Deep Learning for Type 1 Diabetes Mellitus Diagnosis Using Infrared Quantum Cascade Laser Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
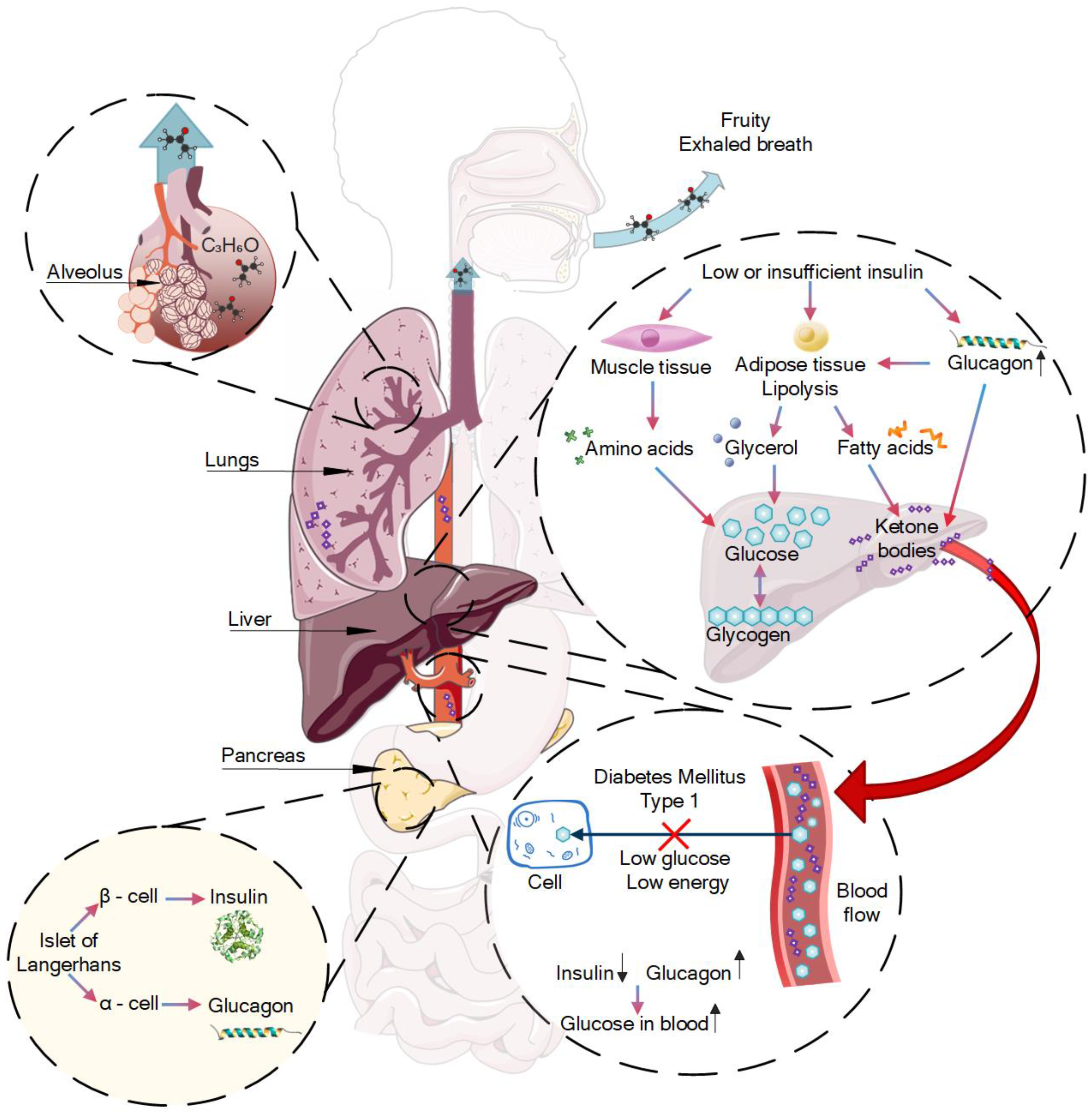
2.1. Diabetes Fruity Exhaled Breath
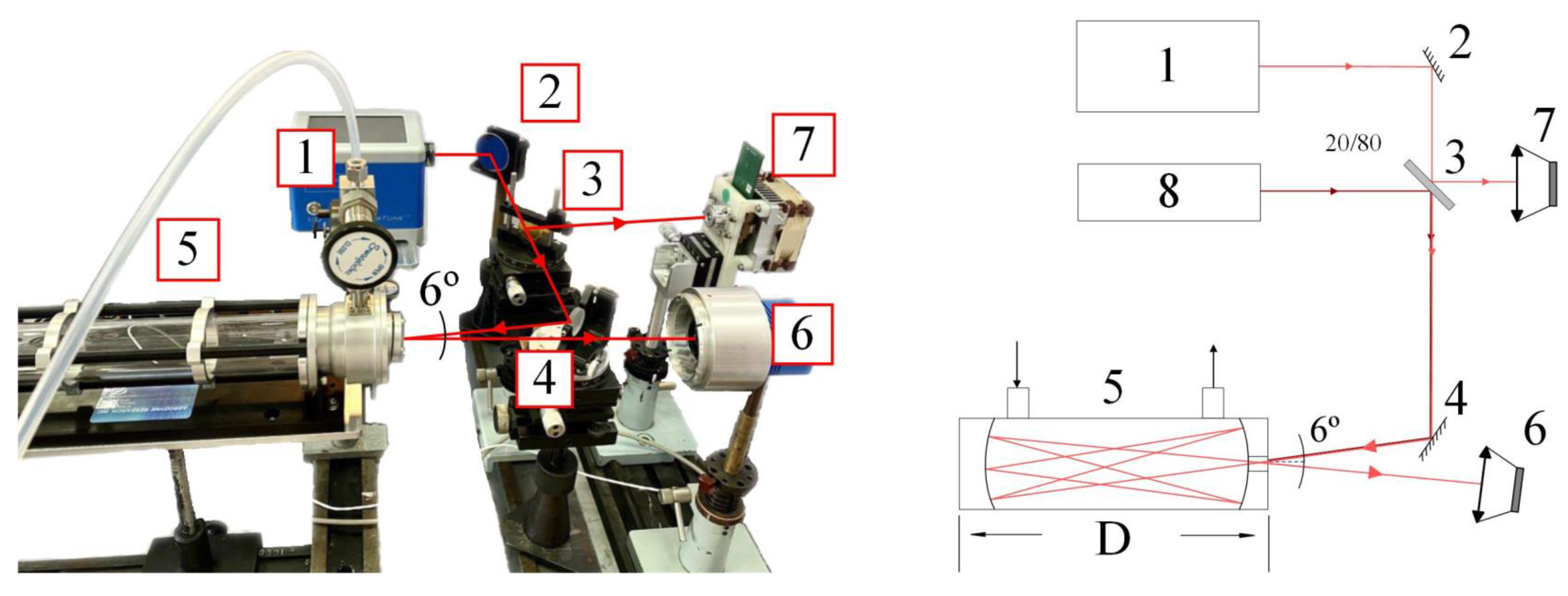
2.2. Experimental Setup
2.3. Neural Network
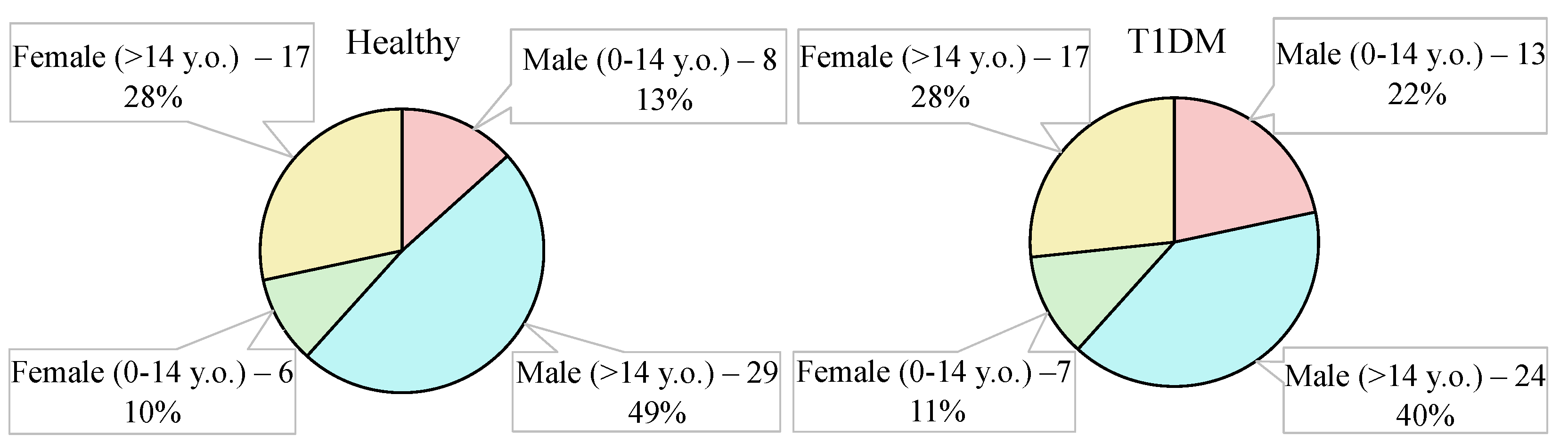
2.4. Groups under the Study
2.5. Sampling Protocol
3. Results
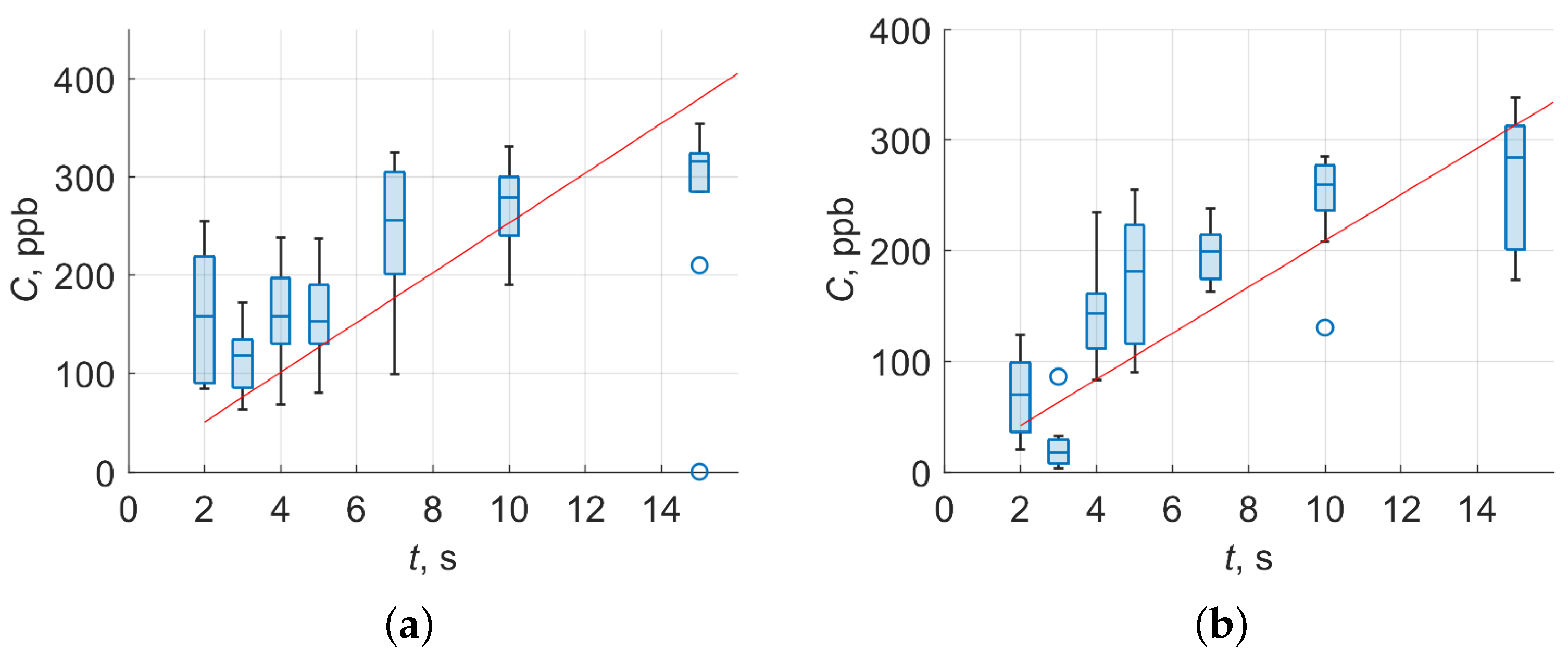
3.1. Sensitivity of Experimental Setup
3.2. Classification of Volunteers by Infrared Breath Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CNN | Convolutional neural network |
| DFB | Distributed feedback |
| DKA | Diabetic ketoacidosis |
| IDF | International Diabetes Federation |
| IR | Infrared |
| FCL | Fully connected layer |
| MIR | Mid-infrared |
| MCT-TE | Mercury Cadmium Telluride thermoelectrically cooled |
| MFC | Mass flow controller |
| MLP | Multilayer perceptron |
| QCL | Quantum cascade laser |
| ppb | Parts per billion |
| ppbv | parts per billion by volume |
| ppm | Parts per million |
| RGS-CV | Random grid search cross-validation framework |
| sccm | standard cubic centimetres per minute |
| SGD | Stochastic gradient descent |
| T1DM | Type 1 diabetes mellitus |
| VOC | Volatile organic compound |
| y.o. | Years old |
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Bayrakli, I. Breath analysis using external cavity diode lasers: A review. J. Biomed. Opt. 2017, 22, 040901. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. Potential of breath and skin analysis for monitoring blood glucose concentration in diabetes. Expert Rev. Mol. Diagn. 2011, 11, 497–503. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Manolis, A. The diagnostic potential of breath analysis. Clin. Chem. 1983, 29, 5–15. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Selvaraj, R.; Vasa, N.J.; Nagendra, S.M.S.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy-Based Sensing Techniques for Exhaled Breath Diagnostics. Molecules 2020, 25, 2227. [Google Scholar] [CrossRef]
- Vaks, V.L.; Domracheva, E.G.; Sobakinskaya, E.A.; Chernyaeva, M.B. Exhaled breath analysis: Physical methods, instruments and medical diagnostics. Uspekhi Fiz. Nauk 2014, 184, 739–758. [Google Scholar] [CrossRef] [Green Version]
- van Mastrigt, E.; Reyes-Reyes, A.; Brand, K.; Bhattacharya, N.; Urbach, H.P.; Stubbs, A.P.; de Jongste, J.C.; Pijnenburg, M.W. Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J. Breath Res. 2016, 10, 026003. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012, 6, 036008. [Google Scholar] [CrossRef] [Green Version]
- Erhart, S.; Amann, A.; Haberlandt, E.; Edlinger, G.; Schmid, A.; Filipiak, W.; Schwarz, K.; Mochalski, P.; Rostasy, K.; Karall, D.; et al. 3-Heptanone as a potential new marker for valproic acid therapy. J. Breath Res. 2009, 3, 016004. [Google Scholar] [CrossRef]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath Acetone Sensors as Non-Invasive Health Monitoring Systems: A Review. IEEE Sensors J. 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Ruzsányi, V.; Kalapos, M.P. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017, 11, 024002. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Prazeller, P.; Mayr, D.; Jordan, A.; Rieder, J.; Fall, R.; Lindinger, W. Human breath isoprene and its relation to blood cholesterol levels: New measurements and modeling. J. Appl. Physiol. 2001, 91, 762–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharitonov, S.; Barnes, P. Exhaled Markers of Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1693–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.E.; Hayes, J.M. Isotope-ratio-monitoring gas chromatography-mass spectrometry. Anal. Chem. 1978, 50, 1465–1473. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, W.; Wang, L.; Xu, N.; Ding, Q.; Cao, C. Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2695–2705. [Google Scholar] [CrossRef] [Green Version]
- Fink, T.; Baumbach, J.I.; Kreuer, S. Ion mobility spectrometry in breath research. J. Breath Res. 2014, 8, 027104. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, Z.; He, Y.; Zhu, Z.; Wang, L.; Yin, K. Femtosecond laser textured porous nanowire structured glass for enhanced thermal imaging. Chin. Opt. Lett. 2022, 20, 033801. [Google Scholar] [CrossRef]
- Nadeem, F.; Mandon, J.; Khodabakhsh, A.; Cristescu, S.; Harren, F. Sensitive Spectroscopy of Acetone Using a Widely Tunable External-Cavity Quantum Cascade Laser. Sensors 2018, 18, 2050. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Zhu, F.; Kolomenskii, A.A.; Bounds, J.; Zhang, S.; Amani, M.; Fernyhough, L.J.; Schuessler, H.A. Sensitive acetone detection with a mid-IR interband cascade laser and wavelength modulation spectroscopy. OSA Contin. 2019, 2, 640. [Google Scholar] [CrossRef]
- Goyal, A.K.; Kotidis, P.; Deutsch, E.R.; Zhu, N.; Norman, M.; Ye, J.; Zafiriou, K.; Mazurenko, A. Detection of chemical clouds using widely tunable quantum cascade lasers. In Proceedings of the Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XVI, Baltimore, MD, USA, 22 May 2015. [Google Scholar] [CrossRef]
- Richard, L.; Romanini, D.; Ventrillard, I. Nitric Oxide Analysis Down to ppt Levels by Optical-Feedback Cavity-Enhanced Absorption Spectroscopy. Sensors 2018, 18, 1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, R.; Schmidt, F.M. ICL-based TDLAS sensor for real-time breath gas analysis of carbon monoxide isotopes. Opt. Express 2017, 25, 12743. [Google Scholar] [CrossRef] [PubMed]
- McManus, J.B.; Zahniser, M.S.; Nelson, D.D., Jr.; Shorter, J.H.; Herndon, S.C.; Wood, E.C.; Wehr, R. Application of quantum cascade lasers to high-precision atmospheric trace gas measurements. Opt. Eng. 2010, 49, 1–11. [Google Scholar] [CrossRef]
- Jimenez, R.; Herndon, S.; Shorter, J.H.; Nelson, D.D.; McManus, J.B.; Zahniser, M.S. Atmospheric trace gas measurements using a dual quantum-cascade laser mid-infrared absorption spectrometer. In Novel In-Plane Semiconductor Lasers IV; Mermelstein, C., Bour, D.P., Eds.; SPIE: Bellingham, DC, USA, 2005. [Google Scholar] [CrossRef]
- Reyes-Reyes, A.; Horsten, R.C.; Urbach, H.P.; Bhattacharya, N. Study of the Exhaled Acetone in Type 1 Diabetes Using Quantum Cascade Laser Spectroscopy. Anal. Chem. 2014, 87, 507–512. [Google Scholar] [CrossRef]
- Tuzson, B.; Looser, H.; Felder, F.; Bovey, F.; Tappy, L.; Emmenegger, L. Human Breath Acetone Analysis by Mid-IR Laser Spectroscopy: Development and Application. In High-Brightness Sources and Light-Driven Interactions; OSA: Jawa Tengah, Indonesia, 2018. [Google Scholar] [CrossRef]
- Trefz, P.; Obermeier, J.; Lehbrink, R.; Schubert, J.K.; Miekisch, W.; Fischer, D.C. Exhaled volatile substances in children suffering from type 1 diabetes mellitus: Results from a cross-sectional study. Sci. Rep. 2019, 9, 15707. [Google Scholar] [CrossRef]
- Skarysz, A.; Alkhalifah, Y.; Darnley, K.; Eddleston, M.; Hu, Y.; McLaren, D.B.; Nailon, W.H.; Salman, D.; Sykora, M.; Thomas, C.L.P.; et al. Convolutional neural networks for automated targeted analysis of raw gas chromatography-mass spectrometry data. In Proceedings of the 2018 International Joint Conference on Neural Networks (IJCNN), Rio de Janeiro, Brazil, 8–13 July 2018. [Google Scholar] [CrossRef]
- Stepanov, E.V.; Kasoev, S.G. Multicomponent Analysis of Biomarkers in Exhaled Air Using Diode Laser Spectroscopy. Opt. Spectrosc. 2019, 126, 736–744. [Google Scholar] [CrossRef]
- Morozov, A.; Kochikov, I.; Novgorodskaya, A.; Sologub, A.; Fufurin, I. Statistical estimation of the probability of the correct substance detection in FTIR spectroscopy. Comput. Opt. 2015, 39, 614–621. [Google Scholar] [CrossRef]
- Kochikov, I.V.; Morozov, A.N.; Svetlichnyi, S.I.; Fufurin, I.L. Substance recognition in the open atmosphere from a single Fourier transform spectroradiometer interferogram. Opt. Spectrosc. 2009, 106, 666–671. [Google Scholar] [CrossRef]
- Martínez, R.A.S.; Hernández, J.M.P.; Terol, G.L.; Gallego-Jara, J.; García-Marcos, L.; Díaz, M.C.; de Diego Puente, T. Data preprocessing workflow for exhaled breath analysis by GC/MS using open sources. Sci. Rep. 2020, 10, 22008. [Google Scholar] [CrossRef]
- Stönner, C.; Edtbauer, A.; Derstroff, B.; Bourtsoukidis, E.; Klüpfel, T.; Wicker, J.; Williams, J. Proof of concept study: Testing human volatile organic compounds as tools for age classification of films. PLoS ONE 2018, 13, e0203044. [Google Scholar] [CrossRef]
- Kistenev, Y.V.; Borisov, A.V.; Titarenko, M.A.; Baydik, O.D.; Shapovalov, A.V. Diagnosis of oral lichen planus from analysis of saliva samples using terahertz time-domain spectroscopy and chemometrics. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, K.; Herrero, P.; Georgiou, P. Deep Learning for Diabetes: A Systematic Review. IEEE J. Biomed. Health Inform. 2021, 25, 2744–2757. [Google Scholar] [CrossRef]
- Song, B.; Li, S.; Sunny, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Gurudath, S.; Raghavan, S.; Tsusennaro, I.; et al. Classification of imbalanced oral cancer image data from high-risk population. J. Biomed. Opt. 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, S.; Zou, Y.; Zhu, Q. Deep learning-based method to accurately estimate breast tissue optical properties in the presence of the chest wall. J. Biomed. Opt. 2021, 26, 106004. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, Z.; Kuen, J.; Ma, L.; Shahroudy, A.; Shuai, B.; Liu, T.; Wang, X.; Wang, G.; Cai, J.; et al. Recent advances in convolutional neural networks. Pattern Recognit. 2018, 77, 354–377. [Google Scholar] [CrossRef] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Zhang, X.; Wu, C.; Lin, T.; Ying, Y. Deep learning for vibrational spectral analysis: Recent progress and a practical guide. Anal. Chim. Acta 2019, 1081, 6–17. [Google Scholar] [CrossRef]
- Acquarelli, J.; van Laarhoven, T.; Gerretzen, J.; Tran, T.N.; Buydens, L.M.; Marchiori, E. Convolutional neural networks for vibrational spectroscopic data analysis. Anal. Chim. Acta 2017, 954, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.; Zhang, Z.; Niu, S.; Sha, H.; Yang, R.; Yun, Y.; Lu, H. Deep-Learning-Based Drug–Target Interaction Prediction. J. Proteome Res. 2017, 16, 1401–1409. [Google Scholar] [CrossRef]
- Fan, X.; Ming, W.; Zeng, H.; Zhang, Z.; Lu, H. Deep learning-based component identification for the Raman spectra of mixtures. Anal. 2019, 144, 1789–1798. [Google Scholar] [CrossRef]
- Fufurin, I.L.; Golyak, I.S.; Anfimov, D.R.; Tabalina, A.S.; Kareva, E.R.; Morozov, A.N.; Demkin, P.P. Machine learning applications for spectral analysis of human exhaled breath for early diagnosis of diseases. In Optics in Health Care and Biomedical Optics X; Luo, Q., Li, X., Gu, Y., Zhu, D., Eds.; SPIE: Bellingham, DC, USA, 2020. [Google Scholar] [CrossRef]
- Tabalina, A.S.; Anfimov, D.R.; Fufurin, I.L.; Golyak, I.S. Infrared quantum cascade laser spectroscopy as non-invasive diagnostic tests for human diseases. In Biomedical Spectroscopy, Microscopy, and Imaging; Popp, J., Gergely, C., Eds.; SPIE: Bellingham, DC, USA, 2020; Volume 11359, pp. 233–242. [Google Scholar] [CrossRef]
- Fufurin, I.L.; Anfimov, D.R.; Kareva, E.R.; Scherbakova, A.V.; Demkin, P.P.; Morozov, A.N.; Golyak, I.S. Numerical techniques for infrared spectra analysis of organic and inorganic volatile compounds for biomedical applications. Opt. Eng. 2021, 60, 082016. [Google Scholar] [CrossRef]
- Coppieters, K.T.; von Herrath, M.G. Histopathology of Type I Diabetes: Old Paradigms and New Insights. Rev. Diabet. Stud. 2009, 6, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flier, J.S.; Underhill, L.H.; Eisenbarth, G.S. Type I Diabetes Mellitus. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef]
- Kalapos, M. Possible physiological roles of acetone metabolism in humans. Med. Hypotheses 1999, 53, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Saxby, N.; Beggs, S.; Kariyawasam, N.; Battersby, M.; Lawn, S. Do guidelines provide evidence-based guidance to health professionals on promoting developmentally appropriate chronic condition self-management in children? A systematic review. Chronic Illn. 2018, 16, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Hinton, G. Rectified Linear Units Improve Restricted Boltzmann Machines Vinod Nair. Citeseer 2010, 27, 807–814. [Google Scholar]
- Bottou, L. Large-Scale Machine Learning with Stochastic Gradient Descent. In Proceedings of COMPSTAT’2010; Lechevallier, Y., Saporta, G., Eds.; Physica-Verlag HD: Heidelberg, Hermany, 2010; pp. 177–186. [Google Scholar]
- Glorot, X.; Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. J. Mach. Learn. Res. Proc. Track 2010, 9, 249–256. [Google Scholar]
- Matsumoto, M.; Nishimura, T. Mersenne twister. ACM Trans. Model. Comput. Simul. 1998, 8, 3–30. [Google Scholar] [CrossRef] [Green Version]
- Bergstra, J.; Bengio, Y. Random Search for Hyper-Parameter Optimization. J. Mach. Learn. Res. 2012, 13, 281–305. [Google Scholar]
- Diskin, A.M.; Panl, P.; Smith, D. Time variation of ammonia, acetone, isoprene and ethanol in breath: A quantitative SIFT-MS study over 30 days. Physiol. Meas. 2003, 24, 107–119. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Simic, M.; Ajdukovic, N.; Veselinovic, I.; Mitrovic, M.; Djurendic-Brenesel, M. Endogenous ethanol production in patients with Diabetes Mellitus as a medicolegal problem. Forensic Sci. Int. 2012, 216, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Shorten, C.; Khoshgoftaar, T.M. A survey on Image Data Augmentation for Deep Learning. J. Big Data 2019, 6. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath Ethanol and Acetone as Indicators of Serum Glucose Levels: An Initial Report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Mörk, A.K.; Johanson, G. A human physiological model describing acetone kinetics in blood and breath during various levels of physical exercise. Toxicol. Lett. 2006, 164, 6–15. [Google Scholar] [CrossRef]
- Kundu, S.K.; Bruzek, J.A.; Nair, R.; Judilla, A.M. Breath acetone analyzer: Diagnostic tool to monitor dietary fat loss. Clin. Chem. 1993, 39, 87–92. [Google Scholar] [CrossRef]


| Parameter | Value | Value Range |
|---|---|---|
| Number of kernels of the convolutional layer | kernels | |
| Size of kernels of the convolutional layer | N | |
| Stride for the convolution and max-pooling | s | ; |
| Momentum in the SGD updating rule | momentum | |
| Number of neurons in FCL | neurons | |
| Learning rate | lr | |
| Number of epochs | epoches |
| No | Substance | Wavenumber, cm | Cross-Section, 10 cm |
|---|---|---|---|
| 1 | Ammonia | 930 | 4.28 |
| 2 | Acetone | 1217 | 3.37 |
| 3 | Methanol | 1033 | 7.16 |
| 4 | Ethanol | 1065 | 2.58 |
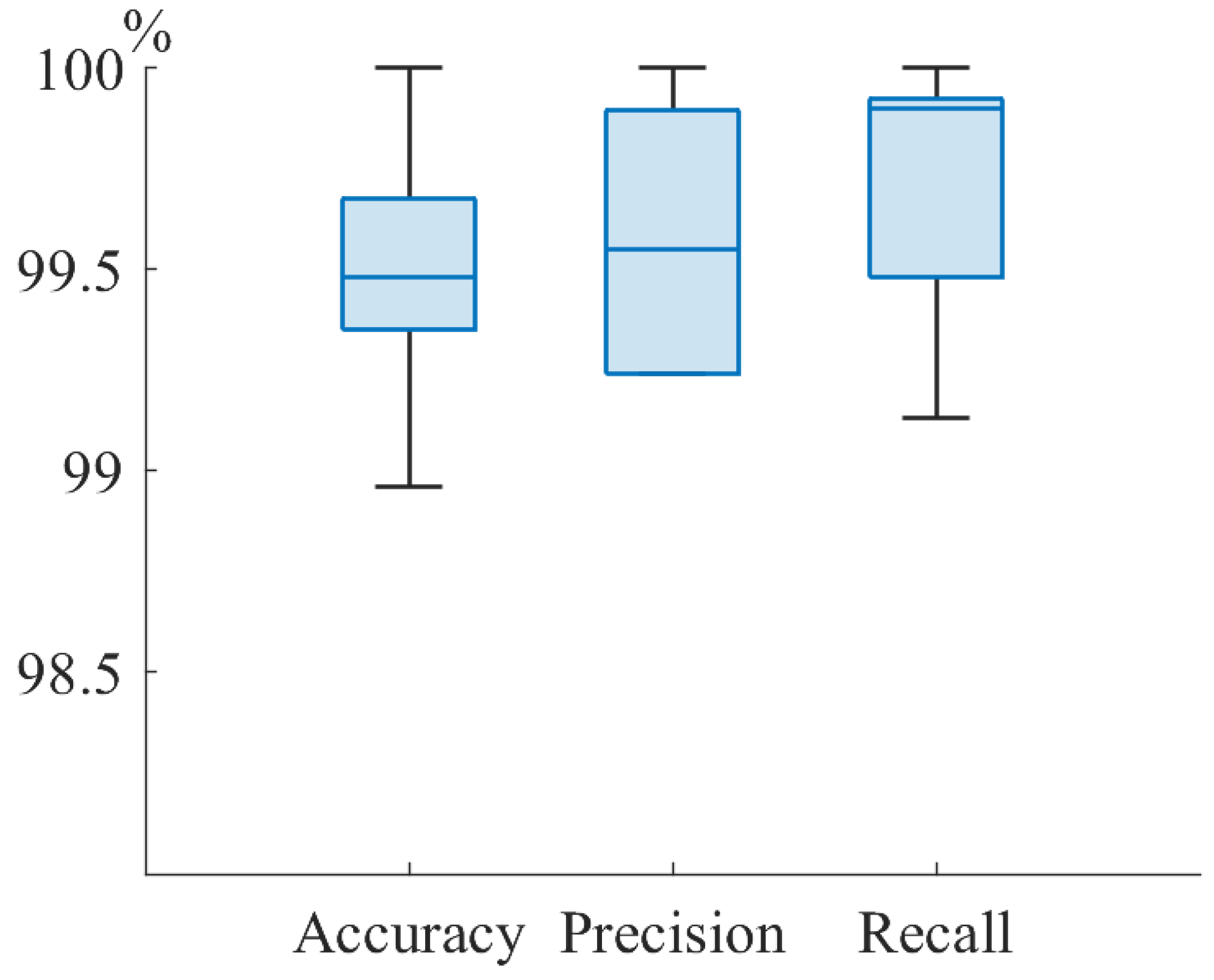
| Group | Accuracy | Precision | Recall |
|---|---|---|---|
| All | 99.7 | 99.5 | 99.6 |
| Male | 99.5 | 98.6 | 100 |
| Female | 99.7 | 99.9 | 99.8 |
| Minors (less 14 y.o.) | 99.5 | 99.6 | 99.5 |
| Adults (more 14 y.o.) | 98.9 | 99.3 | 99.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fufurin, I.; Berezhanskiy, P.; Golyak, I.; Anfimov, D.; Kareva, E.; Scherbakova, A.; Demkin, P.; Nebritova, O.; Morozov, A. Deep Learning for Type 1 Diabetes Mellitus Diagnosis Using Infrared Quantum Cascade Laser Spectroscopy. Materials 2022, 15, 2984. https://doi.org/10.3390/ma15092984
Fufurin I, Berezhanskiy P, Golyak I, Anfimov D, Kareva E, Scherbakova A, Demkin P, Nebritova O, Morozov A. Deep Learning for Type 1 Diabetes Mellitus Diagnosis Using Infrared Quantum Cascade Laser Spectroscopy. Materials. 2022; 15(9):2984. https://doi.org/10.3390/ma15092984
Chicago/Turabian StyleFufurin, Igor, Pavel Berezhanskiy, Igor Golyak, Dmitriy Anfimov, Elizaveta Kareva, Anastasiya Scherbakova, Pavel Demkin, Olga Nebritova, and Andrey Morozov. 2022. "Deep Learning for Type 1 Diabetes Mellitus Diagnosis Using Infrared Quantum Cascade Laser Spectroscopy" Materials 15, no. 9: 2984. https://doi.org/10.3390/ma15092984
APA StyleFufurin, I., Berezhanskiy, P., Golyak, I., Anfimov, D., Kareva, E., Scherbakova, A., Demkin, P., Nebritova, O., & Morozov, A. (2022). Deep Learning for Type 1 Diabetes Mellitus Diagnosis Using Infrared Quantum Cascade Laser Spectroscopy. Materials, 15(9), 2984. https://doi.org/10.3390/ma15092984






