Review on Preparation Technology and Properties of Refractory High Entropy Alloys
Abstract
:1. Introduction
2. Preparation Technology of RHEAs
2.1. Preparation Technology of Bulk RHEAs
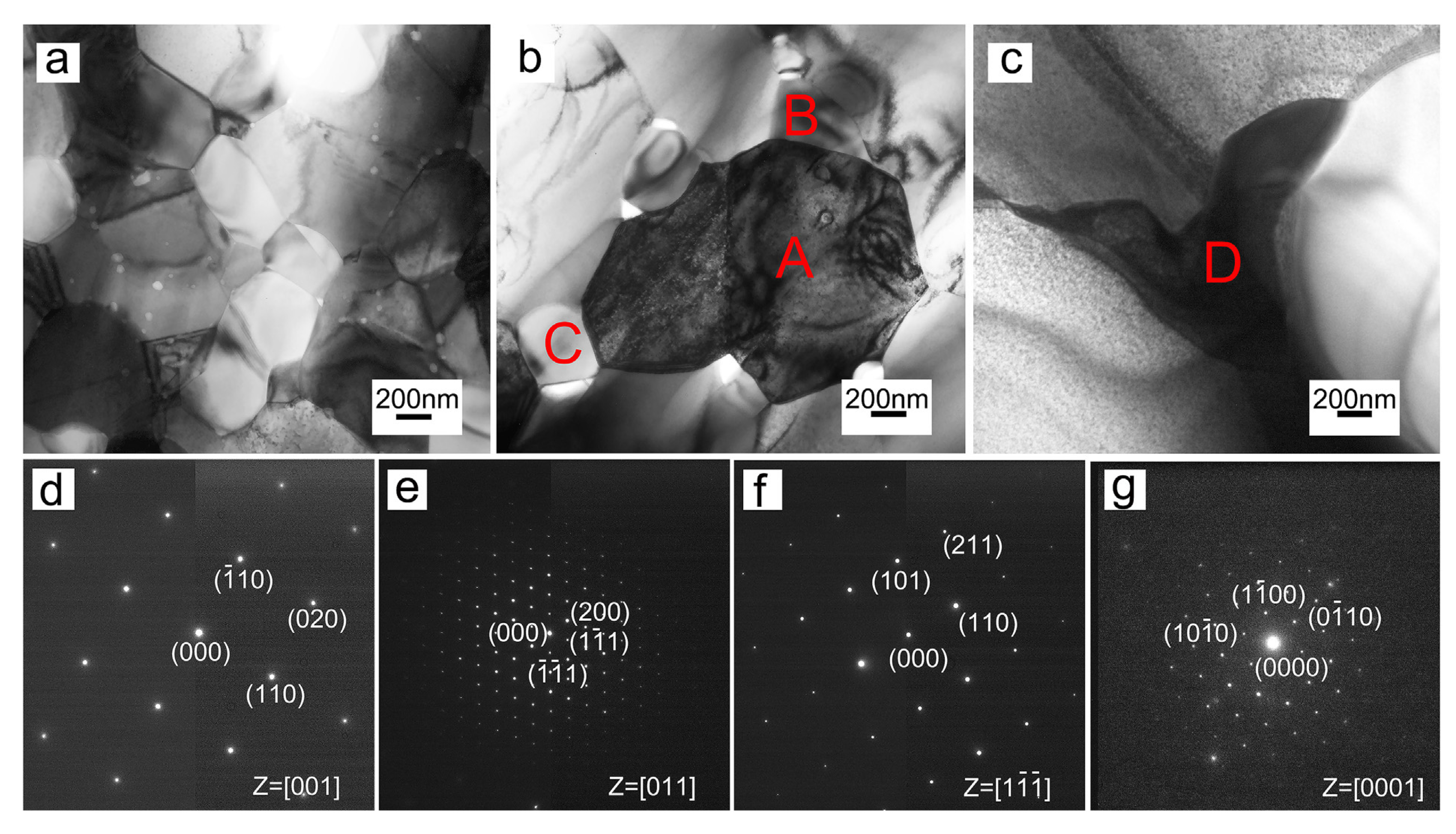
2.1.1. Arc Melting
2.1.2. Powder Metallurgy
2.2. Preparation Technology of Coating/Film RHEAs
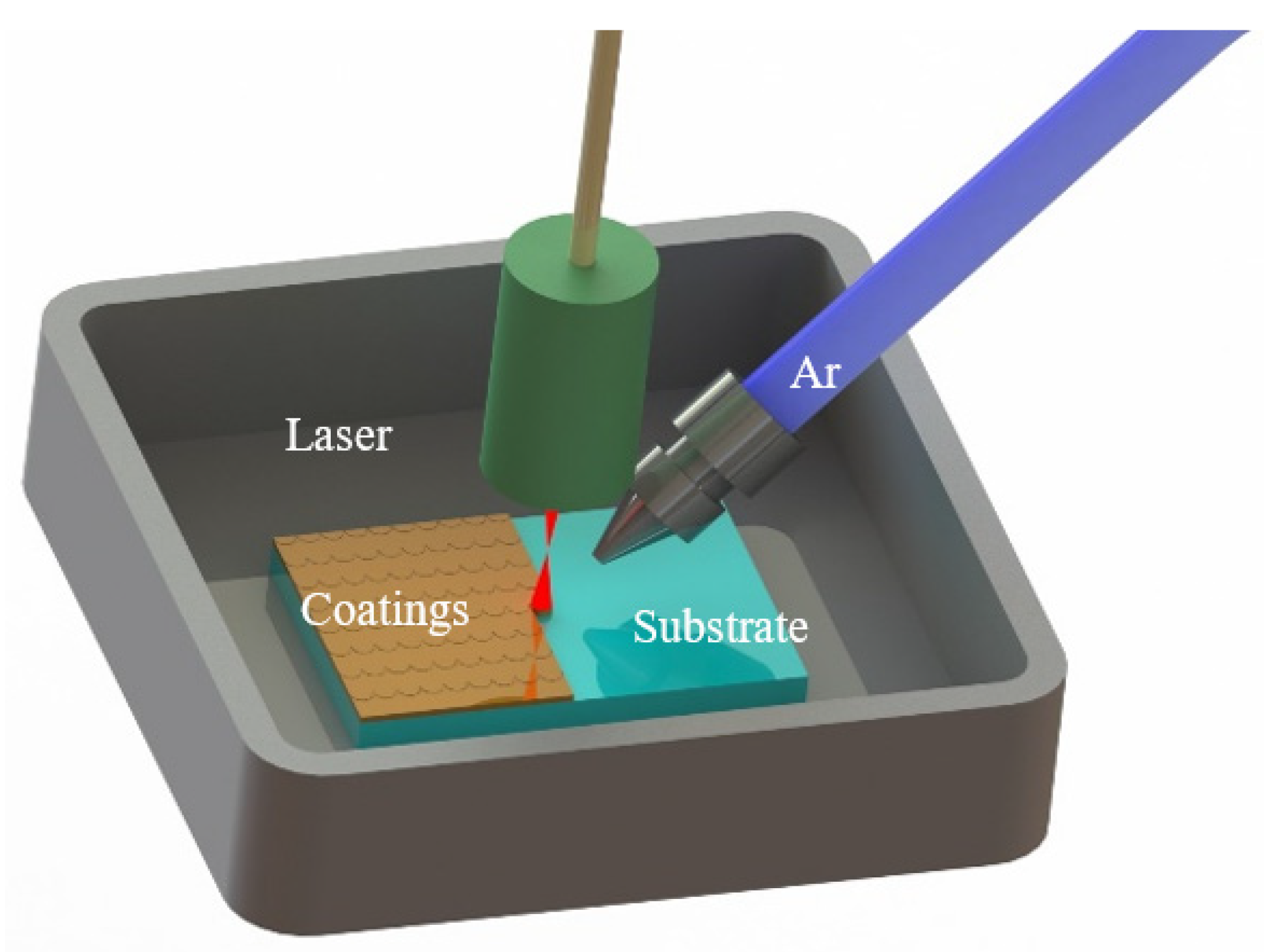
2.2.1. Laser Cladding
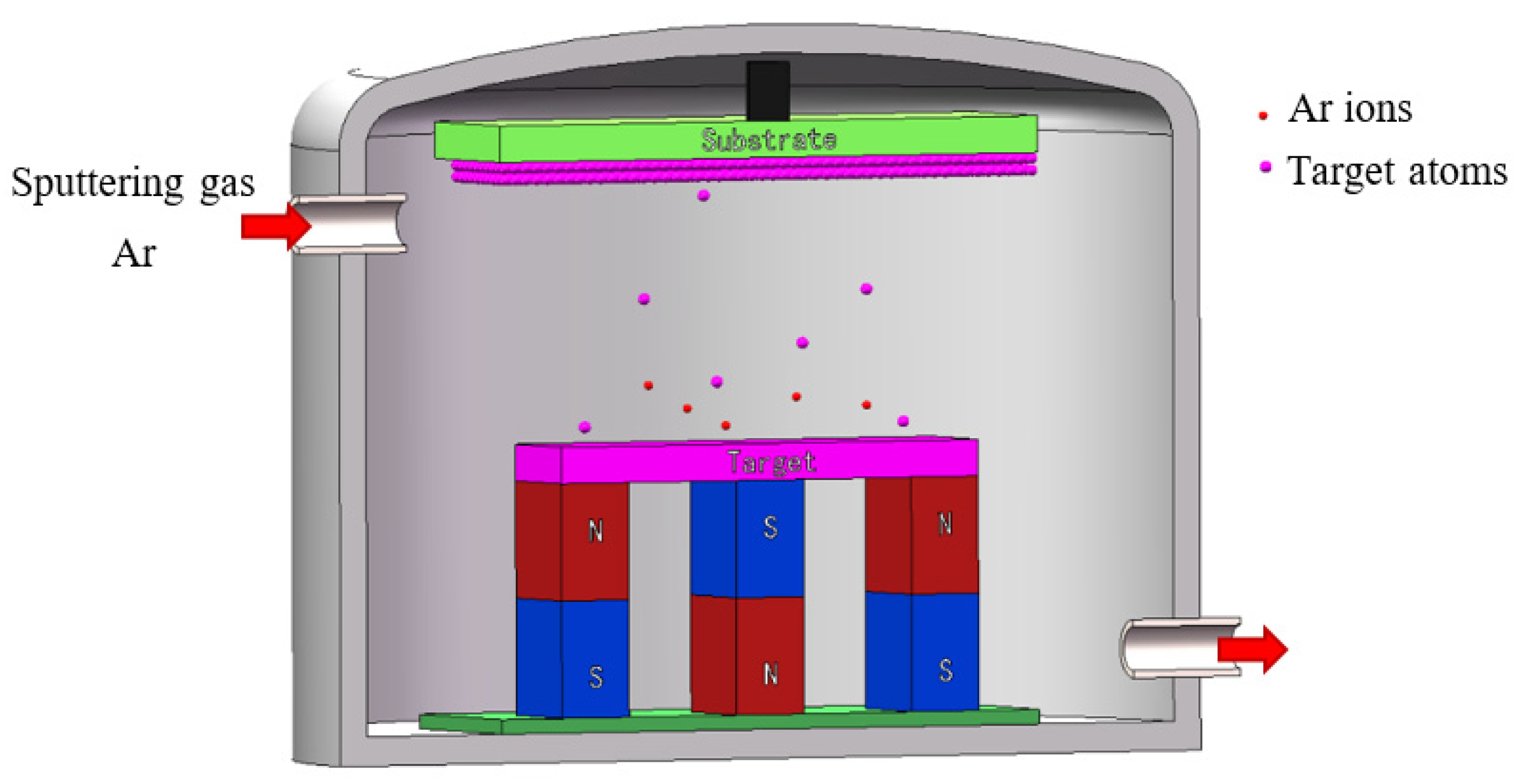
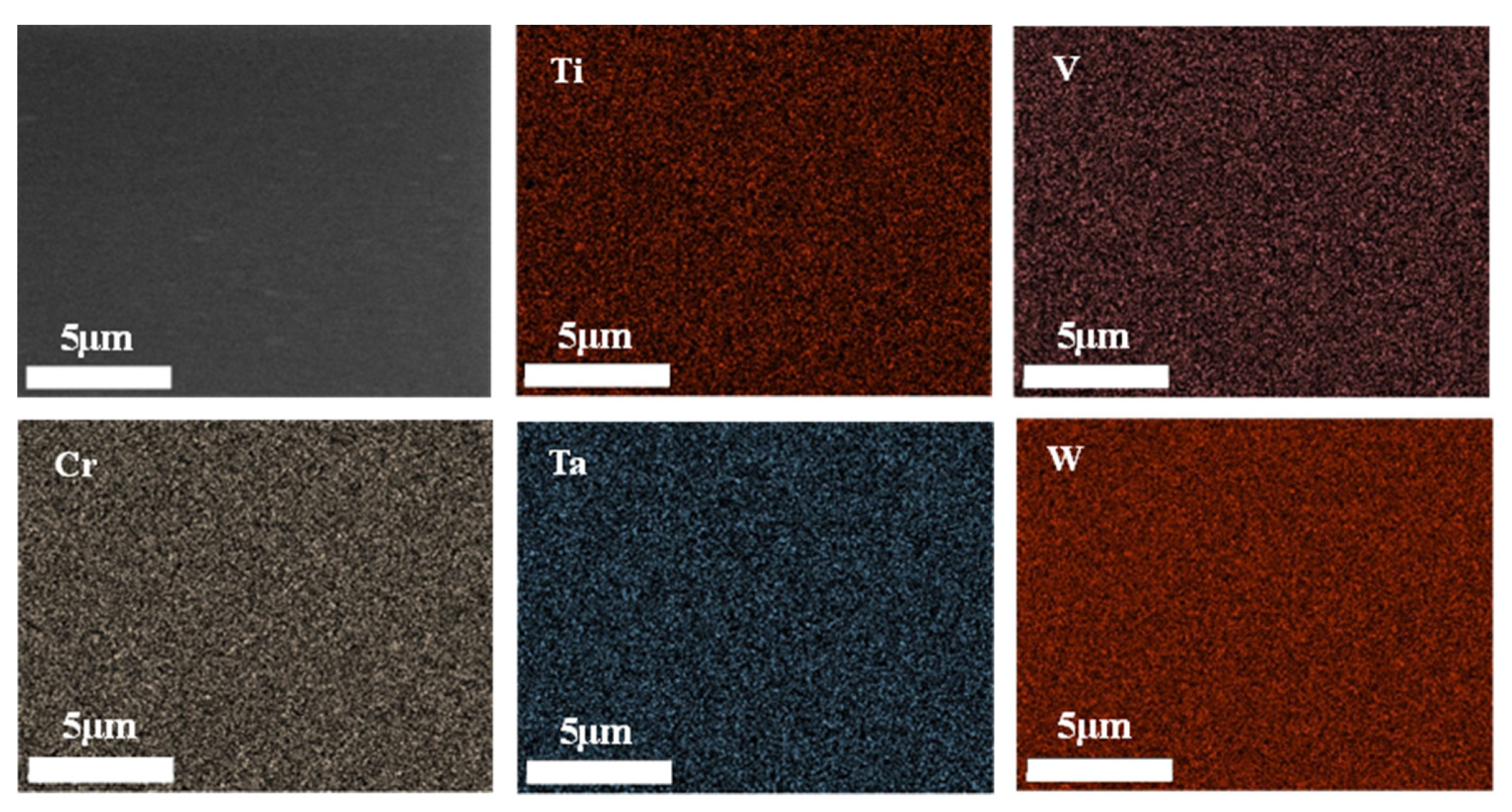
2.2.2. Magnetron Sputtering
3. Properties of RHEAs
3.1. Mechanical Properties
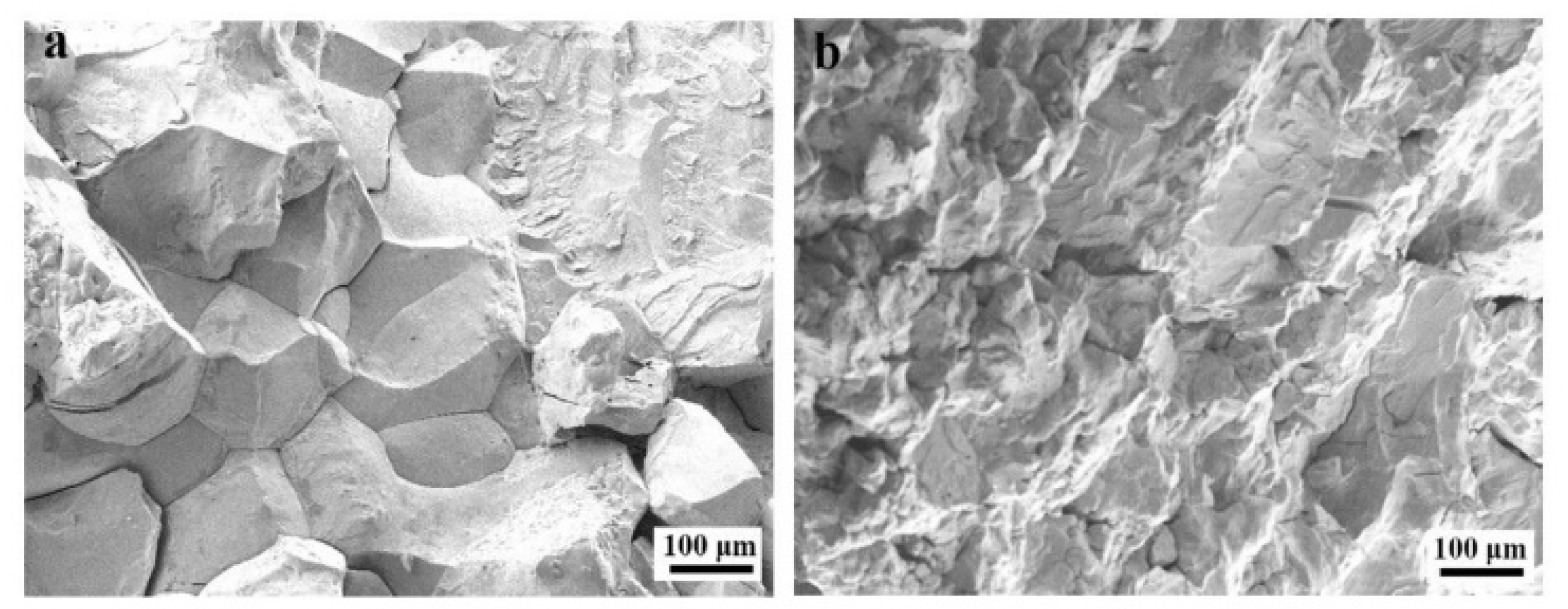
3.2. Wear Resistance
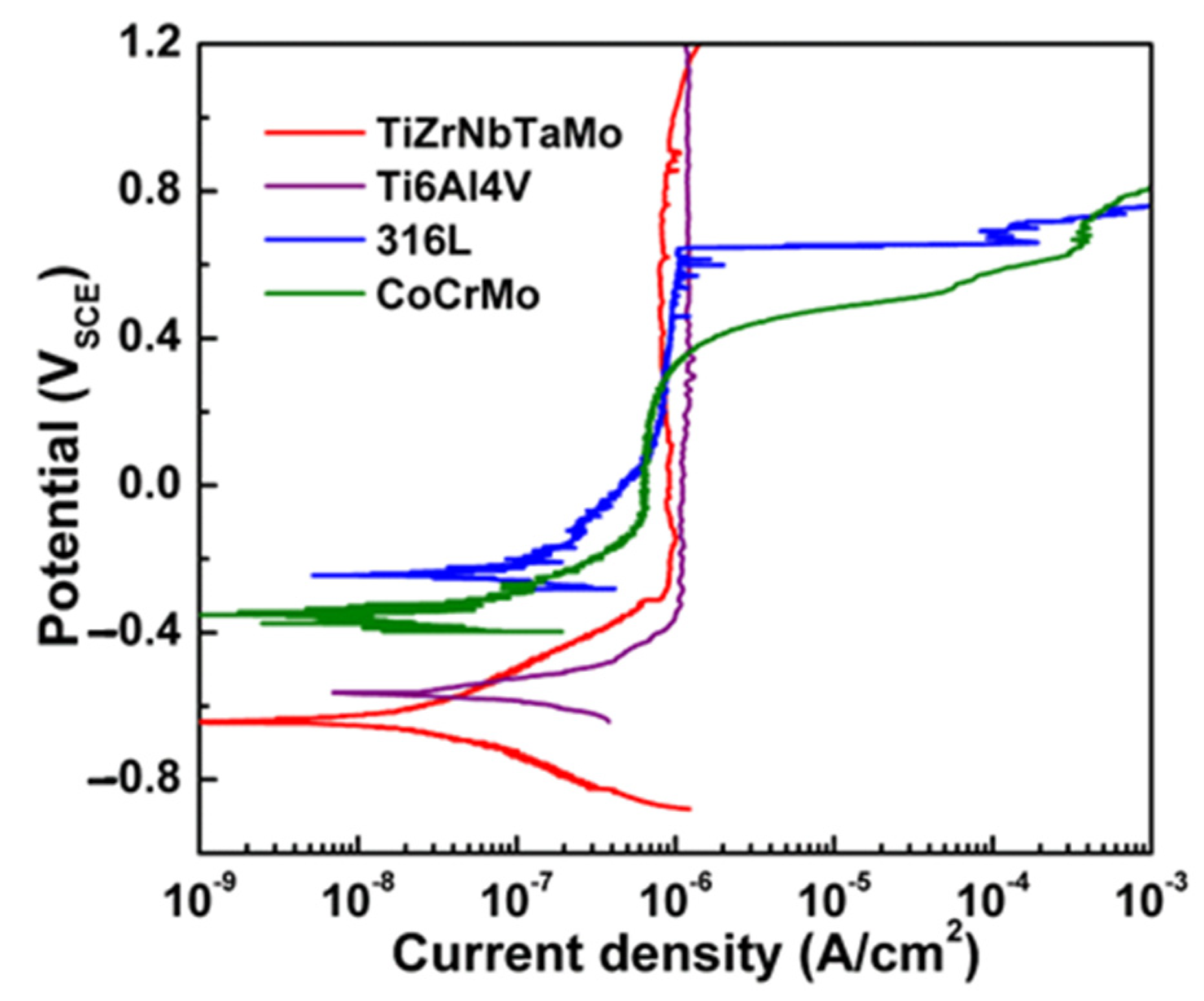
3.3. Corrosion Resistance
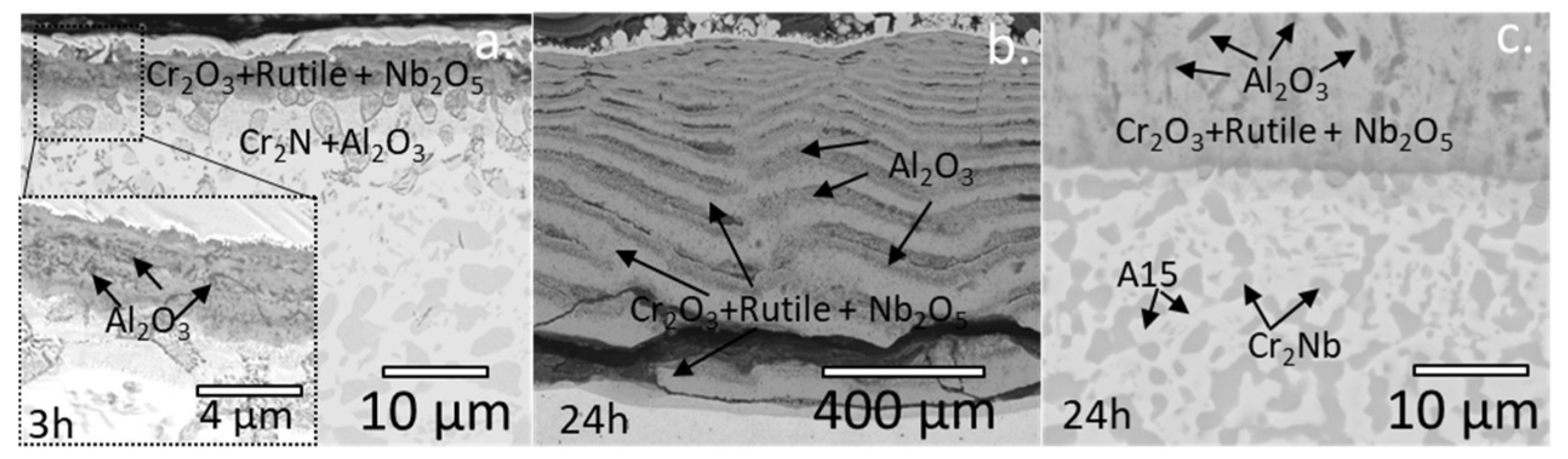
3.4. Oxidation Resistance
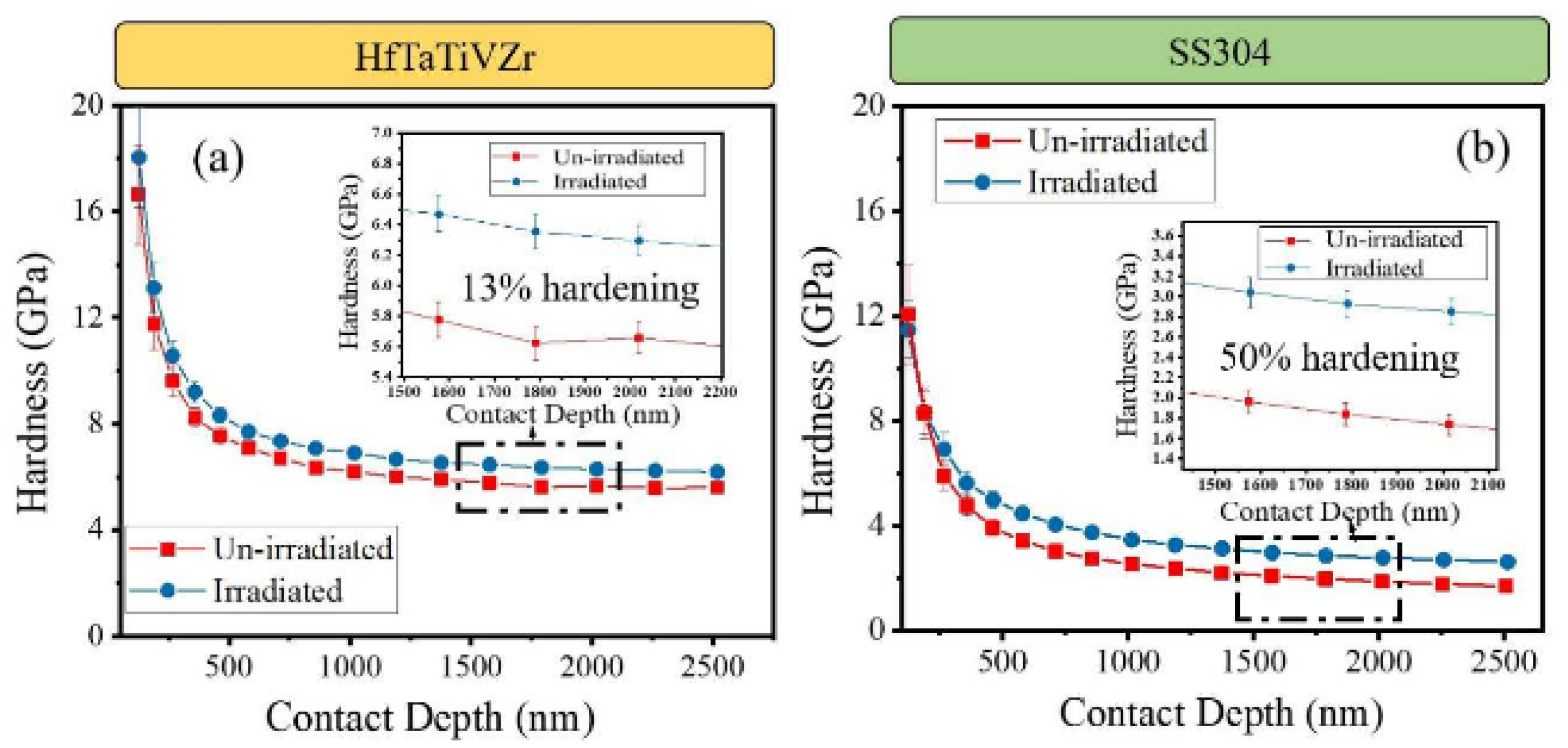
3.5. Radiation Resistance
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.W.; Li, X.G. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowa, J.; Zajusz, M.; Kucza, W.; Cieślak, G.; Berent, K.; Czeppe, T.; Kulik, T.; Danielewski, M. Demystifying the sluggish diffusion effect in high entropy alloys. J. Alloys Compd. 2019, 783, 193–207. [Google Scholar] [CrossRef]
- Varvenne, C.; Luque, A.; Curtin, W.A. Theory of strengthening in fcc high entropy alloys. Acta Mater. 2016, 118, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Bei, H.; Pharr, G.M.; George, E.P. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 2014, 81, 428–441. [Google Scholar] [CrossRef]
- Gorsse, S.; Couzinié, J.P.; Miracle, D.B. From high-entropy alloys to complex concentrated alloys. Comptes Rendus Phys. 2018, 19, 721–736. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2019, 188, 435–474. [Google Scholar] [CrossRef]
- Li, J.G.; Huang, R.R.; Zhang, Q.; Li, X.Y. Mechnical properties and behaviors of high entropy alloys. Chin. J. Theor. Appl. Mech. 2020, 52, 333–359. [Google Scholar]
- Mehta, A.; Sohn, Y.H. Fundamental core effects in transition metal high-entropy alloys: “High-entropy” and “sluggish diffusion” effects. Diffus. Found. 2021, 29, 75–93. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys-a review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Luan, H.W.; Zhao, W.; Yao, K.F. Recent developments on mechanical and functional properties of high entropy alloys. Trans. Mater. Heat Treat. 2020, 41, 1–11. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, Y.K.; Cheng, X.W.; Wu, C.; Cheng, B. High-temperature mechanical properties and microstructure of ZrTiHfNbMox (x = 0.5, 1.0, 1.5) refractory high entropy alloys. IOP Conf. Ser. Mater. Sci. Eng. 2018, 359, 012033. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.C.; Tseng, K.K.; Hsu, W.L.; Tsai, M.H.; Tsai, C.W.; Lin, C.M.; Chen, S.K.; Lin, S.J.; Yeh, J.W. Solution strengthening of ductile refractory HfMoxNbTaTiZr high-entropy alloys. Mater. Lett. 2016, 175, 284–287. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Kang, B.; Kong, T.; Raza, A.; Ryu, H.J.; Hong, S.H. Fabrication, microstructure and mechanical property of a novel Nb-rich refractory high-entropy alloy strengthened by in-situ formation of dispersoids. Int. J. Refract. Met. Hard Mater. 2019, 81, 15–20. [Google Scholar] [CrossRef]
- Yao, H.W.; Miao, J.W.; Liu, Y.M.; Guo, E.Y.; Huang, H.; Lu, Y.P.; Wang, T.M.; Li, T.J. Effect of Ti and Nb contents on microstructure and mechanical properties of HfZrVTaMoWTixNby refractory high-entropy alloys. Adv. Eng. Mater. 2021, 23, 2100225. [Google Scholar] [CrossRef]
- Shao, L.; Yang, M.; Ma, L.; Tang, B.Y. Effect of Ta and Ti content on high temperature elasticity of HfNbZrTa1-xTix refractory high-entropy alloys. Int. J. Refract. Met. Hard Mater. 2021, 95, 105451. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.L.; Xu, Z.Q.; Cheng, X.W. Designing VxNbMoTa refractory high-entropy alloys with improved properties for high-temperature applications. Scr. Mater. 2021, 191, 131–136. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.L.; Xu, Z.Q.; Cheng, X.W. Effects of vanadium concentration on mechanical properties of VxNbMoTa refractory high-entropy alloys. Mater. Sci. Eng. A 2021, 808, 140848. [Google Scholar] [CrossRef]
- Chen, C.L. Influence of V and heat treatment on characteristics of WMoNbTaV refractory high-entropy alloy coatings by mechanical alloying. Coatings 2021, 11, 265. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.P. Effects of tungsten addition on the microstructure and mechanical properties of near-eutectic AlCoCrFeNi2 high-entropy alloy. J. Mater. Eng. Perform. 2018, 27, 109–115. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.L.; Xu, Z.Q.; Cheng, X.W. Microstructures and mechanical properties of HfNbTaTiZrW and HfNbTaTiZrMoW refractory high-entropy alloys. J. Alloys Compd. 2019, 803, 778–785. [Google Scholar] [CrossRef]
- Ham, G.S.; Kim, Y.K.; Na, Y.S.; Lee, K.A. Effect of Ti addition on the microstructure and high-temperature oxidation property of AlCoCrFeNi high-entropy alloy. Met. Mater. Int. 2021, 27, 156–165. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, C.H.; Lee, C.F. The effects of secondary elemental Mo or Ti addition in Al0.3CoCrFeNi high-entropy alloy on age hardening at 700 °C. J. Alloys Compd. 2010, 495, 55–58. [Google Scholar] [CrossRef]
- Han, Z.D.; Chen, N.; Zhao, S.F.; Fan, L.W. Effect of Ti additions on mechanical properties of NbMoTaW and VNbMoTaW refractory high entropy alloys. Intermetallics 2017, 84, 153–157. [Google Scholar] [CrossRef]
- Jiao, Z.M.; Ma, S.G.; Chu, M.Y.; Yang, H.J.; Wang, Z.H.; Zhang, Y.; Qiao, J.M. Superior mechanical properties of AlCoCrFeNiTix high-entropy alloys upon dynamic loading. J. Mater. Eng. Perform. 2016, 25, 451–456. [Google Scholar] [CrossRef]
- Sheikh, S.; Gan, L.; Ikeda, A.; Murakami, H.; Guo, S. Alloying effect on the oxidation behavior of a ductile Al0.5Cr0.25Nb0.5Ta0.5Ti1.5 refractory high-entropy alloy. Mater. Today Adv. 2020, 7, 100104. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhang, L.; Liu, X.Y.; Chen, Q.; Xu, Y. Effect of Zr addition on the microstructure and mechanical properties of CoCrFeNiMn high-entropy alloy synthesized by spark plasma sintering. Entropy 2018, 20, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenko, N.Y.; Stepanov, N.D.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and mechanical properties of B2 ordered refractory AlNbTiVZrx (x = 0–1.5) high-entropy alloys. Mater. Sci. Eng. A 2017, 704, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Shek, C.H. Effects of Hf on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. J. Alloys Compd. 2020, 827, 154159. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Combinatorial synthesis and analysis of AlxTayVz-Cr20Mo20Nb20Ti20Zr10 and Al10CrMoxNbTiZr10 refractory high-entropy alloys: Oxidation behavior. J. Alloys Compd. 2020, 828, 154427. [Google Scholar] [CrossRef]
- Yan, J.H.; Li, M.J.; Li, K.L.; Qiu, J.W.; Guo, Y.J. Effects of Cr content on microstructure and mechanical properties of WMoNbTiCr high-entropy alloys. J. Mater. Eng. Perform. 2020, 29, 2125–2133. [Google Scholar] [CrossRef]
- Yan, X.L.; Guo, H.; Yang, W.; Pang, S.J.; Wang, Q.; Liu, Y.; Liaw, P.K.; Zhang, T. Al0.3CrxFeCoNi high-entropy alloys with high corrosion resistance and good mechanical properties. J. Alloys Compd. 2021, 860, 158436. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, L.; Guo, N.N.; Luo, L.S.; Zhu, G.M.; Shi, C.C.; Su, Y.Q.; Guo, J.J. Microstructure characteristics and mechanical properties of Hf0.5Mo0.5NbTiZr refractory high entropy alloy with Cr addition. Int. J. Refract. Met. Hard Mater. 2021, 95, 105405. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, Z.F.; Liang, X.B.; Cheng, Y.H. Research progress in antioxidation of high entropy alloys at high temperatures. Surf. Technol. 2021, 50, 162–172. [Google Scholar] [CrossRef]
- Schellert, S.; Gorr, B.; Christ, H.-J.; Pritzel, C.; Laube, S.; Kauffmann, A.; Heilmaier, M. The effect of Al on the formation of a CrTaO4 layer in refractory high entropy alloys Ta-Mo-Cr-Ti-xAl. Oxid. Met. 2021, 96, 333–345. [Google Scholar] [CrossRef]
- Lin, C.M.; Juan, C.C.; Chang, C.H.; Tsai, C.W.; Yeh, J.W. Effect of Al addition on mechanical properties and microstructure of refractory AlxHfNbTaTiZr alloys. J. Alloys Compd. 2015, 624, 100–107. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Zhou, Q.; Zhang, F.; Han, W.C.; Du, Y.; Hua, K. Wang, H.F. Effect of Al addition on the microstructure, mechanical and wear properties of TiZrNbHf refractory high entropy alloys. Tribol. Int. 2021, 160, 107031. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb-20Mo-20Cr-20Ti-20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.M.; Zhang, A.J.; Han, J.S.; Meng, J.H. Effect of Si additions on microstructure and mechanical properties of refractory NbTaWMo high-entropy alloys. J. Mater. Sci. 2019, 54, 5844–5851. [Google Scholar] [CrossRef]
- Lee, K.; Jung, Y.; Han, J.; Hong, S.H.; Kim, K.B.; Liaw, P.K.; Lee, C.; Song, G. Development of precipitation-strengthened Al0.8NbTiVM (M = Co, Ni) light-weight refractory high-entropy alloys. Materials 2021, 14, 2085. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Li, Z.W.; Zhang, Y.F.; Wang, W. Effect of Co content on microstructure and properties of high entropy alloying cladding layer on surface of T10 steel. Hot Work. Technol. 2016, 45, 143–145. [Google Scholar] [CrossRef]
- Li, A.M.; Huang, Y.W.; Chen, R.H.; Ouyang, Z.F.; Zheng, Q.F. Microstructure and properties of NixAlTiCrFeCoCu High entropy alloys. Mater. Mech. Eng. 2017, 41, 53–58. [Google Scholar] [CrossRef]
- Tu, C.H.; Wu, S.K.; Lin, C. A study on severely cold-rolled and intermediate temperature aged HfNbTiZr refractory high-entropy alloy. Intermetallics 2020, 126, 106935. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Zhang, H.; Tang, Y.; Zhu, L.A.; Ye, Y.C.; Li, S.; Bai, S.X. Microstructure, mechanical properties and energetic characteristics of a novel high-entropy alloy HfZrTiTa0.53. Mater. Des. 2017, 133, 435–443. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.T.; Chen, Z.; Zhang, J.Y.; Shen, B.L. Oxidation response of a vacuum arc melted NbZrTiCrAl refractory high entropy alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Qiao, J.W. Research progress of refractory high-entropy alloys. Mater. China 2019, 38, 768–774. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.K.; Wu, W.Q.; Song, W.; Zhang, W.; Liu, B. Progress of powder metallurgical high entropy alloys. Chin. J. Nonferrous Met. 2019, 29, 2155–2184. [Google Scholar] [CrossRef]
- Gao, N.; Long, Y.; Peng, H.Y.; Zhang, W.H.; Peng, L. Microstructure and mechanical properties of TiVNbTa refractory high-entropy alloy prepared by powder metallurgy. Chin. J. Mater. Res. 2019, 33, 572–578. [Google Scholar] [CrossRef]
- Long, Y.; Liang, X.B.; Su, K.; Peng, H.Y.; Li, X.Z. A fine-grained NbMoTaWVCr refractory high-entropy alloy with ultra-high strength: Microstructural evolution and mechanical properties. J. Alloys Compd. 2019, 780, 607–617. [Google Scholar] [CrossRef]
- Cao, Y.K.; Liu, Y.; Liu, B.; Zhang, W.D.; Wang, J.W.; Du, M. Effects of Al and Mo on high temperature oxidation behavior of refractory high entropy alloys. Trans. Nonferrous Metal. Soc. 2019, 29, 1476–1483. [Google Scholar] [CrossRef]
- Cao, Y.K.; Liu, Y.; Li, Y.P.; Liu, B.; Wang, J.W.; Du, M.; Liu, R.P. Precipitation strengthening in a hot-worked TiNbTa0.5ZrAl0.5 refractory high entropy alloy. Mater. Lett. 2019, 246, 186–189. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Y.; Hao, X.H.; Zhang, X.W.; Liu, H.X. Lightweight refractory high entropy alloy coating by laser cladding on Ti-6Al-4V surface. Vacuum 2020, 183, 109823. [Google Scholar] [CrossRef]
- Guo, Y.X.; Wang, H.L.; Liu, Q.B. Microstructure evolution and strengthening mechanism of laser-cladding MoFexCrTiWAlNby refractory high-entropy alloy coatings. J. Alloys Compd. 2020, 834, 155147. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhang, H.; Li, D.C.; Chen, Z.H.; Huang, S.; Lu, Z.L.; Yan, H.Q. WxNbMoTa refractory high-entropy alloys fabricated by laser cladding deposition. Materials 2019, 12, 533. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.N.; Zhou, X.L.; Yu, X.N.; Li, J.H. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding. Surf. Coat. Technol. 2017, 311, 321–329. [Google Scholar] [CrossRef]
- Sahasrabudhe, H.; Soderlind, J.; Bandyopadhyay, A. Laser processing of in situ TiN/Ti composite coating on titanium. J. Mech. Behav. Biomed. 2016, 53, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Dutta Majumdar, J.; Galun, R.; Mordike, B.L.; Manna, I. Effect of laser surface melting on corrosion and wear resistance of a commercial magnesium alloy. Mater. Sci. Eng. A 2003, 361, 119–129. [Google Scholar] [CrossRef]
- Li, R.B.; Huang, T.; Jiang, C.X.; Zhang, R.L. Study on preparation, microstructure and mechanical properties of TaWTiVCr high entropy alloy thin film. Surf. Technol. 2020, 49, 159–167. [Google Scholar] [CrossRef]
- Song, B.R.; Li, Y.H.; Wang, K.H.; Cong, Z.H.; Gao, B.; Song, Z.X.; Chen, J. Nano-mechanical properties of TaNbHfZr metallic glass films. Surf. Eng. 2019, 35, 728–735. [Google Scholar] [CrossRef]
- Alvi, S.; Jarzabek, D.M.; Kohan, M.G.; Hedman, D.; Jenczyk, P.; Natile, M.M.; Vomiero, A.; Akhtar, F. Synthesis and mechanical characterization of CuMoTaWV high entropy film by magnetron sputtering. ACS Appl. Mater. Interfaces 2020, 12, 21070–21079. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Kuang, S.F.; Yu, X.; Wang, L.Q.; Huang, W.J. Tribo-mechanical properties of CrNbTiMoZr high-entropy alloy film synthesized by direct current magnetron sputtering. Surf. Coat. Technol. 2020, 403, 126374. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Roh, A.; Son, M.; Ham, M.H.; Kim, J.H.; Choi, H. Mechanical and electrical properties of NbMoTaW refractory high-entropy alloy thin films. Int. J. Refract. Met. Hard Mater. 2019, 80, 286–291. [Google Scholar] [CrossRef]
- Li, T.X.; Lu, Y.P.; Cao, Z.Q.; Wang, T.M.; Li, Y.J. Opportunity and challenge of refractory high-entropy alloys in the field of reactor structural materials. Acta Metall. Sin. 2021, 57, 42–54. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of TixNbMoTaW refractory high-entropy alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H.F.; Zhang, Y. NbTaV-(Ti, W) Refractory high-entropy alloys: Experiments and modeling. Mater. Sci. Eng. A 2016, 674, 203–211. [Google Scholar] [CrossRef]
- Lee, C.; Chou, Y.; Kim, G.; Gao, M.C.; An, K.; Brechtl, J.; Zhang, C.; Chen, W.; Poplawsky, J.D.; Song, G.; et al. Lattice-distortion-enhanced yield strength in a refractory high-entropy alloy. Adv. Mater. 2020, 32, 2004029. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Hu, Z.F.; Yuan, X.T.; Yin, Y.X. Microstructure and mechanical properties of TiZrHfNbSc refractory high entropy alloy. Rare Metal Mater. Eng. 2020, 49, 2820–2824. [Google Scholar]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63. [Google Scholar] [CrossRef]
- Mathiou, C.; Poulia, A.; Georgatis, E.; Karantzalis, A.E. Microstructural features and dry-sliding wear response of MoTaNbZrTi high entropy alloy. Mater. Chem. Phys. 2018, 210, 126–135. [Google Scholar] [CrossRef]
- Jia, Y.J.; Chen, H.N.; Liang, X.D. Microstructure and wear resistance of CoCrNbNiW high-entropy alloy coating prepared by laser melting deposition. Rare Meter. 2019, 38, 1153–1159. [Google Scholar] [CrossRef]
- Huang, Y.M.; Wang, Z.Y.; Xu, Z.Z.; Zang, X.M.; Chen, X.G. Microstructure and properties of TiNbZrMo high entropy alloy coating. Mater. Lett. 2020, 285, 129004. [Google Scholar] [CrossRef]
- Guo, Z.M.; Zhang, A.J.; Han, J.S.; Meng, J.H. The effect of Si addition on tribological properties of NbTaWMo refractory high entropy alloy at high temperature. Tribology 2021, 41, 197–205. [Google Scholar] [CrossRef]
- Wen, X.; Jin, G.; Pang, X.J.; Cai, S.B.; Zhang, Z.H.; Cui, X.F.; Wang, H.D.; Xu, B.S. Effect of heat treatment on microstructure and corrosion resistance of NiCrCoTiV high-entropy alloy prepared by vacuum hot-pressing sintering. Mater. Rep. B 2017, 31, 79–83. [Google Scholar] [CrossRef]
- Yan, D.L.; Song, K.K.; Sun, H.G.; Wu, S.; Zhao, K.; Zhang, H.Z.; Yuan, S.Z.; Kim, J.T.; Chawake, N.; Renk, O.; et al. Microstructures, mechanical properties, and corrosion behaviors of refractory high-entropy ReTaWNbMo alloys. J. Mater. Eng. Perform. 2020, 29, 399–409. [Google Scholar] [CrossRef]
- Huang, S.B.; Wang, C.J.; Chen, Y.Y.; Lee, J.W.; Li, C.L. Thermal and corrosion properties of V-Nb-Mo-Ta-W and V-Nb-Mo-Ta-W-Cr-B high entropy alloy coatings. Surf. Coat. Technol. 2019, 375, 802–809. [Google Scholar] [CrossRef]
- Wang, S.P.; Xu, J. TiZrNbTaMo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Mater. Sci. Eng. C 2017, 73, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.K.; Sanchez, F.; Moncayo, S.; Ramana, C.V. Static and cyclic oxidation of Nb-Cr-V-W-Ta high entropy alloy in air from 600 to 1400 °C. J. Mater. Sci. Technol. 2020, 38, 189–196. [Google Scholar] [CrossRef]
- Chang, C.H.; Titus, M.S.; Yeh, J.W. Oxidation behavior between 700 and 1300°C of Refractory TiZrNbHfTa high-entropy alloys containing aluminum. Adv. Eng. Mater. 2018, 20, 1700948. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.J.; Chen, H.; Kauffmann, A.; Heilmaier, M. Effect of micro alloying with silicon on high temperature oxidation resistance of novel refractory high-entropy alloy Ta-Mo-Cr-Ti-Al. Mater. High Temp. 2018, 35, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.; Gorr, B.; Christ, H.J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the oxidation mechanism of refractory high entropy alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Ayyagari, A.; Muskeri, S.; Hasannaeimi, V.; Salloom, R.; Chen, W.Y.; Mukherjee, S. Ion irradiation response and mechanical behavior of reduced activity high entropy alloy. J. Nucl. Mater. 2020, 529, 151955. [Google Scholar] [CrossRef]
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.; Schneider, M.M.; Sobieraj, D.; Wróbel, J.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding radiation resistance of tungsten-based high entropy alloys. Sci. Adv. 2019, 5, eaav2002. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Tseng, K.K.; Yang, T.Y.; Chao, D.S.; Yeh, J.W.; Liang, J.H. Irradiation-induced swelling and hardening in HfNbTaTiZr refractory high-entropy alloy. Mater. Lett. 2020, 272, 127832. [Google Scholar] [CrossRef]
- Moschetti, M.; Xu, A.; Schuh, B.; Hohenwarter, A.; Couzinié, J.P.; Kruzic, J.J.; Bhattacharyya, D.; Gludovatz, B. On the room-temperature mechanical properties of an ion-irradiated TiZrNbHfTa refractory high entropy alloy. JOM 2020, 72, 130–138. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dudarev, S.L.; Nguyen-Manh, D.; Zheng, S.; Packer, L.M.; Sublet, J.-C. Neutron-induced dpa, transmutations, gas production, and helium embrittlement of fusion materials. J. Nucl. Mater. 2013, 442, S755–S760. [Google Scholar] [CrossRef] [Green Version]
- Eshed, E.; Larianovsky, N.; Kovalevsky, A.; Popov, V., Jr.; Gorbachev, I.; Popov, V.; Katz-Demyanetz, A. Microstructural evolution and phase formation in 2nd-generation refractory-based high entropy alloys. Materials 2018, 11, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkov, O.N.; Senkova, S.V.; Miracle, D.B.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr-Nb-Ti-V-Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr-Nb-Ti-V-Zr system: Microstructure and Phase Analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Li, C.L.; Ma, Y.; Hao, J.M.; Yan, Y.; Wang, Q. Research progress and application of refractory high entropy alloys. J. Netshape Form. Eng. 2017, 9, 117–124. [Google Scholar]

| Element Composition of RHEAs | Melting Point (°C) | Density at RT (g/cm3) | Main Performance | |
|---|---|---|---|---|
| Refractory metal elements | Mo | 2610 | 10.22 | Hardness [15], Strength [16,17] |
| Nb | 2468 | 8.57 | Hardness [18,19], Yield strength [20] | |
| Ta | 2996 | 16.65 | Strength [21] | |
| V | 1902 | 6.11 | Strength [22], Ductility [23], Hardness [24] | |
| W | 3410 | 19.35 | Hardness [25], Yield strength [26] | |
| Ti | 1660 | 4.51 | Oxidation resistance [27], Hardness [28], Yield strength [29], Plasticity [30], Ductility [21] | |
| Zr | 1852 | 6.51 | Oxidation resistance [31], Yield strength [32], Plasticity [33] | |
| Hf | 2227 | 13.31 | Hardness, Yield strength [34] | |
| Cr | 1857 | 7.19 | Oxidation resistance [35], Hardness [36], Corrosion resistance [37], Plasticity, Strength [38] | |
| Re | 3180 | 21.04 | Plasticity, Creep resistance [39] | |
| Non-refractory metal elements | Al | 660 | 2.7 | Oxidation resistance [40], Strength, reduce density [41], Wear resistance, Yield strength [42] |
| Si | 1414 | 2.33 | Oxidation resistance [43], Hardness, Strength [44] | |
| Co | 1495 | 8.9 | Yield strength [45], Wear resistance [46] | |
| Ni | 1453 | 8.9 | corrosion resistance [47], Yield strength [45] | |
| Nominal Composition | 1500 °C | 1600 °C | |||||
|---|---|---|---|---|---|---|---|
| BCC PHASE | t-(Ta,V)O2 | C15 Laves | BCC Phase | t-(Ta,V)O2 | C14 Laves | ||
| Nb | 16.67 | 16.04 | 2.45 | 17.83 | 16.37 | 2.16 | 16.65 |
| Mo | 16.67 | 17.28 | - | 3.59 | 16.70 | - | 3.01 |
| Ta | 16.67 | 15.38 | 24 | 18.92 | 16.18 | 22.43 | 22.31 |
| W | 16.67 | 17.91 | - | 7.47 | 17.68 | - | - |
| V | 16.67 | 16.17 | 12.96 | 11.58 | 16.02 | 13.41 | 13.17 |
| Cr | 16.67 | 17.21 | - | 40.58 | 17.04 | - | 44.82 |
| O | - | - | 60.57 | - | - | 61.97 | - |
| RHEAs | σ0.2 (MPa) | σp (MPa) | εp (%) | |
|---|---|---|---|---|
| TixNbMoTaW | Ti0 | 996 | 1148 | 1.9 |
| Ti0.25 | 1109 | 1197 | 2.5 | |
| Ti0.5 | 1211 | 1578 | 5.9 | |
| Ti0.75 | 1304 | 1593 | 8.4 | |
| Ti1 | 1455 | 1910 | 11.5 | |
| Materials | TiZrNbTaMo | Ti6Al4V | 316 L SS | CoCrMo |
|---|---|---|---|---|
| Ecorr (mVSCE) | −607 ± 55 | −571 ± 11 | −234 ± 13 | −320 ± 30 |
| Ip (μA/cm2) | 0.89 ± 0.06 | 0.96 ± 0.21 | 0.83 ± 0.03 | 0.42 ± 0.19 |
| Epit (mVSCE) | - | - | 675 ± 30 | 435 ± 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Li, Y.; Qi, Y.; Wang, B. Review on Preparation Technology and Properties of Refractory High Entropy Alloys. Materials 2022, 15, 2931. https://doi.org/10.3390/ma15082931
Ren X, Li Y, Qi Y, Wang B. Review on Preparation Technology and Properties of Refractory High Entropy Alloys. Materials. 2022; 15(8):2931. https://doi.org/10.3390/ma15082931
Chicago/Turabian StyleRen, Xiqiang, Yungang Li, Yanfei Qi, and Bo Wang. 2022. "Review on Preparation Technology and Properties of Refractory High Entropy Alloys" Materials 15, no. 8: 2931. https://doi.org/10.3390/ma15082931






