Radiographic Analysis of Graft Dimensional Changes in Transcrestal Maxillary Sinus Augmentation: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedure
2.3. Biomaterials
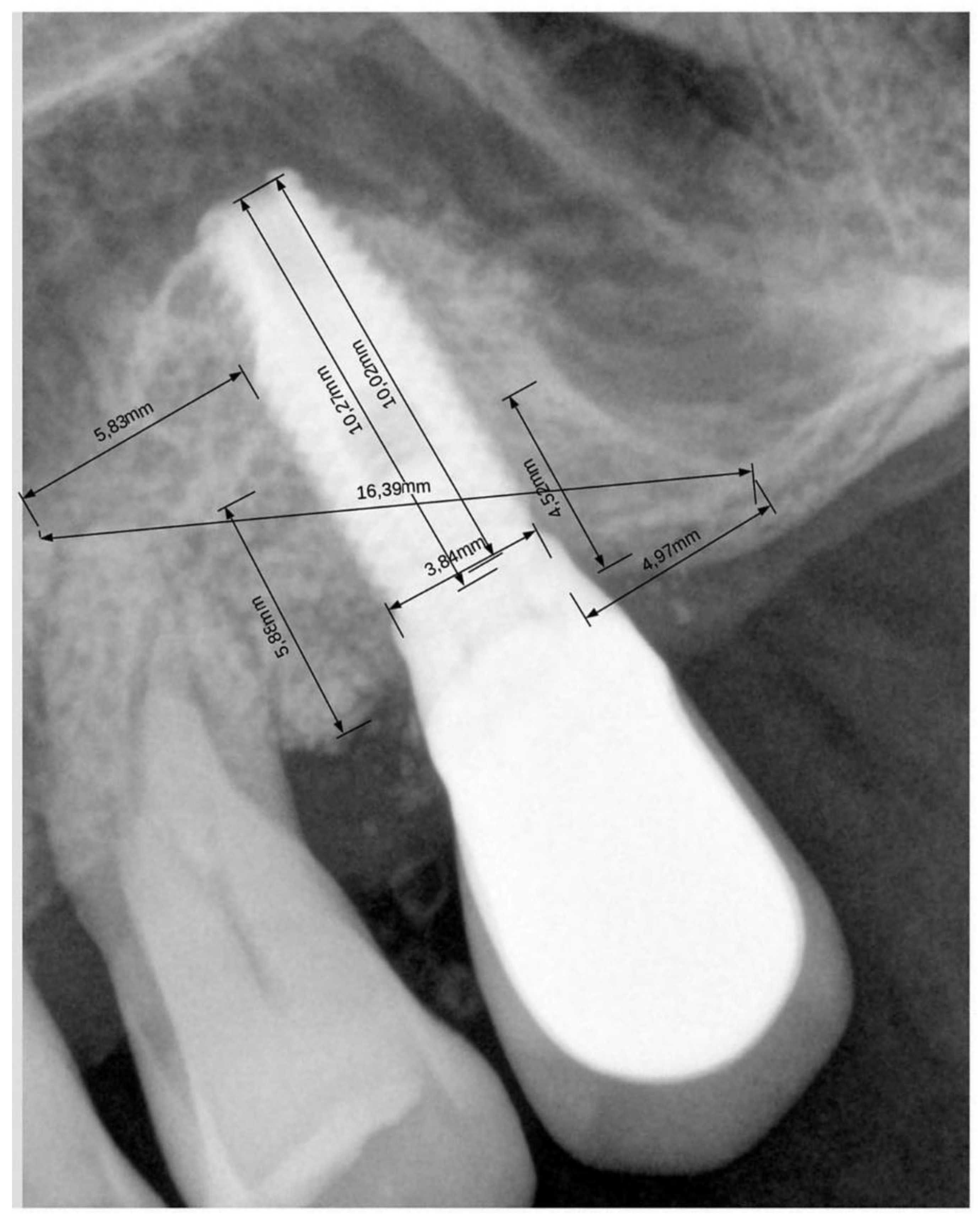
2.4. Radiographic Evaluation
2.5. Statistical Analysis
3. Results
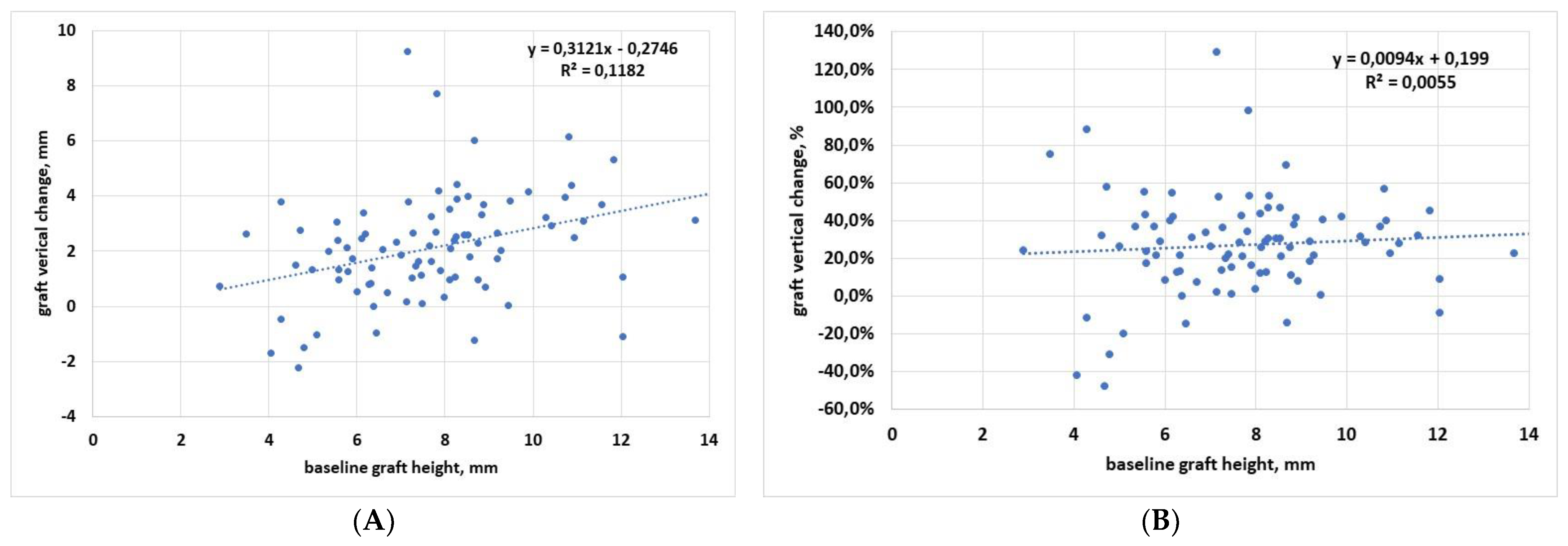
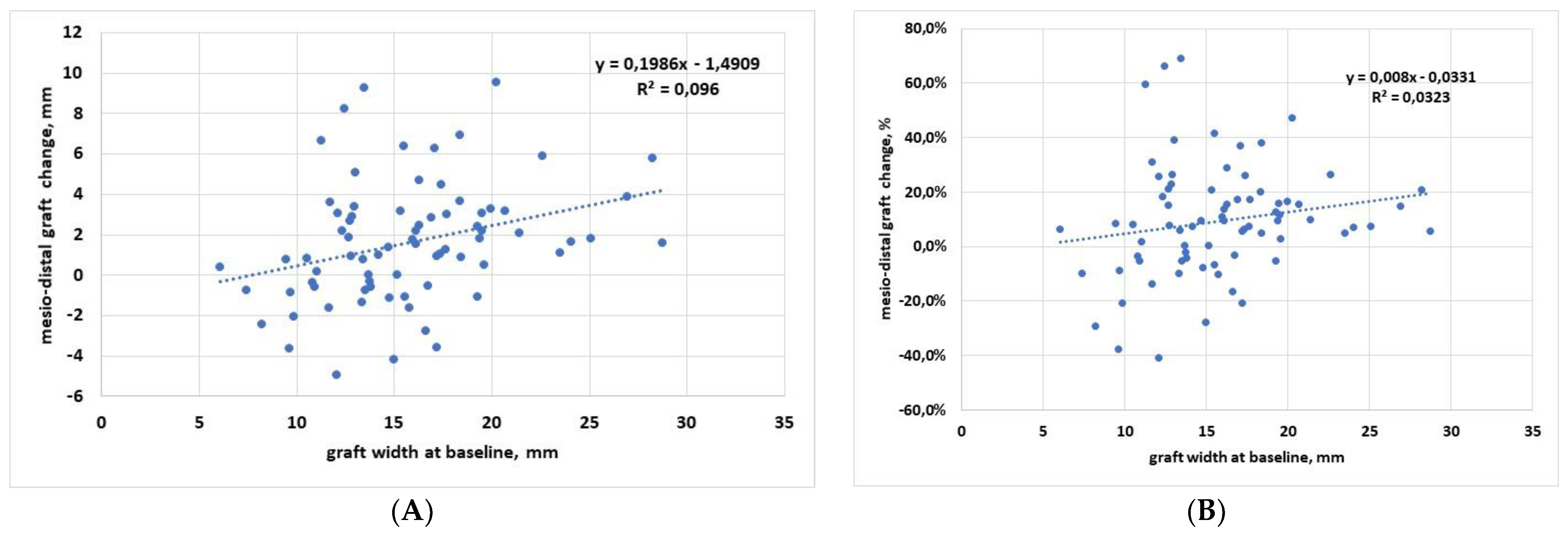
3.1. Regression Analysis
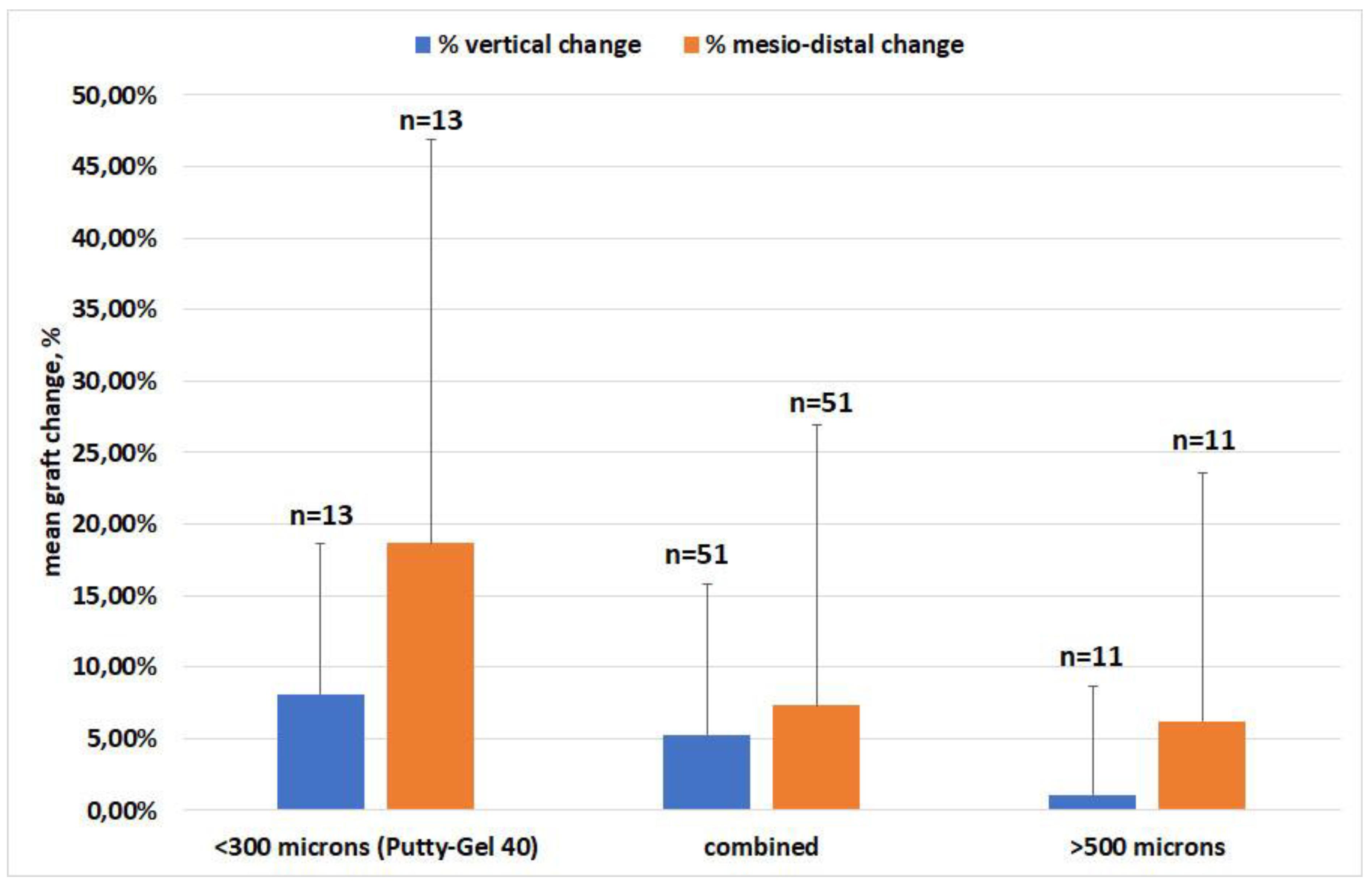
3.2. Effect of Material
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossetti, P.H.O.; Bonachela, W.C.; Rossetti, L.M.N. Relevant Anatomic and Biomechanical Studies for Implant Possibilities on the Atrophic Maxilla: Critical Appraisal and Literature Review. J. Prosthodont. 2010, 19, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, G.; Piersanti, L.; Piattelli, A.; Iezzi, G.; Pieri, F.; Marchetti, C. Augmentation of the Floor of the Maxillary Sinus with Recombinant Human Bone Morphogenetic Protein-7: A Pilot Radiological and Histological Study in Humans. Br. J. Oral Maxillofac. Surg. 2013, 51, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Aldelaimi, T.N.; Khalil, A.A. Maxillary Sinus Augmentation. J. Craniofac. Surg. 2016, 27, e557–e559. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Wolf, J.; Dym, H. Maxillary Sinus Augmentation. Dent. Clin. N. Am. 2015, 59, 375–388. [Google Scholar] [CrossRef]
- La Barbera, L.; Corbella, S.; Taschieri, S.; Galbusera, F.; Giannì, A.B.; Francetti, L. Augmentation of the Atrophic Maxillary Sinus Floor: Graft Stiffness, Implant Shape and Length. J. Biol. Regul. Homeost. Agents 2018, 32, 1295–1301. [Google Scholar]
- Del Fabbro, M.; Rosano, G.; Taschieri, S. Implant survival rates after maxillary sinus augmentation. Eur. J. Oral Sci. 2008, 116, 497–506. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Wallace, S.S.; Testori, T. Long-Term Implant Survival in the Grafted Maxillary Sinus: A Systematic Review. Int. J. Periodontics Restor. Dent. 2013, 33, 773–783. [Google Scholar] [CrossRef]
- Tatum, H., Jr. Maxillary and Sinus Implant Reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar]
- Iezzi, G.; Scarano, A.; Mangano, C.; Cirotti, B.; Piattelli, A. Histologic Results from a Human Implant Retrieved Due to Fracture 5 Years after Insertion in a Sinus Augmented with Anorganic Bovine Bone. J. Periodontol. 2008, 79, 192–198. [Google Scholar] [CrossRef]
- Huwais, S.; Mazor, Z.; Ioannou, A.L.; Gluckman, H.; Neiva, R. A Multicenter Retrospective Clinical Study with Up-to-5-Year Follow-up Utilizing a Method That Enhances Bone Density and Allows for Transcrestal Sinus Augmentation Through Compaction Grafting. Int. J. Oral Maxillofac. Implant. 2018, 33, 1305–1311. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Arcangelo, M.; D’Arcangelo, C.; Celletti, R.; de Oliveira, P.S. Lateral Sinus Floor Elevation Performed with Trapezoidal and Modified Triangular Flap Designs: A Randomized Pilot Study of Post-Operative Pain Using Thermal Infrared Imaging. Int. J. Environ. Res. Public Health 2018, 15, 1277. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Traini, T.; Scarano, A.; Degidi, M.; Perrotti, V.; Piccirilli, M.; Piattelli, A. Maxillary Sinus Augmentation with Bio-Oss Particles: A Light, Scanning, and Transmission Electron Microscopy Study in Man. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Botticelli, D.; Nakajima, Y.; Sakuma, S.; Baba, S. Anatomical Analyses for Maxillary Sinus Floor Augmentation with a Lateral Approach: A Cone Beam Computed Tomography Study. Ann. Anat. Anat. Anz. 2019, 226, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Contessi, M.; Gambardella, U.; Schmitz, J.; Giacometti, E.; Celko, M.; Trisi, P. Autologous Tooth Graft for Maxillary Sinus Augmentation: A Multicenter Clinical Study. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 45. [Google Scholar] [CrossRef]
- Kuo, P.-Y.; Lin, C.-Y.; Chang, C.-C.; Wang, Y.-M.; Pan, W.-L. Grafted Bone Remodeling Following Transcrestal Sinus Floor Elevation: A Cone-Beam Computed Tomography Study. Biomed. J. 2021, 44, 627–635. [Google Scholar] [CrossRef]
- Testori, T.; Panda, S.; Clauser, T.; Scaini, R.; Zuffetti, F.; Capelli, M.; Taschieri, S.; Mortellaro, C.; Del Fabbro, M. Short Implants and Platelet-Rich Fibrin for Transcrestal Sinus Floor Elevation: A Prospective Multicenter Clinical Study. J. Biol. Regul. Homeost. Agents 2019, 33, 121–135. [Google Scholar]
- Tan, W.C.; Lang, N.P.; Zwahlen, M.; Pjetursson, B.E. A Systematic Review of the Success of Sinus Floor Elevation and Survival of Implants Inserted in Combination with Sinus Floor Elevation. Part II: Transalveolar Technique. J. Clin. Periodontol. 2008, 35, 241–254. [Google Scholar] [CrossRef]
- Summers, R.B. The Osteotome Technique: Part 3—Less Invasive Methods of Elevating the Sinus Floor. Compendium 1994, 15, 698, 700, 702–704 passim, quiz 710. [Google Scholar]
- Checchi, L.; Felice, P.; Antonini, E.S.; Cosci, F.; Pellegrino, G.; Esposito, M. Crestal Sinus Lift for Implant Rehabilitation: A Randomised Clinical Trial Comparing the Cosci and the Summers Techniques. A Preliminary Report on Complications and Patient Preference. Eur. J. Oral Implantol. 2010, 3, 221–232. [Google Scholar]
- Kühl, S.; Kirmeier, R.; Platzer, S.; Bianco, N.; Jakse, N.; Payer, M. Transcrestal Maxillary Sinus Augmentation: Summers’ versus a Piezoelectric Technique--an Experimental Cadaver Study. Clin. Oral Implants Res. 2016, 27, 126–129. [Google Scholar] [CrossRef]
- Salgar, N. Osseodensified Crestal Sinus Window Augmentation: An Alternative Procedure to the Lateral Window Technique. J. Oral Implantol. 2021, 47, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Sohn, D.-S.; Bae, M.-S.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Flapless Transcrestal Sinus Augmentation Using Hydrodynamic Piezoelectric Internal Sinus Elevation with Autologous Concentrated Growth Factors Alone. Implant Dent. 2014, 23, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Erasmi, A.; Sievers, H.-H.; Scharfschwerdt, M.; Eckel, T.; Misfeld, M. In Vitro Hydrodynamics, Cusp-Bending Deformation, and Root Distensibility for Different Types of Aortic Valve-Sparing Operations: Remodeling, Sinus Prosthesis, and Reimplantation. J. Thorac. Cardiovasc. Surg. 2005, 130, 1044–1049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catros, S.; Montaudon, M.; Bou, C.; Da Costa Noble, R.; Fricain, J.C.; Ella, B. Comparison of Conventional Transcrestal Sinus Lift and Ultrasound-Enhanced Transcrestal Hydrodynamic Cavitational Sinus Lift for the Filling of Subantral Space: A Human Cadaver Study. J. Oral. Implantol. 2015, 41, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Cayón, R.; Romero-Ruiz, M.-M.; Torres-Lagares, D.; Pérez-Dorao, B.; Wainwright, M.; Abalos-Labruzzi, C.; Gutiérrez-Pérez, J.-L. Hydrodynamic Ultrasonic Maxillary Sinus Lift: Review of a New Technique and Presentation of a Clinical Case. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e271–e275. [Google Scholar] [CrossRef]
- Cordioli, G.; Majzoub, Z.; Piattelli, A.; Scarano, A. Removal Torque and Histomorphometric Investigation of 4 Different Titanium Surfaces: An Experimental Study in the Rabbit Tibia. Int. J. Oral Maxillofac. Implant. 2000, 15, 668–674. [Google Scholar]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary Sinus Augmentation with Different Biomaterials: A Comparative Histologic and Histomorphometric Study in Man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef]
- Wagner, F.; Dvorak, G.; Nemec, S.; Pietschmann, P.; Figl, M.; Seemann, R. A Principal Components Analysis: How Pneumatization and Edentulism Contribute to Maxillary Atrophy. Oral Dis. 2017, 23, 55–61. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcome after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin Oral Implants Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Ignjatovic, D.; Matuliene, G.; Brägger, U.; Schmidlin, K.; Lang, N.P. Transalveolar Maxillary Sinus Floor Elevation Using Osteotomes with or without Grafting Material. Part II: Radiographic Tissue Remodeling. Clin. Oral Implants Res. 2009, 20, 677–683. [Google Scholar] [CrossRef]
- Scarano, A.; de Oliveira, P.S.; Traini, T.; Lorusso, F. Sinus Membrane Elevation with Heterologous Cortical Lamina: A Randomized Study of a New Surgical Technique for Maxillary Sinus Floor Augmentation without Bone Graft. Materials 2018, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Ciccarese, S.; Amuso, D.; Mortellaro, C.; Lorusso, F. Cortical Bone Lamina Approach for Mandibular Large Cystic Defect: A Case Report. J. Biol. Regul. Homeost. Agents 2019, 33, 85–91. [Google Scholar] [PubMed]
- Malchiodi, L.; Quaranta, A.; D’Addona, A.; Scarano, A.; Quaranta, M. Jaw Reconstruction with Grafted Autologous Bone: Early Insertion of Osseointegrated Implants and Early Prosthetic Loading. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2006, 64, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Eser, C.; Gencel, E.; Gökdoğan, M.; Kesiktaş, E.; Yavuz, M. Comparison of Autologous and Heterologous Bone Graft Stability Effects for Filling Maxillary Bone Gap after Le Fort I Osteotomy. Adv. Clin. Exp. Med. 2015, 24, 341–348. [Google Scholar] [CrossRef]
- Bathla, S.C.; Fry, R.R.; Majumdar, K. Maxillary Sinus Augmentation. J. Indian Soc. Periodontol. 2018, 22, 468–473. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef]
- Franceschetti, G.; Farina, R.; Minenna, L.; Riccardi, O.; Stacchi, C.; Di Raimondo, R.; Maietti, E.; Trombelli, L. The Impact of Graft Remodeling on Peri-Implant Bone Support at Implants Placed Concomitantly with Transcrestal Sinus Floor Elevation: A Multicenter, Retrospective Case Series. Clin. Oral Implants Res. 2020, 31, 105–120. [Google Scholar] [CrossRef]
- Froum, S.J.; Tarnow, D.P.; Wallace, S.S.; Rohrer, M.D.; Cho, S.C. Sinus Floor Elevation Using Anorganic Bovine Bone Matrix (OsteoGraf/N) with and without Autogenous Bone: A Clinical, Histologic, Radiographic, and Histomorphometric Analysis--Part 2 of an Ongoing Prospective Study. Int. J. Periodontics Restor. Dent. 1998, 18, 528–543. [Google Scholar]
- Hatano, N.; Shimizu, Y.; Ooya, K. A Clinical Long-Term Radiographic Evaluation of Graft Height Changes after Maxillary Sinus Floor Augmentation with a 2:1 Autogenous Bone/Xenograft Mixture and Simultaneous Placement of Dental Implants. Clin. Oral Implants Res. 2004, 15, 339–345. [Google Scholar] [CrossRef]
- Wanschitz, F.; Figl, M.; Wagner, A.; Rolf, E. Measurement of Volume Changes after Sinus Floor Augmentation with a Phycogenic Hydroxyapatite. Int. J. Oral Maxillofac. Implant. 2006, 21, 433–438. [Google Scholar]
- Zijderveld, S.A.; Schulten, E.A.J.M.; Aartman, I.H.A.; ten Bruggenkate, C.M. Long-Term Changes in Graft Height after Maxillary Sinus Floor Elevation with Different Grafting Materials: Radiographic Evaluation with a Minimum Follow-up of 4.5 Years. Clin. Oral Implant. Res. 2009, 20, 691–700. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ko, M.-J.; Lee, J.-H.; Jeong, S.-N. A Radiographic Evaluation of Graft Height Changes after Maxillary Sinus Augmentation. J. Periodontal Implant Sci. 2018, 48, 174. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhang, J.; Chen, Y.; Han, J.; Yu, M.; Zhou, Y. Changes in Bone Graft Height and Influencing Factors after Sinus Floor Augmentation by Using the Lateral Window Approach: A Clinical Retrospective Study of 1 to 2 Years. J. Prosthet. Dent. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Deluiz, D.; Vitenson, J.; Bruun, N.H.; Tinoco, E.M.B. Maxillary Sinus Floor Augmentation with Autogenous Bone Graft Compared with a Composite Grafting Material or Bone Substitute Alone: A Systematic Review and Meta-Analysis Assessing Volumetric Stability of the Grafting Material. JOMR 2021, 12, e1. [Google Scholar] [CrossRef] [PubMed]
- Aaboe, M.; Pinholt, E.M.; Hjorting-Hansen, E. Healing of Experimentally Created Defects: A Review. Br. J. Oral Maxillofac. Surg. 1995, 33, 312–318. [Google Scholar] [CrossRef]
- Haas, R.; Donath, K.; Födinger, M.; Watzek, G. Bovine Hydroxyapatite for Maxillary Sinus Grafting: Comparative Histomorphometric Findings in Sheep: Sinuslift in Sheep: Histomorphometric Results. Clin. Oral Implant. Res. 1998, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Kim, I.-K.; Cho, H.-Y.; Pae, S.-P.; Jung, B.-S.; Cho, H.-W.; Seo, J.-H. Assessment of the Autogenous Bone Graft for Sinus Elevation. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 274. [Google Scholar] [CrossRef]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone Reactions to Anorganic Bovine Bone (Bio-Oss) Used in Sinus Augmentation Procedures: A Histologic Long-Term Report of 20 Cases in Humans. Int. J. Oral Maxillofac. Implant. 1999, 14, 835–840. [Google Scholar]
- Hallman, M.; Cederlund, A.; Lindskog, S.; Lundgren, S.; Sennerby, L. A Clinical Histologic Study of Bovine Hydroxyapatite in Combination with Autogenous Bone and Fibrin Glue for Maxillary Sinus Floor Augmentation: Results after 6 to 8 Months of Healing. Clin. Oral Implant. Res. 2001, 12, 135–143. [Google Scholar] [CrossRef]
- Gosain, A.K. Hydroxyapatite Cement Paste Cranioplasty for the Treatment of Temporal Hollowing After Cranial Vault Remodeling in a Growing Child. J. Craniofacial Surg. 1997, 8, 506–511. [Google Scholar] [CrossRef]
- Scarano, A.; Cholakis, A.K.; Piattelli, A. Histologic Evaluation of Sinus Grafting Materials After Peri-implantitis-Induced Failure: A Case Series. Int. J. Oral Maxillofac. Implant. 2017, 32, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Wang, H.-L.; Wallace, S.S.; Piattelli, A.; Iezzi, G.; Tavelli, L.; Tumedei, M.; Vinci, R.; Del Fabbro, M. Late maxillary sinus graft infections due to peri-implantitis: Case reports with histologic analysis. Int. J. Periodontics Restor. Dent. 2021, 41, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Wallace, S.S.; Trisi, P.; Capelli, M.; Zuffetti, F.; Del Fabbro, M. Effect of xenograft (ABBM) particle size on vital bone formation following maxillary sinus augmentation: A multicenter, randomized, controlled, clinical histomorphometric trial. Int. J. Periodontics Restor. Dent. 2013, 33, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, P.; Mattheos, N.; Yodsanga, S.; Jansisyanont, P. The impact of deproteinized bovine bone particle size on histological and clinical bone healing outcomes in the augmented sinus: A randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2022, in press. [Google Scholar] [CrossRef]
- De Molon, R.S.; Magalhaes-Tunes, F.S.; Semedo, C.V.; Furlan, R.G.; de Souza, L.G.L.; de Souza Faloni, A.P.; Marcantonio, E., Jr.; Faeda, R.S. A randomized clinical trial evaluating maxillary sinus augmentation with different particle sizes of demineralized bovine bone mineral: Histological and immunohistochemical analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 810–823. [Google Scholar] [CrossRef]
- Pignaton, T.B.; Spin-Neto, R.; Ferreira, C.E.A.; Martinelli, C.B.; de Oliveira, G.J.P.L.; Marcantonio, E. Remodelling of Sinus Bone Grafts According to the Distance from the Native Bone: A Histomorphometric Analysis. Clin. Oral Implants Res. 2020, 31, 959–967. [Google Scholar] [CrossRef]
- Beck, F.; Reich, K.M.; Lettner, S.; Heimel, P.; Tangl, S.; Redl, H.; Ulm, C. The Vertical Course of Bone Regeneration in Maxillary Sinus Floor Augmentations: A Histomorphometric Analysis of Human Biopsies. J. Periodontol. 2021, 92, 263–272. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Canullo, L.; Khijmatgar, S.; Modenese, L.; Gallifante, G.; Del Fabbro, M. Radiographic and Histomorphometric Evaluation of Biomaterials Used for Lateral Sinus Augmentation: A Systematic Review on the Effect of Residual Bone Height and Vertical Graft Size on New Bone Formation and Graft Shrinkage. J. Clin. Med. 2021, 10, 4996. [Google Scholar] [CrossRef]
| Gender | 24 Males/42 Females |
|---|---|
| Age, years | 67.9 ± 10.6 (range 43 to 84) |
| Smoking habits | 47 nonsmokers |
| 9 patients 5 cig/day | |
| 1 patient 8 cig/day | |
| 7 patients 10 cig/day | |
| 2 patients 15 cig/day | |
| Follow-up, months | 82.3 ± 54.7 (range 14 to 240) |
| Grafting material | 11 patients: OsteoBiol® mp3® + OsteoBiol® Gel 40 |
| 7 patients: OsteoBiol® Putty + Bioresorb | |
| 13 patients: OsteoBiol® Putty + autogenous bone | |
| 3 patients: OsteoBiol® Putty + Bioresorb + autogenous bone | |
| 7 patients: OsteoBiol® Putty | |
| 8 patients: OsteoBiol® Gel 40 + autogenous bone | |
| 3 patients: OsteoBiol® Putty + OsteoBiol® Gel 40 | |
| 6 patients: OsteoBiol® GTO® | |
| 2 patients: OsteoBiol® Apatos® + OsteoBiol® Gel 40 + autogenous bone | |
| 1 patient: autogenous bone + Bioresorb | |
| 2 patients: OsteoBiol® Gel 40 + OsteoBiol® Apatos® | |
| 4 patients: OsteoBiol® GTO® + autogenous bone | |
| 3 patients: OsteoBiol® Gel 40 + Bioresorb |
| Length, mm | Diameter, mm | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.5 | 3.75 | 3.8 | 4 | 4.1 | 4.2 | 4.3 | 4.5 | 5.0 | ||
| 8.5 | - | - | - | 1 | - | - | - | - | - | 1 |
| 9.0 | - | - | - | - | - | - | - | 1 | 9 | 10 |
| 10.0 | 1 | - | 4 | 3 | 2 | 1 | 5 | 15 | 1 | 32 |
| 11.0 | - | - | 1 | 6 | - | - | - | 10 | 8 | 25 |
| 11.5 | 1 | - | - | 7 | - | 2 | 1 | 4 | - | 15 |
| 13 | - | 1 | - | 3 | 1 | - | - | 1 | - | 6 |
| Total | 2 | 1 | 5 | 20 | 3 | 3 | 6 | 31 | 18 | 89 |
| Implant Site | Number of Implants |
|---|---|
| Right first premolar | 2 |
| Right second premolar | 11 |
| Right first molar | 31 |
| Right second molar | 11 |
| Left first premolar | 1 |
| Left second premolar | 5 |
| Left first molar | 20 |
| Left second molar | 8 |
| Total | 89 |
| Distance Measured | Baseline Mean ± SD (Range), mm | Follow-Up Mean ± SD (Range), mm | Change Mean ± SD (Range), % | p-Value | Total Number | Unit |
|---|---|---|---|---|---|---|
| Mesiodistal graft width | 15.7 ± 4.6 (6.0, 28.7) | 14.1 ± 4.67 (4.0, 27.1) | 9.3% ± 20.7% (−40.9%, 68.8%) | p < 0.001 | 75 | Graft |
| Mesial extension | 4.3 ± 2.1 (0, 9.5) | 3.7 ± 2.2 (0, 9.6) | 13.8% ± 38.1% (−74.6%, 100%) | p < 0.001 | 75 | Graft |
| Distal extension | 5.0 ± 2.4 (0.0, 14.3) | 4.2 ± 2.3 (0, 9.5) | 8.8% ± 46.5% (−126.6%,100%) | p = 0.003 | 75 | Graft |
| Vertical implant apex-graft | 1.8 ± 1.54 (−2.1, 7.6) | 1.0 ± 1.6 (−4.7, 7.5) | 41.5% ± 85.5% (−312.0%, 100%) | p < 0.001 | 86 | Implant |
| Total vertical bone height | 13.4 ± 1.9 (8.7, 19.1) | 12.6 ± 2.1 (6.1, 18.9) | 5.3% ± 9.74% (−23.0%, 31.5%) | p < 0.001 | 86 | implant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comuzzi, L.; Tumedei, M.; Piattelli, A.; Tartaglia, G.; Del Fabbro, M. Radiographic Analysis of Graft Dimensional Changes in Transcrestal Maxillary Sinus Augmentation: A Retrospective Study. Materials 2022, 15, 2964. https://doi.org/10.3390/ma15092964
Comuzzi L, Tumedei M, Piattelli A, Tartaglia G, Del Fabbro M. Radiographic Analysis of Graft Dimensional Changes in Transcrestal Maxillary Sinus Augmentation: A Retrospective Study. Materials. 2022; 15(9):2964. https://doi.org/10.3390/ma15092964
Chicago/Turabian StyleComuzzi, Luca, Margherita Tumedei, Adriano Piattelli, Gianluca Tartaglia, and Massimo Del Fabbro. 2022. "Radiographic Analysis of Graft Dimensional Changes in Transcrestal Maxillary Sinus Augmentation: A Retrospective Study" Materials 15, no. 9: 2964. https://doi.org/10.3390/ma15092964
APA StyleComuzzi, L., Tumedei, M., Piattelli, A., Tartaglia, G., & Del Fabbro, M. (2022). Radiographic Analysis of Graft Dimensional Changes in Transcrestal Maxillary Sinus Augmentation: A Retrospective Study. Materials, 15(9), 2964. https://doi.org/10.3390/ma15092964










