Abstract
Objectives: The present systematic review and meta-analysis aims to analyse the clinical performance of short compared to longer implants inserted in sites without the need for bone augmentation. Methods: The protocol of the present PRISMA-driven meta-analysis was registered on PROSPERO (CRD42021264781). Electronic and manual searches were performed up to January 2022. All Randomized Controlled Trials (RCTs) comparing short (≤6 mm) to longer (≥8.5 mm) implants placed in non-atrophic and non-augmented sites were included. The quality of the included studies was assessed using the Cochrane risk of bias tool for randomized clinical trials (RoB 2) and the quality of evidence was determined with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. A meta-analysis was performed on implant survival rate, marginal bone level change (MBLc), and technical and biological complications at the available follow-up time points. The power of the meta-analytic findings was determined by trial sequential analysis (TSA). Results: From 1485 initial records, 13 articles were finally included. No significant difference was found in the survival rate between short and long implant at any follow-up (moderate quality of evidence). Significantly more bone loss for long implants at 1 and 5 years from implant placement and more technical complications with short implants at 10 years were found. No other significant inter-group differences in terms of MBLc and biological complications were detected. Conclusions: Moderate evidence exists suggesting that short implants perform as well as longer ones in the rehabilitation of edentulous sites without the need for bone augmentation. Further long-term, well-designed RCTs, however, are still needed to provide specific evidence-based clinical recommendations for an extended use of short implants in non-atrophic sites.
1. Introduction
The rehabilitation of edentulous patients with dental implants constitutes a stable and highly predictable acquisition of modern dentistry, corroborated by well-documented clinical results and long survival [1,2]. The presence of an adequate bone volume at the edentulous site still represents a basic requirement for dental implants to be correctly placed. Dimensional reduction of the alveolar process, however, usually happens after tooth extraction, and adjunctive loss of bone may be caused by trauma, periodontal disease, or atrophy [3]. In these cases, additional reconstructive surgeries, e.g., vertical bone augmentation procedures or sinus lift, may be applied to allow standard-length implant placement, although they invariably associate with relevant limitations, such as longer treatment time, additional costs, increased postoperative morbidity, and a higher risk of complications [4,5,6,7,8].
A widely documented alternative to augmentation surgical procedures is represented by the rehabilitation of atrophic edentulous sites with implants of reduced length, which have reached a considerable diffusion in the last few years with very promising clinical results [9,10,11,12].
Several advantages are linked to the use of short implants, including ease of handling, reduced surgical invasiveness, and a low risk of injuring noble anatomical structures, thus sustaining the concept of a “stress minimizing surgery” [9]. Short implants, however, are not free from risks and complications, due to the higher crown-implant ratio and to the lower bone-to-implant contact area with respect to longer fixtures [13].
The clinical performance of short implants has been widely investigated in the recent literature, with not univocal findings. Higher failure rates have been historically associated with short implants compared to longer ones [14,15]. More recent studies showed, for short implants, clinical outcomes comparable to, or even better than, longer implants placed in both native or augmented bone, as confirmed by several systematic reviews [8,12,16,17,18,19,20,21,22,23,24,25].
The reasons for such a great variability may be found in several elements of heterogeneity among studies.
The definition of “short dental implant”, for instance, greatly differs from <10 mm to ≤8 mm and, more recently, ≤7 mm [15,26,27,28]. In this sense, in the 2015 EAO consensus on the use of short implants for dental rehabilitation [11], the authors recommend, for future research, to identify the minimal length for a predictable survival of implants (redefining the concept itself of “short” implant) and to perform clinical trials with an appropriate design to reliably compare these therapeutic concepts in the long term.
Furthermore, one should consider that the greatest part of the existing studies are designed to compare short implants placed in pristine atrophic bone with long implants inserted in augmented sites. Furthermore, short- and medium-term data (1 to 5 years from loading) are prevalent, and longer follow-ups are lacking.
In the 2018 ITI Consensus Conference [12], the authors highlight, in the recommendation for future research, the need for RCTs comparing short and longer implants in pristine bone sites without the need for vertical bone augmentation.
To reliably compare short and long implants, the same clinical scenario should be guaranteed to both groups: similar bone quality and height (which implies the placement of short implants in sites able to receive longer ones), as well as same prosthetic design to rehabilitate the same type of edentulism.
Such strictly designed comparisons would provide, on one hand, further support to the efficacy of short implants in their routine use in case of atrophic bone, and, on the other hand, could also help to extend their clinical indications to their use also in case of non-atrophic sites, instead of long implants.
The aim of the present systematic review and meta-analysis is to analyse the available scientific evidence from randomized controlled trials (RCTs) regarding the clinical performance of short (≤6 mm) compared to longer (≥8.5 mm) implants exclusively placed in sites without the need for bone augmentation.
2. Materials and Methods
2.1. Study Registration
The review protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews with the identification number CRD42021264781.
2.2. Reporting Format
The 27-item Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [29] was followed for the summary and description of the search process results.
Patient, Intervention, Comparison, Outcome, Time (PICOT) question
- The focused question was formulated following the PICOT format [30], where:
- Patients (P): Patients receiving fixed rehabilitations supported by implants placed in sites without bone augmentation procedures in the mandible and/or the maxilla.
- Intervention (I): dental implants with length ≤ 6 mm.
- Comparison (C): dental implants with length ≥ 8.5 mm.
- Outcome (O): implant survival rate as primary outcome, marginal bone level change, and biological and technical complication rate as secondary outcomes.
- Time (T): follow-up ≥ 1 year from prosthetic loading.
2.3. Focused Question
Do short (≤6 mm) implants perform as well as longer dental implants (≥8.5 mm) placed in sites without the need for bone augmentation?
2.4. Information Sources and Search Strategy
A computerized, systematic search of literature was performed using MEDLINE (PubMed, www.ncbi.nlm.nih.gov/pubmed, (accessed on 17 January 2022)), EMBASE, SCOPUS, clinicaltrial.gov, open grey until 17 January 2022. No date or language restriction was applied. The detailed search strategy for each electronic database consulted is presented in Table 1.

Table 1.
Detailed search strategy for electronic databases.
A manual search was performed on the major journals of implantology: Clinical Implant Dentistry and Related Research, Clinical Oral Implant Research, Clinical Oral Investigations, International Journal of Periodontics and Restorative Dentistry, Journal of Clinical Periodontology, Journal of Dental Research, Journal of Dentistry Journal of Periodontal Research, Journal of Periodontology.
Corresponding authors were contacted as needed to obtain information about missing data or unpublished material.
2.5. Outcome Variables
The primary outcome was considered the implant survival rate (SR), defined as the ratio between the number of implants still present at the follow-up and those originally randomized (intention to treat analysis), at implant level.
As secondary outcomes, the following outcomes were considered:
- Marginal bone level change (MBLc), defined as the difference in crestal bone height between baseline and follow-up measures, considered at patient or implant level.
- Technical complication rate, considered at patient level, concerning the number of any technical complication, such as prosthesis fracture, screw loosening or fracture, implant fracture, etc., occurred until the follow-up.
- Biological complication rate, considered at patient level, concerning the number of biological complications, i.e., peri-implant mucositis and peri-implantitis, occurred until the follow-up.
2.6. Eligibility Criteria
Studies were deemed eligible if they met the following inclusion criteria:
- (a)
- RCTs comparing short (≤6 mm) implants in the test group and longer implants (≥8.5 mm) in the control group;
- (b)
- studies with a follow-up period of at least 12 months from prosthetic loading;
- (c)
- studies in which the implants were restored with a fixed prosthesis;
- (d)
- studies where both test and control implants were placed exclusively in sites without the need for bone augmentation in the mandible and/or maxilla.
The following studies were excluded:
- (a)
- preclinical in vitro or animal studies;
- (b)
- case reports and case series;
- (c)
- prospective and retrospective observational studies;
- (d)
- non-randomized controlled trials;
- (e)
- reviews and meta analysis;
- (f)
- studies with insufficient information for any quantitative analysis.
2.7. Population Characteristics
In accordance with the study design, two patient groups were created: patients with short implants (≤6 mm) and longer dental implants (≥8.5 mm).
2.8. Study Selection and Data Extraction
All articles were initially screened by two independent reviewers based on titles and abstracts and imported to a reference manager to remove duplicates. Afterwards, full texts were carefully examined and included or excluded using a predetermined data extraction form based on the aforementioned eligibility criteria. Any disagreement was resolved via discussion between the two reviewers. The level of agreement between the reviewers regarding study inclusion was calculated using kappa coefficient. Data concerning patient and treatment characteristics, as well as clinical outcomes for each available follow-up, were independently extracted from all the eligible studies by two reviewers. When more articles referred to the same study, data and information reported were compared and, if possible, integrated. In the case of conflicting data among articles referring to the same study, only one of them was chosen. When additional information was required, authors were contacted. If no or inconclusive responses were obtained, data were excluded from the analysis.
2.9. Meta-Analysis
A meta-analysis was performed on implant survival rate as primary outcome and MBLc and technical and biological complications as secondary outcomes. A separate analysis was performed on the baseline chosen for MBLc measurements (prosthetic loading or implant placement). Following the principles of “intention-to-treat” analysis, the total number of initially randomized patients (or implants) for each included study was considered for calculation of the overall effect size of implant survival rate and complications. When data at implant and patient level were both reported in the same study, patient-level data were preferred. MBLc data were separately analysed from both implant placement and prosthetic loading as baseline; MBLc data provided by studies performing immediate loading were included in both the analyses. Negative and positive MBLc values were used to indicate bone loss and bone gain, respectively. Effect sizes were displayed as mean difference (MD) or risk ratio (RR) for continuous and dichotomous variables, respectively, with 95% confidence intervals. Forest plots were created to illustrate the effects of the different studies and global estimation.
RevMan 5 software (Review Manager, version 5.4, the Cochrane Collaboration, 2020, London, UK) was used to perform the statistical analyses. Statistical significance was defined as a p-value < 0.05. The study-specific estimates were pooled with the random-effects models if heterogeneity across tested trials with the Chi2 (Cochran Q) test (p < 0.1) and I2 statistics > 50% proved to be high [31]. If the meta-analysis contained a sufficient number of trials to make a visual inspection of the plot meaningful (ten trials minimum), funnel plots were considered as a tool for assessment of publication bias.
2.10. Trial Sequential Analysis
Trial Sequential Analysis (TSA) was performed for the main outcome (implant survival), to evaluate the power of the meta-analysis and to adjust the results for type I and II errors. TSA 0.9.5.10 Beta software was used (Copenhagen Trial Unit Centre for Clinical Intervention Research Department, Copenhagen, Denmark). The fixed-effects model was selected for the meta-analysis. The required information size (RIS) and alpha-spending monitoring boundaries were estimated by setting type I and type II error at 5% and 20% (power of 80%), respectively. To calculate RIS, for both test (short implants) and control (long implants) arms, the incidence (positive events) was estimated according to the findings of the meta-analysis, without applying correction for heterogeneity. The corresponding graphics allowed to determine if the cumulative Z-curve (blue line) crosses the RIS threshold (vertical red line) and the trial sequential monitoring threshold (horizontal red line). If so, it was considered that the studies had an adequate sample size, and their results were valid. Otherwise, it was assumed that the available information was inadequate, and more evidence was needed.
2.11. Risk of Bias and Quality of Evidence
The quality of the included trials was assessed using the Cochrane Risk of Bias Tool for randomized clinical trials (RoB 2) (updated on 22 August 2019) [32] by two calibrated examiners independently to ensure agreement on the scoring system. Each study was judged to be at low, moderate (some concerns), or high risk of bias based on five domains: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; (5) bias in selection of the reported result. The overall risk of bias of each study was considered “low” when the risk of bias was judged low for all domains. It was judged to raise “some concerns” when at least one domain raised some concerns but no domain was at a high risk of bias. It was judged “high” when at least one domain was at high risk or the study was judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result. The outcome assessed for risk of bias was the implant survival rate. The aim of the review team was to investigate the effect of assignment to the interventions at baseline, regardless of whether the interventions were received as intended (‘intention-to-treat effect’). Articles reporting results from the same study were grouped and evaluated together.
Following Grades of Recommendation, Assessment, Development and Evaluation (GRADE) methods [33], a ‘Summary of findings’ table for the primary outcome of the present meta-analysis (survival rate), including all follow-up periods of each comparison group, was developed by the GRADEpro GDT web application, http://gradepro.org, (accessed on 20 February 2020). The quality of the body of evidence was assessed by considering the overall risk of bias of the included trials, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias. The quality of evidence was categorized as high, moderate, low, or very low.
3. Results
3.1. Study Selection
The electronic search retrieved a total of 1525 articles. 766 records were screened after duplicate removal and 18 remained after title and abstract evaluation. One additional article [34] was collected through manual screening and unpublished data of another article were obtained directly from authors [35]. After full-text assessment, another seven articles were excluded (Table 2).

Table 2.
Excluded studies and reasons for exclusion.
The k value for the inter-reviewer agreement for potentially pertinent papers was 0.865 (for the selection of titles and abstracts) and 0.894 (for the selection of full-text articles). Finally, data from 12 articles published between 2013 and 2021 [34,43,44,45,46,47,48,49,50,51,52] and one unpublished article [35] were included in the present systematic review. The selection process is shown in Figure 1.
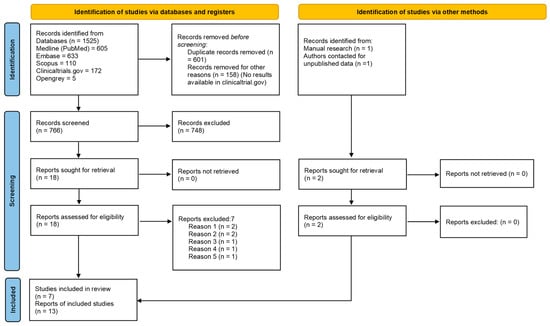
Figure 1.
PRISMA 2020 flowchart of the selection process.
3.2. Characteristics of the Included Articles
The characteristics of the 13 articles [34,35,43,44,45,46,47,48,49,50,51,52,53] included in this systematic review are summarized in Table 3.

Table 3.
Characteristics of the included studies.
Three articles [47,48,52] reported data pertaining to the same cohort at 1, 3, and 5 years of follow-up. Two articles followed the same patients at 1 and 3 years [34,51]. Four articles [35,43,44,49] followed 2 cohorts from 1 to 5 years and another 2 articles [46,53] at 5-year and 10-year follow-ups.
Ten articles [34,43,44,45,46,47,48,51,52,53] reported the outcomes from implants placed both in the upper and lower jaws, whereas 2 studies (3 articles) [35,49,50] considered only mandible implants. Two articles [43,44] reported the outcome of maxillary and mandibular implants separately. A parallel-group design was followed in all the studies but one [45], where some patients were allowed to receive both test and control implants.
The total number of inserted implants was 1066; 540 short implants were inserted (50.7%) with a minimum of 23 implants [50] and a maximum of 124 [34,51] implants; 526 long implants were inserted (49.3%) with a minimum of 23 [50] and a maximum of 116 implants [34,51].
The total number of treated patients was 454, and in one study [45] some patients received both short and long implants; 235 patients were treated with short implants (51.8%) with a minimum of 11 [46,53] and a maximum of 75 patients [34,51]; 234 patients were treated with long implants (51.5%) with a minimum of 13 [46,53] and a maximum of 75 patients [51,53].
Nine articles [35,45,46,47,48,49,50,52,53] considered short implants of 6 mm, 2 articles [43,44] of 5 mm, and 2 articles [34,51] of 4 mm. Two articles [34,51] considered control implants as ≥8.5 mm long; 4 articles as 10 mm long [45,46,50,53]; 5 articles as 11 mm long [35,47,48,49,52]; and 2 as ≥11.5 mm long [43,44].
The majority of the included studies focused on partial edentulism in the posterior jaws rehabilitated by single or 2–3 splinted crowns [34,45,46,47,48,50,51,52,53], while only 2 studies (4 articles) [35,43,44,49] focused on the rehabilitation of total edentulism by full-arch prostheses.
In terms of the loading protocol [54], in three articles (two studies), implants were immediately loaded [43,44,50], six articles (three studies) followed an early loading protocol (1–8 weeks) [45,46,47,48,52,53], and the remaining performed conventional loading (>8 weeks) [34,35,49,51].
In five articles (three studies) [34,43,44,50,51], post-extraction implants were also included, whereas all the other implants were exclusively placed in healed bone (at least 4 months after tooth extraction), although the exact surgical timing [55] was often not specified.
3.3. Risk/Confounding Factors
Factors such as smoking, implant surface, bone quality, primary stability, crown-to-implant ratio, periodontal status, and systemic diseases were screened to ascertain if they had been assessed and analysed in the originally included studies.
Six articles (3 studies) [34,35,43,44,49,51] included heavy smokers (≥10 cigarettes/day) and a similar inter-group distribution was reported. The other studies excluded or avoided the recruitment of heavy smokers [46,47,48,52,53]. In two studies [45,50], smokers were included but not categorized in heavy or light smokers.
All the studies included only systemically healthy patients or patients with controlled systemic diseases, or patients without general contraindications to implant surgery.
All of them evaluated the periodontal status of the patients and excluded patients with active periodontitis or reported that the periodontal treatment was performed if needed. Only one study (3 articles) [47,48,52] reports the number of patients with a history of periodontitis for each study group.
Only two studies (3 articles) assessed the quality of bone [43,44,45], showing no inter-group differences. The majority of them [34,45,47,48,50,51,52] systematically evaluated the primary stability of implants and, where available, no significant inter-group differences were reported. One study reported bone quality only for lost implants [48].
Three studies reported the anatomical crown-to-implant ratio [45,46,47], and 2 studies assessed the presence of patients with bruxism [45,47], but none of them provided information about intergroup-distribution, while severe bruxism was an exclusion criteria of other two studies (3 articles) [35,49,50].
3.4. Risk of Bias and Quality of Evidence
A total of 7 studies (13 articles) were evaluated.
Risk of bias assessment expressed as percentage of the included studies according to domain is presented in Figure 2. Most of the domains were fulfilled by all the studies. For some of them, however, some concerns were expressed for possible biases arising from the randomization process (about 30% of the studies), deviation from intended interventions (about 15%), and in the selection of the reported results (about 70% of the studies).
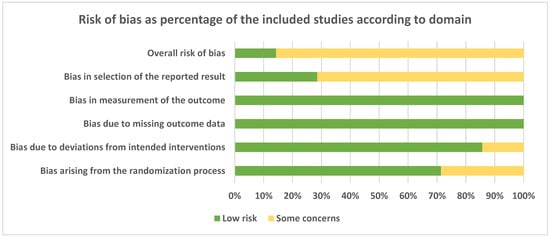
Figure 2.
Risk of bias assessment expressed as a percentage of the included studies according to domain.
According to the GRADE system, pooling of studies from 1 to 10 years of follow-up [34,35,44,45,48,50,53] provided moderate-quality evidence (Table 4) for a comparable survival rate between short and long implants.

Table 4.
Grades of recommendation, assessment, development, and evaluation (GRADE) approach summarizing the evidence. Question: Short implants (≤6 mm) compared to longer implants (≥8.5 mm) in edentulous sites without the need for bone augmentation. Moderate certainty: The authors are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Generated by GRADEpro GDT web application, http://gradepro.org, (accessed on 20 February 2020). CI: confidence interval; RR: risk ratio; MD: mean difference. a Small simple size (less than optimal information size), CI of RR included 1.
3.5. Meta-Analysis
3.5.1. Survival Rate
The overall survival rates of the reported implants for the short and long implants, respectively, were 96.85% and 98.48% at 1 year (7 articles), 96.14% and 98.76% at 3 years (4 articles), 95.40% and 98.44% at 5 years (5 articles), and 96.15% and 100% at 10 years (one article).
Forest plots of the survival rate (RR) comparing short and long implants at different follow-ups are shown in Figure 3. Mantel–Haenszel (MH)-weighted RR < 1 indicated a lower survival rate of short implants than the long implants. Data were analysed at implant level. The estimates were pooled using a random effect model for 3- and 5-years of follow-up analysis, due to the high heterogeneity found (I2 = 56%, p < 0.1 and I2 = 57%, p < 0.1, respectively), while for all the other analyses, a fixed-effect model was used.
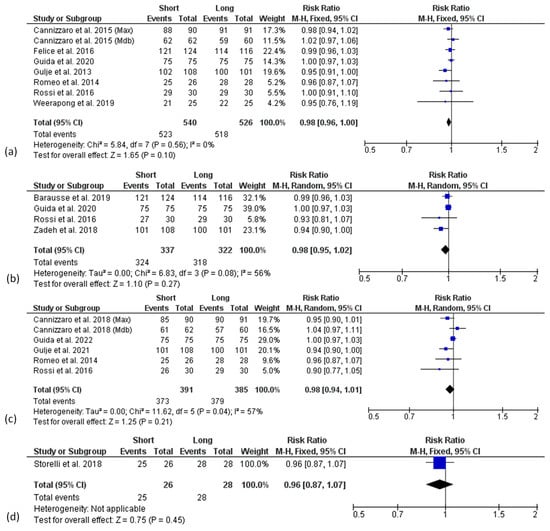
Figure 3.
Forest plots (risk ratio, RR) of the survival rate comparing short and long implant groups in (a) 1-year, (b) 3-year, (c) 5-year and (d) 10-years results. RR < 1 indicated a survival rate of short implants lower than the long implants.
The meta-analysis of 7 studies (8 data sets) at 1-year follow-up revealed no significant difference of survival rate between short and long implants with a risk ratio of 0.98 (95% CI: 0.96–1.00; p = 0.10) (Figure 3a).
A similar finding was observed in the analysis of 3-year results from 4 studies [34,45,49,52], with a risk ratio of 0.98 (95% CI: 0.95–1.02; p = 0.27) (Figure 3b), as well as in the analysis of 5-year results from 5 studies (6 data sets) [35,44,45,46,48] with a risk ratio of 0.98 (95% CI: 0.94–1.01; p = 0.21) (Figure 3c).
The 10-years follow-up analysis included only one study [53] and showed no significant difference between short and long implants in terms of survival rate with a risk ratio of 0.96 (95% CI: 0.87–1.07; p = 0.45) (Figure 3d).
Although not statistically significant, a trend for higher survival rates in the longer implant group at all follow-up times was found.
Trial Sequential analysis was carried out considering, for each study, only the results of the latest follow-up (from 1 to 10 years) [34,35,44,45,48,50,53]. TSA showed (Figure 4) a trend for better outcomes in favour of the long implants, without achieving significance. The cumulative Z-curve (blue line) kept below the trial sequential monitoring threshold (horizontal red line), revealing the presence of a not significant effect. The total sample size of the meta-analysis was below the required information size (vertical red line, n = 1804 implants), indicating the need for more trials, as the meta-analysis had not sufficient power to detect a better performance of long over short implants.
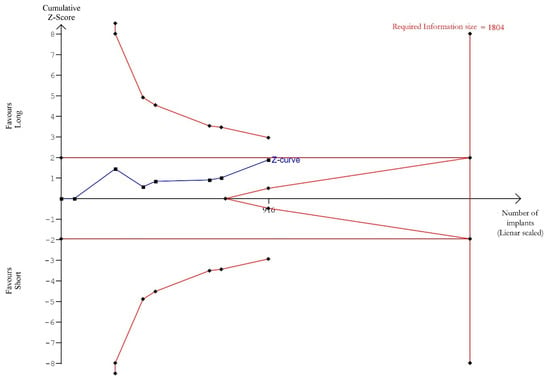
Figure 4.
Trial Sequential Analysis (TSA) of implant survival rate comparing short with long implants (follow-up range 1 to 10 years). Two-sided graph. The cumulative Z-curve showing treatment effect (blue line) does not cross the trial sequential monitoring boundaries (horizontal red line), revealing a not significant effect. Additionally, the Z-curve does not surpass the required information size threshold (vertical red line), revealing weak power of evidence.
3.5.2. Peri-Implant Marginal Bone Level Change (MBLc)
Two different analyses were conducted based on the baseline considered in each article for the measurements of MBLc: implant placement or prosthetic loading.
Three articles chose implant placement [34,51,52] and six articles chose implant loading [35,45,46,48,49,53] as a baseline for peri-implant MBLc measurements, and they were separately analysed (Figure 4 and Figure 5, respectively); in two studies (three articles) [43,44,50] implants were immediately loaded after placement; thus, they were included in both analyses. Only one article [47] reported values for both implant placement and prosthetic loading as the baseline. The majority of the studies considered MBLc at patient level, only 2 articles reported MBLc at implant level [45,48], while one study [52] reported values both at implant and patient level. In one case, data were both patient and implant level, since one implant per patient was considered [50].
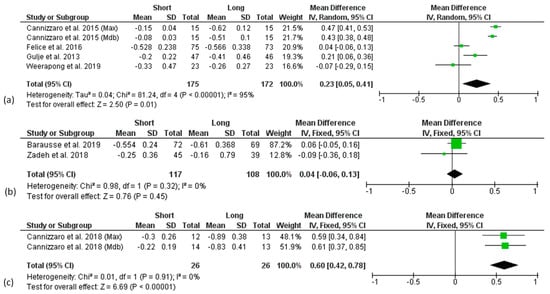
Figure 5.
Forest plots reporting difference in means for Marginal Bone Level change (MBLc) between short and long implant groups at 1 (a), 3 (b), and 5 (c) years considering implant placement (IP) as baseline.
Due to the limited number of the available articles, studies reporting data at implant level were analysed together with studies reporting data at patient level.
A high heterogeneity was found in the 1- and 5-year follow-up analysis considering prosthetic loading as baseline and in the 1-year analysis considering implant placement as baseline, so that a random effect model was used, whereas for all the other analyses, a fixed-effect model was applied.
No difference in the overall effect size was found 3 years after implant placement (Figure 5b) as well as at 1, 3, 5, and 10 years after prosthetic loading (Figure 6a–d).
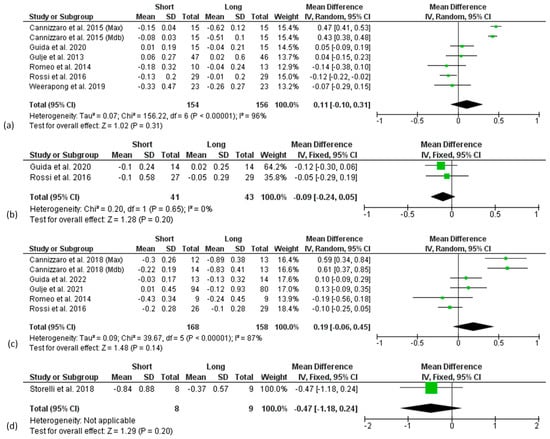
Figure 6.
Forest plots reporting difference in means for Marginal Bone Level change (MBLc) between short and long implant groups at 1 (a), 3 (b), 5 (c), and 10 (d) years considering prosthetic loading (PL) as baseline.
There was significantly higher marginal bone loss at the 1- and 5-year follow-ups after implant placement for long implants, with a mean difference compared to short implants of 0.23 mm (p = 0.01) and 0.60 mm (p < 0.00001), respectively (Figure 5a,c), although the analysis was strongly affected by one study [43,44].
3.5.3. Biological and Technical Complications
A meta-analysis regarding the biological complications (mucositis and peri-implantitis), and prosthetic complications (implant, prosthesis, abutment or screw fractures, screw loosening, and denture renewal), registered in the included studies was performed. Other reported complications, such as pain, soft tissue ulcerations and wound dehiscence, or loosening of cover/healing screws, were not included among the biological and technical complications, respectively. Data were analysed at the patient level. Three articles provided only implant-level data and were excluded from the analysis of biological [45,48] and technical [45,52] complications. A fixed effect model was used for all the analysis at each follow-up point. None of the 1-year follow-up articles reported patients suffering from mucositis or peri-implantitis. No difference for biological complications between short and long implant groups from 3 to 10 years of follow-up was found (Figure 7a–c).
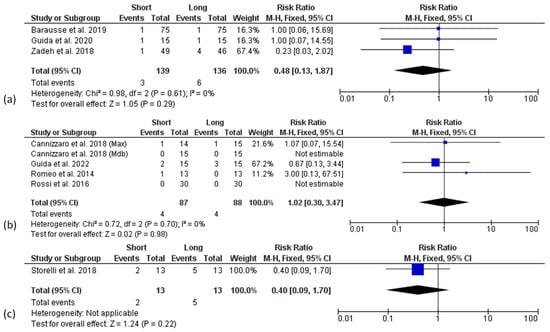
Figure 7.
Forest plots (risk ratio, RR) for biological complication rate comparing short and long implant groups at 3 (a), 5 (b), and 10 years (c) of follow-up. RR > 1 indicated a higher complication rate for short than the long implants.
Similarly, no significant differences in terms of technical complication rates between groups were found at the 1-year, 3-year, and 5-year follow-ups (Figure 8a–c). Only at 10 years was a higher technical complication rate found in the short implant group, although the analysis included only one article [53].
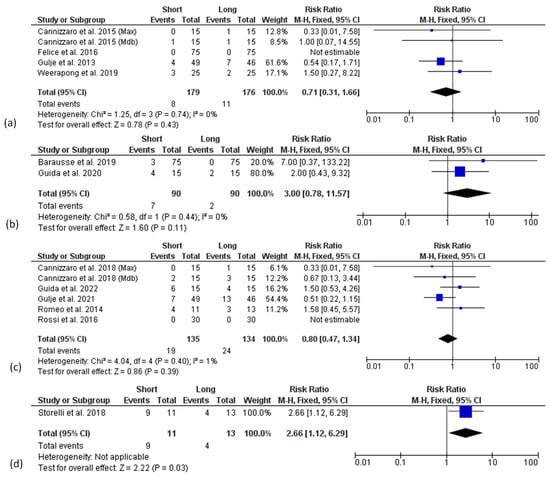
Figure 8.
Forest plots (risk ratio, RR) for technical complication rate comparing short and long implant groups at 1- (a), 3- (b), 5- (c) and 10-year follow-up (d). RR > 1 indicated a higher complication rate for short rather than long implants.
4. Discussion
The present meta-analysis exclusively included randomized controlled trials comparing short (≤6 mm) and longer (≥8.5 mm) implants placed in sites without the need for bone augmentation.
Universally accepted definitions of a “short”, “standard”, or “long” implant in the literature does not yet exist, and these concepts continuously evolved throughout the years [27,45,56]. In accordance with the 2018 ITI Consensus Report [12], the authors considered truly “short” implants of length ≤ 6 mm, in comparison to control implants with a length ≥ 8.5 mm.
Short implants have been extensively proposed in atrophic sites as an alternative to longer implants associated with bone augmentation, finding in this treatment their natural term of comparison.
Dental implants placed in vertically augmented bone showed a high survival rate (98%, range 95–100%), comparably to short implants placed in atrophic sites (96%, range 86.7–100%) after periods of 1 to 5 years in function, although they were associated with a higher number of complications, surgical time, and treatment costs [12].
The authors believe, however, that comparison of short and long implants in the same clinical scenario could be the only ones able to correctly evaluate their performance avoiding the effect of confounding factors related to the different characteristics of the edentulous sites, especially those related to the augmentation procedure itself.
Only two other meta-analyses, among those available in the literature, addressed this issue, trying to analyse, by specific sub-analyses, the influence on the reported outcomes of the variable “bone augmentation” among the included trials [8,25], thus representing the main term of comparison for our results. With respect to those meta-analyses, however, the authors were able to include a higher number of more recent RCTs [34,35,44,48,49,50,52,53] with longer follow-up, as well as further additional data directly obtained by the authors themselves.
The first relevant finding of the present meta-analysis is the comparable survival rate found between test and control groups at all the available follow-up times, although the low number of available studies limits the strength of the evidence for this outcome. In fact, the available sample size is smaller than the optimal information size and this affects the rating of evidence by the GRADE methods, indicating a moderate quality, as well as the TSA, which revealed the weak power of the meta-analysis findings. Recent systematic reviews on short implants separately performed additional analyses focused on those RCTs comparing short and longer implants placed in non-augmented bone [8,25]. Ravidà et al. (2019) showed homogeneous results in terms of survival rates between trials with or without bone augmentation procedures, thus suggesting no influence of this variable on this outcome. It must be underlined that just one study of only one follow-up period (one year) was included in the “non-augmentation” group by the authors. Yu et al. (2021) performed a similar sub-group analysis on a larger number of studies and follow-up periods (1, 3, and 5 years). In this case, accordingly with our analysis, a trend for a higher risk of failure for short implants at 1 and 3 years was found, which reached the significance, differently from our results, at 5 years of follow-up with an RR of 0.955 (95% CI 0.912–0.999, p < 0.05). Several factors must be considered for a correct interpretation of such different findings, including the type of edentulism, the loading time, and the prosthetic design. Indeed, if we look at the trials reporting higher risk of failure for short implants at 5 years in the study by Yu et al. (2021), the authors find that all the implants were early loaded and supported single crowns or 2/3-units fixed partial prostheses. In contrast, two over the three additional data sets (from two studies [35,44]) included in our analysis regarded splinted implants, conventionally loaded, supporting full-arch rehabilitations of mandibular edentulism. Moreover, details on other important variables (such as bruxism, smoke habit, bone quality and implant stability) in the patients experiencing implant loss were rarely provided by the primary studies, making it more difficult to correctly analyse possible causes and risk factors of implant failures.
Peri-implant marginal bone level maintenance over time is of pivotal importance for the long-term success of dental implants. The present meta-analysis showed that only 1- and 5-year follow-up data from implant placement showed a significantly higher bone loss for long implant group. However, such results must be considered with caution due to the very low number of included studies and the high heterogeneity found, with a significant impact on the overall evaluation given by two articles [43,44] derived from the same study. This latter result was the only one included in the “no augmentation” group by Ravidà et al. [8] in their meta-analysis, resulting in a significantly higher bone loss for the long implant group at the 1-year follow-up. Also, Yu et al. [25] performed a sub-group analysis for MBLc on “augmentation” vs. “no augmentation” studies one year after two baseline points (implant placement and prosthetic loading). They did not find a difference between long and short implants both one year after prosthetic loading and, different from the study results, also one year after implant placement. Also in this case, the low number of included studies and their heterogeneity impose caution.
In addition, the incidence of the reported biological and technical complications did not show a statistically significant difference between short and long implants, in line with the analyses performed by previous similar reviews up to 5 years of follow-up [8,25]. At longer follow-ups, a statistically significant higher number of technical complications was found after 10 years [53], although the value of this finding was sustained by only one trial. It is hard, however, to compare the results with those from other similar studies, in light of the different biological complications considered (only the occurrence of peri-implant mucositis and peri-implantitis was analysed in our study), and, moreover, of the different diagnostic criteria adopted for such diseases among the studies.
Looking at the main limitations of the present meta-analysis, the low number of includible studies and their mainly short/medium follow-ups must be cited. Furthermore, the variety of experimental conditions among studies limit the power of the obtained results, and no further analyses could be performed based on variables including implant location (mandible vs. maxilla), type of edentulism (total vs. partial), implant type, smoke habit, implant loading, periodontal health, systemic conditions due to the reduced number of available studies, or the lack of information retrievable about these variables. No subgroup analysis could be performed for confounding factors, such as smoking and periodontal status, or for the level of analysis (implant or patient level). Implant-level and patient-level data, were pooled together in the MBLc meta-analysis, with a consequent possible underestimation of the confidence intervals for the pooled estimate.
Further, well-designed RCTs comparing clinical and radiological outcomes of short and long implants placed in similar conditions (location, type of edentulism, prosthetic rehabilitation, etc.) and with an adequate analysis of confounding factors should be performed to obtain more solid evidence about the efficacy of short implants used in atrophic sites.
This approach would also help to extend their clinical indications, supporting the hypothesis of their routine use in non-atrophic edentulous sites instead of traditionally long implants, in the groove of a minimally invasive, low-stress, simplified implant therapy, with benefits for both patients and clinicians.
The ideal length of implants supporting prosthetic rehabilitations, indeed, is a relative concept that underwent a progressive reduction throughout the years from the origin of modern implantology to the present [27,45,56], also thanks to the outstanding progress made in the last decades in terms of constitutive materials and macro-design of dental implants, as well as of micro/nano-topographic and chemical modifications of modern implant surfaces [57,58].
The advantages of using short implants in non-atrophic sites compared to longer ones include a simplified surgical management during placement, with a lower risk of involving noble anatomic structures, as well as a simplified and less invasive surgical procedure, if biological complications, over time, might require fixture removal, with the chance to leave an amount of residual bone enough for a new rehabilitation. On the other hand, due to the reduced fixture length, some conditions, such as low-density bone and operative protocols, e.g., post-extraction placement, and immediate/early loading, could reduce the reliability and predictability of this rehabilitative approach.
This is the reason, in the authors’ opinion, because further RCTs should be performed in which all the possible relevant variables and risks are considered, analysed, and controlled in order to obtain more solid evidence on the performance of short implants in each specific clinical scenario, starting from the “low-risk” one (healed sites, good bone quality, conventional loading, splinted implants), to the others.
5. Conclusions
Moderate evidence exists suggesting that short implants perform, in the medium term, as well as longer ones in the rehabilitation of edentulous sites without the need for bone augmentation. However, long-term data are lacking and the experimental conditions among the analysed studies are heterogeneous. Further data from long-term, well-designed RCTs conducted under comparable clinical conditions, in terms of location and type of edentulism, prosthetic design, surgical and loading timing, and with a proper analysis of potential confounding factors, are needed. In this way it will be possible to provide specific, evidence-based, clinical recommendations and reliable indications for an extended use of short implants, also in the case of non-atrophic sites.
Author Contributions
Conceptualization: M.A. and L.G.; literature search: M.A. and A.D.V.; data extraction: M.A. and G.C.; qualitative assessment: G.C. and A.D.V.; data analysis: M.A., E.B., M.D.F. and L.G.; writing—original draft preparation: M.A., E.B., G.C., M.D.F., L.G. and A.D.V.; writing—review and editing: M.A., E.B., G.C., M.D.F. and L.G.; supervision: L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000 2017, 73, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Needleman, I.; Salvi, G.E.; Pjetursson, B.E. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int. J. Oral Maxillofac. Implant. 2014, 29, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Rees, J.; Karasoulos, D.; Alissa, R.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Augmentation procedures of the maxillary sinus. Cochrane Database Syst. Rev. 2010, 3, CD008397. [Google Scholar]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Thoma, D.S.; Haas, R.; Tutak, M.; Garcia, A.; Schincaglia, G.P.; Hämmerle, C.H. Randomized controlled multicentre study comparing short dental implants (6 mm) versus longer dental implants (11–15 mm) in combination with sinus floor elevation procedures. Part 1: Demographics and patient-reported outcomes at 1 year of loading. J. Clin. Periodontol. 2015, 42, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ravidà, A.; Wang, I.-C.; Barootchi, S.; Askar, H.; Tavelli, L.; Gargallo-Albiol, J.; Wang, H.L. Meta-analysis of randomized clinical trials comparing clinical and patient-reported outcomes between extra-short (≤6 mm) and longer (≥10 mm) implants. J. Clin. Periodontol. 2019, 46, 118–142. [Google Scholar] [CrossRef]
- Nisand, D.; Renouard, F. Short implant in limited bone volume. Periodontology 2000 2014, 66, 72–96. [Google Scholar] [CrossRef] [PubMed]
- Nisand, D.; Picard, N.; Rocchietta, I. Short implants compared to implants in vertically augmented bone: A systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. 1), 170–179. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Donos, N.; Alcoforado, G.; Balmer, M.; Gurzawska, K.; Mardas, N.; Milinkovic, I.; Nisand, D.; Rocchietta, I.; Stavropoulos, A.; et al. Therapeutic concepts and methods for improving dental implant outcomes. Summary and consensus statements. The 4th EAO Consensus Conference 2015. Clin. Oral Implant. Res. 2015, 26 (Suppl. 1), 202–206. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Al-Nawas, B.; Araujo, M.; Avila-Ortiz, G.; Barter, S.; Brodala, N.; Chappuis, V.; Chen, B.; De Souza, A.; Almeida, R.F.; et al. Group 1 ITI Consensus Report: The influence of implant length and design and medications on clinical and patient-reported outcomes. Clin. Oral Implant. Res. 2018, 29, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hingsammer, L.; Watzek, G.; Pommer, B. The influence of crown-to-implant ratio on marginal bone levels around splinted short dental implants: A radiological and clincial short term analysis. Clin. Implant Dent. Relat. Res. 2017, 19, 1090–1098. [Google Scholar] [CrossRef]
- Bahat, O. Treatment planning and placement of implants in the posterior maxillae: Report of 732 consecutive Nobelpharma implants. Int. J. Oral Maxillofac. Implant. 1993, 8, 151–161. [Google Scholar] [CrossRef][Green Version]
- Pommer, B.; Frantal, S.; Willer, J.; Posch, M.; Watzek, G.; Tepper, G. Impact of dental implant length on early failure rates: A meta-analysis of observational studies. J. Clin. Periodontol. 2011, 38, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Burgueño-Barris, G.; Figueiredo, R.; Jung, R.E.; Gay-Escoda, C.; Valmaseda-Castellón, E. Interventions for Dental Implant Placement in Atrophic Edentulous Mandibles: Vertical Bone Augmentation and Alternative Treatments. A Meta-Analysis of Randomized Clinical Trials. J. Periodontol. 2016, 87, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.A.A.; Ferro-Alves, M.L.; Okamoto, R.; Mendonça, M.R.; Pellizzer, E.P. Short dental implants versus standard dental implants placed in the posterior jaws: A systematic review and meta-analysis. J. Dent. 2016, 47, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sánchez, J.-L.; García-Sala-Bonmatí, F.; Martínez-González, A.; García-Dalmau, C.; Mañes-Ferrer, J.F.; Brotons-Oliver, A. Predictability of short implants (<10 mm) as a treatment option for the rehabilitation of atrophic maxillae. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e392–e402. [Google Scholar] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hüsler, J.; Hämmerle, C.H.; Jung, R.E. EAO Supplement Working Group 4—EAO CC 2015 Short implants versus sinus lifting with longer implants to restore the posterior maxilla: A systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. 1), 154–169. [Google Scholar] [CrossRef]
- Tong, Q.; Zhang, X.; Yu, L. Meta-analysis of Randomized Controlled Trials Comparing Clinical Outcomes Between Short Implants and Long Implants with Bone Augmentation Procedure. Int. J. Oral Maxillofac. Implant. 2017, 32, e25–e34. [Google Scholar] [CrossRef][Green Version]
- Toti, P.; Marchionni, S.; Menchini-Fabris, G.B.; Marconcini, S.; Covani, U.; Barone, A. Surgical techniques used in the rehabilitation of partially edentulous patients with atrophic posterior mandibles: A systematic review and meta-analysis of randomized controlled clinical trials. J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2017, 45, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alemany, A.; Fernández-Estevan, L.; Agustín-Panadero, R.; Montiel-Company, J.M.; Labaig-Rueda, C.; Mañes-Ferrer, J.F. Clinical Behavior of Short Dental Implants: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3271. [Google Scholar] [CrossRef]
- Palacios, J.A.V.; Garcia, J.J.; Caramês, J.M.M.; Quirynen, M.; da Silva Marques, D.N. Short implants versus bone grafting and standard-length implants placement: A systematic review. Clin. Oral Investig. 2018, 22, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Suarez, F.; Galindo-Moreno, P.; García-Nogales, A.; Fu, J.H.; Wang, H.L. A systematic review on marginal bone loss around short dental implants (<10 mm) for implant-supported fixed prostheses. Clin. Oral Implant. Res. 2014, 25, 1119–1124. [Google Scholar]
- Yu, X.; Xu, R.; Zhang, Z.; Yang, Y.; Deng, F. A meta-analysis indicating extra-short implants (≤6 mm) as an alternative to longer implants (≥8 mm) with bone augmentation. Sci. Rep. 2021, 11, 8152. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Li, Y.; Deng, W.-W.; Wu, T.; Zhang, W. Short Implants (5 to 8 mm) Versus Longer Implants (>8 mm) with Sinus Lifting in Atrophic Posterior Maxilla: A Meta-Analysis of RCTs. Clin. Implant Dent. Relat. Res. 2017, 19, 207–215. [Google Scholar] [CrossRef]
- Renouard, F.; Nisand, D. Impact of implant length and diameter on survival rates. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 35–51. [Google Scholar] [CrossRef]
- Telleman, G.; Raghoebar, G.M.; Vissink, A.; den Hartog, L.; Huddleston Slater, J.J.; Meijer, H.J. A systematic review of the prognosis of short (<10 mm) dental implants placed in the partially edentulous patient. J. Clin. Periodontol. 2011, 38, 667–676. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stone, P.W. Popping the (PICO) question in research and evidence-based practice. Appl. Nurs. Res. 2002, 15, 197–198. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, I4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Barausse, C.; Felice, P.; Pistilli, R.; Buti, J.; Esposito, M. Posterior jaw rehabilitation using partial prostheses supported by implants 4.0 × 4.0 mm or longer: Three-year postloading results of a multicentre randomised controlled trial. Clin. Trials Dent. 2019, 1, 25–36. [Google Scholar]
- Guida, L.; Annunziata, M.; Esposito, U.; Sirignano, M.; Torrisi, P.; Cecchinato, D. 6-mm implants for the full-arch rehabilitation of edentulous mandibles: 5-year results from a multicenter randomized controlled trial. J. Clin. Periodontol. 2022, 49. Special issue: Abstracts EuroPerio 10, accepted. [Google Scholar]
- Sahrmann, P.; Naenni, N.; Jung, R.E.; Held, U.; Truninger, T.; Hämmerle, C.H.; Attin, T.; Schmidlin, P.R. Success of 6-mm Implants with Single-Tooth Restorations: A 3-year Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 623–628. [Google Scholar] [CrossRef]
- Naenni, N.; Sahrmann, P.; Schmidlin, P.R.; Attin, T.; Wiedemeier, D.B.; Sapata, V.; Hämmerle, C.H.F.; Jung, R.E. Five-Year Survival of Short Single-Tooth Implants (6 mm): A Randomized Controlled Clinical Trial. J. Dent. Res. 2018, 97, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Schoen, P.; Naenni, N.; Jung, R.; Attin, T.; Schmidlin, P.R. Peri-implant bone density around implants of different lengths: A 3-year follow-up of a randomized clinical trial. J. Clin. Periodontol. 2017, 44, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Sluka, B.; Naenni, N.; Jung, R.E.; Attin, T.; Schmidlin, P.R.; Sahrmann, P. Changes of radiopacity around implants of different lengths: Five-year follow-up data of a randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Della Vecchia, M.P.; Leles, C.R.; Cunha, T.R.; Ribeiro, A.B.; Sorgini, D.B.; Muglia, V.A.; Reis, A.C.; Albuquerque, R.F., Jr.; de Souza, R.F. Mini-Implants for Mandibular Overdentures: Cost-Effectiveness Analysis alongside a Randomized Trial. JDR Clin. Transl. Res. 2018, 3, 47–56. [Google Scholar] [CrossRef]
- Esposito, M.; Barausse, C.; Pistilli, R.; Checchi, V.; Diazzi, M.; Gatto, M.R.; Felice, P. Posterior jaws rehabilitated with partial prostheses supported by 4.0 × 4.0 mm or by longer implants: Four-month post-loading data from a randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 221–230. [Google Scholar]
- Al-Hashedi, A.A.; Taiyeb-Ali, T.B.; Yunus, N. Outcomes of placing short implants in the posterior mandible: A preliminary randomized controlled trial. Aust. Dent. J. 2016, 61, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, G.; Felice, P.; Buti, J.; Leone, M.; Ferri, V.; Esposito, M. Immediate loading of fixed cross-arch prostheses supported by flapless-placed supershort or long implants: 1-year results from a randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 27–36. [Google Scholar] [PubMed]
- Cannizzaro, G.; Felice, P.; Ippolito, D.R.; Velasco-Ortega, E.; Esposito, M. Immediate loading of fixed cross-arch prostheses supported by flapless-placed 5 mm or 11.5 mm long implants: 5-year results from a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 295–306. [Google Scholar]
- Rossi, F.; Botticelli, D.; Cesaretti, G.; De Santis, E.; Storelli, S.; Lang, N.P. Use of short implants (6 mm) in a single-tooth replacement: A 5-year follow-up prospective randomized controlled multicenter clinical study. Clin. Oral Implant. Res. 2016, 27, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Storelli, S.; Casano, G.; Scanferla, M.; Botticelli, D. Six-mm versus 10-mm long implants in the rehabilitation of posterior edentulous jaws: A 5-year follow-up of a randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 371–381. [Google Scholar]
- Guljé, F.; Abrahamsson, I.; Chen, S.; Stanford, C.; Zadeh, H.; Palmer, R. Implants of 6 mm vs. 11 mm lengths in the posterior maxilla and mandible: A 1-year multicenter randomized controlled trial. Clin. Oral Implant. Res. 2013, 24, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Guljé, F.L.; Meijer, H.J.A.; Abrahamsson, I.; Barwacz, C.A.; Chen, S.; Palmer, P.J.; Zadeh, H.; Stanford, C.M. Comparison of 6-mm and 11-mm dental implants in the posterior region supporting fixed dental prostheses: 5-year results of an open multicenter randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 15–22. [Google Scholar] [CrossRef]
- Guida, L.; Annunziata, M.; Esposito, U.; Sirignano, M.; Torrisi, P.; Cecchinato, D. 6-mm-short and 11-mm-long implants compared in the full-arch rehabilitation of the edentulous mandible: A 3-year multicenter randomized controlled trial. Clin. Oral Implant. Res. 2020, 31, 64–73. [Google Scholar] [CrossRef]
- Weerapong, K.; Sirimongkolwattana, S.; Sastraruji, T.; Khongkhunthian, P. Comparative Study of Immediate Loading on Short Dental Implants and Conventional Dental Implants in the Posterior Mandible: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implant. 2019, 34, 141–149. [Google Scholar] [CrossRef]
- Felice, P.; Checchi, L.; Barausse, C.; Pistilli, R.; Sammartino, G.; Masi, I.; Ippolito, D.R.; Esposito, M. Posterior jaws rehabilitated with partial prostheses supported by 4.0 × 4.0 mm or by longer implants: One-year post-loading results from a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2016, 9, 35–45. [Google Scholar]
- Zadeh, H.H.; Guljé, F.; Palmer, P.J.; Abrahamsson, I.; Chen, S.; Mahallati, R.; Stanford, C.M. Marginal bone level and survival of short and standard-length implants after 3 years: An Open Multi-Center Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2018, 29, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Storelli, S.; Abbà, A.; Scanferla, M.; Botticelli, D.; Romeo, E. 6 mm vs. 10 mm-long implants in the rehabilitation of posterior jaws: A 10-year follow-up of a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 283–292. [Google Scholar]
- Weber, H.-P.; Morton, D.; Gallucci, G.O.; Roccuzzo, M.; Cordaro, L.; Grutter, L. Consensus statements and recommended clinical procedures regarding loading protocols. Int. J. Oral Maxillofac. Implant. 2009, 24, 180–183. [Google Scholar]
- Chen, S.T.; Beagle, J.; Jensen, S.S.; Chiapasco, M.; Darby, I. Consensus statements and recommended clinical procedures regarding surgical techniques. Int. J. Oral Maxillofac. Implant. 2009, 24, 272–278. [Google Scholar]
- Friberg, B.; Jemt, T.; Lekholm, U. Early failures in 4641 consecutively placed Brånemark dental implants: A study from stage 1 surgery to the connection of completed prostheses. Int. J. Oral Maxillofac. Implant. 1991, 6, 142–146. [Google Scholar]
- Aljateeli, M.; Wang, H.-L. Implant microdesigns and their impact on osseointegration. Implant Dent. 2013, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Guida, L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. Front. Oral Biol. 2015, 17, 62–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).