The New High-Pressure Phases of Nitrogen-Rich Ag–N Compounds
Abstract
:1. Introduction
2. Computation Details
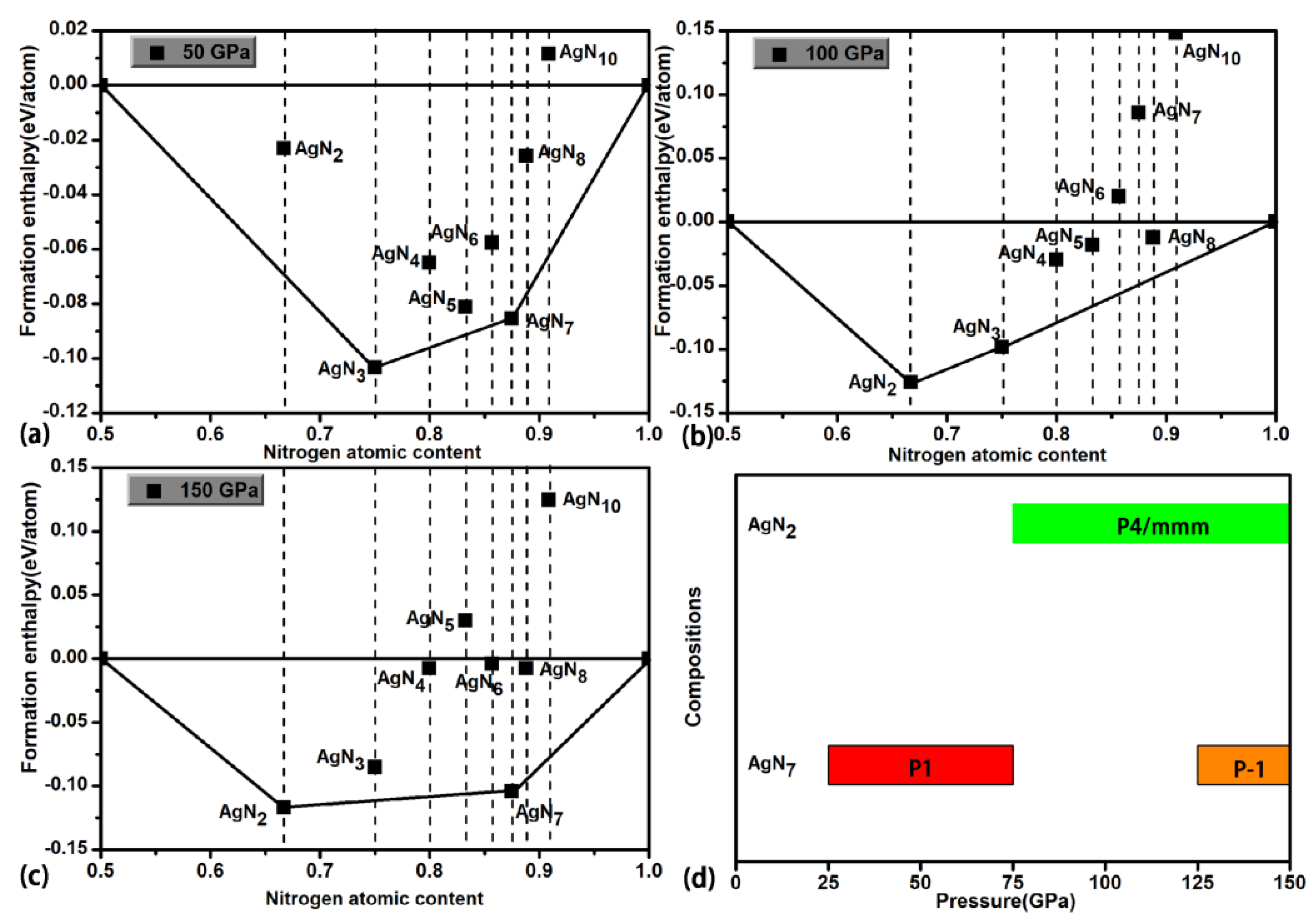
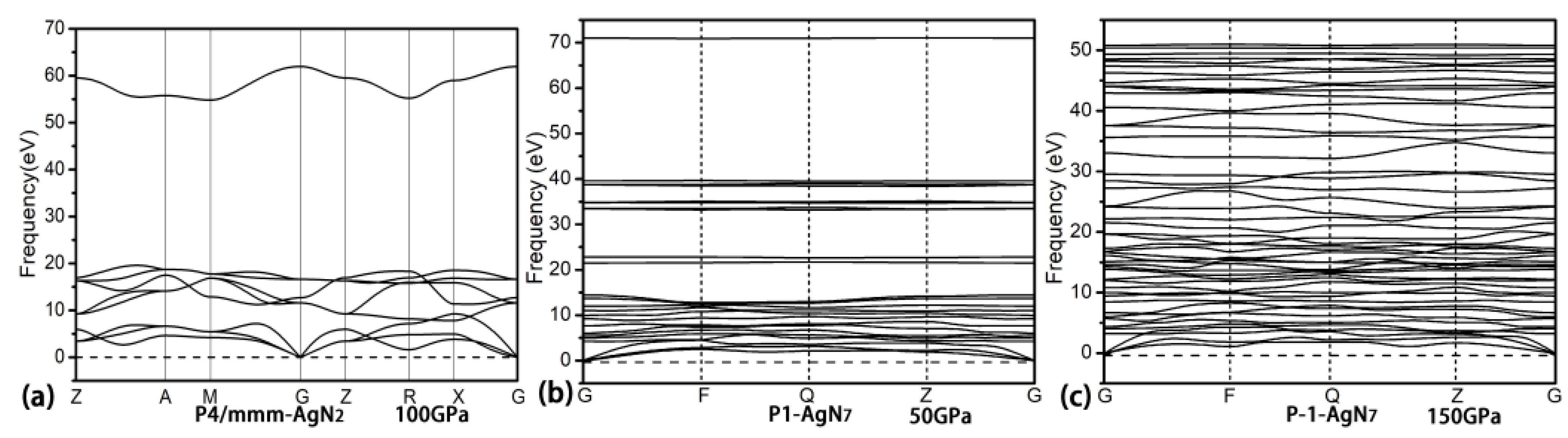
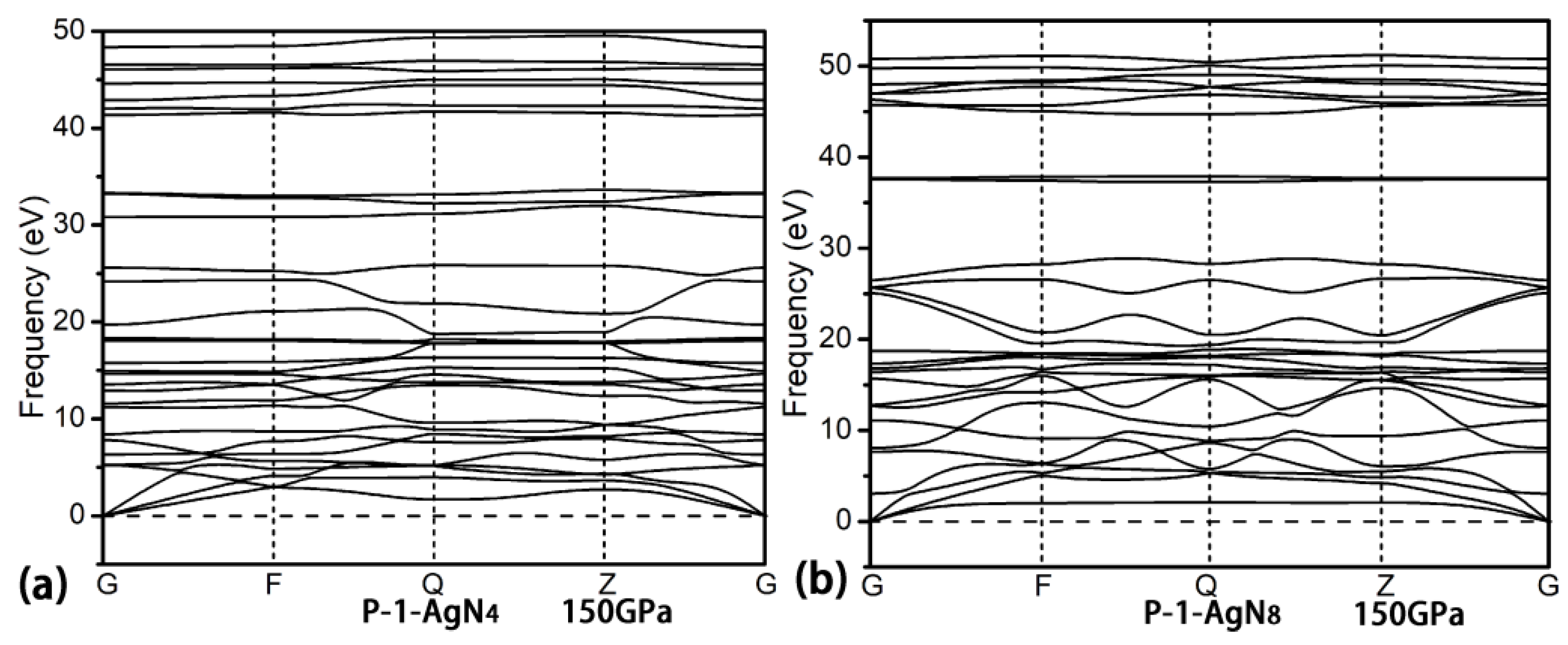
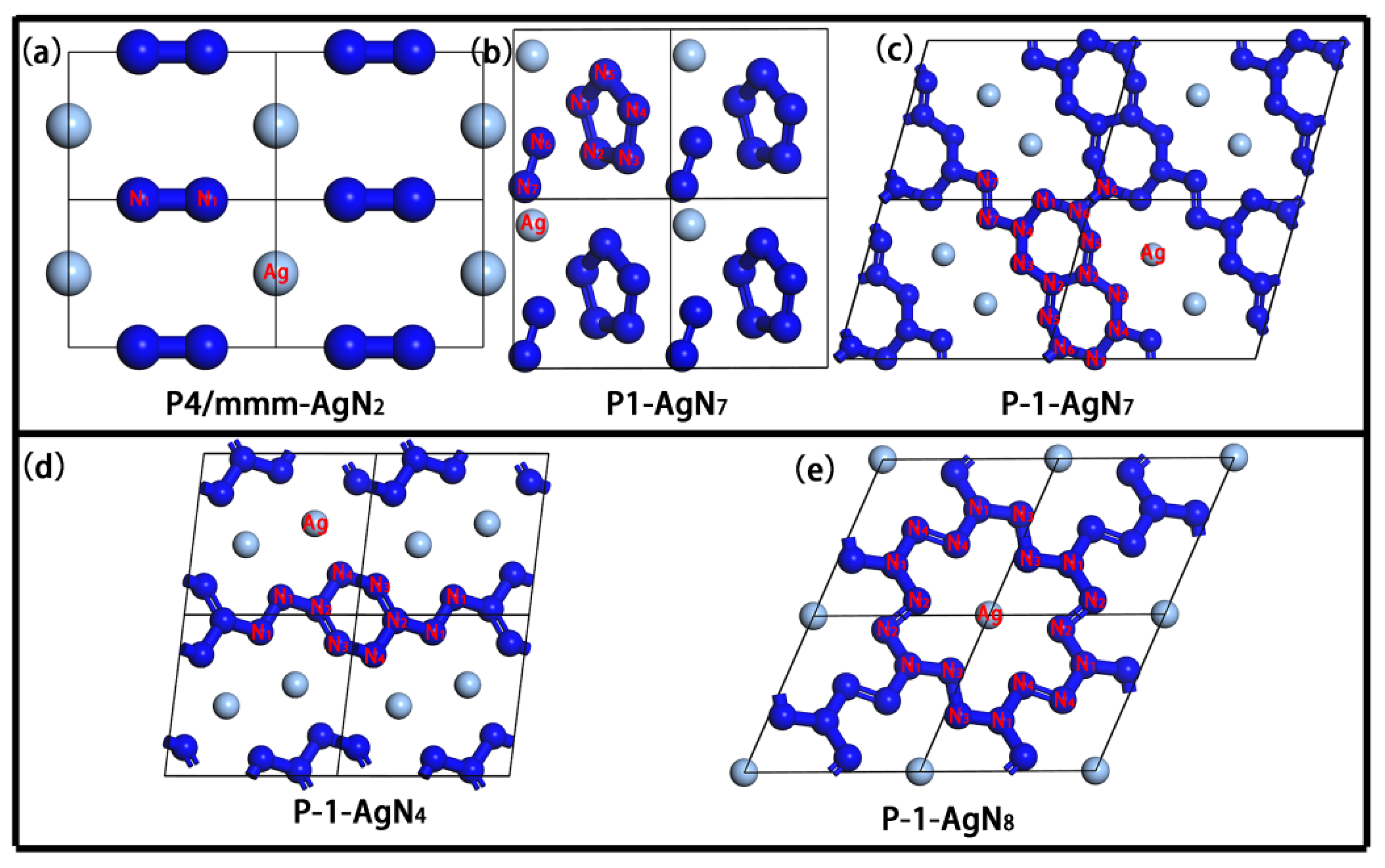
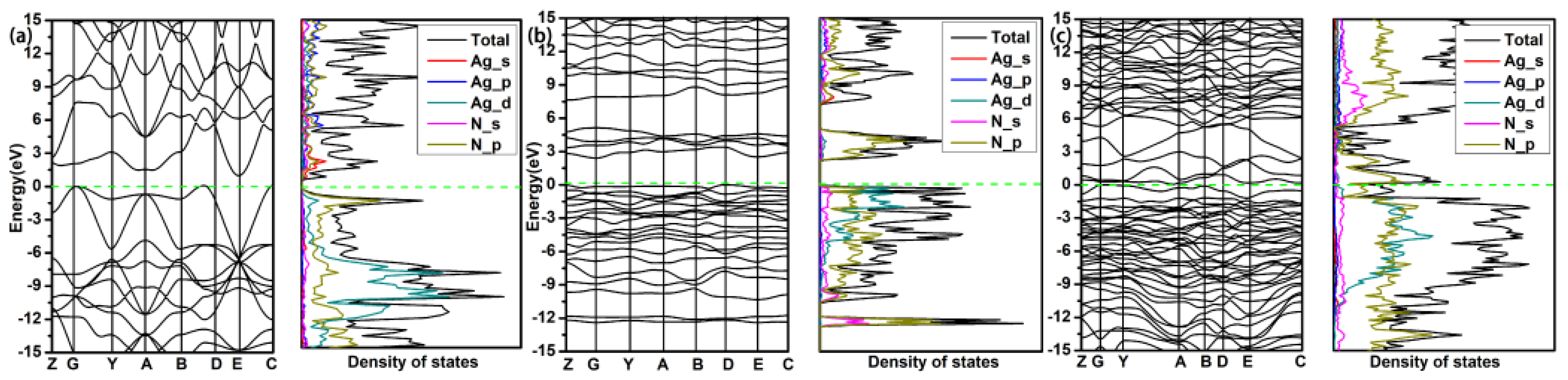
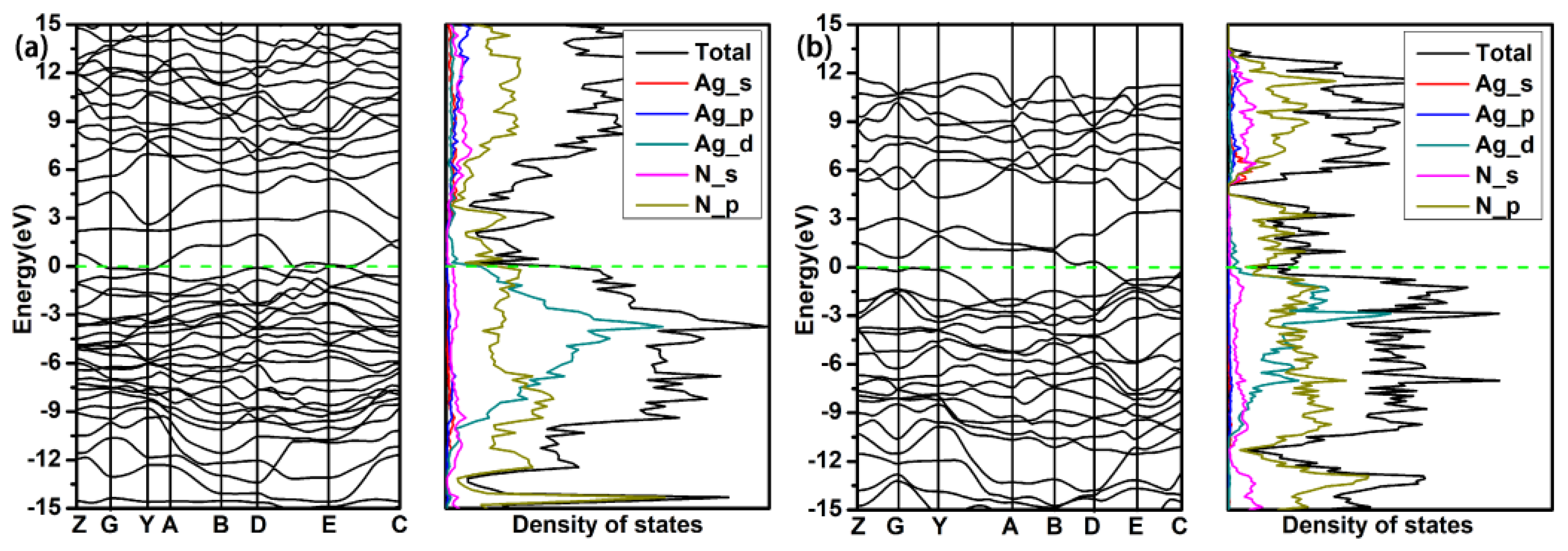
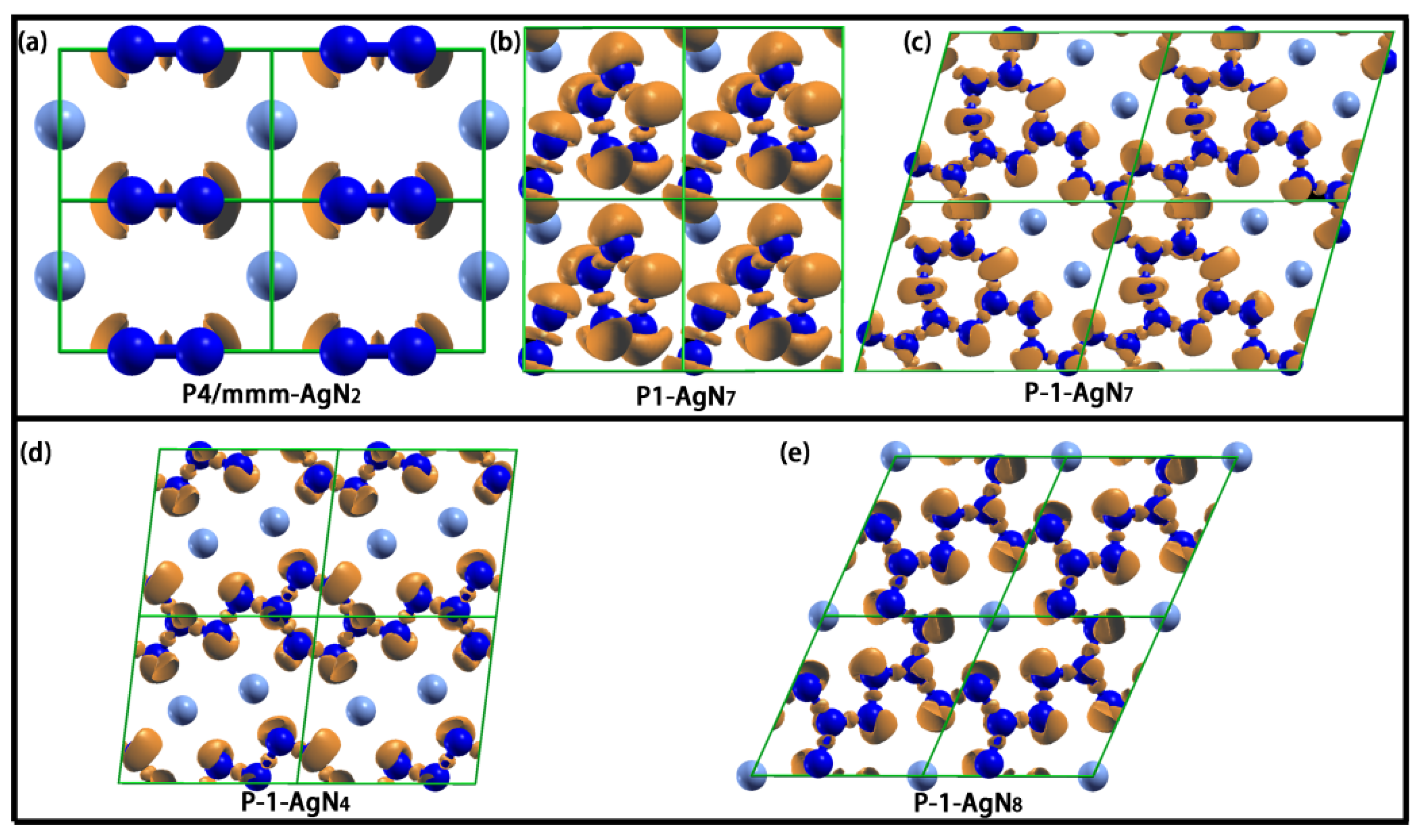
3. Results and Discussion
C33C55-C352 > 0, C44C66-C462 > 0, C22 + C33-2C23 > 0,
C22 (C33C55-C352) + 2C23C25C35-(C232) C55-(C252) C33 > 0,
2 [C15C25 (C33C12-C13C23) + C15C35 (C22C13-C12C23) + C25C35 (C11C23-C12C13)]-[C152 (C22C33-C232) + C25C25 (C11C33-C132) + C35C35 (C11C22-C122)] + C55g > 0.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.L.; Tian, F.B.; Wang, L.C.; Cui, T.; Liu, B.B.; Zou, G.T. Structural stability of polymeric nitrogen: A first-principles investigation. J. Chem. Phys. 2010, 132, 024502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; He, Z.; Ma, Y.M.; Cui, T.; Liu, Z.M.; Liu, B.B.; Li, J.F.; Zou, G.T. Prediction of a new layered phase of nitrogen from first-principles simulations. J. Phys. Condens. Matter. 2007, 19, 425226. [Google Scholar] [CrossRef]
- Alemany, M.M.G.; Martins, J.L. Density-functional study of nonmolecular phases of nitrogen: Metastable phase at low pressure. Phys. Rev. B 2003, 68, 024110. [Google Scholar] [CrossRef]
- Ma, Y.M.; Oganov, A.R.; Li, Z.W.; Yu, X.; Kotakoski, J. Novel high pressure structures of polymeric nitrogen. Phys. Rev. Lett. 2009, 102, 065501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahariev, F.; Hu, A.; Hooper, J.; Zhang, F.; Woo, T. Layered single-bonded nonmolecular phase of nitrogen from first-principles simulation. Phys. Rev. B 2005, 72, 214108. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, Y.C.; Miao, M.S.; Zhong, X.; Lv, J.; Cui, T.; Li, J.F.; Chen, L.; Pickard, C.J.; Ma, Y.M. Cagelike diamondoid nitrogen at high pressures. Phys. Rev. Lett. 2012, 109, 175502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, C.J.; Needs, R.J. High-pressure phases of nitrogen. Phys. Rev. Lett. 2009, 102, 125702. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Yin, Y.Y.; Pang, Z.K. Predicted new structures of polymeric nitrogen under 100–600 GPa. Comput. Mater. Sci. 2015, 98, 399–404. [Google Scholar] [CrossRef]
- Zahariev, F.; Hooper, J.; Alavi, S.; Zhang, F.; Woo, T.K. Low-pressure metastable phase of single-bonded polymeric nitrogen from a helical structure motif and first-principles calculations. Phys. Rev. B 2007, 75, 140101. [Google Scholar] [CrossRef]
- Mattson, W.D.; Sanchez-Portal, D.; Chiesa, S.; Martin, R.M. Prediction of new phases of nitrogen at high pressure from first-principles simulations. Phys. Rev. Lett. 2004, 93, 125501. [Google Scholar] [CrossRef]
- Greschner, M.J.; Zhang, M.; Majumdar, A.; Liu, H.Y.; Peng, F.; Tse, J.S.; Yao, Y.S. A New Allotrope of Nitrogen as High-Energy Density Material. J. Phys. Chem. A 2016, 120, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, B.; Gerber, R.B.; Krylov, A.I. Calculations predict a stable molecular crystal of N8. Nat. Chem. 2014, 6, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.M.; Liu, H.Y.; Gan, Y.D.; Kuang, A.; Tian, C.L. A novel low-pressure structure of polymeric nitrogen. Chin. Sci. Bull. 2021, 66, 2908–2914. [Google Scholar] [CrossRef]
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A.; Dzivenko, D.A.; Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 2004, 3, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, D.; Kim, M.; Smith, J.; Yoo, C.S. Pressure-induced symmetry-lowering transition in dense nitrogen to layered polymeric nitrogen (LP-N) with colossal Raman intensity. Phys. Rev. Lett. 2014, 113, 205502. [Google Scholar] [CrossRef] [Green Version]
- Laniel, D.; Geneste, G.; Weck, G.; Mezouar, M.; Loubeyre, P. Hexagonal Layered Polymeric Nitrogen Phase Synthesized near 250 GPa. Phys. Rev. Lett. 2019, 122, 066001. [Google Scholar] [CrossRef]
- Ji, C.; Adeleke, A.A.; Yang, L.X.; Wang, B.; Gou, H.Y.; Yao, Y.S.; Li, B.; Meng, Y.; Smith, J.S.; Prakapenda, V.B.; et al. Nitrogen in black phosphorus structure. Sci. Adv. 2020, 6, edba9206. [Google Scholar] [CrossRef]
- Laniel, D.; Winkler, B.; Fedotenko, T.; Pakhomova, A.; Chariton, S.; Milman, V.; Prakapenka, V.; Dubrovinsky, L.; Dubrovinskaia, N. High-Pressure Polymeric Nitrogen Allotrope with the Black Phosphorus Structure. Phys. Rev. Lett. 2020, 124, 216001. [Google Scholar] [CrossRef]
- Niu, S.; Xu, D.; Li, H.Y.; Yao, Z.; Liu, S.; Zhai, C.G.; Hu, K.; Shi, X.H.; Wang, P.; Liu, B.B. Pressure-stabilized polymerization of nitrogen in manganese nitrides at ambient and high pressures. Phys. Chem. Chem. Phys. 2022, 24, 5738–5747. [Google Scholar] [CrossRef]
- Du, H.F.; Ge, Y.F.; Zhu, J.L.; Guo, W.; Yao, Y.G. Pressure-induced novel nitrogen-rich aluminum nitrides: AlN6, Al2N7 and AlN7 with polymeric nitrogen chains and rings. Phys. Chem. Chem. Phys. 2021, 23, 12350–12359. [Google Scholar] [CrossRef]
- Bondarchuk, S.V. Recoverability of N4x– Anions to Ambient Pressure: A First Principles Study of cyclo- and syn-Tetranitrogen Units. J. Phys. Chem. C 2021, 125, 7368–7377. [Google Scholar] [CrossRef]
- Li, L.; Bao, K.; Zhao, X.B.; Cui, T. Bonding Properties of Manganese Nitrides at High Pressure and the Discovery of MnN4 with Planar N4 Rings. J. Phys. Chem. C 2021, 125, 24605–24612. [Google Scholar] [CrossRef]
- Peng, F.; Yao, Y.S.; Liu, H.Y.; Ma, Y.M. Crystalline LiN5 Predicted from First Principles as a Possible High-Energy Material. J. Phys. Chem. Lett. 2015, 6, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.A.; Oleynik, I.I. Sodium pentazolate: A nitrogen rich high energy density material. Chem. Phys. Lett. 2016, 643, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.S.; Steele, B.A.; Oleynik, I.I. Novel rubidium poly-nitrogen materials at high pressure. J. Chem. Phys. 2017, 147, 234701. [Google Scholar] [CrossRef]
- Peng, F.; Han, Y.X.; Liu, H.Y.; Yao, Y.S. Exotic stable cesium polynitrides at high pressure. Sci. Rep. 2015, 5, 16902. [Google Scholar] [CrossRef]
- Zhu, S.S.; Peng, F.; Liu, H.Y.; Majumdar, A.; Gao, T.; Yao, Y.S. Stable Calcium Nitrides at Ambient and High Pressures. Inorg. Chem. 2016, 55, 7550–7555. [Google Scholar] [CrossRef]
- Wei, S.L.; Liu, Y.; Li, D.; Liu, Z.; Wang, W.J.; Tian, F.B.; Bao, K.; Duan, D.F.; Liu, B.B.; Cui, T. Pressure-Stabilized Polymerization of Nitrogen in Alkaline-Earth Metal Strontium Nitrides. Phys. Chem. Chem. Phys. 2020, 22, 5242. [Google Scholar] [CrossRef]
- Li, J.F.; Sun, L.; Wang, X.L.; Zhu, H.Y.; Miao, M.S. Simple Route to Metal cyclo-N5–Salt: High-Pressure Synthesis of CuN5. J. Chem. Phys. C 2018, 122, 22339–22344. [Google Scholar] [CrossRef]
- Xia, K.; Zheng, X.X.; Yuan, J.N.; Liu, C.; Gao, H.; Wu, Q.; Sun, J. Pressure-Stabilized High-Energy-Density Alkaline-Earth-Metal Pentazolate Salts. J. Phys. Chem. C 2019, 123, 10205–10211. [Google Scholar] [CrossRef]
- Huang, B.W.; Frapper, G. Barium–Nitrogen Phases under Pressure: Emergence of Structural Diversity and Nitrogen-Rich Compound. Chem. Mater. 2018, 30, 7623–7636. [Google Scholar] [CrossRef]
- Xia, K.; Yuan, J.N.; Zheng, X.X.; Liu, C.; Gao, H.; Wu, Q.; Sun, J. Predictions on High-Power Trivalent Metal Pentazolate Salts. J. Phys. Chem. Lett. 2019, 10, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.W.; Wang, B.S.; Wu, S.H.; Guégan, F.; Hu, W.Y.; Frapper, G. Predicted Polymeric and Layered Covalent Networks in Transition Metal Pentazolate M(cyclo-N5)x Phases at Ambient and High Pressure: Up to 20 Nitrogen Atoms per Metal. Chem. Mater. 2021, 33, 5298–5307. [Google Scholar] [CrossRef]
- Zhang, M.G.; Yan, H.Y.; Wei, Q.; Liu, H.Y. A new high-pressure polymeric nitrogen phase in potassium azide. RSC Adv. 2015, 5, 11825–11830. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, Z.; Lin, H.Q.; Li, Y.L. Pressure-induced planar N6 rings in potassium azide. Sci. Rep. 2014, 4, 4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.Y.; Huang, B.W.; Zeng, Q.F.; Oganov, A.R.; Zhang, L.T.; Frapper, G. Emergence of Novel Polynitrogen Molecule-like Species, Covalent Chains, and Layers in Magnesium–Nitrogen MgxNy Phases under High Pressure. J. Phys. Chem. C 2017, 121, 11037–11046. [Google Scholar] [CrossRef]
- Wei, S.L.; Li, D.; Liu, Z.; Li, X.; Tian, F.B.; Duan, D.F.; Liu, B.B.; Cui, T. Alkaline earth metal (Mg) polynitrides at high pressure as possible high-energy materials. Phys. Chem. Chem. Phys. 2017, 19, 9246–9252. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.G.; Lian, L.L.; Cai, Y.M.; Liu, B.; Wang, B.; Wei, S.L.; Li, D. Structural phase transition and bonding properties of high-pressure polymeric CaN3. RSC Adv. 2018, 8, 4314–4320. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Sha, L.; Zhu, C.; Yao, Y.S. New multifunctional tungsten nitride with energetic N6 and extreme hardness predicted from first principles. EPL 2017, 118, 46001. [Google Scholar] [CrossRef]
- Xia, K.; Gao, H.; Liu, C.; Yuan, J.N.; Sun, J.; Wang, H.-T.; Xing, D.Y. A novel superhard tungsten nitride predicted by machine-learning accelerated crystal structure search. Sci. Bull. 2018, 63, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.T.; Zhao, Z.Y.; Liu, L.L.; Yang, G.C. Pressure-induced stable BeN4 as a high energy density material. J. Power Sources 2017, 365, 155–161. [Google Scholar] [CrossRef]
- Zhang, X.J.; Xie, X.; Dong, H.F.; Zhang, X.; Wu, F.G.; Mu, Z.F.; Wen, M.R. Pressure-Induced High-Energy-Density BeN4 Materials with Nitrogen Chains: First Principles Study. J. Phys. Chem. C 2021, 125, 25376–25382. [Google Scholar] [CrossRef]
- Niu, S.; Li, Z.H.; Li, H.Y.; Shi, X.H.; Yao, Z.; Liu, B.B. New Cadmium-Nitrogen Compounds at High Pressures. Inorg. Chem. 2021, 60, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Tian, R.F.; Wan, B.; Liu, H.Y.; Gong, N.; Chen, P.; Shen, T.; Yao, Y.S.; Gou, H.Y.; Gao, F. Prediction of Stable Iron Nitrides at Ambient and High Pressures with Progressive Formation of New Polynitrogen Species. Chem. Mater. 2018, 30, 8476–8485. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, D.H.; Zhang, S.T.; Zhang, H.J. Pressure-stabilized GdN6 with an armchair–antiarmchair structure as a high energy density material. J. Mater. Chem. A 2021, 9, 16751–16758. [Google Scholar] [CrossRef]
- Aydin, S.; Ciftci, Y.O.; Tatar, A. Superhard transition metal tetranitrides: XN4(X. = Re, Os, W). J. Mater. Res. 2012, 27, 1705–1715. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wu, L.L.; Wan, B.; Lin, Y.Z.; Hu, Q.Y.; Zhao, Y.; Gao, R.; Li, Z.P.; Zhang, J.W.; Gou, H.Y. Diverse ruthenium nitrides stabilized under pressure: A theoretical prediction. Sci. Rep. 2016, 6, 33506. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.H.; Yao, Z.; Liu, B.B. New High-Pressure Phases of the Zn–N System. J. Phys. Chem. C 2020, 124, 4044–4049. [Google Scholar] [CrossRef]
- Wu, L.L.; Zhou, P.Y.; Li, Y.G.; Wan, B.; Sun, S.H.; Xu, J.J.; Sun, J.; Liao, B.; Gou, H.Y. Ultra-incompressibility and high energy density of ReN8 with infinite nitrogen chains. J. Mater. Sci. 2020, 56, 3814–3826. [Google Scholar] [CrossRef]
- Zhang, J.; Oganov, A.R.; Li, X.F.; Niu, H.Y. Pressure-stabilized hafnium nitrides and their properties. Phys. Rev. B 2017, 95, 020103. [Google Scholar] [CrossRef] [Green Version]
- Steele, B.A.; Oleynik, I.I. Novel Potassium Polynitrides at High Pressures. J. Phys. Chem. A 2017, 121, 8955–8961. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.L.; Li, D.; Liu, Z.; Wang, W.J.; Tian, F.B.; Bao, K.; Duan, D.F.; Liu, B.B.; Cui, T. A Novel Polymerization of Nitrogen in Beryllium Tetranitride at High Pressure. J. Phys. Chem. C 2017, 121, 9766–9772. [Google Scholar] [CrossRef]
- Liu, S.; Liu, R.; Li, H.Y.; Yao, Z.; Shi, X.H.; Wang, P.; Liu, B.B. Cobalt-Nitrogen Compounds at High Pressure. Inorg. Chem. 2021, 60, 14022–14030. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Feng, X.L.; Liu, H.Y.; Hao, J.; Redfern, S.A.T.; Lei, W.W.; Liu, D.; Ma, Y.M. Route to high-energy density polymeric nitrogen t-N via He-N compounds. Nat. Commun. 2018, 9, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.Y.; Weng, X.-J.; Oganov, A.R.; Shao, X.; Gao, G.Y.; Dong, X.; Wang, H.-T.; Tian, Y.J.; Zhou, X.-F. Helium-nitrogen mixtures at high pressure. Phys. Rev. B 2021, 103, L060102. [Google Scholar] [CrossRef]
- Laniel, D.; Weck, G.; Gaiffe, G.; Garbarino, G.; Loubeyre, P. High-Pressure Synthesized Lithium Pentazolate Compound Metastable under Ambient Conditions. J. Phys. Chem. Lett. 2018, 9, 1600–1604. [Google Scholar] [CrossRef]
- Bykov, M.; Bykova, E.; Chariton, S.; Prakapenka, V.B.; Batyrev, I.G.; Mahmood, M.F.; Goncharov, A.F. Stabilization of pentazolate anions in the high-pressure compounds Na2N5 and NaN5 and in the sodium pentazolate framework NaN5•N2. Dalton. Trans. 2021, 50, 7229–7237. [Google Scholar] [CrossRef]
- Steele, B.A.; Stavrou, E.; Crowhurst, J.C.; Zaug, J.M.; Prakapenka, V.B.; Oleynik, I.I. High-Pressure Synthesis of a Pentazolate Salt. Chem. Mater. 2017, 29, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Salke, N.P.; Xia, K.; Fu, S.Y.; Zhang, Y.J.; Greenberg, E.; Prakapenka, V.B.; Liu, J.; Sun, J.; Lin, J.F. Tungsten Hexanitride with Single-Bonded Armchairlike Hexazine Structure at High Pressure. Phys. Rev. Lett. 2021, 126, 065702. [Google Scholar] [CrossRef]
- Bykov, M.; Khandarkhaeva, S.; Fedotenko, T.; Sedmak, P.; Dubrovinskaia, N.; Dubrovinsky, L. Synthesis of FeN4 at 180 GPa and its crystal structure from a submicron-sized grain. Acta. Cryst. 2018, 74, 1392–1395. [Google Scholar]
- Laniel, D.; Winkler, B.; Koemets, E.; Fedotenko, T.; Bykov, M.; Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N. Synthesis of magnesium-nitrogen salts of polynitrogen anions. Nat. Commun. 2019, 10, 4515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, D.B.; Zhang, F.X.; Ji, C.; Hannon, T.; Zhu, H.Y.; Wu, J.Z.; Levitas, V.I.; Ma, Y.Z. Phase transition and structure of silver azide at high pressure. J. Appl. Phys. 2011, 110, 023524. [Google Scholar] [CrossRef] [Green Version]
- Li, D.M.; Zhu, P.F.; Wang, Y.J.; Liu, B.B.; Jiang, J.R.; Huang, X.L.; Wang, X.L.; Zhu, H.Y.; Cui, Q.L. High-pressure spectroscopic study of silver azide. RSC Adv. 2016, 6, 82270–82276. [Google Scholar] [CrossRef]
- Zhu, W.H.; Xiao, H.M. First-principles study of structural and vibrational properties of crystalline silver azide under high pressure. J. Solid-State Chem. 2007, 180, 3521–3528. [Google Scholar] [CrossRef]
- Niu, S.F.; Liu, R.; Shi, X.H.; Yao, Z.; Liu, B.B.; Lu, S.C. High-pressure new phase of AgN3. Mod. Phys. Lett. B 2021, 35, 2150386. [Google Scholar] [CrossRef]
- Williams, A.S.; Cong, K.N.; Gonzalez, J.M.; Oleynik, I.I. Crystal structure of silver pentazolates AgN5 and AgN6. Dalton Trans. 2021, 50, 16364. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lv, J.; Zhu, L.; Ma, Y.M. CLYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 2012, 183, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G. Furthmüller, Efficient iterative schemes for ab initio total-energy calculating using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.L.; Burke, K. Restoring the Density-Gradient Exxpansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
| AgNx | (GPa) | C11 | C22 | C33 | C44 | C55 | C66 | C12 | C13 | C15 | C23 | C25 | C35 | C46 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P4/mmm-AgN2 | 100 | 563 | - | 648 | 85 | - | 22 | 313 | 310 | - | - | - | - | - |
| P1-AgN7 | 50 | 215 | 294 | 231 | 70 | 68 | 42 | 160 | 154 | −3 | 173 | −5 | 8 | 24 |
| P-1-AgN7 | 150 | 943 | 942 | 800 | 221 | 207 | 279 | 513 | 396 | −85 | 377 | −113 | −19 | −85 |
| P-1-AgN4 | 150 | 910 | 912 | 710 | 127 | 148 | 270 | 593 | 354 | −42 | 338 | −39 | 49 | 27 |
| P-1-AgN8 | 150 | 1163 | 1114 | 861 | 193 | 56 | 250 | 604 | 152 | −17 | 228 | −24 | 12 | 0.24 |
| Structures | Pressure | Element | σ(e)/Atom |
|---|---|---|---|
| P4/mmm-AgN2 | 100 | Ag | −0.70 |
| N | +0.35 | ||
| P1-AgN7 | 50 | Ag | −0.82 |
| N | +0.117 | ||
| P-1-AgN7 | 150 | Ag | −0.623 |
| N | +0.089 | ||
| P-1-AgN4 | 150 | Ag | −0.64 |
| N | +0.16 | ||
| P-1-AgN8 | 150 | Ag | −0.76 |
| N | 0.095 |
| Compounds | Energy Densities (kJ/g) | Detonation Velocity (km/s) | Detonation Pressure (GPa) |
|---|---|---|---|
| P-1-AgN7 | 3.90 | 13.58 | 115.5 |
| P-1-AgN8 | 3.90 | 17.59 | 210.7 |
| TNT | 4.30 | 6.90 | 19.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Xu, D.; Yao, Z.; Niu, S.; Liu, B. The New High-Pressure Phases of Nitrogen-Rich Ag–N Compounds. Materials 2022, 15, 4986. https://doi.org/10.3390/ma15144986
Liu R, Xu D, Yao Z, Niu S, Liu B. The New High-Pressure Phases of Nitrogen-Rich Ag–N Compounds. Materials. 2022; 15(14):4986. https://doi.org/10.3390/ma15144986
Chicago/Turabian StyleLiu, Ran, Dan Xu, Zhen Yao, Shifeng Niu, and Bingbing Liu. 2022. "The New High-Pressure Phases of Nitrogen-Rich Ag–N Compounds" Materials 15, no. 14: 4986. https://doi.org/10.3390/ma15144986
APA StyleLiu, R., Xu, D., Yao, Z., Niu, S., & Liu, B. (2022). The New High-Pressure Phases of Nitrogen-Rich Ag–N Compounds. Materials, 15(14), 4986. https://doi.org/10.3390/ma15144986






