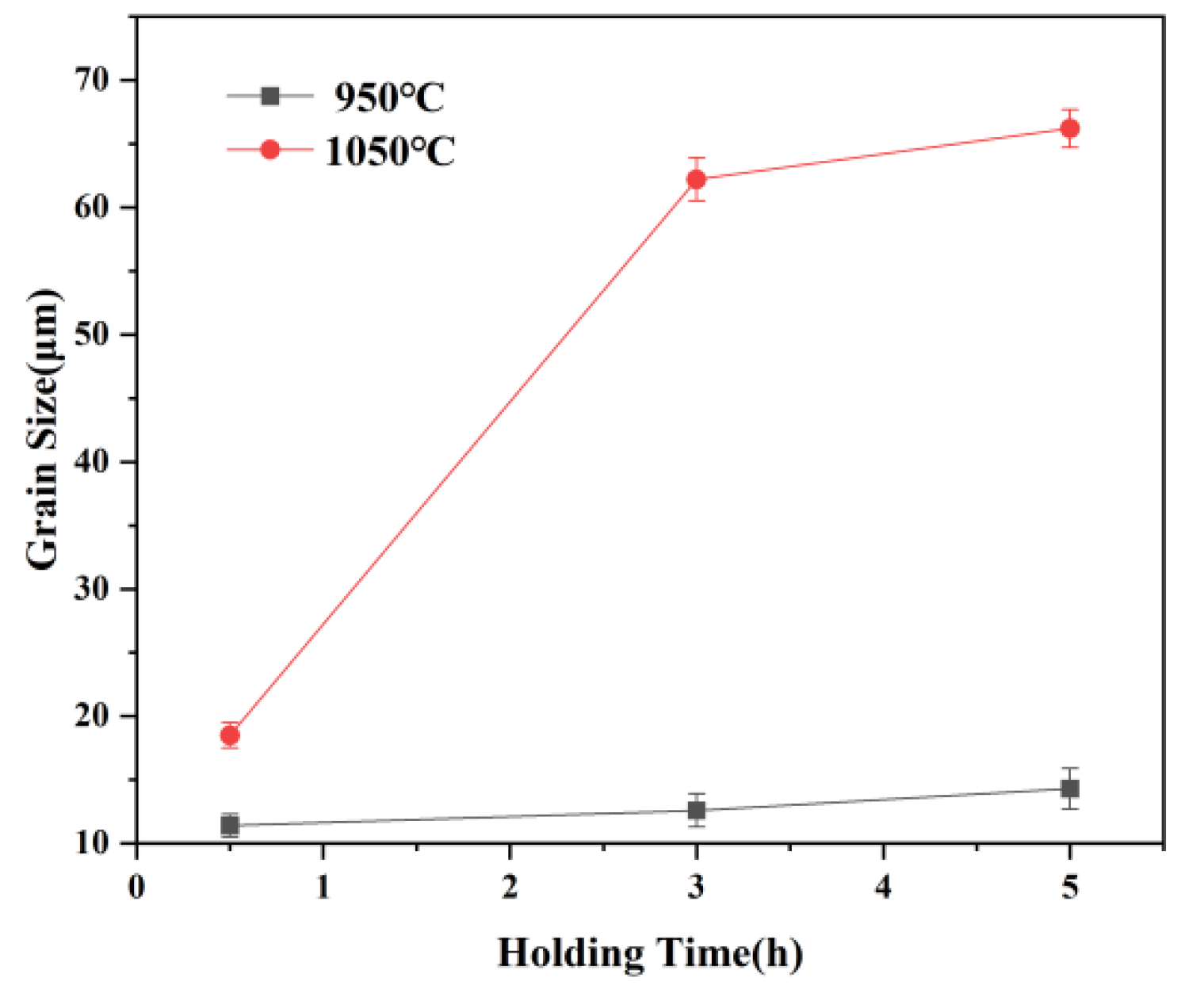
3.1. Evolution of the Austenite Grain Size against Temperature and Times
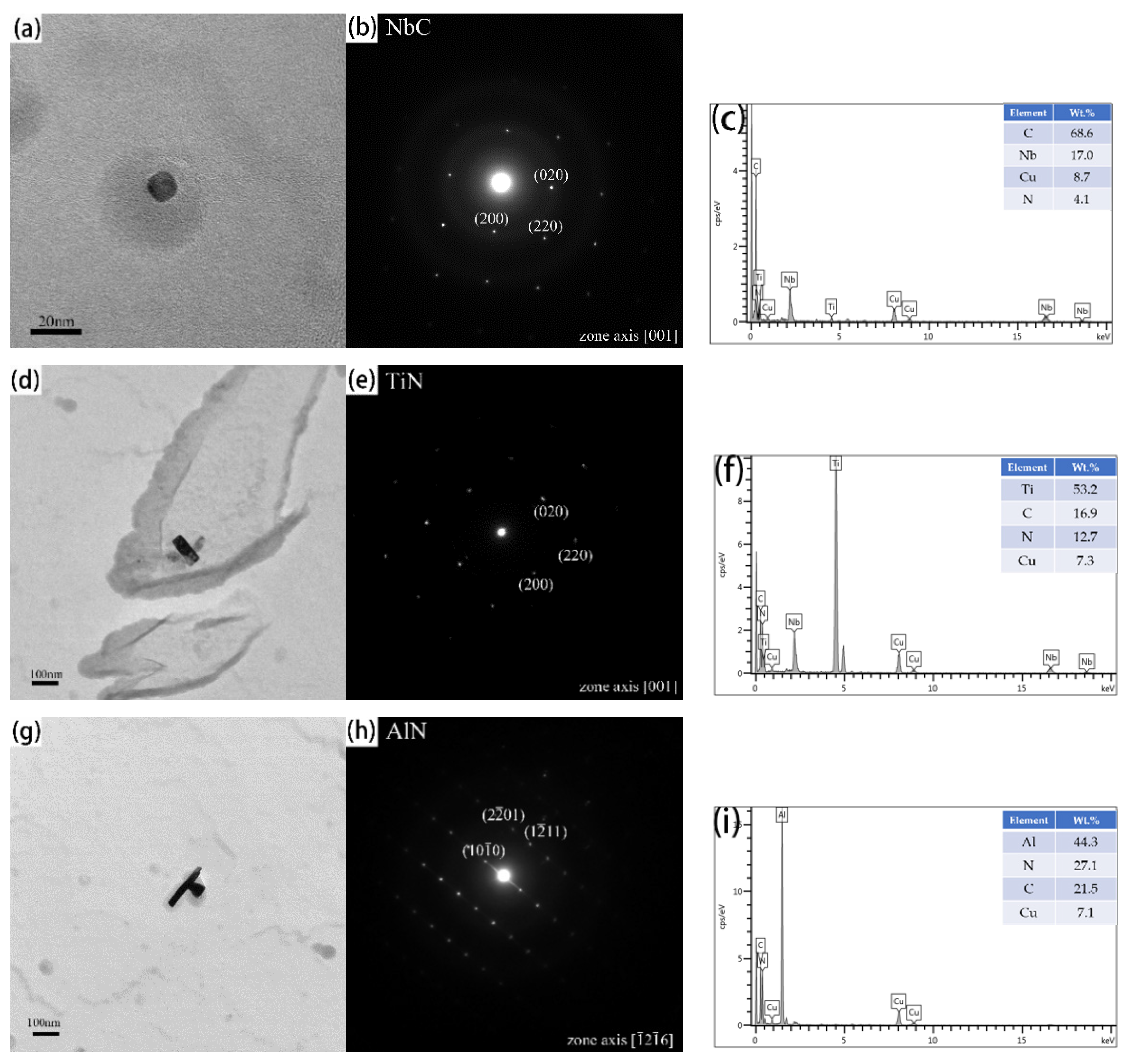
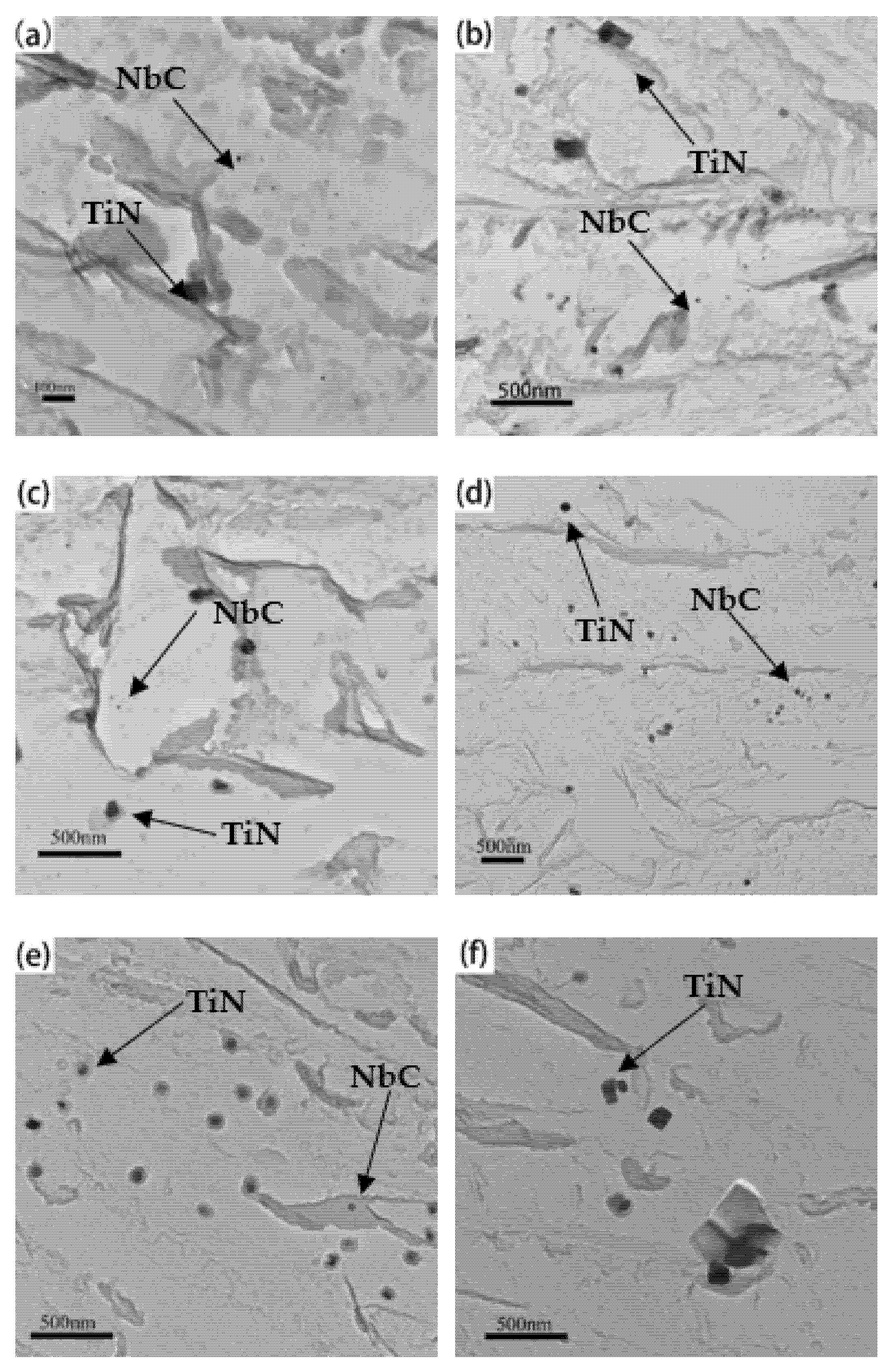
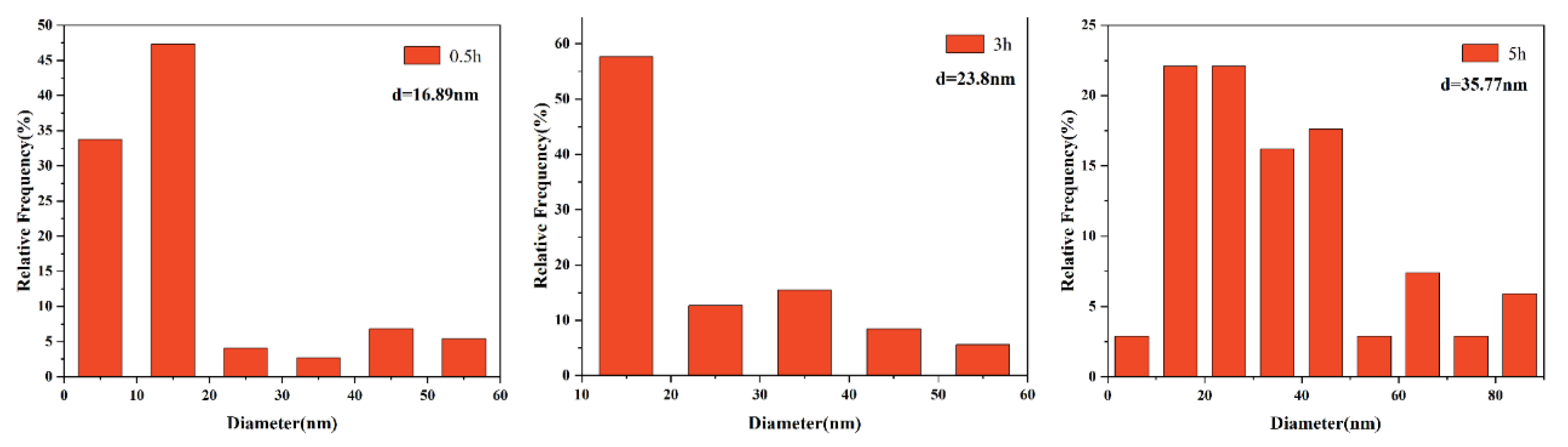
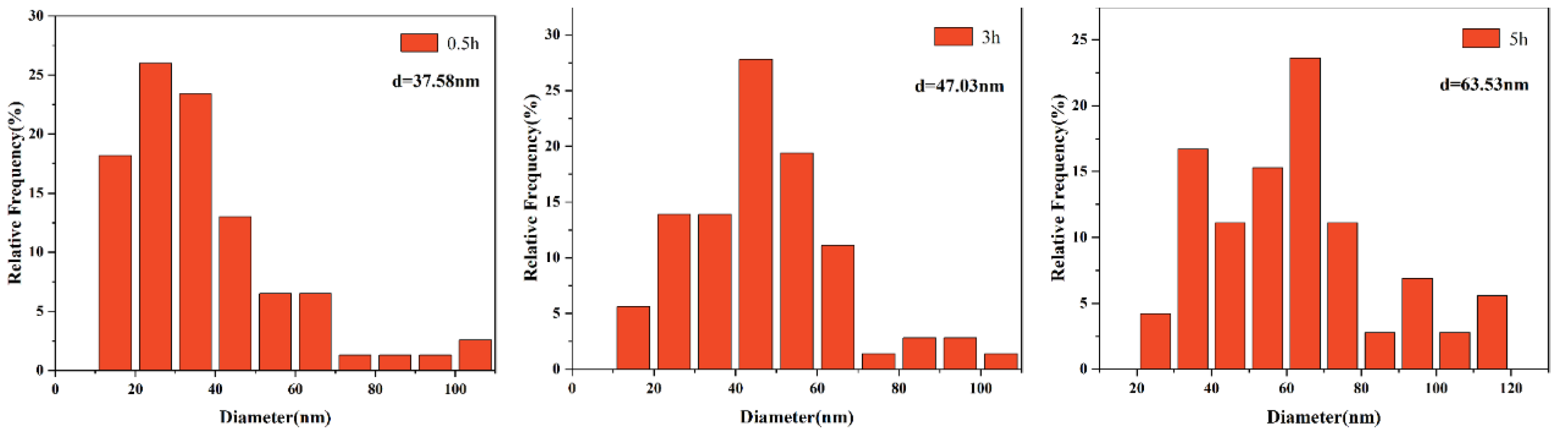
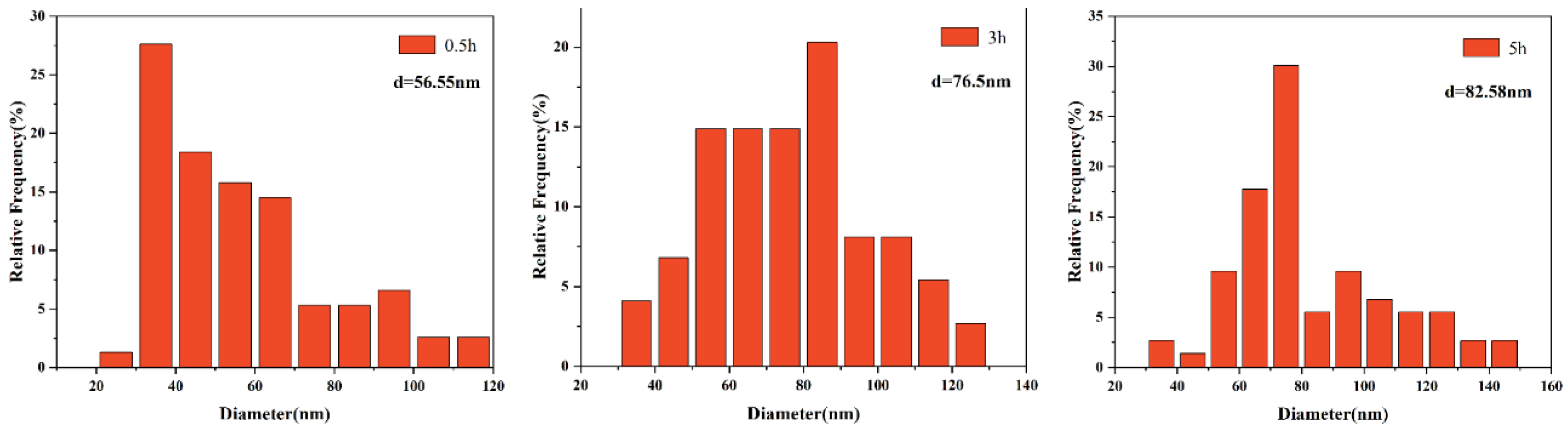
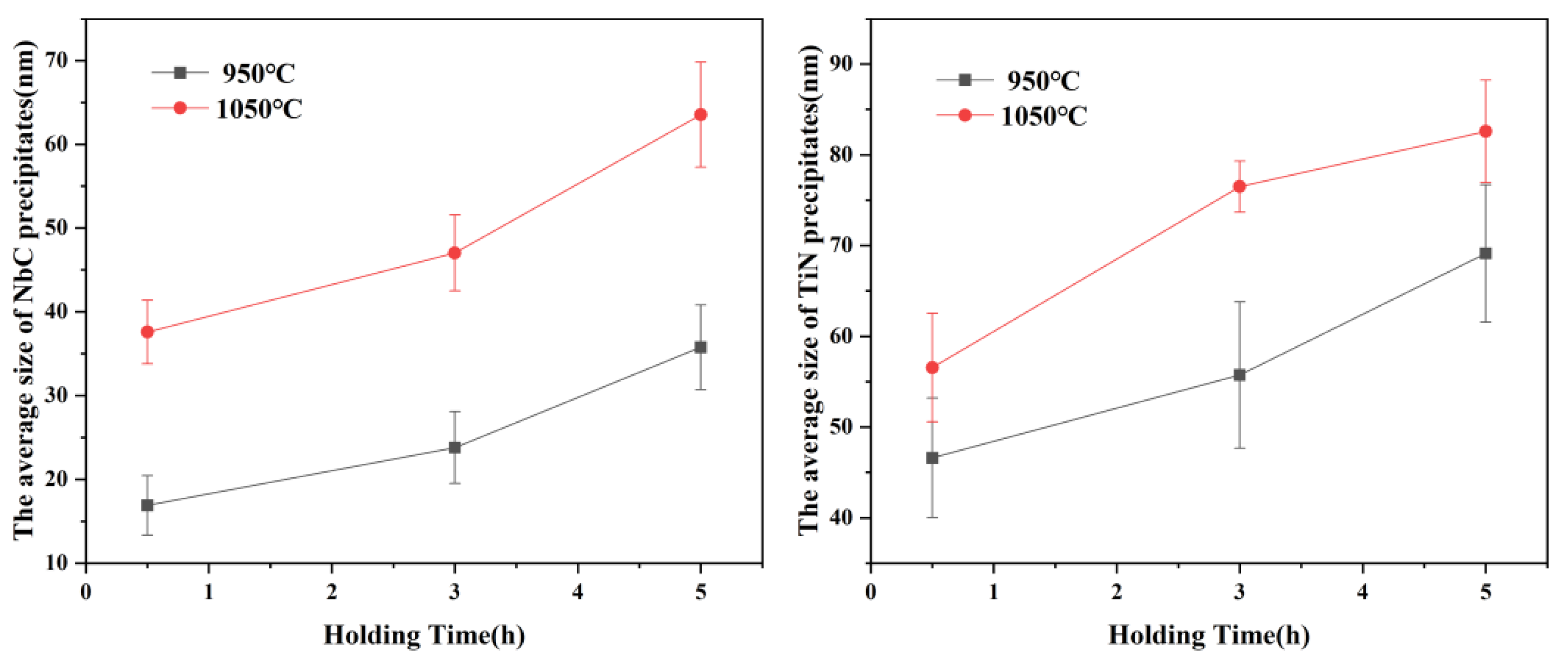
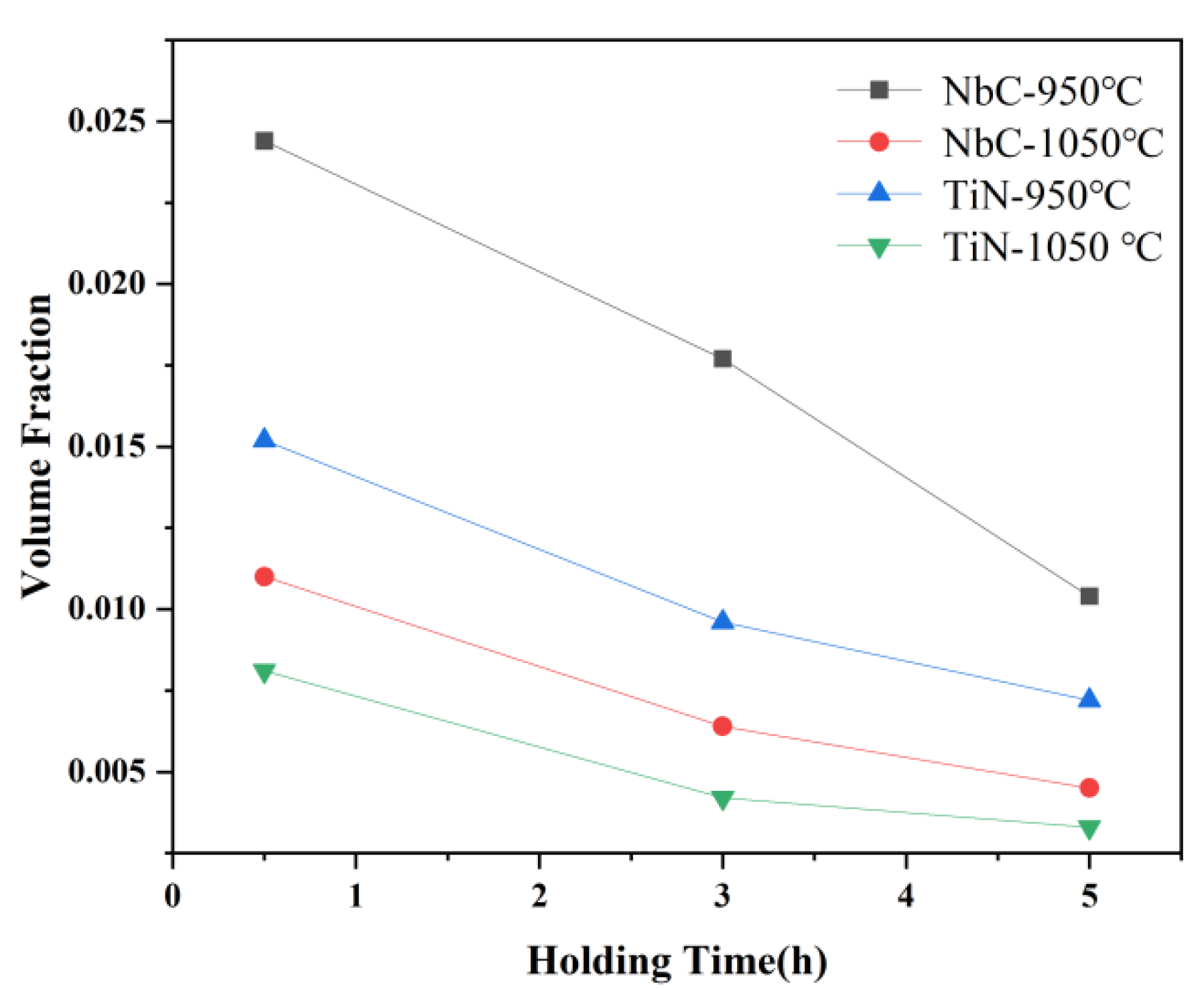
3.2. The Morphological Evolution of Precipitates
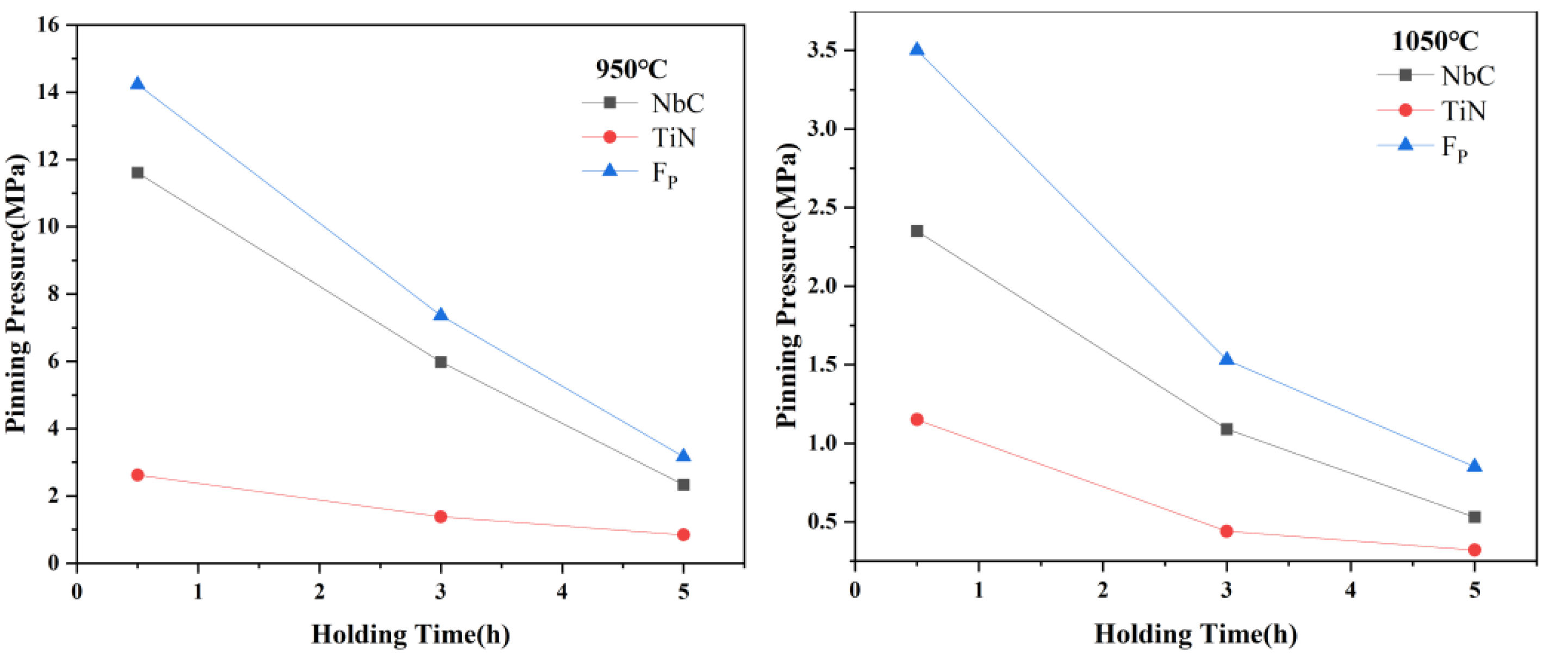
3.3. Effects of Precipitates on the Growth of Austenite Grain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, J.I.; Moren, A.E. Diffusion modeling of the carburization process. Metall. Trans. A 1978, 9A, 1515–1525. [Google Scholar] [CrossRef]
- Kubota, M.; Ochi, T. Development of Anti-Coarsening Steel for Carburizing. Mater. Sci. Forum 2007, 539–543, 4855–4860. [Google Scholar] [CrossRef]
- De Morais, R.F.; Reguly, A.; de Almeida, L.H. Transmission Electron Microscopy Characterization of a Nb Microalloyed Steel for Carburizing at High Temperatures. J. Mater. Eng. Perform. 2006, 15, 494–498. [Google Scholar] [CrossRef]
- Białobrzeska, B.; Konat, Ł.; Jasínski, R. The Influence of Austenite Grain Size on the Mechanical Properties of Low-Alloy Steel with Boron. Metals 2017, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Alogab, K.A.; Matlock, D.K.; Speer, J.G.; Kleebe, H.J. The Influence of Niobium Microalloying on Austenite Grain Coarsening Behavior of Ti-modified SAE 8620 Steel. ISIJ Int. 2007, 47, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Gladman, T. Grain Size Control; Institute of Materials, Minerals & Mining: London, UK, 2004; p. 99. [Google Scholar]
- Saito, G.; Sakaguchi, N.; Ohno, M.; Matsuura, K.; Takeuchi, M.; Sano, T.; Minoguchi, K.; Yamaoka, T. Effects of Cooling Rate after Hot Forging on Precipitation of Fine Particles during Subsequent Normalizing and Austenite Grain Growth during Carburization of Al- and Nb-microalloyed Case-hardening Steel. ISIJ Int. 2021, 61, 1964–1970. [Google Scholar] [CrossRef]
- Zhang, L.C.; Wen, X.L.; Liu, Y.Z. Effect of Precipitates on Austenite Grain Growth Behavior in a Low-Carbon Nb-V Microalloyed Steel. Mater. Sci. Forum. 2017, 898, 783–790. [Google Scholar] [CrossRef]
- Dong, J.; Li, C.; Liu, C.; Huang, Y.; Yu, L.; Li, H.; Liu, Y. Microstructural and mechanical properties development during quenching-partitioning-tempering process of Nb-V-Ti microalloyed ultra-high strength steel. Mater. Sci. Eng. A 2017, 705, 249–256. [Google Scholar] [CrossRef]
- Staśko, R.; Adrian, H.; Adrian, A. Effect of nitrogen and vanadium on austenite grain growth kinetics of a low alloy steel. Mater. Charact. 2006, 56, 340–347. [Google Scholar] [CrossRef]
- Khalid, F.A.; Edmonds, D.V. Interphase Precipitation in Microalloyed Engineering Steels and Model Alloy. Mater. Sci. Technol. 1993, 9, 384–396. [Google Scholar] [CrossRef]
- Han, K.; Mottishaw, T.D.; Smith, G.D.W.; Edmonds, D.V. Effects of Vanadium Addition on Nucleation and Growth of Pearlite in High-Carbon Steel. Mater. Sci. Technol. 1994, 10, 955–963. [Google Scholar] [CrossRef]
- Han, K.; Smith, G.D.W.; Edmonds, D.V. Pearlite Phase-Transformation in Si and V Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1995, 26, 1617–1631. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, D.V. Innovation in the processing of tonnage materials: Examples from the steel and aluminium industries. J. Mater. Process. Technol. 1998, 83, 1–13. [Google Scholar] [CrossRef]
- Han, K.; Edmonds, D.V.; Smith, G.D.W. Optimization of mechanical properties of high-carbon pearlitic steels with Si and V additions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 1313–1324. [Google Scholar] [CrossRef] [Green Version]
- Inque, K.; Onhuma, I.; Ohtani, H.; Ishida, K.; Nishizawa, T. Solubility product of TiN in austenite. ISIJ Int. 1998, 38, 991–997. [Google Scholar] [CrossRef]
- Saito, G.; Sakaguchi, N.; Ohno, M.; Matsuura, K.; Takeuchi, M.; Sano, T.; Minoguchi, K.; Yamaoka, T. Effects of Concentrations of Micro-alloying Elements and Hot-forging Temperature on Austenite Grain Structure Formed during Carburization of Case-hardening Steel. ISIJ Int. 2020, 60, 2549–2557. [Google Scholar] [CrossRef]
- Wada, H.; Pehilke, R.D. Nitrogen Solubility and Nitride Formation in Austenitic Fe-Ti Alloys. Metall. Trans. B 1985, 16B, 815–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Liu, Y.; Liu, C.; Dong, J.; Yu, L.; Li, H. Study of the kinetics of austenite grain growth by dynamic Ti-rich and Nb-rich carbonitride dissolution in HSLA steel: In-situ observation and modeling. Mater. Charact. 2020, 169, 110612. [Google Scholar] [CrossRef]
- Sha, Q.; Sun, Z. Grain growth behavior of coarse-grained austenite in a Nb–V–Ti microalloyed steel. Mater. Sci. Eng. A 2009, 523, 77–84. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Graux, A.; Cazottes, S.; De Castro, D.; San Martín, D.; Capdevila, C.; Cabrera, J.M.; Molas, S.; Schreiber, S.; Mirković, D.; Danoix, F.; et al. Precipitation and grain growth modelling in Ti-Nb microalloyed steels. Materialia 2019, 5, 100233. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Liu, W.; Xu, Y.; Chen, C.; Yang, Y.; Luo, J.; Zhang, K. B2 Grain Growth Behavior of a Ti-22Al-25Nb Alloy Fabricated by Hot Pressing Sintering. J. Mater. Eng. Perform. 2018, 27, 2288–2297. [Google Scholar] [CrossRef]
- Maalekian, M.; Radis, R.; Militzer, M.; Moreau, A.; Poole, W.J. In situ measurement and modelling of austenite grain growth in a Ti/Nb microalloyed steel. Acta Mater. 2012, 60, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Koyama, S.; Teruo, I.; Kiichi, N. Solubility of Niobium Carbide and Nitride in Austenite of Low Alloy Steel. Nihon Kinzoku Gakkai Shi 1972, 36, 775–778. [Google Scholar] [CrossRef]
- Yan, B.; Liu, Y.; Wang, Z.; Liu, C.; Si, Y.; Li, H.; Yu, J. The Effect of Precipitate Evolution on Austenite Grain Growth in RAFM Steel. Materials 2017, 10, 1017. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.W.; Karjalainen, L.P.; Qian, B.; Chen, X. Nonuniform Distribution of Carbonitride Particles and Its Effect on Prior Austenite Grain Size in the Simulated Coarse-grained Heat-affected Zone of Thermomechanical Control-processed Steels. Metall. Mater. Trans. A 1996, 27, 4031–4038. [Google Scholar] [CrossRef]
- Rohrer, G.S. Introduction to Grains, Phases, and Interfaces—An Interpretation of Microstructure. Trans. AIME, 1948, vol. 175, pp. 15–51, by C.S. Smith. Metall. Mater. Trans. A 2010, 41, 1063–1100. [Google Scholar] [CrossRef] [Green Version]
- Militzer, M.; Giumelli, A.; Hawbolt, E.B.; Meadowcroft, T.R. Austenite Grain Growth Kinetics in Al-killed Plain Carbon Steels. Metall. Mater. Trans. A 1996, 27, 3399–3409. [Google Scholar] [CrossRef]
- Banerjee, K.; Militzer, M.; Perez, M.; Wang, X. Nonisothermal Austenite Grain Growth Kinetics in a Microalloyed X80 Linepipe Steel. Metall. Mater. Trans. A 2010, 41, 3161–3172. [Google Scholar] [CrossRef]

| Element | C | Si | Mn | Cr | Nb | Ti | Al | [N] |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 0.23 | 0.23 | 0.70 | 1.10 | 0.055 | 0.030 | 0.036 | 0.0134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Xiao, Y.; Min, N.; Li, W.; Zhao, S. The Influence of Precipitate Morphology on the Growth of Austenite Grain in Nb-Ti-Al Microalloyed Steels. Materials 2022, 15, 3176. https://doi.org/10.3390/ma15093176
Yuan J, Xiao Y, Min N, Li W, Zhao S. The Influence of Precipitate Morphology on the Growth of Austenite Grain in Nb-Ti-Al Microalloyed Steels. Materials. 2022; 15(9):3176. https://doi.org/10.3390/ma15093176
Chicago/Turabian StyleYuan, Junfeng, Yang Xiao, Na Min, Wei Li, and Sixin Zhao. 2022. "The Influence of Precipitate Morphology on the Growth of Austenite Grain in Nb-Ti-Al Microalloyed Steels" Materials 15, no. 9: 3176. https://doi.org/10.3390/ma15093176
APA StyleYuan, J., Xiao, Y., Min, N., Li, W., & Zhao, S. (2022). The Influence of Precipitate Morphology on the Growth of Austenite Grain in Nb-Ti-Al Microalloyed Steels. Materials, 15(9), 3176. https://doi.org/10.3390/ma15093176






