Size and Shape’s Effects on the High-Pressure Behavior of WS2 Nanomaterials
Abstract
:1. Introduction
2. Experimental Section
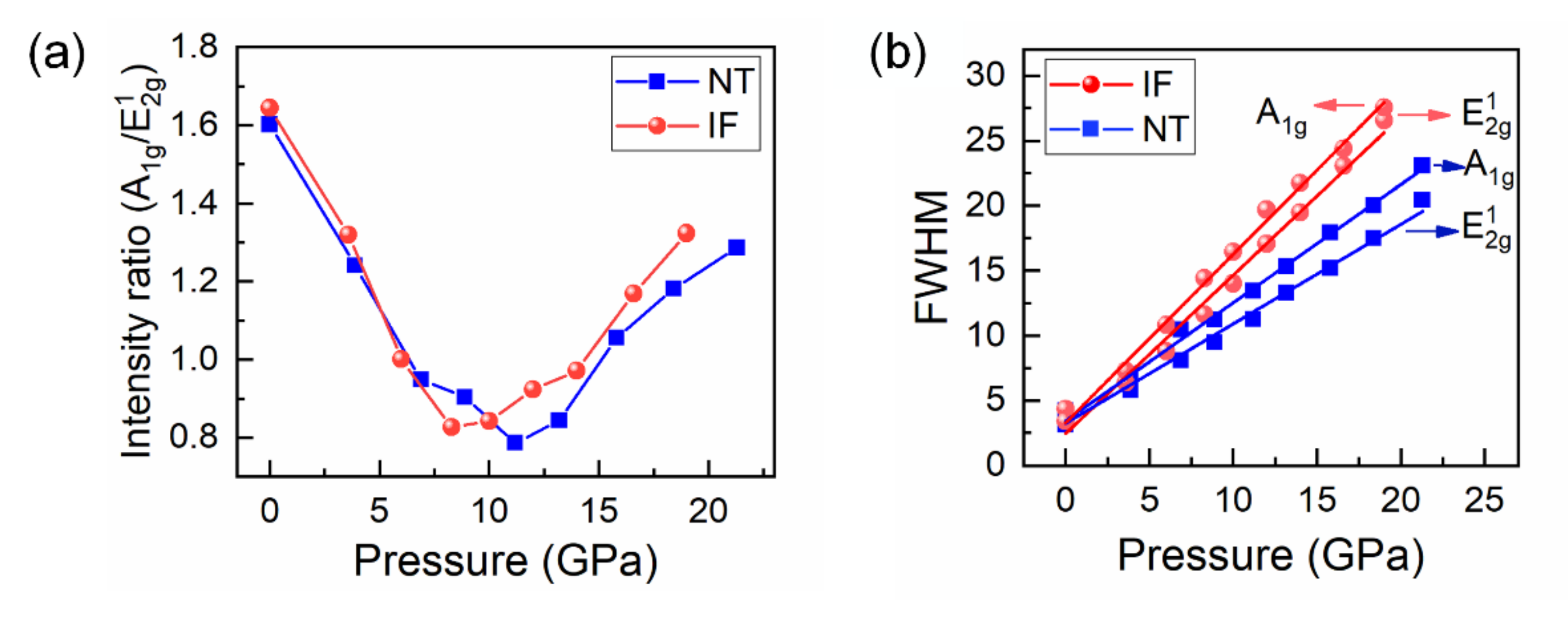
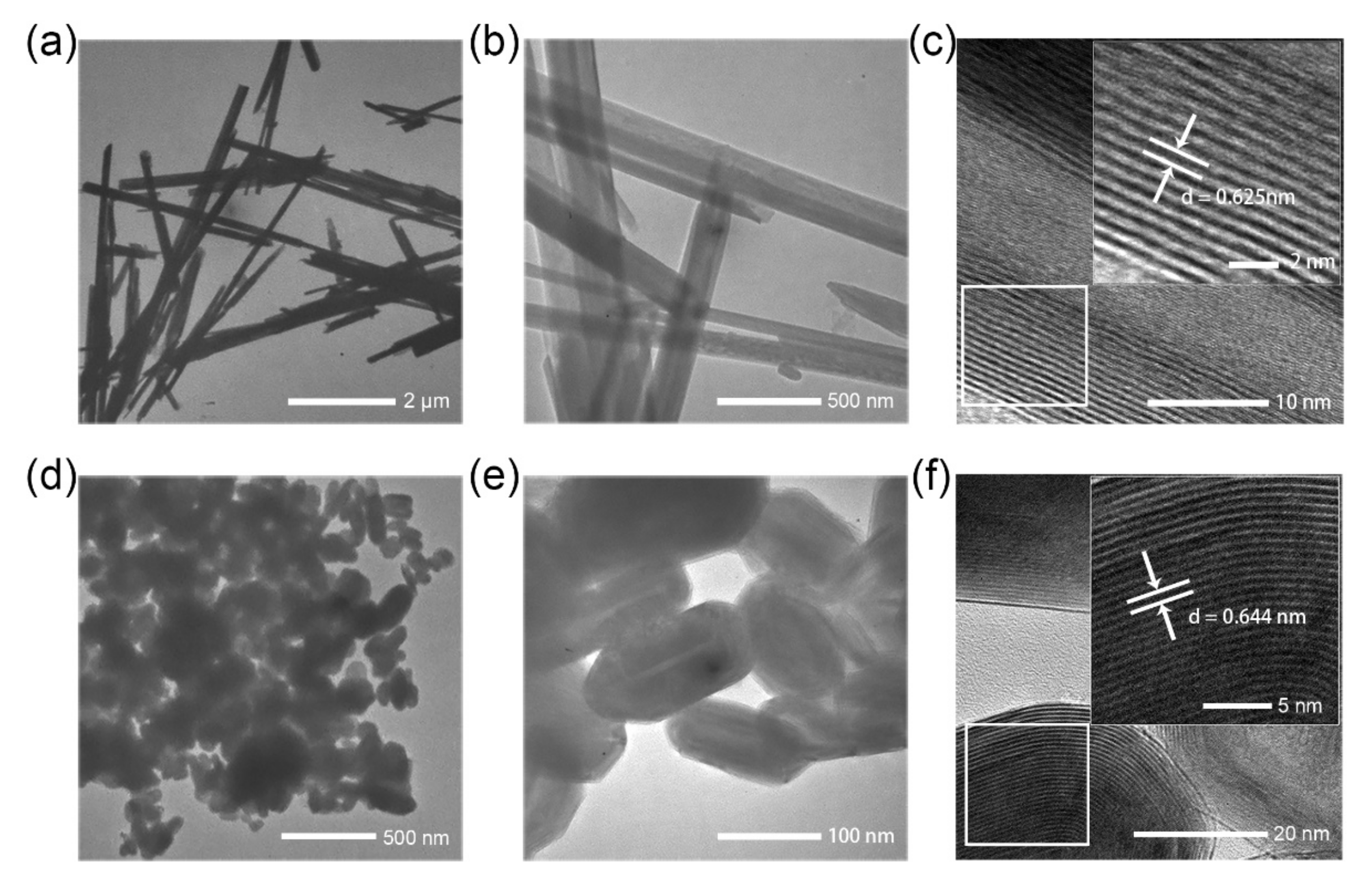
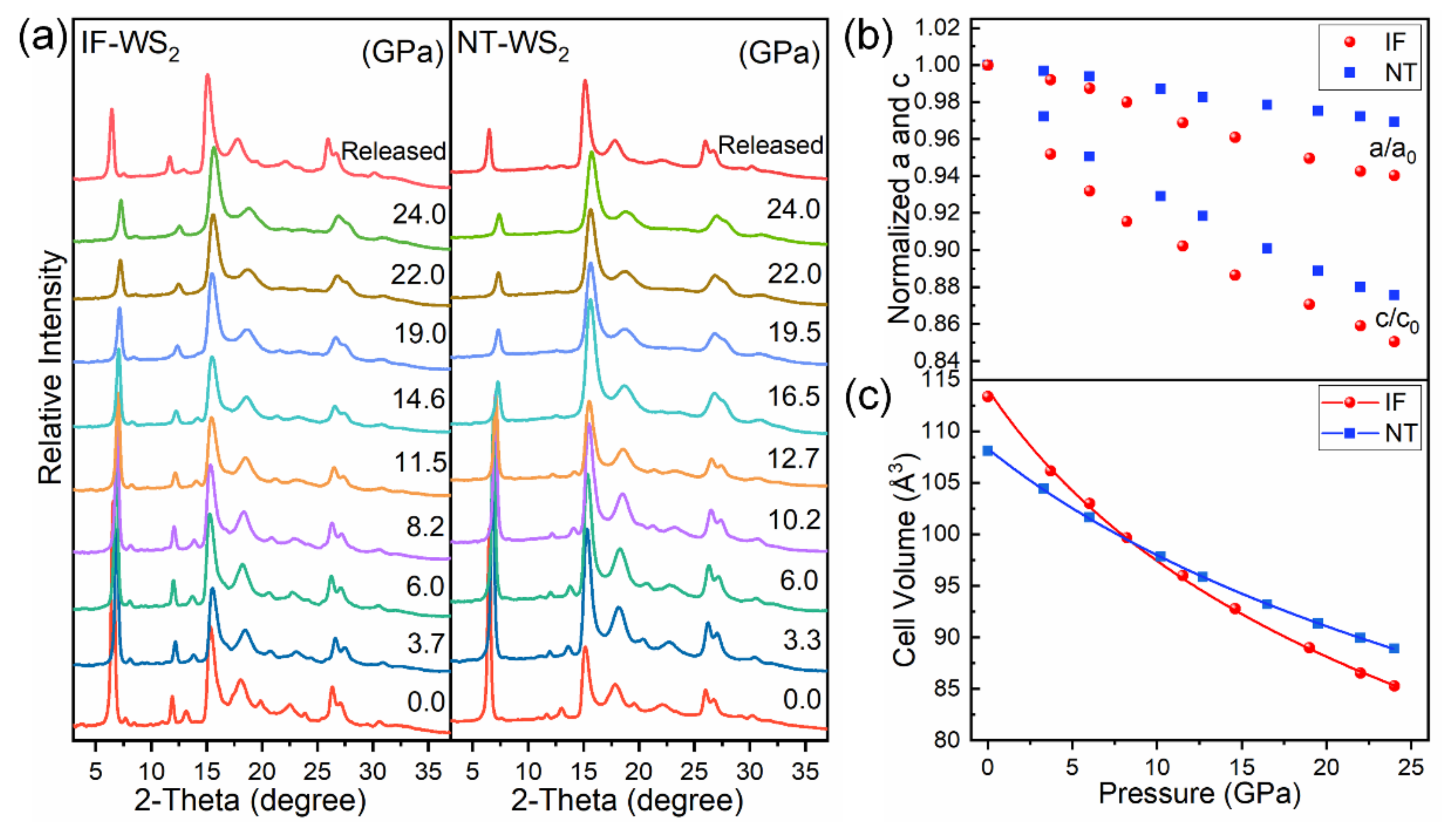
3. Results and Discussion
| Samples | a = b [Å] | c [Å] | V [Å3] | K0 (GPa) | Reference |
|---|---|---|---|---|---|
| Bulk WS2 | 3.138 | 12.416 | 105.870 | 56.7 | Theoretical [33] |
| Bulk WS2 | 3.158 | 12.375 | 107.450 | 63.0 | Experimental [34] |
| NT-WS2 | 3.153 | 12.558 | 108.083 | 81.7 | This work |
| IF-WS2 | 3.190 | 12.861 | 113.350 | 46.3 | This work |
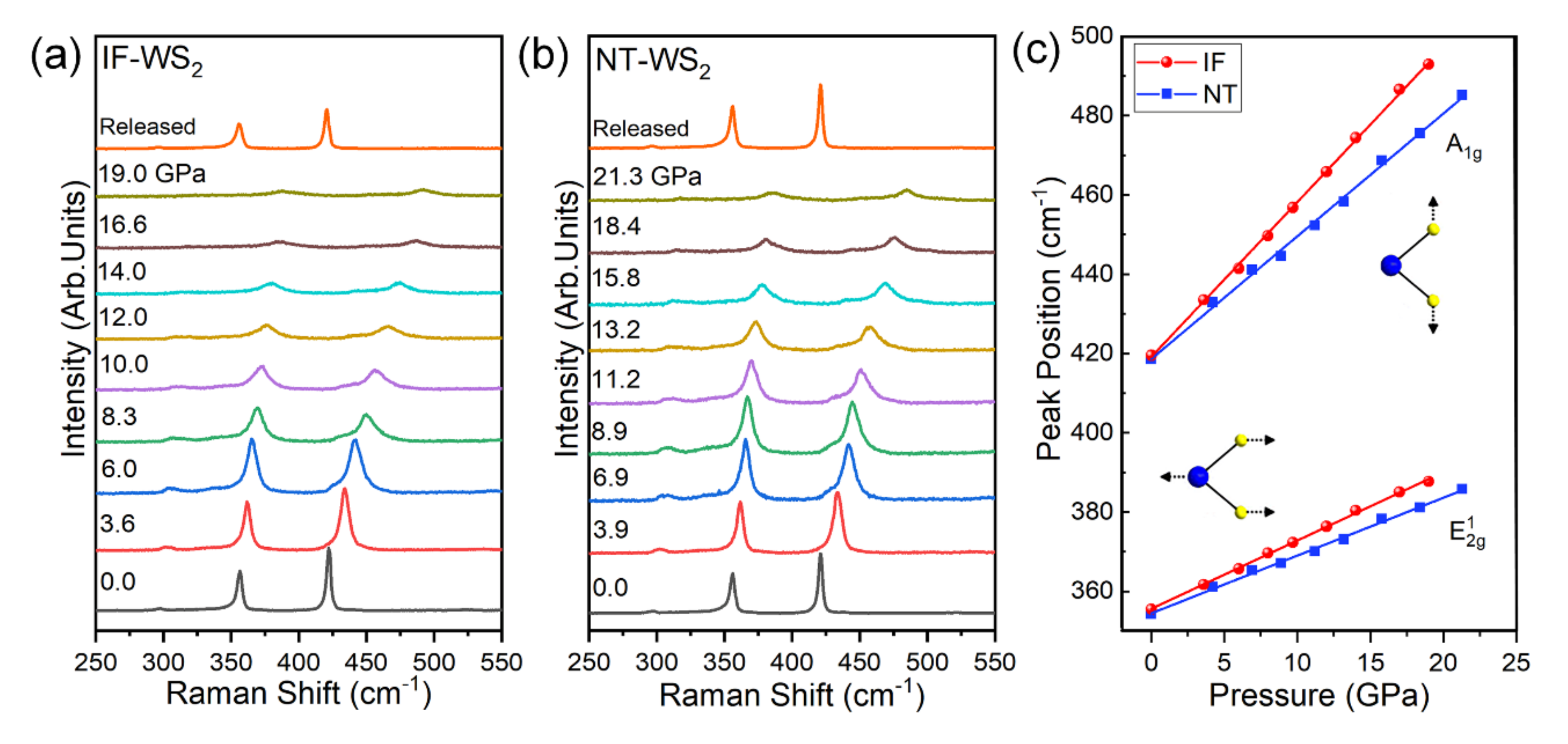
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. TEM Image of the Released NT-WS2 and IF-WS2 after Compression
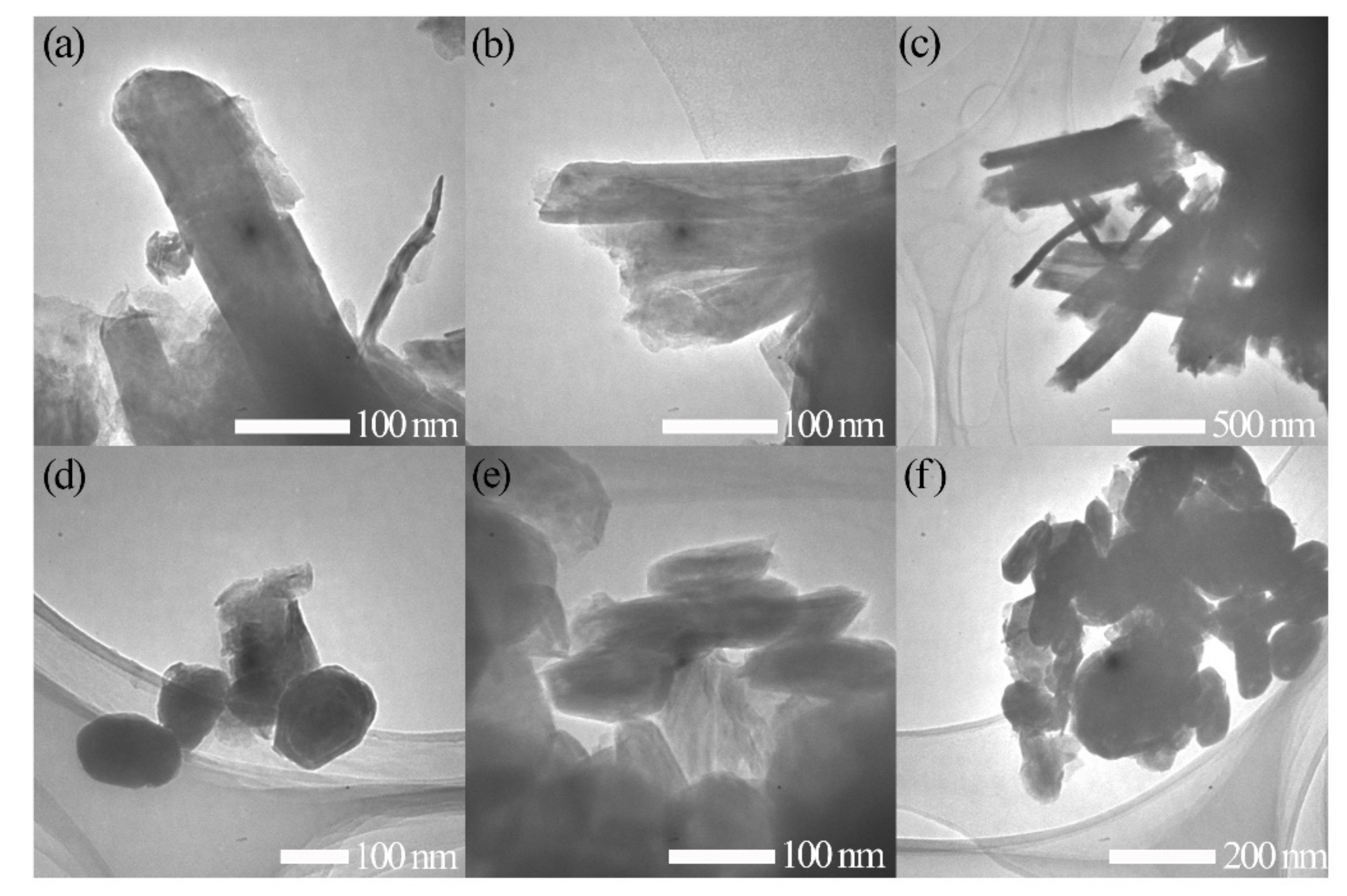
Appendix A.2. Synthesis of NT-WS2 and IF-WS2
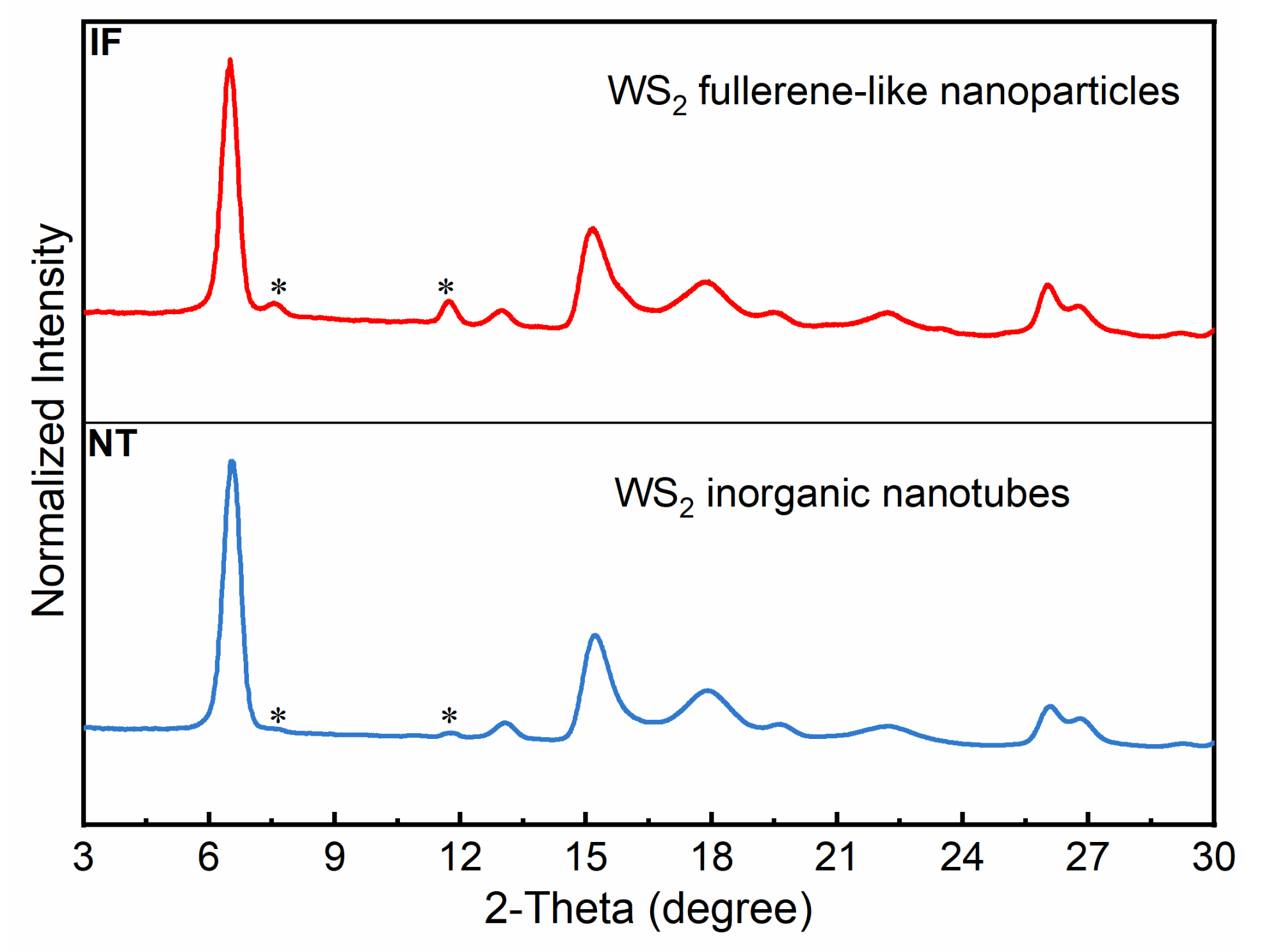
Appendix A.3. XRD Pattern of NT-WS2 and IF-WS2 at Ambient Pressure
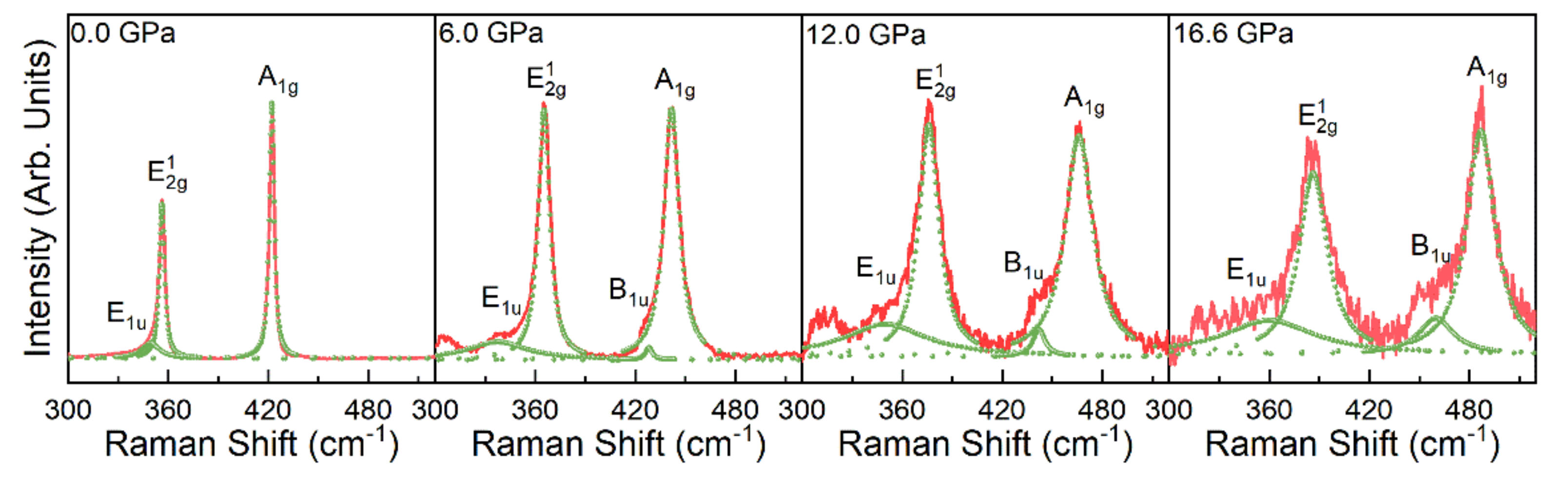
Appendix A.4. Raman Analysis

References
- Bai, F.; Bian, K.; Huang, X.; Wang, Z.; Fan, H. Pressure induced nanoparticle phase behavior, property, and applications. Chem. Rev. 2019, 119, 7673–7717. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lane, J.M.D.; Baca, L.; Tafoya, J.; Ao, T.; Stoltzfus, B.; Knudson, M.; Morgan, D.; Austin, K.; Park, C. Shape dependence of pressure-induced phase transition in CdS semiconductor nanocrystals. J. Am. Chem. Soc. 2020, 142, 6505–6510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, B.; Dong, J.; Li, C.; Dong, Q.; Lin, T.; Liu, R.; Wang, P.; Shen, P.; Li, Q. Size and morphology effects on the high pressure behaviors of Mn3O4 nanorods. Nanoscale Adv. 2020, 2, 5841–5847. [Google Scholar] [CrossRef]
- San-Miguel, A. Nanomaterials under high-pressure. Chem. Soc. Rev. 2006, 35, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Krishnamoorti, R. Nanocomposites: Structure, phase behavior, and properties. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Herhold, A.B.; Johnson, C.S.; Alivisatos, A.P. Size dependence of structural metastability in semiconductor nanocrystals. Science 1997, 276, 398–401. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, B.; Yang, X.; Liu, R.; Liu, B.; Liu, J.; Chen, Z.; Zou, B.; Cui, T.; Liu, B. Morphology-tuned phase transitions of anatase TiO2 nanowires under high pressure. J. Phys. Chem. C 2013, 117, 8516–8521. [Google Scholar] [CrossRef]
- Li, Z.; Liu, B.; Yu, S.; Wang, J.; Li, Q.; Zou, B.; Cui, T.; Liu, Z.; Chen, Z.; Liu, J. The study of structural transition of ZnS nanorods under high pressure. J. Phys. Chem. C 2011, 115, 357–361. [Google Scholar] [CrossRef]
- Dong, Q.; Li, Q.; Li, S.; Shi, X.; Niu, S.; Liu, S.; Liu, R.; Liu, B.; Luo, X.; Si, J. Structural phase transition and superconductivity hierarchy in 1T-TaS2 under pressure up to 100 GPa. npj Quantum Mater. 2021, 6, 20. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Singh, E.; Singh, P.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Flexible molybdenum disulfide (MoS2) atomic layers for wearable electronics and optoelectronics. ACS Appl. Mater. Interfaces 2019, 11, 11061–11105. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wu, Z.; Han, C.; He, J.; Ni, Z.; Chen, W. Two-dimensional transition metal dichalcogenides: Interface and defect engineering. Chem. Soc. Rev. 2018, 47, 3100–3128. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Malic, E. Exciton physics and device application of two-dimensional transition metal dichalcogenide semiconductors. npj 2D Mater. Appl. 2018, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Gong, Z.; Li, D. Nonlinear optics of two-dimensional transition metal dichalcogenides. InfoMat 2019, 1, 317–337. [Google Scholar] [CrossRef] [Green Version]
- Duerloo, K.-A.N.; Li, Y.; Reed, E.J. Structural phase transitions in two-dimensional Mo-and W-dichalcogenide monolayers. Nat. Commun. 2014, 5, 4214. [Google Scholar] [CrossRef] [Green Version]
- Gandi, A.N.; Schwingenschlögl, U. WS2 as an excellent high-temperature thermoelectric material. Chem. Mater. 2014, 26, 6628–6637. [Google Scholar] [CrossRef]
- Ovchinnikov, D.; Allain, A.; Huang, Y.-S.; Dumcenco, D.; Kis, A. Electrical transport properties of single-layer WS2. ACS Nano 2014, 8, 8174–8181. [Google Scholar] [CrossRef]
- Hu, J. Preparation and Optical Absorption Properties of Tungsten Disulfide Nanomaterials. Acta Microsc. 2019, 28, 1459–1466. [Google Scholar]
- Wu, F.; Xia, Y.; Sun, M.; Xie, A. Two-dimensional (2D) few-layers WS2 nanosheets: An ideal nanomaterials with tunable electromagnetic absorption performance. Appl. Phys. Lett. 2018, 113, 052906. [Google Scholar]
- Hu, J.J.; Zabinski, J.S.; Sanders, J.H.; Bultman, J.E.; Voevodin, A.A. Pulsed Laser Syntheses of Layer-Structured WS2 Nanomaterials in Water. J. Phys. Chem. B 2006, 110, 8914–8916. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Zan, X.; Sun, A. Synthesis of WS2 nanosheets by a novel mechanical activation method. Mater. Lett. 2010, 64, 856–858. [Google Scholar] [CrossRef]
- Levi, R.; Bitton, O.; Leitus, G.; Tenne, R.; Joselevich, E. Field-effect transistors based on WS2 nanotubes with high current-carrying capacity. Nano Lett. 2013, 13, 3736–3741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Yang, L.; Liu, Y.; Xu, T.; Ning, Z.; Zak, A.; Zhang, Z.; Tenne, R.; Chen, Q. High-performance photodetectors for visible and near-infrared lights based on individual WS2 nanotubes. Appl. Phys. Lett. 2012, 100, 243101. [Google Scholar] [CrossRef]
- Brown, S.; Musfeldt, J.; Mihut, I.; Betts, J.; Migliori, A.; Zak, A.; Tenne, R. Bulk vs nanoscale WS2: Finite size effects and solid-state lubrication. Nano Lett. 2007, 7, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Sekine, T.; Li, Y.H.; Fay, M.W.; Zhao, Y.M.; Patrick Poa, C.; Wang, W.X.; Roe, M.J.; Brown, P.D.; Fleischer, N. Shock-absorbing and failure mechanisms of WS2 and MoS2 nanoparticles with fullerene-like structures under shock wave pressure. J. Am. Chem. Soc. 2005, 127, 16263–16272. [Google Scholar] [CrossRef]
- O’Neal, K.R.; Cherian, J.; Zak, A.; Tenne, R.; Liu, Z.; Musfeldt, J. High pressure vibrational properties of WS2 nanotubes. Nano Lett. 2016, 16, 993–999. [Google Scholar] [CrossRef]
- Zak, A.; Sallacan-Ecker, L.; Margolin, A.; Feldman, Y.; Popovitz-Biro, R.; Albu-Yaron, A.; Genut, M.; Tenne, R. Scaling up of the WS2 nanotubes synthesis. Fuller. Nanotub. Carbon Nanostruct. 2010, 19, 18–26. [Google Scholar] [CrossRef]
- Feldman, Y.; Frey, G.; Homyonfer, M.; Lyakhovitskaya, V.; Margulis, L.; Cohen, H.; Hodes, G.; Hutchison, J.; Tenne, R. Bulk synthesis of inorganic fullerene-like MS2 (M= Mo, W) from the respective trioxides and the reaction mechanism. J. Am. Chem. Soc. 1996, 118, 5362–5367. [Google Scholar] [CrossRef]
- Mao, H.; Bell, P.; Shaner, J.T.; Steinberg, D. Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R 1 fluorescence pressure gauge from 0.06 to 1 Mbar. J. Appl. Phys. 1978, 49, 3276–3283. [Google Scholar] [CrossRef]
- Ross, N.L. The equation of state and high-pressure behavior of magnesite. Am. Mineral. 1997, 82, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Piermarini, G.J.; Block, S.; Barnett, J.; Forman, R. Calibration of the pressure dependence of the R1 ruby fluorescence line to 195 kbar. J. Appl. Phys. 1975, 46, 2774–2780. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Tenne, R. Inorganic nanotubes and fullerene-like nanoparticles. J. Mater. Res. 2006, 21, 2726–2743. [Google Scholar] [CrossRef]
- Katsura, T.; Tange, Y. A simple derivation of the Birch-Murnaghan equations of state (EOSs) and comparison with EOSs derived from other definitions of finite strain. Minerals 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zeng, Z.-Y.; Liang, T.; Tang, M.; Cheng, Y. Elastic Properties and Electronic Structure of WS2 under Pressure from First-principles Calculations. Z. Nat. A 2017, 72, 295–301. [Google Scholar] [CrossRef]
- Bandaru, N.; Kumar, R.S.; Baker, J.; Tschauner, O.; Hartmann, T.; Zhao, Y.; Venkat, R. Structural stability of WS2 under high pressure. .Int. J. Mod. Phys. 2014, 28, 1450168. [Google Scholar] [CrossRef]
- Del Corro, E.; de la Roza, A.O.; Taravillo, M.; Baonza, V.G. Raman modes and Grüneisen parameters of graphite under compressive biaxial stress. Carbon 2012, 50, 4600–4606. [Google Scholar] [CrossRef]
- Proctor, J.E.; Gregoryanz, E.; Novoselov, K.S.; Lotya, M.; Coleman, J.N.; Halsall, M.P. High-pressure Raman spectroscopy of graphene. Phys. Rev. B 2009, 80, 073408. [Google Scholar] [CrossRef] [Green Version]
- Livneh, T.; Sterer, E. Resonant Raman scattering at exciton states tuned by pressure and temperature in 2H-MoS2. Phys. Rev. B 2010, 81, 195209. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Martin, J.-M.; Dassenoy, F.; Belin, M.; Montagnac, G.; Reynard, B.; Fleischer, N. Pressure-induced exfoliation of inorganic fullerene-like WS2 particles in a Hertzian contact. J. Appl. Phys. 2006, 99, 023524. [Google Scholar] [CrossRef]
- Yu, S.D.; Chang, L.X.; Yang, H.B.; Liu, B.B.; Hou, Y.Y.; Wang, L.; Yao, M.G.; Cui, T.; Zou, G.T. Study of the hydrostatic pressure dependence of the Raman spectrum of W/WS2 fullerene-like nanosphere with core-shell structure. J. Phys. Condens. Matter 2007, 19, 425228. [Google Scholar] [CrossRef]
- Qadri, S.B.; Yang, J.; Ratna, B.; Skelton, E.F.; Hu, J. Pressure induced structural transitions in nanometer size particles of PbS. Appl. Phys. Lett. 1996, 69, 2205–2207. [Google Scholar] [CrossRef]
- Tolbert, S.; Alivisatos, A. Size dependence of a first order solid-solid phase transition: The wurtzite to rock salt transformation in CdSe nanocrystals. Science 1994, 265, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Xu, D.; Wei, Z.; Zhao, T.; Lin, T.; Tenne, R.; Zak, A.; Li, Q.; Liu, B. Size and Shape’s Effects on the High-Pressure Behavior of WS2 Nanomaterials. Materials 2022, 15, 2838. https://doi.org/10.3390/ma15082838
Yue L, Xu D, Wei Z, Zhao T, Lin T, Tenne R, Zak A, Li Q, Liu B. Size and Shape’s Effects on the High-Pressure Behavior of WS2 Nanomaterials. Materials. 2022; 15(8):2838. https://doi.org/10.3390/ma15082838
Chicago/Turabian StyleYue, Lei, Dan Xu, Ziyu Wei, Tingting Zhao, Tao Lin, Reshef Tenne, Alla Zak, Quanjun Li, and Bingbing Liu. 2022. "Size and Shape’s Effects on the High-Pressure Behavior of WS2 Nanomaterials" Materials 15, no. 8: 2838. https://doi.org/10.3390/ma15082838
APA StyleYue, L., Xu, D., Wei, Z., Zhao, T., Lin, T., Tenne, R., Zak, A., Li, Q., & Liu, B. (2022). Size and Shape’s Effects on the High-Pressure Behavior of WS2 Nanomaterials. Materials, 15(8), 2838. https://doi.org/10.3390/ma15082838









