Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review
Abstract
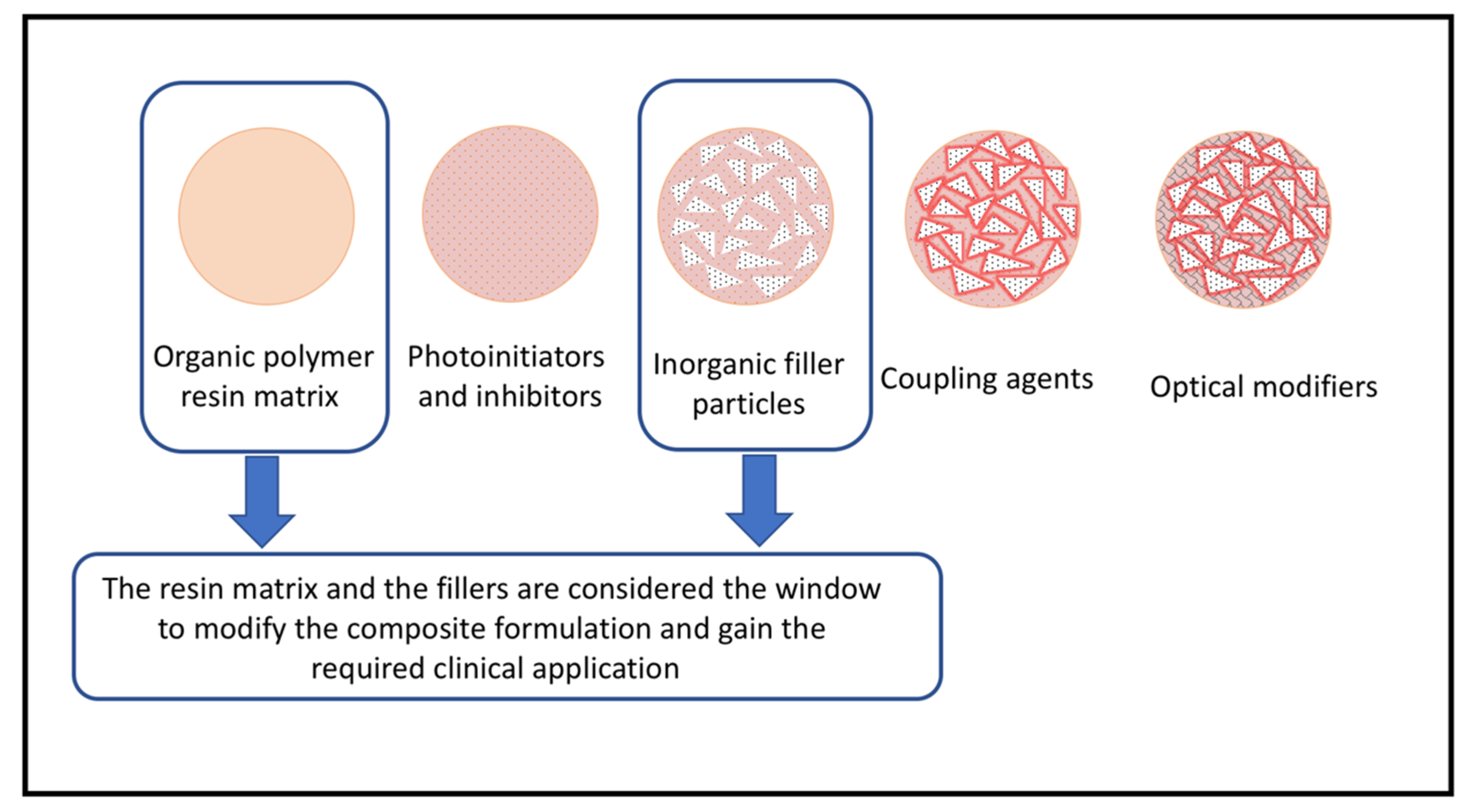
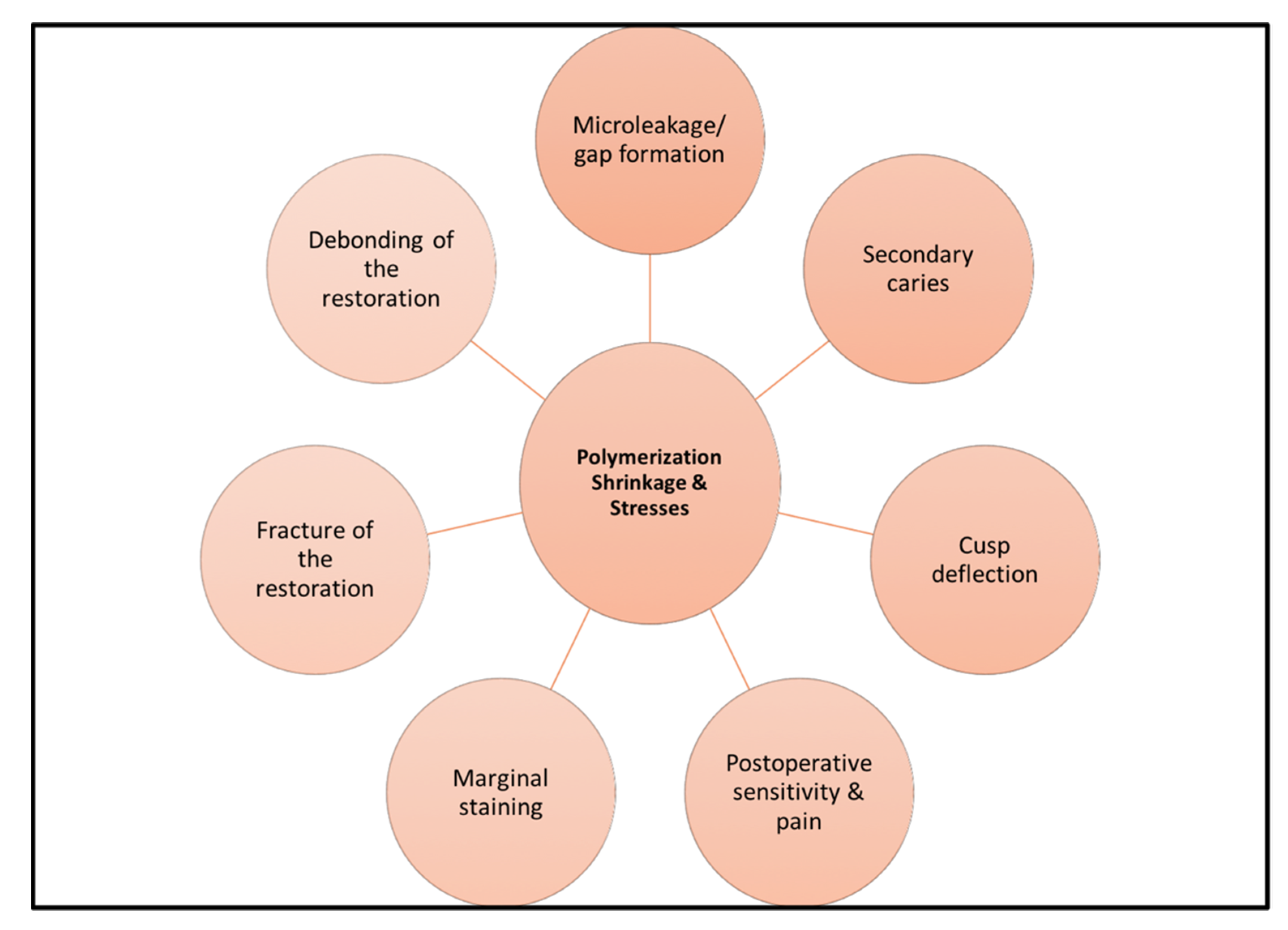
1. Introduction
2. Materials and Methods
3. Approaches for Testing Resin Composite Shrinkage
3.1. Coordinate Measuring Systems
3.2. Optical Coherence Tomography
3.3. Archimedes’ Principle
3.4. Strain Gauge
3.5. Dilatometer Method
3.6. Modified Bonded Disk Method
3.7. Thermomechanical Analyzer Method
3.8. X-ray Microcomputed Tomography
3.9. Video Imaging Methods
3.10. Cantilever Beam-Based Tensometer
4. Resin Formulations Attempt to Reduce Shrinkages and Their Stress
4.1. Silorane-Based Resin Composite
4.2. Nanogels and Functionalized-Nanogels
4.3. Dimethacrylate-Derivative of Dimer Acid
4.4. Tricyclodecane Urethane-Based Resin Composite
4.5. Ormocers
4.6. Thio-Urethane Oligomers
4.7. Thiol-Ene- and Thiol-Norbornene-Based Systems
5. Dynamic Covalent Chemistry and Stress Relaxation
6. Resistance of Dental Resins to Hydrolytic and Enzymatic Degradation in the Oral Environment
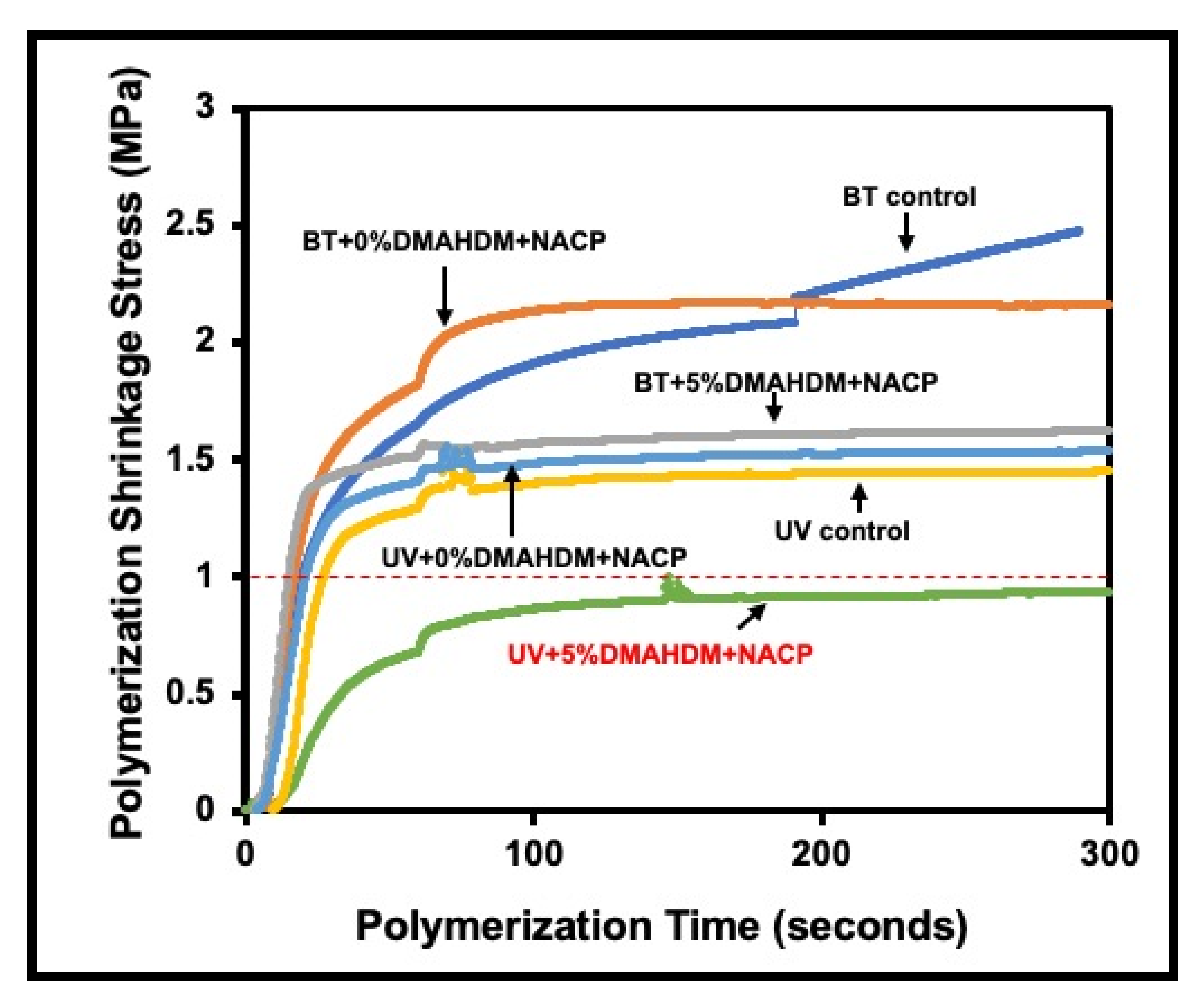
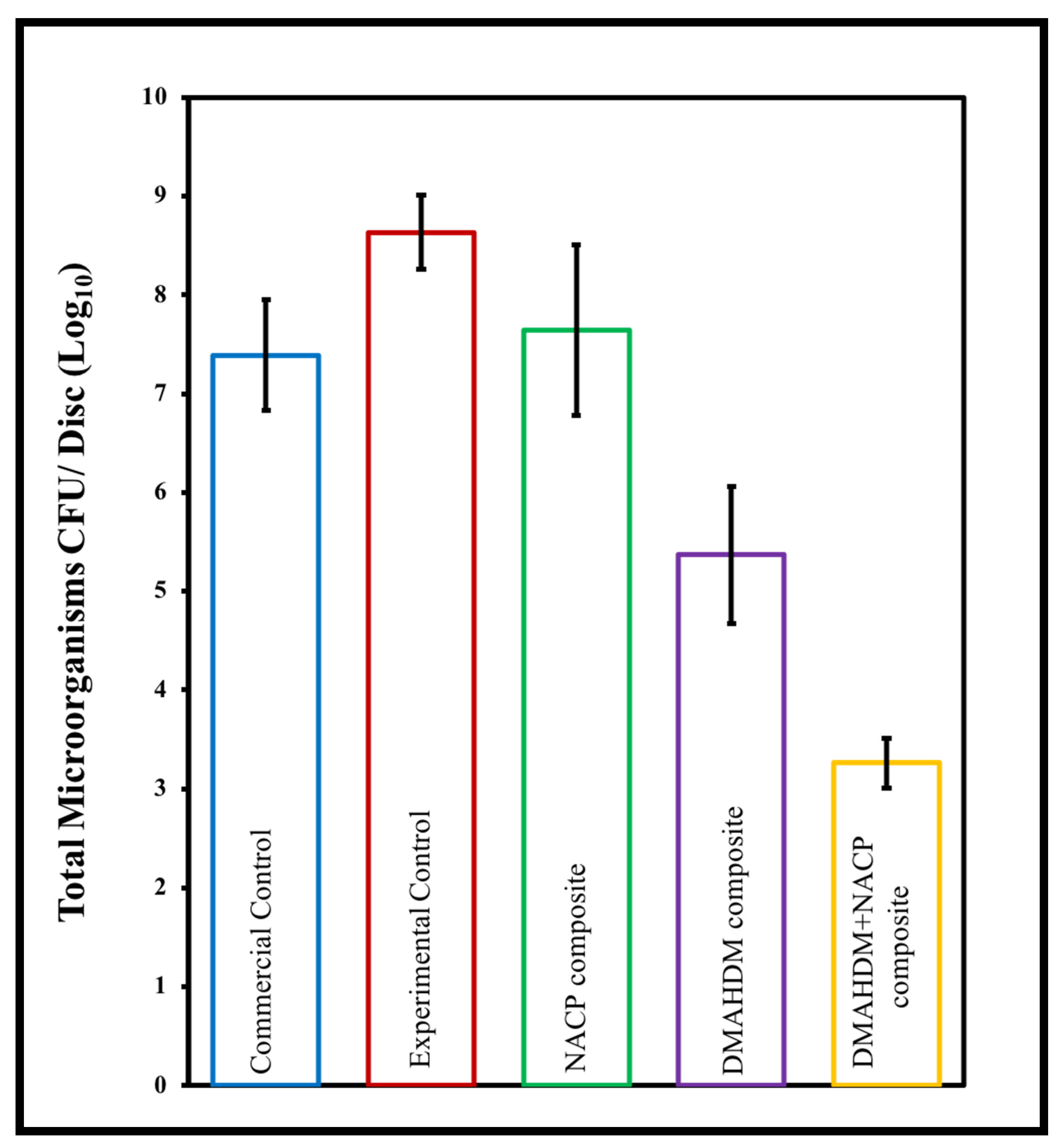
6.1. Using Hydrolytically Stable Monomers with Antibacterial and Remineralizing Properties in Composite Resins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronald, L.; Sakaguchi, J.M. Powers. Craig’s Restorative Dental Materials, 13th ed.; Mosby: London, UK, 2012. [Google Scholar]
- Bayne, S.C.; Ferracane, J.L.; Marshall, G.W.; Marshall, S.J.; van Noort, R. The Evolution of Dental Materials over the Past Century: Silver and Gold to Tooth Color and Beyond. J. Dent. Res. 2019, 98, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hervás-García, A.; Martínez-Lozano, M.A.; Cabanes-Vila, J.; Barjau-Escribano, A.; Fos-Galve, P. Composite resins. A review of the materials and clinical indications. Med. Oral. Patol. Oral. Cir. Bucal. 2006, 11, E215–E220. [Google Scholar] [PubMed]
- Bowen, R. Properties of a silica-reinforced polymer for dental restorations. J. Am. Dent. Assoc. 1963, 66, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.J.; Dickens, S.H. Network structure of Bis-GMA- and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Wolff, D.; Kraus, T.; Schach, C.; Pritsch, M.; Mente, J.; Staehle, H.J.; Ding, P. Recontouring teeth and closing diastemas with direct composite buildups: A clinical evaluation of survival and quality parameters. J. Dent. 2010, 38, 1001–1009. [Google Scholar] [CrossRef]
- Roulet, J.-F. Benefits and disadvantages of tooth-coloured alternatives to amalgam. J. Dent. 1997, 25, 459–473. [Google Scholar] [CrossRef]
- Orilisi, G.; Monterubbianesi, R.; Notarstefano, V.; Tosco, V.; Vitiello, F.; Giuliani, G.; Putignano, A.; Orsini, G. New insights from Raman MicroSpectroscopy and Scanning Electron Microscopy on the microstructure and chemical composition of vestibular and lingual surfaces in permanent and deciduous human teeth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119966. [Google Scholar] [CrossRef]
- Randall, G.C. The Expanded Use of Improved Flowable Composite. Dent Town 2008, 64, 62–72. [Google Scholar]
- Scolavino, S.; Paolone, G.; Orsini, G.; Devoto, W.; Putignano, A. The Simultaneous Modeling Technique: Closing gaps in posteriors. Int. J. Esthet. Dent. 2016, 11, 58–81. [Google Scholar]
- Lucchese, A.; Manuelli, M.; Bassani, L.; Albertini, P.; Matarese, G.; Perillo, L.; Gastaldi, G.; Gherlone, E.F. Fiber reinforced composites orthodontic retainers. Minerva Stomatol. 2015, 64, 323–333. [Google Scholar] [PubMed]
- Ferracane, J.L.; Greener, E.H. Fourier Transform Infrared Analysis of Degree of Polymerization in Unfilled Resins—Methods Comparison. J. Dent. Res. 1984, 63, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Placing Dental Composites—A Stressful Experience. Oper. Dent. 2008, 33, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Ranade, R.; Wunder, S.L.; McCool, J.; Boberick, K.; Baran, G. Interface effects on mechanical properties of particle-reinforced composites. Dent. Mater. 2004, 20, 677–686. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Polizzi, E.; Tetè, G.; Bova, F.; Pantaleo, G.; Gastaldi, G.; Capparè, P.; Gherlone, E. Antibacterial properties and side effects of chlorhexidine-based mouthwashes. a prospective, randomized clinical study. J. Osseointegr. 2020, 12, 2–7. [Google Scholar] [CrossRef]
- Yamauch, S.; Wang, X.; Egusa, E.; Sun, J. High performance dental adhesives containing an ether-based monomer. J. Dent. Res. 2020, 99, 189–195. [Google Scholar] [CrossRef]
- Kleverlaan, C.J.; Feilzer, A.J. Polymerization shrink-age and contraction stress of dental resin composites. Dent. Mater. 2005, 21, 1150–1157. [Google Scholar] [CrossRef]
- Soares, C.J.; Faria-E-Silva, A.L.; Rodrigues, M.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral Res. 2017, 31, e62. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ferracane, J.L. Residual stress in composites with the thin-ring-slitting approach. J. Dent. Res. 2006, 85, 945–949. [Google Scholar] [CrossRef]
- Petrovic, L.M.; Atanackovic, T.M. A model for shrinkage strain in photo polymerization of dental composites. Dent. Mater. 2008, 24, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Tauböck, T.T.; Feilzer, A.J.; Buchalla, W.; Kleverlaan, C.J.; Krejci, I.; Attin, T. Effect of modulated photo-activation on polymerization shrinkage behavior of dental restorative resin composites. Eur. J. Oral Sci. 2014, 122, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E.; Peutzfeld, A. Influence of Pulse-Delay Curing on Softening of Polymer Structures. J. Dent. Res. 2001, 80, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stansbury, J.W.; Bowman, C.N. Impact of Curing Protocol on Conversion and Shrinkage Stress. J. Dent. Res. 2005, 84, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.W.; Pallesen, U. A 7-year randomized prospective study of a one-step self-etching adhesive in non-carious cervical lesions. The effect of curing modes and restorative material. J. Dent. 2012, 40, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Savio, E. Coordinate Measuring Machine. In CIRP Encyclopedia of Production Engineering; Chatti, S., Laperrière, L., Reinhart, G., Tolio, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 364–369. ISBN 978-3-662-53120-4. [Google Scholar]
- Monteiro, G.Q.D.M.; Montes, M.A.J.R.; Rolim, T.V.; Mota, C.C.B.D.O.; Kyotoku, B.D.B.C.; Gomes, A.S.L.; de Freitas, A.Z. Alternative methods for determining shrinkage in restorative resin composites. Dent. Mater. 2011, 27, e176–e185. [Google Scholar] [CrossRef]
- Otis, L.L.; al-Sadhan, R.I.; Meiers, J.; Redford-Badwal, D. Identification of Occlusal Sealants Using Optical Coherence Tomography. J. Clin. Dent. 2003, 14, 7–10. [Google Scholar]
- Koplin, C.; Jaeger, R.; Hahn, P. Kinetic model for the coupled volumetric and thermal behavior of dental composites. Dent. Mater. 2008, 24, 1017–1024. [Google Scholar] [CrossRef]
- Hooshmand, T.; Ghavami-Lahiji, M. Analytical methods for the measurement of polymerization kinetics and stresses of dental resin-based composites: A review. Dent. Res. J. 2017, 14, 225–240. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Wiltbank, B.D.; Shah, N.C. Critical configuration analysis of four methods for measuring polymerization shrinkage strain of composites. Dent. Mater. 2004, 20, 388–396. [Google Scholar] [CrossRef]
- Yu, H.; Mhaisalkar, S.G.; Wong, E.-H. Cure shrinkage measurement of nonconductive adhesives by means of a thermomechanical analyzer. J. Electron. Mater. 2005, 34, 1177–1182. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Rösch, P.; Dabanoglu, A.; Lin, C.-P.; Hickel, R.; Kunzelmann, K.-H. Polymerization composite shrinkage evaluation with 3D deformation analysis from μCT images. Dent. Mater. 2010, 26, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin-Gibson, S. X-ray microcomputed tomography for measuring polymerization shrinkage of polymeric dental composites. Dent. Mater. 2008, 24, 228–234. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Mollica, F.; Prisco, D.; Rengo, S.; Ambrosio, L.; Nicolais, L. A 3D analysis of mechanically stressed dentin–adhesive–composite interfaces using X-ray micro-CT. Biomaterials 2005, 26, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Kakaboura, A.; Rahiotis, C.; Watts, D.; Silikas, N.; Eliades, G. 3D-marginal adaptation versus setting shrinkage in light-cured microhybrid resin composites. Dent. Mater. 2007, 23, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Tiba, A.; Charlton, D.G.; Vandewalle, K.S.; Ragain, J.C. Comparison of two video-imaging instruments for measuring volumetric shrinkage of dental resin composites. J. Dent. 2005, 33, 757–763. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Landis, F.A.; Giuseppetti, A.A.M.; Lin-Gibson, S.; Chiang, M.Y.M. Simultaneous measurement of polymerization stress and curing kinetics for photo-polymerized composites with high filler contents. Dent. Mater. 2014, 30, 1316–1324. [Google Scholar] [CrossRef]
- Larson, T.D. Low Shrinkage Silorane Composites. Northwest Dent. 2017, 96, 15-6–17-9. [Google Scholar]
- Gao, B.-T.; Lin, H.; Zheng, G.; Xu, Y.-X.; Yang, J.-L. Comparison between a silorane-based composite and methacrylate-based composites: Shrinkage characteristics, thermal properties, gel point and vitrification point. Dent. Mater. J. 2012, 31, 76–85. [Google Scholar] [CrossRef]
- Park, J.-K.; Lee, G.-H.; Kim, J.-H.; Park, M.-G.; Ko, C.-C.; Kim, H.-I.; Kwon, Y.H. Polymerization shrinkage, flexural and compression properties of low-shrinkage dental resin composites. Dent. Mater. J. 2014, 33, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Boaro, L.C.C.; Gonçalves, F.; Guimarães, T.C.; Ferracane, J.L.; Versluis, A.; Braga, R.R. Polymerization stress, shrinkage and elastic modulus of current low-shrinkage restorative composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, L.C.; De Vito Moraes, A.G.; Barros, M.; Lewis, S.; Francci, C.; Stansbury, J.W.; Pfeifer, C.S. Polymerization development of “low-shrink” resin composites: Reaction kinetics, polymerization stress and quality of network. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, e169–e179. [Google Scholar] [CrossRef] [PubMed]
- Meereis, C.T.W.; Münchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization shrinkage stress of resin-based dental materials: A systematic review and meta-analyses of composition strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef]
- Ilie, N.; Jelen, E.; Clementino-Luedemann, T.; Hickel, R. Low-shrinkage composite for dental application. Dent. Mater. J. 2007, 26, 149–155. [Google Scholar] [CrossRef]
- Torres, S.A.S.; Silva, G.C.; Maria, D.A.; Campos, W.R.C.; Magalhães, C.S.; Moreira, A.N. Degree of conversion and hardness of a silorane-based composite resin: Effect of light-curing unit and depth. Oper. Dent. 2014, 39, E137–E146. [Google Scholar] [CrossRef]
- Boaro, L.C.; Gonçalves, F.; Guimarães, T.C.; Ferracane, J.L.; Pfeifer, C.S.; Braga, R.R. Sorption, solubility, shrinkage and mechanical properties of “low-shrinkage” commercial resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 398–404. [Google Scholar] [CrossRef]
- Lien, W.; Vandewalle, K.S. Physical properties of a new silorane-based restorative system. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 337–344. [Google Scholar] [CrossRef]
- Krifka, S.; Federlin, M.; Hiller, K.-A.; Schmalz, G. Microleakage of silorane- and methacrylate-based class V composite restorations. Clin. Oral. Investig. 2012, 16, 1117–1124. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Gamba, J.; Forchelet, J.; Krejci, I.; Wataha, J.C. Dynamics of composite polymerization mediates the development of cuspal strain. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2006, 22, 896–902. [Google Scholar] [CrossRef][Green Version]
- Tantbirojn, D.; Pfeifer, C.S.; Braga, R.R.; Versluis, A. Do low-shrink composites reduce polymerization shrinkage effects? J. Dent. Res. 2011, 90, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Van Ende, A.; De Munck, J.; Mine, A.; Lambrechts, P.; Van Meerbeek, B. Does a low-shrinking composite induce less stress at the adhesive interface? Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Giacobbi, M.F.; Vandewalle, K.S. Microtensile bond strength of a new silorane-based composite resin adhesive. Gen Dent. 2012, 60, e148–e152. [Google Scholar] [PubMed]
- Duarte, S.; Phark, J.-H.; Varjão, F.M.; Sadan, A. Nanoleakage, ultramorphological characteristics, and microtensile bond strengths of a new low-shrinkage composite to dentin after artificial aging. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 589–600. [Google Scholar] [CrossRef]
- Almeida e Silva, J.S.; Rolla, J.N.; Baratieri, L.N.; Monteiro, S. The influence of different placement techniques on the microtensile bond strength of low-shrink silorane composite bonded to Class I cavities. Gen Dent. 2011, 59, e233–e237. [Google Scholar]
- Shirai, K.; De Munck, J.; Yoshida, Y.; Inoue, S.; Lambrechts, P.; Suzuki, K.; Shintani, H.; Van Meerbeek, B. Effect of cavity configuration and aging on the bonding effectiveness of six adhesives to dentin. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2005, 21, 110–124. [Google Scholar] [CrossRef]
- Isaac, S.Z.; Bergamin, A.C.P.; Turssi, C.P.; Amaral, F.L.B.D.; Basting, R.T.; França, F.M.G. Evaluation of bond strength of silorane and methacrylate based restorative systems to dentin using different cavity models. J. Appl. Oral Sci. 2013, 21, 452–459. [Google Scholar] [CrossRef][Green Version]
- Ztürk-Bozkurt, F.; Toz, T.; Kara-Tuncer, A.; Gözükara-Bağ, H.; Özcan, M. Clinical Evaluation of Silorane and Nano-hybrid Resin Composite Restorations in Class II Cavities up to 3 Years. Oper. Dent. 2016, 41, 599–606. [Google Scholar] [CrossRef]
- Schmidt, M.; Kirkevang, L.-L.; Hørsted-Bindslev, P.; Poulsen, S. Marginal adaptation of a low-shrinkage silorane-based composite: 1-year randomized clinical trial. Clin. Oral Investig. 2011, 15, 291–295. [Google Scholar] [CrossRef]
- Schmidt, M.; Dige, I.; Kirkevang, L.-L.; Vaeth, M.; Hørsted-Bindslev, P. Five-year evaluation of a low-shrinkage Silorane resin composite material: A randomized clinical trial. Clin. Oral Investig. 2015, 19, 245–251. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Ali, A.K.; Hegazi, H.A.-E.R. A three-year prospective randomized study of silorane- and methacrylate-based composite restorative systems in class II restorations. J. Adhes. Dent. 2014, 16, 285–292. [Google Scholar] [PubMed]
- Walter, R.; Boushell, L.W.; Heymann, H.O.; Ritter, A.V.; Sturdevant, J.R.; Wilder, A.D.; Chung, Y.; Swift, E.J., Jr. Three-year clinical evaluation of a silorane composite resin. J. Esthet. Restor. Dent. 2014, 26, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Yaman, B.C.; Doğruer, I.; Gümüştaş, B.; Efes, B.G. Three-year randomized clinical evaluation of a low-shrinkage silorane-based resin composite in non-carious cervical lesions. Clin. Oral Investig. 2014, 18, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.R.; Garcia, J.W.; Barros, M.D.; Lewis, S.H.; Pfeifer, C.S.; Liu, J.; Stansbury, J.W. Control of polymerization shrinkage and stress in nanogel-modified monomer and composite materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 509–519. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Sun, F.; Stansbury, J.W. Tuning Surface Microstructure and Gradient Property of Polymer by Photopolymerizable Polysiloxane-modified Nanogels. RSC Adv. 2014, 4, 28928–28936. [Google Scholar] [CrossRef]
- Navarro, R.; Bierbrauer, K.; Mijangos, C.; Goiti, E.; Reinecke, H. Modification of poly(vinyl chloride) with new aromatic thiol compounds. Synthesis and characterization. Polym. Degrad. Stab. 2008, 93, 585–591. [Google Scholar] [CrossRef]
- Saraswathy, M.; Stansbury, J.W.; Nair, D.P. Thiol-functionalized nanogels as reactive plasticizers for crosslinked polymer networks. J. Mech. Behav. Biomed. Mater. 2017, 74, 296–303. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M. Effect of the amount of 3-methacyloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 1315–1324. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.K.; McDonough, W.G. Chemistry of Silanes: Interfaces in Dental Polymers and Composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541–558. [Google Scholar] [CrossRef]
- Fronza, B.M.; Lewis, S.; Shah, P.K.; Barros, M.D.; Giannini, M.; Stansbury, J.W. Modification of filler surface treatment of composite resins using alternative silanes and functional nanogels. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 928–936. [Google Scholar] [CrossRef]
- Trujillo-Lemon, M.; Ge, J.; Lu, H.; Tanaka, J.; Stansbury, J.W. Dimethacrylate derivatives of dimer acid. J. Polym. Sci. Part Polym. Chem. 2006, 44, 3921–3929. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Resin composite restorative materials. Aust. Dent. J. 2011, 56, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Ilie, N. The mechanical stability of nano-hybrid composites with new methacrylate monomers for matrix compositions. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, 152–159. [Google Scholar] [CrossRef]
- Marchesi, G.; Breschi, L.; Antoniolli, F.; Di Lenarda, R.; Ferracane, J.; Cadenaro, M. Contraction stress of low-shrinkage composite materials assessed with different testing systems. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Wolter, H.; Storch, W.; Gellermann, C. Novel Carboxy Functionalized Sol-Gel Precursors. 1996. Available online: https://www.osti.gov/biblio/427681-novel-carboxy-functionalized-sol-gel-precursors (accessed on 31 December 1996).
- Moszner, N.; Gianasmidis, A.; Klapdohr, S.; Fischer, U.K.; Rheinberger, V. Sol-gel materials 2. Light-curing dental composites based on ormocers of cross-linking alkoxysilane methacrylates and further nano-components. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 851–856. [Google Scholar]
- Senyurt, A.F.; Hoyle, C.E.; Wei, H.; Piland, S.G.; Gould, T.E. Thermal and Mechanical Properties of Cross-Linked Photopolymers Based on Multifunctional Thiol−Urethane Ene Monomers. Macromolecules 2007, 40, 3174–3182. [Google Scholar] [CrossRef]
- Bacchi, A.; Dobson, A.; Ferracane, J.L.; Consani, R.; Pfeifer, C.S. Thio-urethanes improve properties of dual-cured composite cements. J. Dent. Res. 2014, 93, 1320–1325. [Google Scholar] [CrossRef]
- Bacchi, A.; Nelson, M.; Pfeifer, C.S. Characterization of methacrylate-based composites containing thio-urethane oligomers. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2016, 32, 233–239. [Google Scholar] [CrossRef]
- Cramer, N.B.; Couch, C.L.; Schreck, K.M.; Carioscia, J.A.; Boulden, J.E.; Stansbury, J.W.; Bowman, C.N. Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 21–28. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiolenes: Chemistry of the past with promise for the future. J. Polym. Sci. Part Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Lu, H.; Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Investigations of step-growth thiolene polymerizations for novel dental restoratives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2005, 21, 1129–1136. [Google Scholar]
- Lee, T.Y.; Smith, Z.; Reddy, S.K.; Cramer, N.B.; Bowman, C.N. Thiol-Allyl Ether-Methacrylate Ternary Systems. Polym. Mech. Macromol. 2007, 40, 1466–1472. [Google Scholar] [CrossRef]
- Jacobine, A.F.; Glaser, D.M.; Grabek, P.J.; Mancini, D.; Masterson, M.; Nakos, S.T.; Rakas, M.A.; Woods, J.G. Photocrosslinked norbornene–thiol copolymers: Synthesis, mechanical properties, and cure studies. J. Appl. Polym. Sci. 1992, 45, 471–485. [Google Scholar] [CrossRef]
- Sowan, N.; Cox, L.M.; Shah, P.K.; Song, H.B.; Stansbury, J.W.; Bowman, C.N. Dynamic Covalent Chemistry at Interfaces: Development of Tougher, Healable Composites through Stress Relaxation at the Resin–Silica Nanoparticles Interface. Adv. Mater. Interfaces 2018, 5, 1800511. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Bain, C.D.; Whitesides, G.M. Molecular-Level Control over Surface Order in Self-Assembled Monolayer Films of Thiols on Gold. Science 1988, 240, 62–63. [Google Scholar] [CrossRef]
- Iwan, M.; Andryszewski, T.; Wydryszek, M.; Fialkowski, M. Fabrication of nanocomposites by covalent bonding between noble metal nanoparticles and polymer matrix. RSC Adv. 2015, 5, 70127–70138. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable Photoinduced Self-Healing of Covalently Cross-Linked Polymers through Reshuffling of Trithiocarbonate Units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef]
- Nicolaÿ, R.; Kamada, J.; Van Wassen, A.; Matyjaszewski, K. Responsive Gels Based on a Dynamic Covalent Trithiocarbonate Cross-Linker. Macromolecules 2010, 43, 4355–4361. [Google Scholar] [CrossRef]
- Scott, T.F.; Schneider, A.D.; Cook, W.D.; Bowman, C.N. Photoinduced Plasticity in Cross-Linked Polymers. Science 2005, 308, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Sun, X.; Wang, C.; Sowan, N.; Killgore, J.P.; Long, R.; Wu, H.-A.; Bowman, C.; Ding, Y. Light-Stimulated Permanent Shape Reconfiguration in Cross-Linked Polymer Microparticles. ACS Appl. Mater. Interfaces 2017, 9, 14422–14428. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-Healing of Covalently Cross-Linked Polymers by Reshuffling Thiuram Disulfide Moieties in Air under Visible Light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack Healing in Polymeric Materials via Photochemical [2+2] Cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Lyon, G.B.; Cox, L.M.; Goodrich, J.T.; Baranek, A.D.; Ding, Y.; Bowman, C.N. Remoldable Thiol–Ene Vitrimers for Photopatterning and Nanoimprint Lithography. Macromolecules 2016, 49, 8905–8913. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Bowman, C. Stress Relaxation via Addition−Fragmentation Chain Transfer in a Thiol-ene Photopolymerization. Macromolecules 2009, 42, 2551–2556. [Google Scholar] [CrossRef]
- Park, H.Y.; Kloxin, C.J.; Abuelyaman, A.S.; Oxman, J.D.; Bowman, C. Stress Relaxation via Addition–Fragmentation Chain Transfer in High Tg, High Conversion Methacrylate-Based Systems. Macromolecules 2012, 45, 5640–5646. [Google Scholar] [CrossRef][Green Version]
- Mu, X.; Sowan, N.; Tumbic, J.A.; Bowman, C.N.; Mather, P.T.; Qi, H.J. Photo-induced bending in a light-activated polymer laminated composite. Soft Matter 2015, 11, 2673–2682. [Google Scholar] [CrossRef]
- Park, H.Y.; Kloxin, C.J.; Fordney, M.F.; Bowman, C. Stress Reduction and Tg Enhancement in Ternary Thiol–Yne–Methacrylate Systems via Addition–Fragmentation Chain Transfer. Macromolecules 2012, 45, 5647–5652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sowan, N.; Dobson, A.; Podgorski, M.; Bowman, C.N. Dynamic covalent chemistry (DCC) in dental restorative materials: Implementation of a DCC-based adaptive interface (AI) at the resin–filler interface for improved performance. Dent. Mater. 2020, 36, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Li, S.S.-L. Bisphenol A and phthalates exhibit similar toxicogenomics and health effects. Gene 2012, 494, 85–91. [Google Scholar] [CrossRef]
- Bowen, R.; Cobb, E.; Rapson, J. Adhesive Bonding of Various Materials to Hard Tooth Tissues: Improvement in Bond Strength to Dentin. J. Dent. Res. 1982, 61, 1070–1076. [Google Scholar] [CrossRef]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef]
- Albeshir, E.G.; Balhaddad, A.A.; Mitwalli, H.; Wang, X.; Sun, J.; Melo, M.A.S.; Weir, M.D.; Xu, H.H. Minimally-invasive dentistry via dual-function novel bioactive low-shrinkage-stress flowable nanocomposites. Dent. Mater. 2021, 38, 409–420. [Google Scholar] [CrossRef]
- Bhadila, G.; Wang, X.; Zhou, W.; Menon, D.; Melo, M.A.S.; Montaner, S.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Novel low-shrinkage-stress nanocomposite with remineralization and antibacterial abilities to protect marginal enamel under biofilm. J. Dent. 2020, 99, 103406. [Google Scholar] [CrossRef]
- Bhadila, G.; Wang, X.; Weir, M.D.; Melo, M.A.S.; Martinho, F.; Fay, G.G.; Oates, T.W.; Sun, J.; Xu, H.H.K. Low-shrinkage-stress nanocomposite: An insight into shrinkage stress, antibacterial, and ion release properties. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1124–1134. [Google Scholar] [CrossRef]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Bioactive low-shrinkage-stress nanocomposite suppresses S. mutans biofilm and preserves tooth dentin hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef]
- Filemban, H.; Bhadila, G.; Wang, X.; Melo, M.A.S.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Novel low-shrinkage-stress bioactive nanocomposite with anti-biofilm and remineralization capabilities to inhibit caries. J. Dent. Sci. 2021, 17, 811–821. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albeshir, E.G.; Alsahafi, R.; Albluwi, R.; Balhaddad, A.A.; Mitwalli, H.; Oates, T.W.; Hack, G.D.; Sun, J.; Weir, M.D.; Xu, H.H.K. Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review. Materials 2022, 15, 2951. https://doi.org/10.3390/ma15082951
Albeshir EG, Alsahafi R, Albluwi R, Balhaddad AA, Mitwalli H, Oates TW, Hack GD, Sun J, Weir MD, Xu HHK. Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review. Materials. 2022; 15(8):2951. https://doi.org/10.3390/ma15082951
Chicago/Turabian StyleAlbeshir, Ebtehal G., Rashed Alsahafi, Reem Albluwi, Abdulrahman A. Balhaddad, Heba Mitwalli, Thomas W. Oates, Gary D. Hack, Jirun Sun, Michael D. Weir, and Hockin H. K. Xu. 2022. "Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review" Materials 15, no. 8: 2951. https://doi.org/10.3390/ma15082951
APA StyleAlbeshir, E. G., Alsahafi, R., Albluwi, R., Balhaddad, A. A., Mitwalli, H., Oates, T. W., Hack, G. D., Sun, J., Weir, M. D., & Xu, H. H. K. (2022). Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review. Materials, 15(8), 2951. https://doi.org/10.3390/ma15082951







