Colloidal Processing of Complex-Shaped ZrB2-Based Ultra-High-Temperature Ceramics: Progress and Prospects
Abstract
:1. Introduction
2. Dispersion of ZrB2 Powder
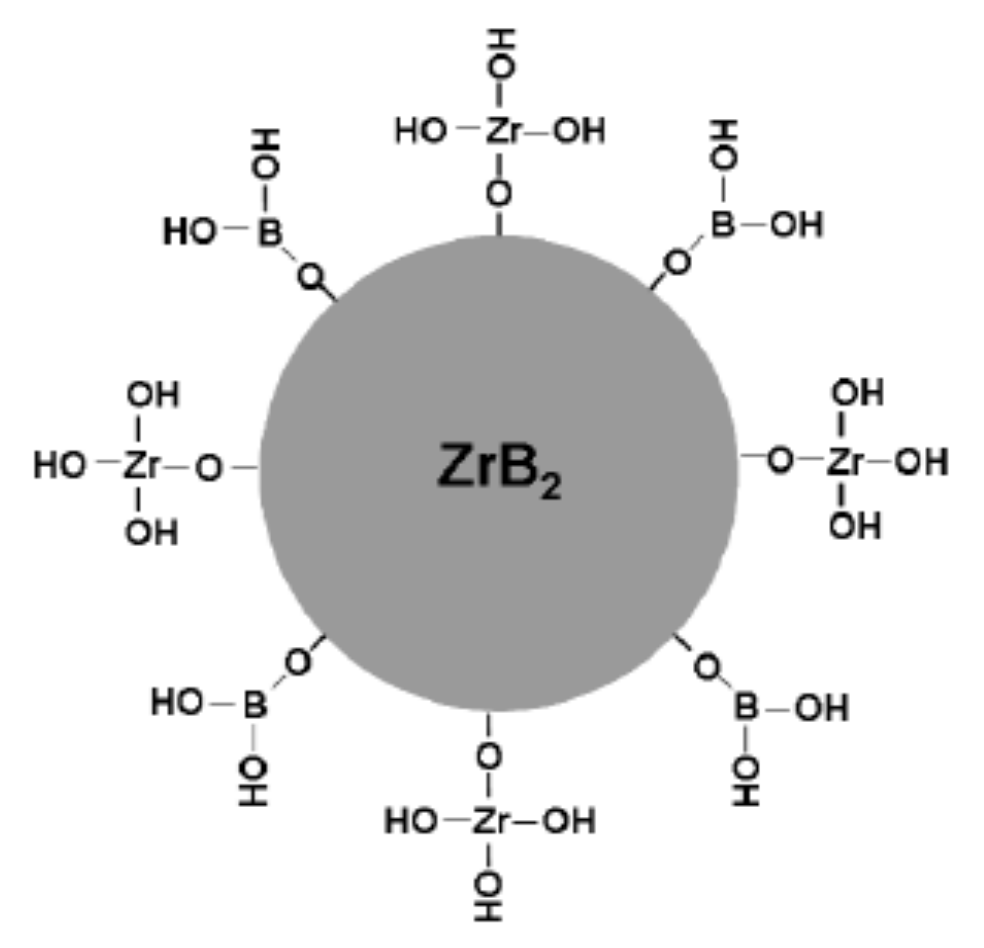
2.1. Corrosion and Hydrolysis of ZrB2 Powders
2.2. Dispersion Mechanism
2.2.1. Van der Waals Force
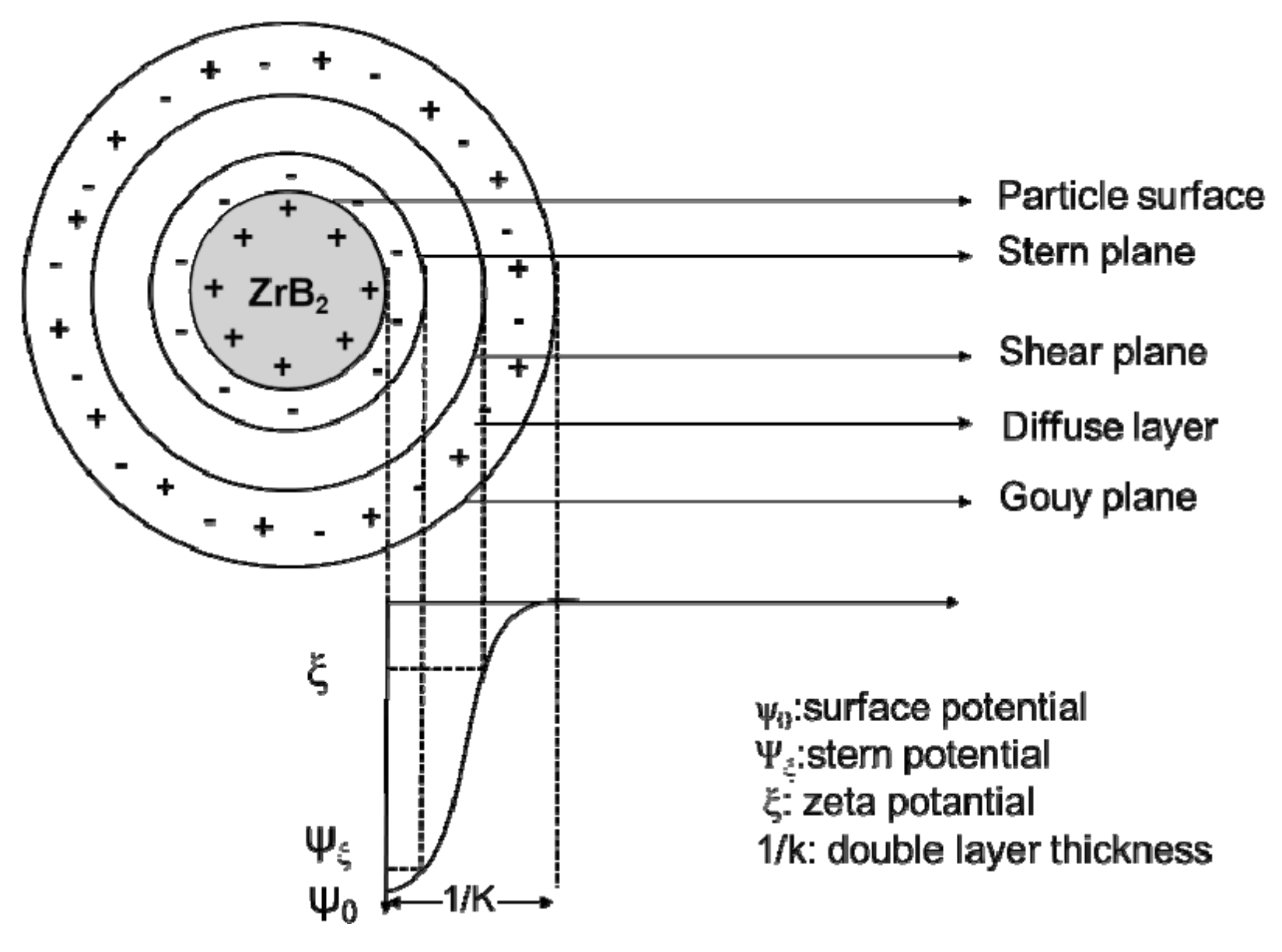
2.2.2. Electrostatic Interaction
2.2.3. Steric Interaction
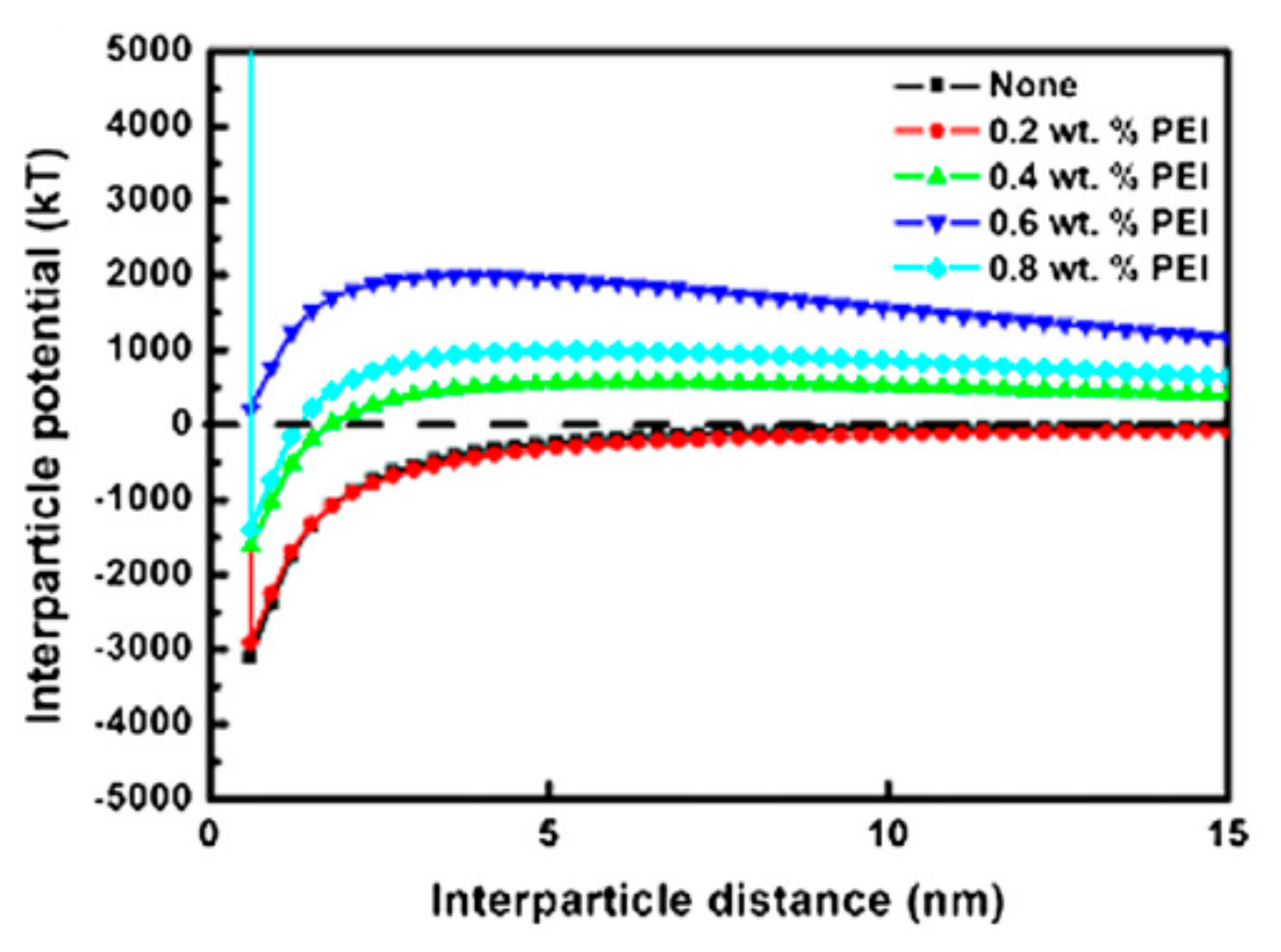
2.3. Dispersion of ZrB2 Powders
3. Colloidal Processing
3.1. Slip-Casting
3.2. Tape-Casting
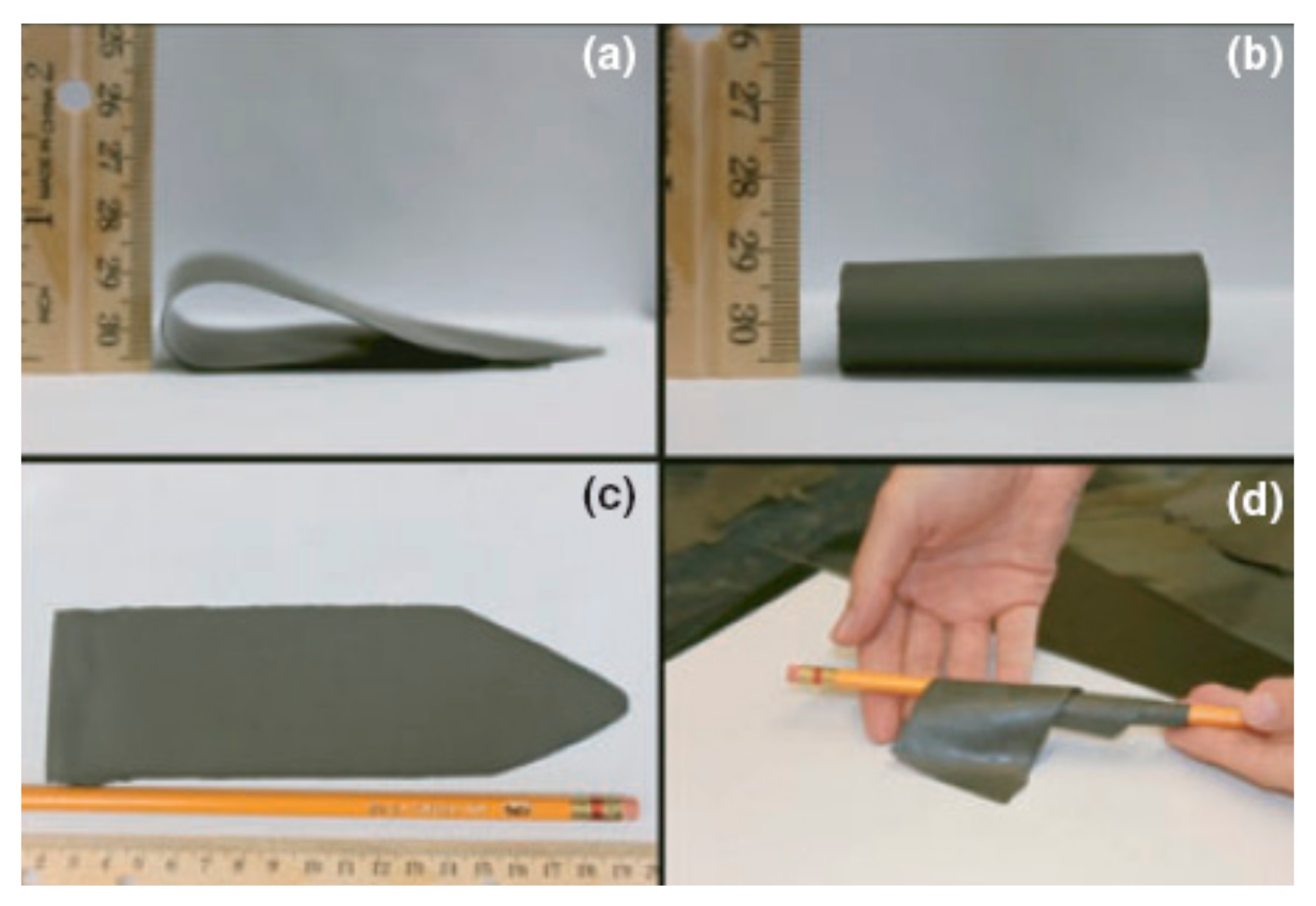
3.3. Gel-Casting
- Capable of producing complex-shaped ceramic components.
- Low equipment and mold costs.
- Capable of mass production.
- High green density and green strength.
- Excellent green machinability.
- Very homogeneous microstructure and material properties.
4. Prospects
5. Summary
- (1)
- The corrosion and hydrolysis mechanisms of ZrB2 particles in aqueous medium have been summarized. The surface of the ZrB2 ceramic particle is mainly covered with an oxide layer composed of Zr-OH and B-OH, which should be controlled during the wet processes, especially aqueous colloidal processing, such as ball-milling, mixing, and suspension preparation.
- (2)
- The dispersion mechanism and dispersion method of ZrB2 ceramic particles (micron-sized and nano-sized) in aqueous medium have been summarized. From the researchers’ great efforts, well-dispersed ZrB2 ceramic suspensions can be obtained, which is in favor of the following colloidal processing.
- (3)
- Various colloidal processing methods, such as slip-casting, tape-casting, freeze-casting, and gel-casting, etc., have been introduced and evaluated. The advantages and disadvantages for each processing method have been summarized, and we provided an evaluation of colloidal processing studies of the ZrB2-based UHTC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alosime, E.M.; Alsuhybani, M.S.; Almeataq, M.S. The oxidation behavior of ZrB2-SiC ceramic composites fabricated by plasma spray process. Materials 2021, 14, 392. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, W.; Hilmas, G. Oxidation of ultra-high temperature transition metal diboride ceramics. Int. Mater. Rev. 2012, 57, 61–72. [Google Scholar] [CrossRef]
- Fahrenholtz, W.; Hilmas, G.; Talmy, I.; Zaykoski, J. Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Sha, J.; Wang, S.; Dai, J.; Zu, Y.; Li, W.; Sha, R. High-temperature mechanical properties and their influence mechanisms of ZrC-modified C-SiC ceramic matrix composites up to 1600 °C. Materials 2020, 13, 1581. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Huang, Y.; Zhang, H.; Yang, Y.; Hong, Y. Microstructure and mechanical properties of multiwalled carbon nanotube toughened spark plasma sintered ZrB2 composites. Adv. Appl. Ceram. 2016, 115, 308–312. [Google Scholar] [CrossRef]
- Orlovskaya, N.; Stadelmann, R.; Lugovy, M.; Subbotin, V.; Subhash, G.; Neubert, M.; Aneziris, C.; Graule, T.; Kuebler, J. Mechanical properties of ZrB2-SiC ceramic composites: Room temperature instantaneous behavior. Adv. Appl. Ceram. 2013, 112, 9–16. [Google Scholar] [CrossRef]
- Nisar, A.; Balani, K. Phase and microstructural correlation of spark plasma sintered HfB2-ZrB2 based ultra-high temperature ceramic composites. Coatings 2017, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Guo, S. Densification of ZrB2-based composites and their mechanical and physical properties: A review. J. Eur. Ceram. Soc. 2009, 29, 995–1011. [Google Scholar] [CrossRef]
- Han, J.; Hu, P.; Zhang, X.; Meng, S.; Han, W. Oxidation-resistant ZrB2-SiC composites at 2200 °C. Comp. Sci. Technol. 2008, 68, 799–806. [Google Scholar] [CrossRef]
- Kakroudi, M.G.; Alvari, M.D.; Asl, M.S.; Vafa, N.P.; Rabizadeh, T. Hot pressing and oxidation behavior of ZrB2-SiC-TaC composites. Ceram. Int. 2020, 46, 3725–3730. [Google Scholar] [CrossRef]
- Tang, Z.; Yi, M.; Xiang, Q.; Du, Y.; Peng, K. Mechanical and ablation properties of a C/C-HfB2-SiC composite prepared by high-solid-loading slurry impregnation combined with precursor infiltration and pyrolysis. J. Eur. Ceram. Soc. 2021, 41, 6160–6170. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, X.; Han, W.; Han, J. Fabrication and evaluation on thermal stability of hafnium diboride matrix composite at severe oxidation condition. Int. J. Refract. Met. Hard Mater. 2009, 27, 711–717. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zhou, N.; Dong, X.; Yuan, F.; He, R. Low temperature fabrication of Cf/BNi/(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C-SiCm high entropy ceramic matrix composite by slurry coating and laminating combined with precursor infiltration and pyrolysis. J. Eur. Ceram. Soc. 2022, 42, 3099–3106. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Moshtaghioun, B.M.; Gómez-García, D.; Domínguez-Rodríguez, A.; Lee, W.E. HfB2 ceramic polycrystals: A low-temperature metal-like ceramic at high temperatures? Scr. Mater. 2021, 203, 114037. [Google Scholar] [CrossRef]
- Jayaseelan, D.D.; Zapata-Solvas, E.; Carney, C.M.; Katz, A.; Brown, P.; Lee, W.E. Microstructural evolution of HfB2 based ceramics during oxidation at 1600–2000 °C. Adv. Appl. Ceram. 2015, 114, 277–295. [Google Scholar] [CrossRef]
- Eakins, E.; Jayaseelan, D.D.; Lee, W.E. Toward oxidation-resistant ZrB2-SiC ultra high temperature ceramics. Metall. Mater. Trans. A. 2011, 42, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, T.A.; Petry, M.D.; Cinibulk, M.K.; Mathur, T.; Gruber, M.R. Thermal and oxidation response of UHTC leading edge samples exposed to simulated hypersonic flight conditions. J. Am. Ceram. Soc. 2013, 96, 907–915. [Google Scholar] [CrossRef]
- Monteverde, F.; Savino, R.; Fumo, M.D.S.; Maso, A.D. Plasma wind tunnel testing of ultra-high temperature ZrB2-SiC composites under hypersonic re-entry conditions. J. Eur. Ceram. Soc. 2010, 30, 2313–2321. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, P.; Han, J.; Meng, S.H. Ablation behavior of ZrB2-SiC ultra high temperature ceramics under simulated atmospheric re-entry conditions. Comp. Sci. Technol. 2008, 68, 1718–1726. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Fattahi, M.; Ershadi, M.N.; Vajdi, M.; Moghanlou, F.S.; Namini, A.S.; Asl, M.S. On the simulation of spark plasma sintered TiB2 ultra high temperature ceramics: A numerical approach. Ceram. Int. 2020, 46, 14787–14795. [Google Scholar] [CrossRef]
- Gu, J.; Zou, J.; Liu, J.; Wang, H.; Zhang, J.; Wang, W.; Fu, Z. Sintering highly dense ultra-high temperature ceramics with suppressed grain growth. J. Eur. Ceram. Soc. 2020, 40, 1086–1092. [Google Scholar] [CrossRef]
- Mungiguerra, S.; Di Martino, G.D.; Cecere, A.; Savino, R.; Zoli, L.; Silvestroni, L.; Sciti, D. Ultra-high-temperature testing of sintered ZrB2-based ceramic composites in atmospheric re-entry environment. Int. J. Heat Mass Transf. 2020, 156, 119910. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Han, J.; Han, W.; Hong, C. Effects of Y2O3 on microstructure and mechanical properties of ZrB2-SiC ceramics. J. Alloys Compd. 2008, 465, 506–511. [Google Scholar] [CrossRef]
- Mallik, M.; Roy, S.; Ray, K.K.; Mitra, R. Effect of SiC content, additives and process parameters on densification and structure-property relations of pressureless sintered ZrB2-SiC composites. Ceram. Int. 2013, 39, 1915–2932. [Google Scholar] [CrossRef]
- Kim, S.; Chae, J.M.; Lee, S.M.; Oh, Y.S.; Kim, H.T.; Jang, B.K. Change in microstructures and physical properties of ZrB2-SiC ceramics hot-pressed with a variety of SiC sources. Ceram. Int. 2014, 40, 3477–3483. [Google Scholar] [CrossRef]
- Popov, O.; Vleugels, J.; Zeynalov, E.; Vishnyakov, V. Reactive hot pressing route for dense ZrB2-SiC and ZrB2-SiC-CNT ultra-high temperature ceramics. J. Eur. Ceram. Soc. 2020, 40, 5012–5019. [Google Scholar] [CrossRef]
- Buinevich, V.S.; Nepapushev, A.A.; Moskovskikh, D.O.; Kuskov, K.V.; Yudin, S.N.; Mukasyan, A.S. Ultra-high-temperature tantalum-hafnium carbonitride ceramics fabricated by combustion synthesis and spark plasma sintering. Ceram. Int. 2021, 47, 30043–30050. [Google Scholar] [CrossRef]
- Liu, Y.; Zu, Y.; Tian, H.; Dai, J.; Sha, J. Microstructure and mechanical properties of continuous carbon fiber-reinforced ZrB2-based composites via combined electrophoretic deposition and sintering. J. Eur. Ceram. Soc. 2021, 41, 1779–1787. [Google Scholar] [CrossRef]
- Feng, B.; Fetzer, A.K.; Ulrich, A.S.; Christian Galetz, M.; Kleebe, H.J.; Ionescu, E. Monolithic ZrB2-based UHTC s using polymer-derived Si (Zr, B) CN as sintering aid. J. Am. Ceram. Soc. 2022, 105, 99–110. [Google Scholar] [CrossRef]
- Nakamura, M.; Shigematsu, I.; Kanayama, K.; Hirai, Y. Surface damage in ZrB2-based composite ceramics induced by electro-discharge machining. J. Mater. Sci. 1991, 26, 6078–6082. [Google Scholar] [CrossRef]
- Cheng, Y.; Eubank, P.T.; Gadalla, A.M. Electraical discharge machining of ZrB2-based ceramics. Mater. Manuf. Process. 1996, 11, 565–574. [Google Scholar] [CrossRef]
- Scatteia, L.; Alfano, D.; Monteverde, F.; Scans, J.L.; Pichelin, M.B. Effect of the machining method on the catalycity and emissivity of ZrB2 and ZrB2-HfB2-Based ceramics. J. Am. Ceram. Soc. 2008, 91, 1461–1468. [Google Scholar] [CrossRef]
- Hirata, Y. Theoretical aspects of colloidal processing. Ceram. Int. 1997, 23, 93–98. [Google Scholar] [CrossRef]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Sigmund, W.M.; Bell, N.S.; Bergstrom, L. Novel powder-processing methods for advanced ceramics. J. Am. Ceram. Soc. 2000, 83, 1557–1574. [Google Scholar] [CrossRef]
- Hotza, D.; Greil, P. Review: Aqueous tape casting of ceramic powders. Mater. Sci. Eng. A 1995, 202, 206–217. [Google Scholar] [CrossRef]
- Lee, S.H.; Sakka, Y.; Kagawa, Y. Corrosion of ZrB2 powder during wet processing-Analysis and control. J. Am. Ceram. Soc. 2008, 91, 1715–1717. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, H.; Yan, Y.; Huang, Z.; Liu, X.; Yang, Y.; Jiang, D. Hydrolysis behavior of zirconium diboride during attrition milling. Mater. Chem. Phys. 2012, 133, 8–15. [Google Scholar] [CrossRef]
- Aschauer, U.; Burgos-Montes, O.; Moreno, R.; Bowen, P. Hamaker 2: A toolkit for the calculation of particle interactions and suspension stability and its application to mullite synthesis by colloidal methods. J. Dispers. Sci. Technol. 2011, 32, 470–479. [Google Scholar] [CrossRef]
- He, R.; Hu, P.; Zhang, X.; Han, W.; Wei, C.; Hou, Y. Preparation of high solid loading, low viscosity ZrB2-SiC aqueous suspensions using PEI as dispersant. Ceram. Int. 2013, 39, 2267–2274. [Google Scholar] [CrossRef]
- Lü, Z.; Jiang, D.; Zhang, J.; Lin, Q. Aqueous tape casting of zirconium diboride. J. Am. Ceram. Soc. 2009, 92, 2212–2217. [Google Scholar] [CrossRef]
- Huang, T.S.; Hilmas, G.E.; Fahrenholtz, W.G.; Leu, M.C. Dispersion of zirconium diboride in an aqueous, high-solids paste. Int. J. Appl. Ceram. Technol. 2007, 4, 470–479. [Google Scholar] [CrossRef]
- Lee, S.H.; Sakka, Y.; Kagawa, Y. Dispersion behavior of ZrB2 powder in aqueous solution. J. Am. Ceram. Soc. 2007, 90, 3455–3459. [Google Scholar] [CrossRef]
- Medri, V.; Capiani, C.; Bellosi, A. Properties of slip-cast and pressureless sintered ZrB2-SiC composites. Int. J. Appl. Ceram. Technol. 2011, 8, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Medri, V.; Capiani, C.; Gardini, D. Slip casting of ZrB2-SiC composites aqueous suspensions. Adv. Eng. Mater. 2010, 12, 210–215. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, Y.; Huang, Z.; Liu, X.; Jiang, D. Preparation and characterization of stable ZrB2-based ultra-high temperature ceramics slurry by aqueous gelcasting. Key Eng. Mater. 2008, 369–372, 1756–1757. [Google Scholar] [CrossRef]
- Yin, J.; Liu, X.; Zhang, H.; Yang, Y.; Jiang, D. Dispersion and gelcasting of zirconium diboride through aqueous route. Int. J. Appl. Ceram. Technol. 2012, 9, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Kan, Y.; Zhang, G.; Wang, P. Slip casting and pressureless sintering of ZrB2-SiC ceramic. J. Inorg. Mater. 2009, 24, 831–835. [Google Scholar] [CrossRef]
- Natividad, S.L.; Marotto, V.R.; Walker, L.S.; Pham, D.; Pinc, W.; Corral, E.L. Tape casting thin, continuous, homogenous, and flexible tapes of ZrB2. J. Am. Ceram. Soc. 2011, 94, 2749–2753. [Google Scholar] [CrossRef]
- He, R.; Hu, P.; Zhang, X.; Han, W.; Wang, Q. Dispersion and rheology of aqueous zirconium diboride nanosuspensions. Int. J. Appl. Ceram. Technol. 2013, 10, 706–713. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Hu, P.; Han, W.; Luo, J. Dispersion and co-dispersion of ZrB2 and SiC nanopowders in ethanol. Ceram. Int. 2012, 38, 2733–2741. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Hu, P.; Hong, C. Dispersion and interaction of ZrB2 nanopowders with gallic acid in n-butanol. J. Eur. Ceram. Soc. 2012, 32, 3463–3468. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.; Liu, X.; Zhang, H.; Yan, Y.; Huang, Z.; Jiang, D. Co-dispersion behavior of ZrB2-SiC-B4C-C powders with polyethyleneimine. Materials 2013, 6, 4249–4258. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Jiang, D.; Peter, G. Tape casting of aqueous Al2O3 slurries. J. Eur. Ceram. Soc. 2000, 20, 1691–1697. [Google Scholar]
- Vozdecky, P.; Roosen, A.; Knieke, C.; Peukert, W. Direct tape casting of nanosized Al2O3 slurries derived from autogenous nanomilling. J. Am. Ceram. Soc. 2010, 93, 1313–1319. [Google Scholar]
- Snijkers, F.; Wilde, A.; Mullens, S.; Luyten, J. Aqueous tape casting of yttria stabilised zirconia using natural product binder. J. Eur. Ceram. Soc. 2004, 24, 1107–1110. [Google Scholar] [CrossRef]
- Mukherjee, A.; Maiti, B.; Das Sharma, A.; Basu, R.N.; Maiti, H.S. Correlation between slurry rheology, green density and sintered density of tape cast yttria stabilised zirconia. Ceram. Int. 2001, 27, 731–739. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, T.; Jiang, D.; Zhang, J.; Lin, Q. Aqueous tape casting process for SiC. Ceram. Int. 2009, 35, 1889–1895. [Google Scholar] [CrossRef]
- Yang, W.; Fuso, L.; Biamino, S.; Vasquez, D.; Vega Bolivar, C.; Fino, P.; Badini, C. Fabrication of short carbon fibre reinforced SiC multilayer composites by tape casting. Ceram. Int. 2012, 38, 1011–1018. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, F.; Jiang, D.; Iwasa, M. Preparation of bulk Si3N4 from tape casting and lamination. Ceram. Int. 2006, 32, 277–282. [Google Scholar] [CrossRef]
- Lü, Z.; Jiang, D.; Zhang, J.; Lin, Q. Processing and properties of ZrB2-SiC composites obtained by aqueous tape casting and hot pressing. Ceram. Int. 2011, 37, 293–301. [Google Scholar] [CrossRef]
- Medri, V.; Pinasco, P.; Sanson, A.; Roncari, E.; Guicciardi, S.; Bellosi, A. ZrB2-based laminates produced by tape casting. Int. J. Appl. Ceram. Technol. 2012, 9, 349–357. [Google Scholar] [CrossRef]
- Lü, Z.; Jiang, D.; Zhang, J.; Lin, Q. Microstructure and mechanical properties of zirconium diboride obtained by aqueous tape casting process and hot pressing. J. Am. Ceram. Soc. 2010, 93, 4153–4157. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, Z.; Huang, S.; Cheng, X. Fabrication of laminated ZrB2-SiC ceramics via tape casting and vacuum hot-pressing sintering and their mechanical properties. Ceram. Int. 2015, 41, 11555–11561. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, X.; Li, S. Laminated ZrB2-SiC/graphite ceramics with simultaneously improved flexural strength and fracture toughness. Ceram. Int. 2014, 40, 5001–5006. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, X.; Hu, P.; Han, W.; Tian, G. The fabrication and mechanical properties of bionic laminated ZrB2-SiC/BN ceramic prepared by tape casting and hot pressing. Scr. Mater. 2011, 65, 791–794. [Google Scholar] [CrossRef]
- Young, A.C.; Omatete, O.O.; Janney, M.A.; Menchhofer, P.A. Gelcasting of alumina. J. Am. Ceram. Soc. 1991, 74, 612–618. [Google Scholar] [CrossRef]
- Omatete, O.O.; Janney, M.A.; Nunn, S.D. Gelcasting: From laboratory development toward industrial production. J. Eur. Ceram. Soc. 1997, 17, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Janney, M.A.; Omatete, O.O.; Walls, C.A.; Nunn, S.D.; Ogle, R.J.; Westmoreland, G. Development of low-toxicity gelcasting systems. J. Am. Ceram. Soc. 1998, 81, 581–589. [Google Scholar] [CrossRef]
- Bengisu, M.; Yilmaz, E. Gelcasting of alumina and zirconia using chitosan gels. Ceram. Int. 2002, 28, 431–438. [Google Scholar] [CrossRef]
- Adolfsson, E. Gelcasting of zirconia using agarose. J. Am. Ceram. Soc. 2006, 89, 1897–1902. [Google Scholar] [CrossRef]
- Vlajic, M.D.; Krstic, V.D. Strength and machining of gelcast SiC ceramics. J. Mater. Sci. 2002, 37, 2943–2947. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Zhang, J.; Jiang, D.; Lin, Q. Preparation of SiC ceramics by aqueous gelcasting and pressureless sintering. Mater. Sci. Eng. A 2007, 443, 257–261. [Google Scholar] [CrossRef]
- Gilissen, R.; Erauw, J.P.; Smolders, A.; Vanswijgenhoven, E.; Luyten, J. Gelcasting, a near net shape technique. Mater. Design 2000, 21, 251–257. [Google Scholar] [CrossRef]
- He, R.; Zhang, X.; Hu, P.; Liu, C.; Han, W. Aqueous gelcasting of ZrB2-SiC ultra high temperature ceramics. Ceram. Int. 2012, 38, 5411–5418. [Google Scholar] [CrossRef]
- He, R.; Hu, P.; Zhang, X.; Liu, C. Gelcasting of complex-shaped ZrB2-SiC ultra high temperature ceramic components. Mater. Sci. Eng. A 2012, 556, 494–499. [Google Scholar] [CrossRef]
- He, R.; Zhang, X.; Hu, P.; Han, W.; Hong, C. Preparation of YAG gel coated ZrB2-SiC composite prepared by gelcasting and pressureless sintering. Comp. B 2013, 54, 307–312. [Google Scholar] [CrossRef]
- Zhang, R.; He, R.; Zhang, X.; Fang, D. Microstructure and mechanical properties of ZrB2-SiC composites prepared by gelcasting and pressureless sintering. Int. J. Refract. Met. Hard Mater. 2014, 43, 84–88. [Google Scholar] [CrossRef]
- He, R.; Zhang, R.; Zhu, X.; Wei, K.; Qu, Z.; Pei, Y.; Fang, D. Improved green strength and green machinability of ZrB2-SiC through gelcasting based on a double gel network. J. Am. Ceram. Soc. 2014, 97, 2401–2404. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.; Liu, X.; Huang, Z.; Lee, S.H. Aqueous gelcasting and pressureless sintering of zirconium diboride foams. Ceram. Int. 2014, 40, 6325–6330. [Google Scholar] [CrossRef]
- He, R.; Zhang, X.; Han, W.; Hu, P.; Hong, C. Effects of solids loading on microstructure and mechanical properties of HfB2-20 vol.% MoSi2 ultra high temperature ceramic composites through aqueous gelcasting route. Mater. Design 2013, 47, 35–40. [Google Scholar] [CrossRef]
- Du, J.; Zhang, X.; Hong, C.; Han, W. Microstructure and mechanical properties of ZrB2-SiC porous ceramic by camphene-based freeze casting. Ceram. Int. 2013, 39, 953–957. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal additive manufacturing: A review. J. Mater. Eng. Perf. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modelling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, M.; Scutelnicu, E.; Rusu, C.C.; Mistodie, L.R.; Mihailescu, D.; Subbiah, A.V. Wire arc additive manufacturing: Review on recent findings and challenges in industrial applications and materials characterization. Metals 2021, 11, 939. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Parandoush, P.; Lin, D. A review on additive manufacturing of polymer-fiber composites. Comp. Struct. 2017, 182, 36–53. [Google Scholar] [CrossRef]
- Goh, G.D.; Yap, Y.L.; Tan, H.K.J.; Sing, S.L.; Goh, G.L.; Yeong, W.Y. Process-structure-properties in polymer additive manufacturing via material extrusion: A review. Crit. Rev. Solid State Mater. Sci. 2020, 45, 113–133. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive manufacturing of metallic and ceramic components by the material extrusion of highly-filled polymers: A review and future perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, J.J.; Seifi, M. Metal additive manufacturing: A review of mechanical properties. Annu. Rev. Mater. Res. 2016, 46, 151–186. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.S.; Vulimiri, P.S.; To, A.C.; Zhang, X.; Brice, C.A.; Kappes, B.B.; Stebner, A.P. Machine learning for materials developments in metals additive manufacturing. Addit. Manuf. 2020, 36, 101641. [Google Scholar]
- Bandyopadhyay, A.; Traxel, K.D. Metal-additive manufacturing-Modeling strategies for application-optimized designs. Addit. Manuf. 2018, 22, 758–774. [Google Scholar] [PubMed]
- Hegab, H.A. Design for additive manufacturing of composite materials and potential alloys: A review. Manuf. Rev. 2016, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- He, R.; Zhou, N.; Zhang, K.; Zhang, X.; Zhang, L.; Wang, W.; Fang, D. Progress and challenges towards additive manufacturing of SiC ceramic. J. Adv. Ceram. 2021, 10, 637–674. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, C.; Wang, G.; He, R.; Ding, G.; Wang, M.; Dai, D.; Fang, D. High solid loading, low viscosity photosensitive Al2O3 slurry for stereolithography based additive manufacturing. Ceram. Int. 2019, 45, 203–208. [Google Scholar] [CrossRef]
- Mishra, G.K.; Paul, C.P.; Rai, A.K.; Agrawal, A.K.; Rai, S.K.; Bindra, K.S. Experimental investigation on laser directed energy deposition based additive manufacturing of Al2O3 bulk structures. Ceram. Int. 2021, 47, 5708–5720. [Google Scholar] [CrossRef]
- Conti, L.; Bienenstein, D.; Borlaf, M.; Graule, T. Effects of the layer height and exposure energy on the lateral resolution of zirconia parts printed by lithography-based additive manufacturing. Materials 2020, 13, 1317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Meng, Q.; Zhang, X.; Qu, Z.; Jing, S.; He, R. Roles of solid loading in stereolithography additive manufacturing of ZrO2 ceramic. Int. J. Refract. Met. Hard Mater. 2021, 99, 105604. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.; Zhang, L.; Jing, S.; Shen, C.; He, R. Additive manufacturing of Csf/SiC composites with high fiber content by direct ink writing and liquid silicon infiltration. Ceram. Int. 2022, 48, 3895–3903. [Google Scholar] [CrossRef]
- Zhou, P.; Qi, H.; Zhu, Z.; Qin, H.; Li, H.; Chu, C.; Yan, M. Development of SiC/PVB composite powders for selective laser sintering additive manufacturing of SiC. Materials 2018, 11, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polozov, I.; Razumov, N.; Masaylo, D.; Silin, A.; Lebedeva, Y.; Popovich, A. Fabrication of silicon carbide fiber-reinforced silicon carbide matrix composites using binder jetting additive manufacturing from irregularly-shaped and spherical powders. Materials 2020, 13, 1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; He, C.; Zhang, P.; Han, W.; Guo, F.; Wu, F.; Du, M. Additive manufacturing of silicon nitride ceramic floatation spheres with excellent mechanical properties. Materials 2019, 12, 2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Dantec, M.; Abdulstaar, M.; Leparoux, M.; Hoffmann, P. Epitaxial growth of silicon on silicon wafers by direct laser melting. Materials 2020, 13, 4728. [Google Scholar] [CrossRef]
- Huang, K.; Elsayed, H.; Franchin, G.; Colombo, P. Additive Manufacturing of SiOC scaffolds with tunable structure-performance relationship. J. Eur. Ceram. Soc. 2021, 41, 7552–7559. [Google Scholar] [CrossRef]
- He, C.; Ma, C.; Li, X.; Hou, F.; Yan, L.; Guo, A.; Liu, J. Continuous fast 3D printing of SiOC ceramic components. Addit. Manuf. 2021, 46, 102111. [Google Scholar] [CrossRef]
- Azarnoush, S.; Laubscher, F.; Zoli, L.; Raj, R. Additive manufacturing of SiCN ceramic matrix for SiC fiber composites by flash pyrolysis of nanoscale polymer films. J. Am. Ceram. Soc. 2016, 99, 1855–1858. [Google Scholar] [CrossRef]
- Jana, P.; Santoliquido, O.; Ortona, A.; Colombo, P.; Sorarù, G.D. Polymer-derived SiCN cellular structures from replica of 3D printed lattices. J. Am. Ceram. Soc. 2018, 101, 2732–2738. [Google Scholar] [CrossRef] [Green Version]
- Feilden, E.; Glymond, D.; Saiz, E.; Vandeperre, L. High temperature strength of an ultra high temperature ceramic produced by additive manufacturing. Ceram. Int. 2019, 45, 18210–18214. [Google Scholar] [CrossRef]
- Kemp, J.W.; Diaz, A.A.; Malek, E.C.; Croom, B.P.; Apostolov, Z.D.; Kalidindi, S.R.; Compton, B.G.; Rueschhoff, L.M. Direct ink writing of ZrB2-SiC chopped fiber ceramic composites. Addit. Manuf. 2021, 44, 102049. [Google Scholar] [CrossRef]

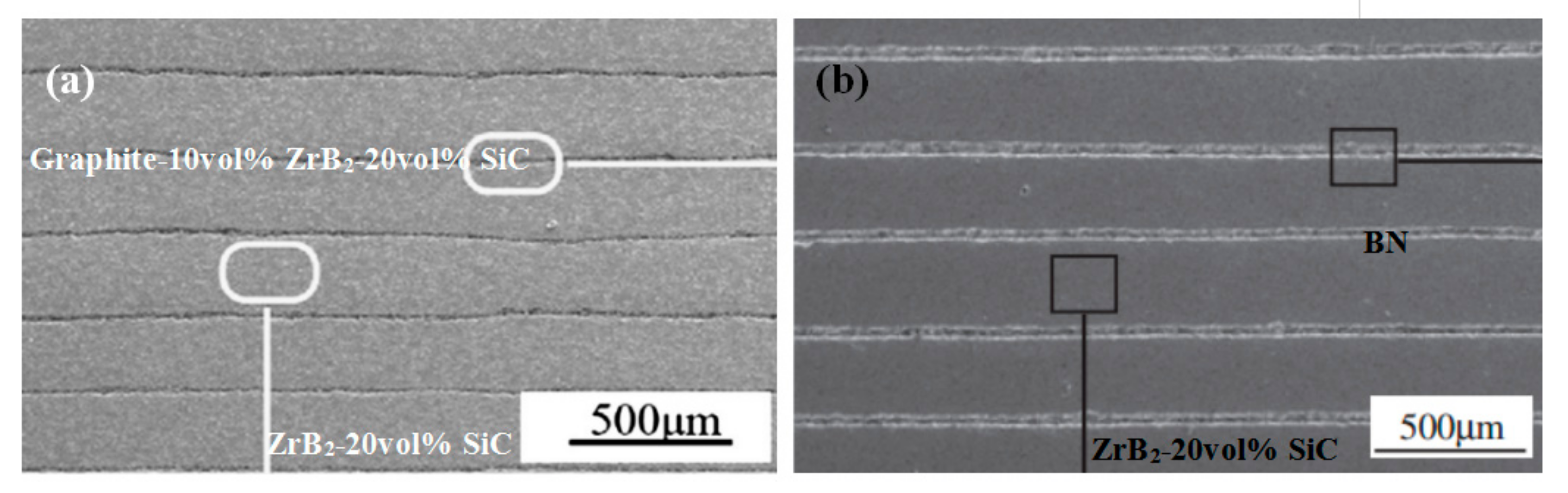

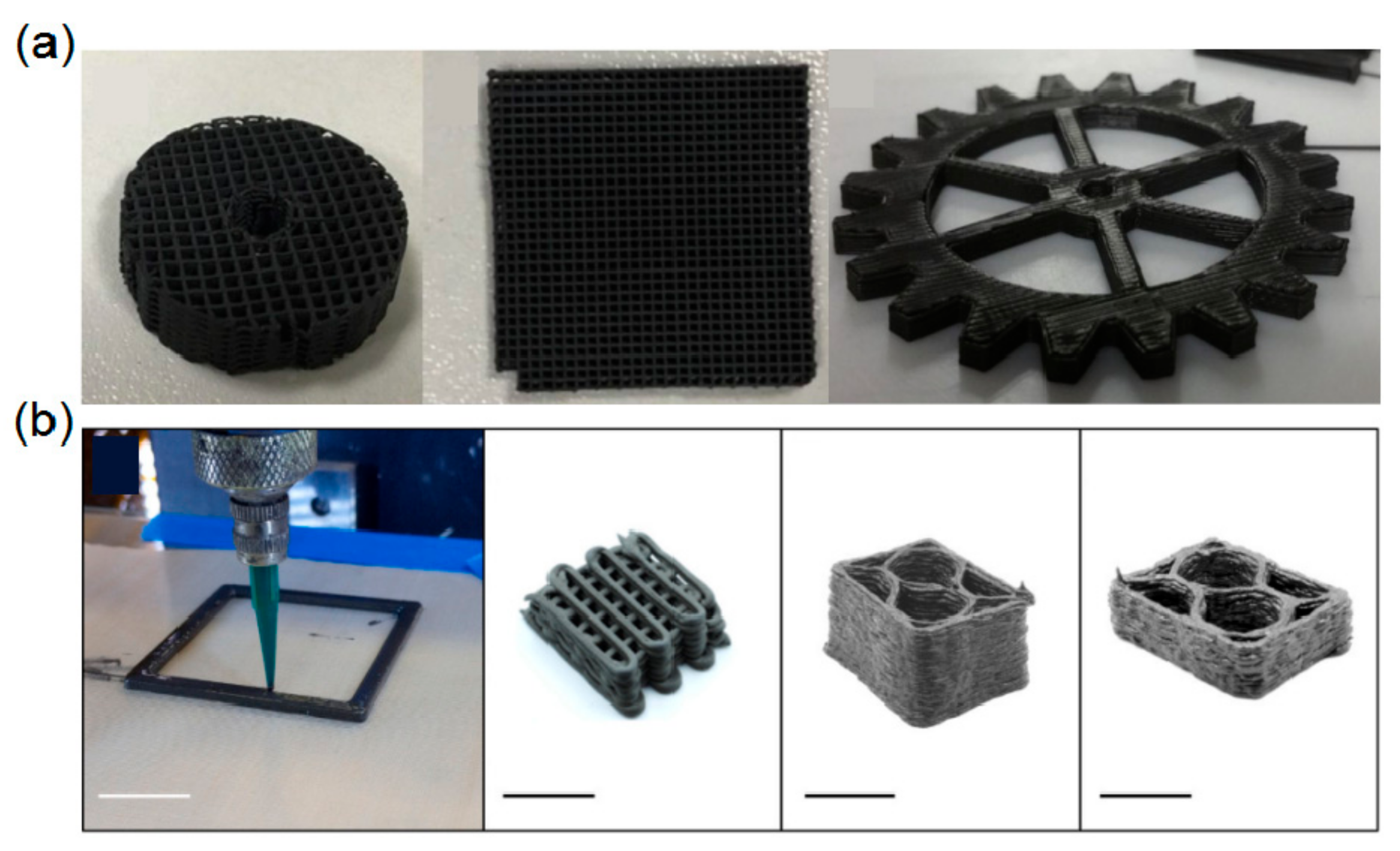
| Powder | Dispersant | Optimum Dispersant Concentration | Optimum pH Value | Medium | Ref. |
|---|---|---|---|---|---|
| Micron-sized ZrB2 | Lopon 885 | 0.4 wt.% a | 9 | Water | [42] |
| Zetasperse 1200 | 4.0 mg/m2 b | 10 | Water | [43] | |
| Dynol607 | 4.0 mg/m2 | 10 | Water | [43] | |
| WA1 | 2.6 mg/m2 | 11 | Water | [43] | |
| Darvan821A | 1.25 mg/m2 | 9 | Water | [43] | |
| PEI10000 | 1.5 wt.% | 11 | Water | [44] | |
| Duramax D3005 | 2.9 wt.% | 6–7 | Water | [45] | |
| Dolapix PC33 | 1.5–2.5 wt.% | 9–10 | Water | [46] | |
| Ammonium Citrate Tribasic | 0.5 wt.% | 9–10 | Water | [47] | |
| SD-07 | 0.60 mg/m2 | 7–12 | Water | [48] | |
| PEI | – | 8 | Water | [41,49] | |
| Blown Menhaden Fish Oil | 1 wt.% | – | Organic solvent | [50] | |
| Nano-sized ZrB2 | PAA3000 | 1 wt.% | 10 | Water | [51] |
| PEI10000 | 0.7 wt.% | 10 | Ethanol | [52] | |
| Gallic acid | 6 wt.% | – | n-butanol | [53,54] |
| Additive Manufacturing Technology | Forming Accuracy |
|---|---|
| Selective laser sintering (SLS) | μm–mm |
| Selective laser melting (SLM) | μm–mm |
| Binder jetting (BJ) | μm–mm |
| Fused deposition modeling (FDM) | mm |
| Laminated object manufacturing (LOM) | mm |
| Robocasting | μm–mm |
| Extrusion-free forming (EFF) | μm–mm |
| Direct ink writing (DIW) | μm–mm |
| Stereolithography (SL) | nm–μm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Yan, C.; Jin, H. Colloidal Processing of Complex-Shaped ZrB2-Based Ultra-High-Temperature Ceramics: Progress and Prospects. Materials 2022, 15, 2886. https://doi.org/10.3390/ma15082886
Liu G, Yan C, Jin H. Colloidal Processing of Complex-Shaped ZrB2-Based Ultra-High-Temperature Ceramics: Progress and Prospects. Materials. 2022; 15(8):2886. https://doi.org/10.3390/ma15082886
Chicago/Turabian StyleLiu, Guoqian, Changhai Yan, and Hua Jin. 2022. "Colloidal Processing of Complex-Shaped ZrB2-Based Ultra-High-Temperature Ceramics: Progress and Prospects" Materials 15, no. 8: 2886. https://doi.org/10.3390/ma15082886
APA StyleLiu, G., Yan, C., & Jin, H. (2022). Colloidal Processing of Complex-Shaped ZrB2-Based Ultra-High-Temperature Ceramics: Progress and Prospects. Materials, 15(8), 2886. https://doi.org/10.3390/ma15082886






