Research on SR/Frit Composites: A Novel Low-Temperature Ceramifiable Expandable Flame-Retardant Material
Abstract
:1. Introduction
2. Experiments
2.1. Experimental Raw Materials
2.2. Experimental Instruments and Apparatus
2.3. Performance Tests
3. Results and Discussion
3.1. LOI and UL-94 Tests of the SR/Frit Composites
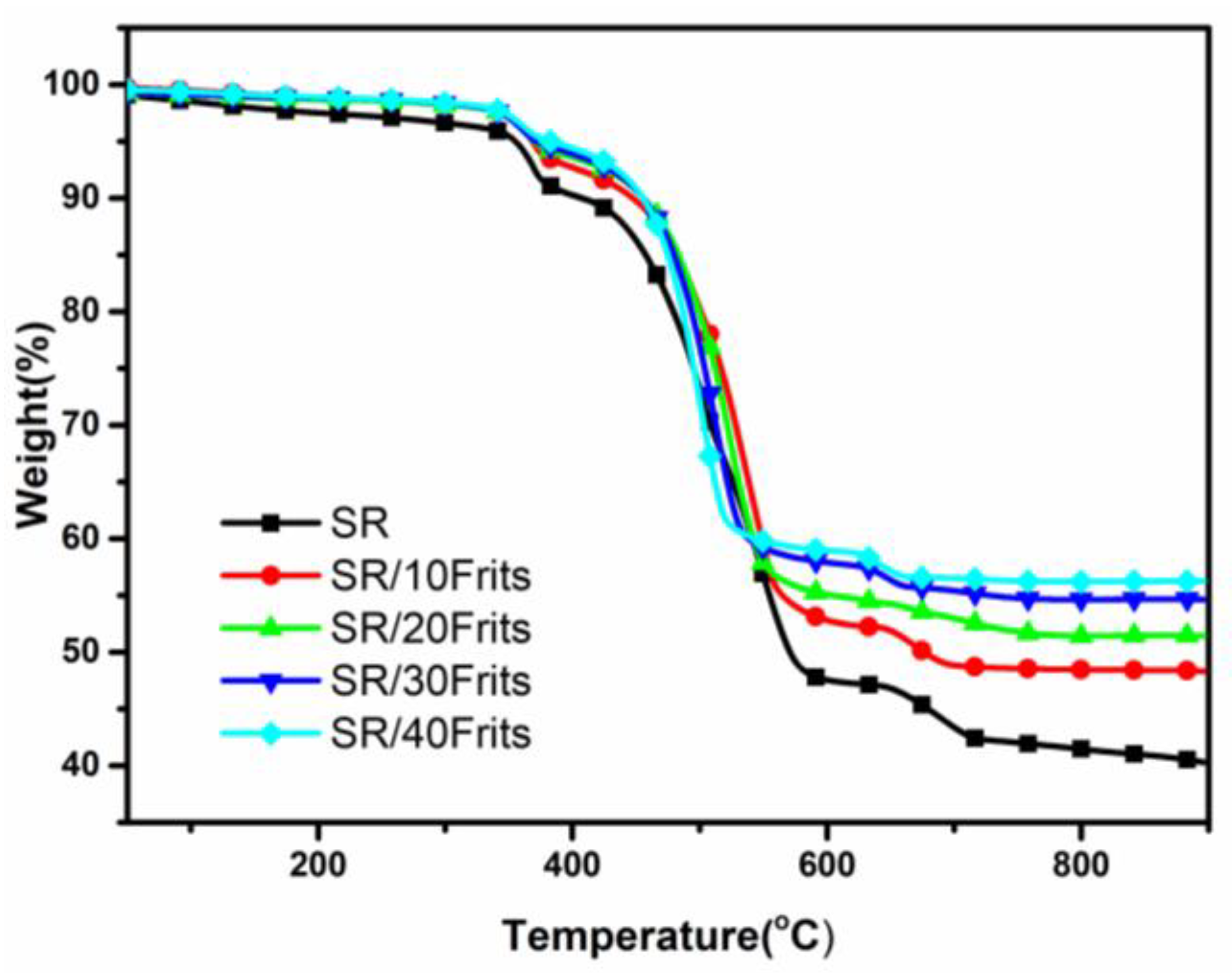
3.2. Thermogravimetric Analysis of SR/Frits Composites
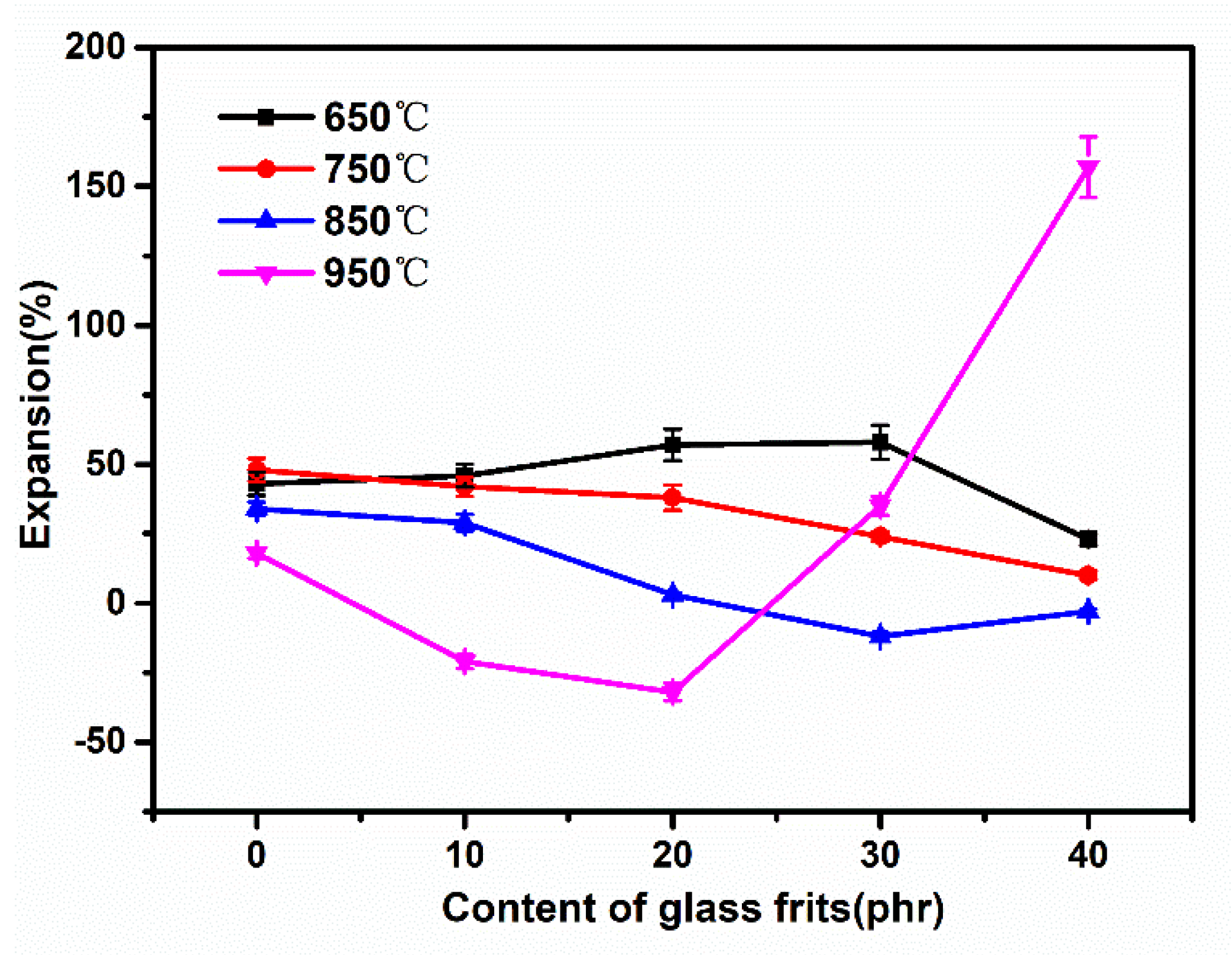
3.3. Influence of Low-Melting-Point Frit Content on the Macroscopic Morphology of Ablation Residues
3.4. Influence of Low-Melting-Point Frit Content on the Microscopic Volume of Ablation Residues
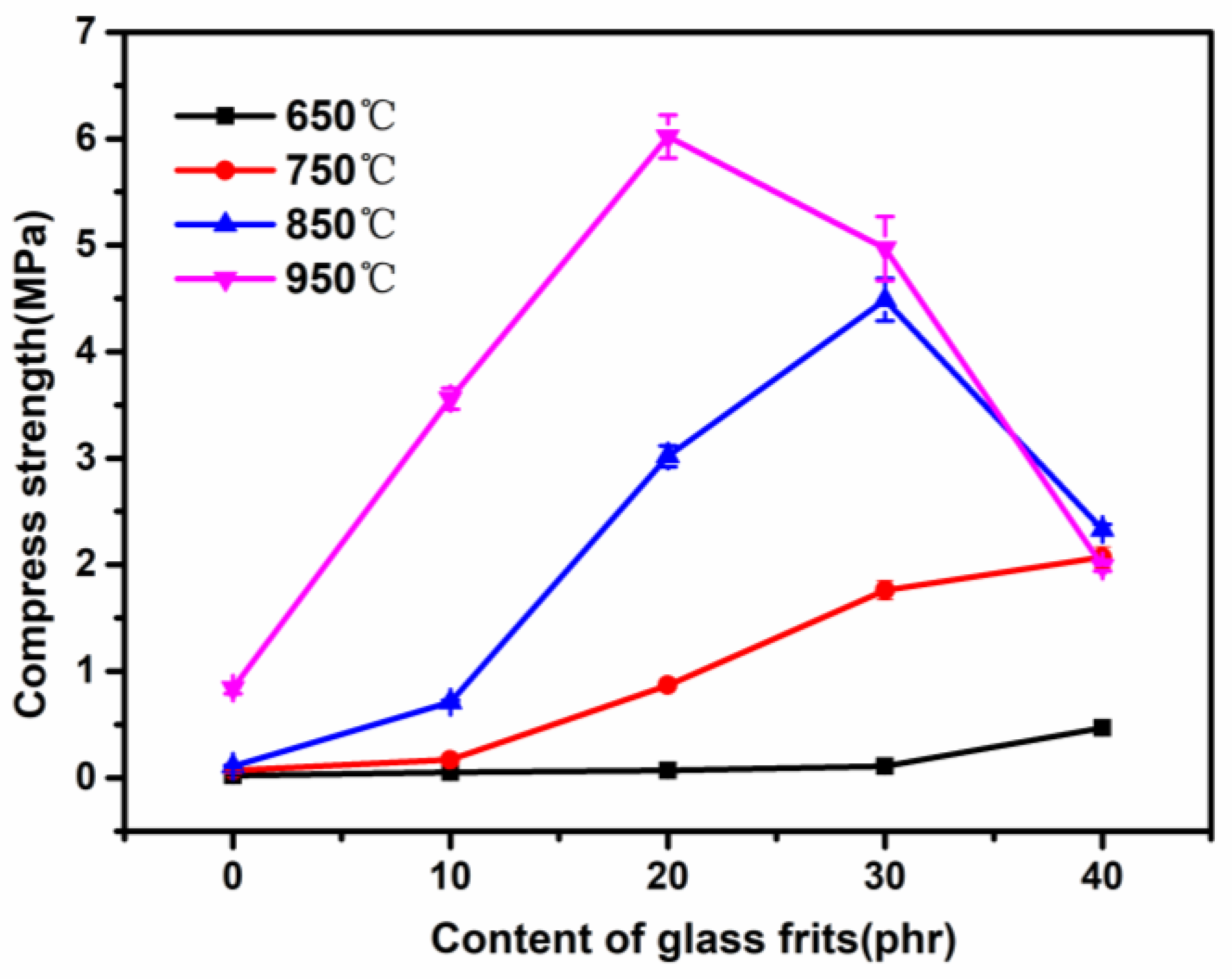
3.5. Influence of Low-Melting-Point Frit Content on the Compressive Strength of Ablation Residues
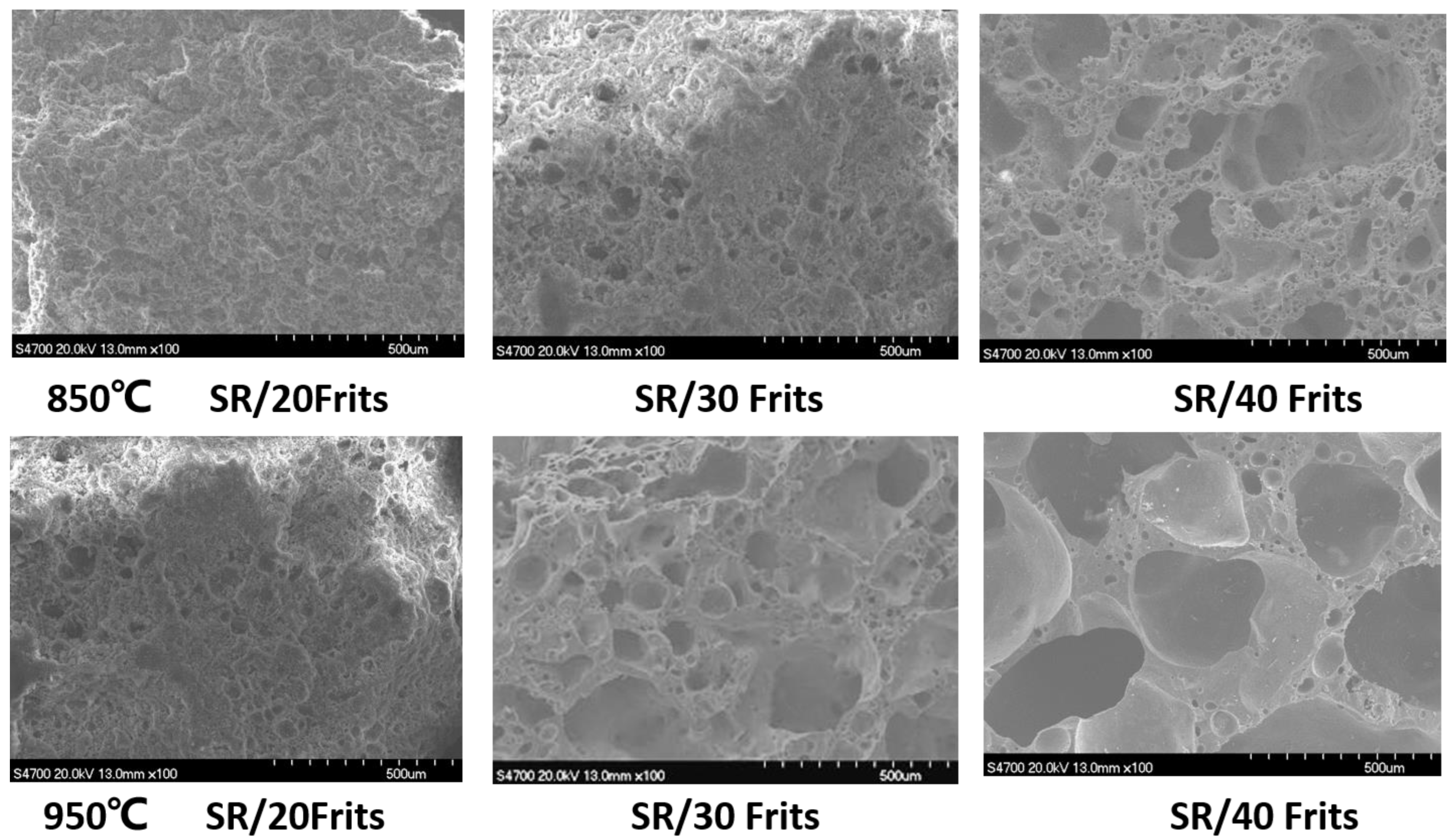
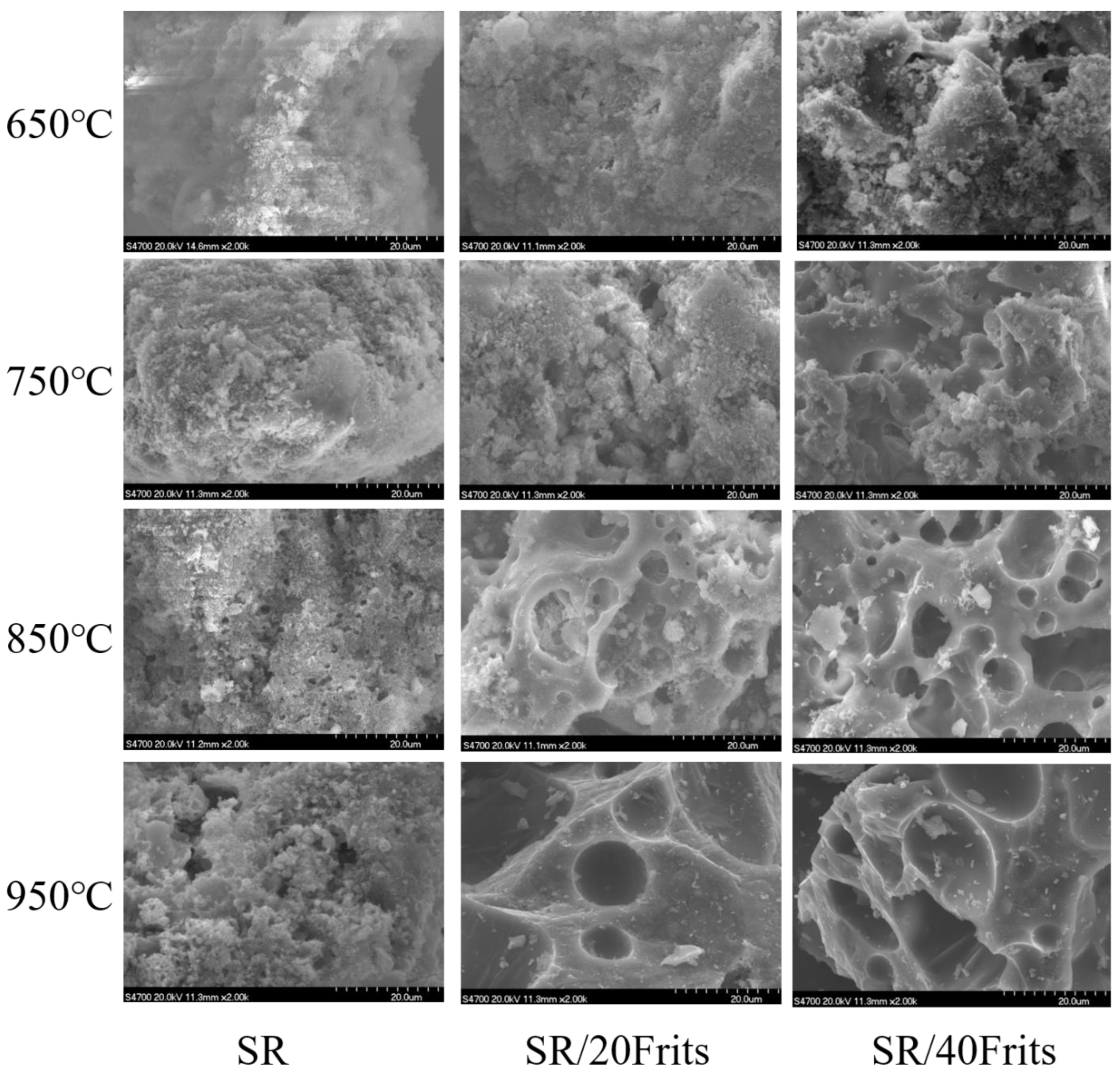
3.6. Influence of Low-Melting-Point Frit Content on the Microscopic Morphology of Ablation Residues
3.7. Mechanical Performance Tests of SR/Frit Composites
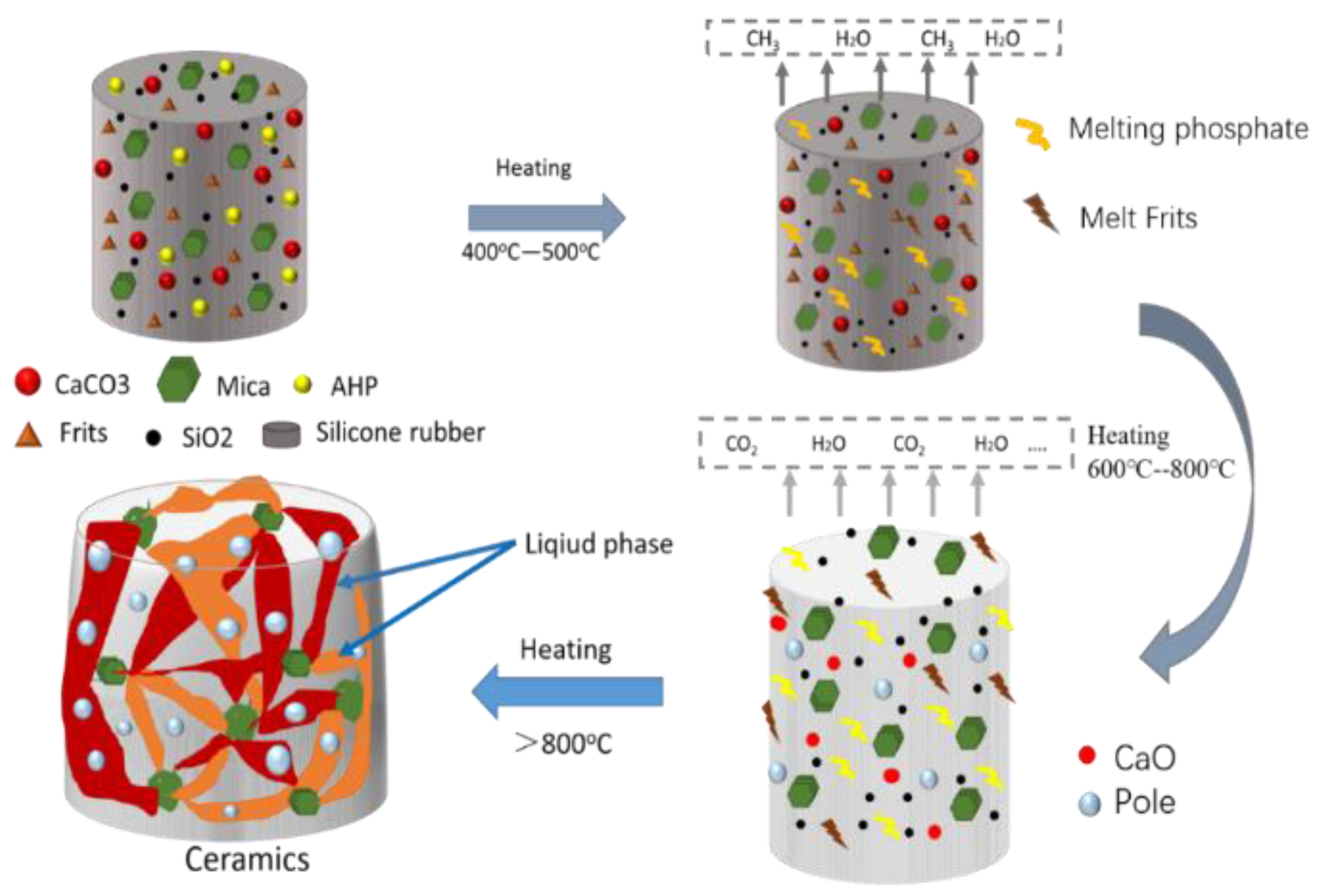
3.8. Expansion and Ceramization Mechanism of SR/Frit Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanu, L.G.; Simon, G.P.; Mansouri, J.; Burford, R.P.; Cheng, Y.B. Development of polymer–ceramic composites for improved fire resistance. J. Mater. Process. Technol. 2004, 153, 401–407. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Preferential orientation of muscovite in ceramifiable silicone composites. Mat. Sci. Eng. A Struct. 2005, 398, 180–187. [Google Scholar] [CrossRef]
- Anyszka, R.; Bielin, D.M.; Pedzich, S.; Szumera, M.J. Influence of surface-modified montmorillonites on properties of silicone rubber-based ceramizable composites. Therm. Anal. Calorim. 2015, 119, 11–121. [Google Scholar] [CrossRef] [Green Version]
- Verdejo, R.; Barroso-Bujans, F.; Rodríguez-Pérez, M.; De Saja, J.A.; Arroyo, M.; Lopez-Manchado, M.A. Carbon nanotubes provide self-extinguishing grade to silicone-based foams. J. Mater. Chem. 2008, 43, 3933–3939. [Google Scholar] [CrossRef]
- Mansouri, J.; Wood, C.A.; Roberts, K.; Cheng, Y.B.; Burford, R.P. Investigation of the ceramifying process of modified silicone–silicate compositions. J. Mater. Sci. 2007, 42, 6046–6055. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B. Pyrolysis behaviour of silicone-based ceramifying composites. Mat. Sci. Eng. A Struct. 2006, 425, 7–14. [Google Scholar] [CrossRef]
- Pędzich, Z.; Bieliński, D.M.; Dul, J.; Zarzecka-Napierała, M. Optimisation of the Ceramic Phase for Ceramizable Silicone Rubber Based Composites. Adv. Sci. Technol. Res. J. 2010, 66, 162–167. [Google Scholar]
- Wang, Y.; Lai, X.; Li, H.; Liu, T.; Zeng, X. Significantly improve fire safety of silicone rubber by efficiently catalyzing ceramization on fluorophlogopite. Compos. Commun. 2021, 25, 100683. [Google Scholar] [CrossRef]
- Li, J.H. Research status and development trend of ceramifiable silicone rubber composites: A brief review. Mater. Res. Express 2022, 9, 012001. [Google Scholar] [CrossRef]
- Niu, H.; Ren, Y.; Guo, H.; Małycha, K.; Orzechowski, K.; Bai, S.L. Recent progress on thermally conductive and electrical insulating rubber composites: Design, processing and applications. Compos. Commun. 2020, 22, 100430. [Google Scholar] [CrossRef]
- Mehmood, B.; Akbar, M.; Ullah, R. Accelerated aging effect on high temperature vulcanized silicone rubber composites under DC voltage with controlled environmental conditions. Eng. Fail. Anal. 2020, 118, 104870. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Wang, L.; Li, J.; Feng, T.; Fan, J.; Chen, L.; Gu, J. Superior wave-absorbing performances of silicone rubber composites via introducing covalently bonded SnO2@ MWCNT absorbent with encapsulation structure. Compos. Commun. 2020, 22, 100486. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.Y.; Yang, R.J. The effects of phosphorus-based flame retardants and octaphenyl polyhedral oligomeric silsesquioxane on the ablative and flame-retardation properties of room temperature vulcanized silicone rubber insulating composites. Polym. Degrad. Stab. 2016, 125, 140–147. [Google Scholar] [CrossRef]
- Li, J.H. Effect of expandable graphite on expansion and combustion performance of modified silicone rubber. Fire Sci. Technol. 2020, 39, 1573–1576. [Google Scholar]
- Li, J.H. Improving the mechanical and ceramifiable properties of low temperature prepared silicone rubber composites. Mater. Res. Express 2021, 8, 095203. [Google Scholar] [CrossRef]
- Li, H.Y.; Tao, S.; Huang, Y.H.; Su, Z.T.; Zheng, J.P. The improved thermal oxidative stability of silicone rubber by using iron oxide and carbon nanotubes as thermal resistant additives. Compos. Sci. Technol. 2013, 76, 52–60. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, T.; Shao, H.; Xin-hao, G. Application of Glass Frits in Ceramifying Silicone Rubber. Polym. Bull. 2014, 7, 30–35. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, Q.; Wu, L.; Wang, T.J. Preparation and properties of ceramifying silicone rubber. Nanjing Univ. Technol. 2011, 33, 48–51. [Google Scholar] [CrossRef]
- Li, P.; Jin, H.; Wei, S.; Liu, H.; Gao, N.; Shi, Z. Ceramization mechanism of ceramizable silicone rubber composites with nano silica at low temperature. Materials 2020, 13, 3708. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of ageing on the fire behaviour of flame-retarded polymers: A review. Polym. Int. 2015, 64, 313–328. [Google Scholar] [CrossRef]
- Li, H. Study on the Preparation, Structure, and Performance of Ceramifiable Silicone Rubber; South China University of Technology: Guangzhou, China, 2016. [Google Scholar]
- Yang, H.; Wang, X.; Song, L.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Aluminum hypophosphite in combination with expandable graphite as a novel flame retardant system for rigid polyurethane foams. Polym. Adv. Technol. 2015, 25, 1034–1043. [Google Scholar] [CrossRef]
- Owen, M.J. Coupling agents: Chemical bonding at interfaces. Adhes. Sci. Eng. 2002, 2, 403–431. [Google Scholar]
- Guo, J.; Gao, W.; Wang, Y.; Liang, D.; Li, H.; Zhang, X. Effect of glass frit with low softening temperature on the properties, microstructure and formation mechanism of polysiloxane elastomer-based ceramizable composites. Polym. Degrad. Stab. 2017, 136, 71. [Google Scholar] [CrossRef]
- Li, B.; Long, Q.; Duan, D.J. Effect of sintering temperatures on properties of BaO-B2O3 SiO2-Al2O3, glass/silica composites for CBGA package. Mater. Sci. Mater. Electron. 2016, 27, 2206. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Thermal stability and flammability of silicone polymer composites. Polym. Degrad. Stab. 2006, 91, 1373–1379. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Shan, Z.; Wang, S.; Xiao, Y. Surface modification of magnesium hydroxide by wet process and effect on the thermal stability of silicone rubber. Appl. Surf. Sci. 2019, 465, 740. [Google Scholar] [CrossRef]

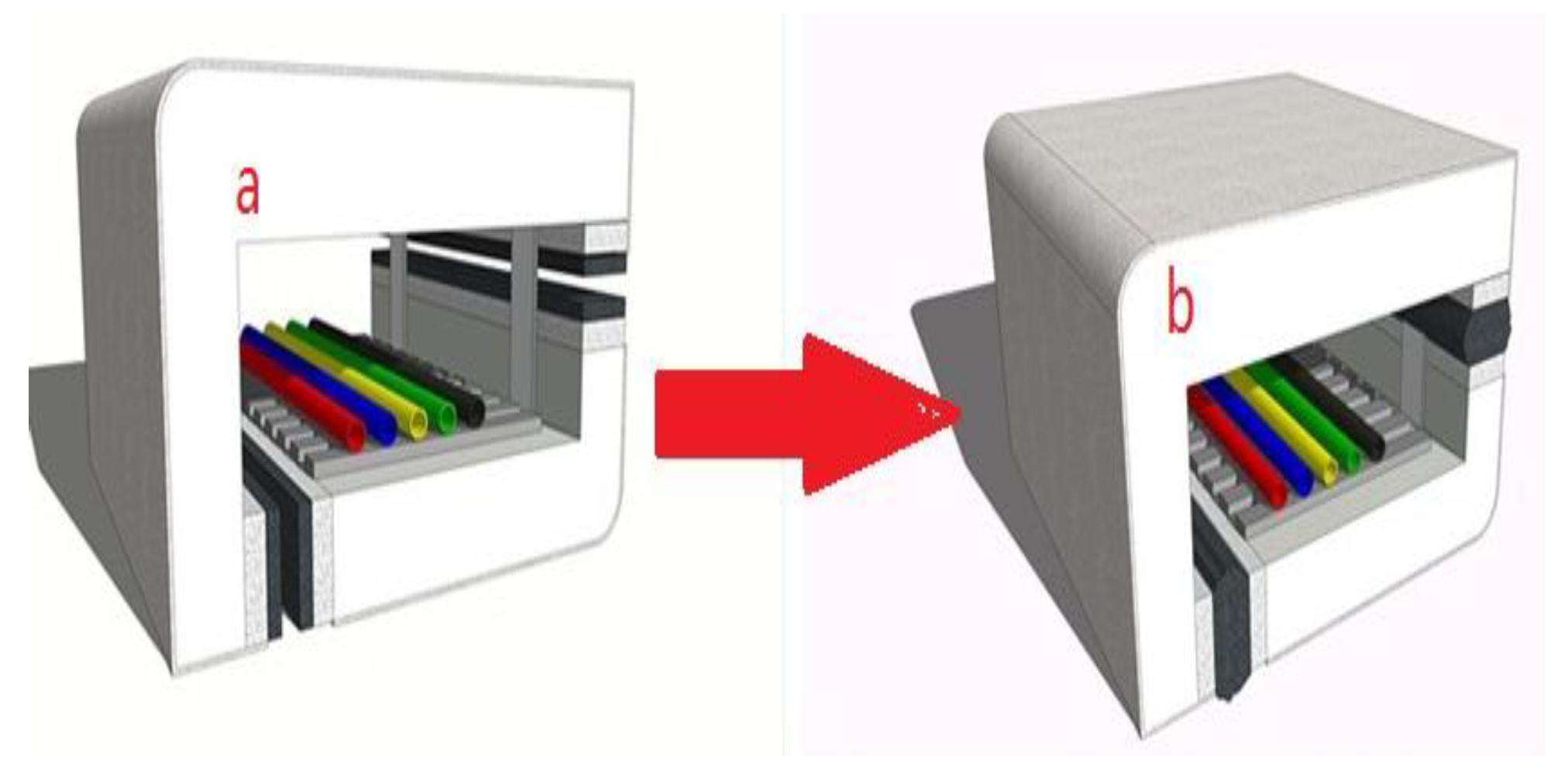

| Samples | SR | AHP | Mica | CaCO3 | SiO2 | Frits |
|---|---|---|---|---|---|---|
| SR | 100 | 10 | 5 | 10 | 20 | 0 |
| SR/10Frits | 100 | 10 | 5 | 10 | 20 | 10 |
| SR/20Frits | 100 | 10 | 5 | 10 | 20 | 20 |
| SR/30Frits | 100 | 10 | 5 | 10 | 20 | 30 |
| SR/40Frits | 100 | 10 | 5 | 10 | 20 | 40 |
| Instrument/Apparatus | Model/Specification | Manufacturer |
|---|---|---|
| Vertical Burning Tester | JF-3 | Nanjing Jiangning Analytical Instrument Co., Ltd., Nanjing, China |
| Oxygen Index Tester | JF-3 | Nanjing Jiangning Analytical Instrument Co., Ltd., Nanjing, China |
| Universal Electronic Tensile Machine | WDW-50E | Jinan Shijin Group Company Ltd., Jinan, China |
| Thermal Gravimetric Analyzer | TAQ-500 | Shimadzu, Kyoto, Japan |
| Scanning Electron Microscope | HITACHI-S4800 | Hitachi, Tokyo, Japan |
| Shore Hardness Tester | Shore A | Shanghai Luchuan Measuring Tools Co., Ltd., Shanghai, China |
| Samples | LOI | UL-94 | ||
|---|---|---|---|---|
| T1 | T2 | |||
| SR | 30.9 ± 0.2 | - | - | None |
| SR/10Frits | 31.7 ± 0.1 | 0 | 6 ± 1 | None |
| SR/20Frits | 32.0 ± 0.3 | 0 | 0 | None |
| SR/30Frits | 32.3 ± 0.2 | 0 | 0 | None |
| SR/40Frits | 32.4 ± 0.1 | 0 | 0 | None |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Hardness |
|---|---|---|---|
| SR | 4.7 ± 0.5 | 302 ± 30 | 68 ± 1 |
| SR/10Frits | 3.9 ± 0.4 | 257 ± 25 | 70 ± 2 |
| SR/20Frits | 3.6 ± 0.3 | 235 ± 25 | 72 ± 1 |
| SR/30Frits | 2.9 ± 0.3 | 218 ± 20 | 74 ± 1 |
| SR/40Frits | 2.6 ± 0.3 | 176 ± 20 | 75 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Li, J. Research on SR/Frit Composites: A Novel Low-Temperature Ceramifiable Expandable Flame-Retardant Material. Materials 2022, 15, 2961. https://doi.org/10.3390/ma15092961
Zhu H, Li J. Research on SR/Frit Composites: A Novel Low-Temperature Ceramifiable Expandable Flame-Retardant Material. Materials. 2022; 15(9):2961. https://doi.org/10.3390/ma15092961
Chicago/Turabian StyleZhu, Hongwei, and Jianhua Li. 2022. "Research on SR/Frit Composites: A Novel Low-Temperature Ceramifiable Expandable Flame-Retardant Material" Materials 15, no. 9: 2961. https://doi.org/10.3390/ma15092961
APA StyleZhu, H., & Li, J. (2022). Research on SR/Frit Composites: A Novel Low-Temperature Ceramifiable Expandable Flame-Retardant Material. Materials, 15(9), 2961. https://doi.org/10.3390/ma15092961





