Mechanical Performance and Precipitation Behavior in Al-Si-Cu-Mg Cast Alloys: Effect of Prolonged Thermal Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Alloys
2.2. Mechanical Testing
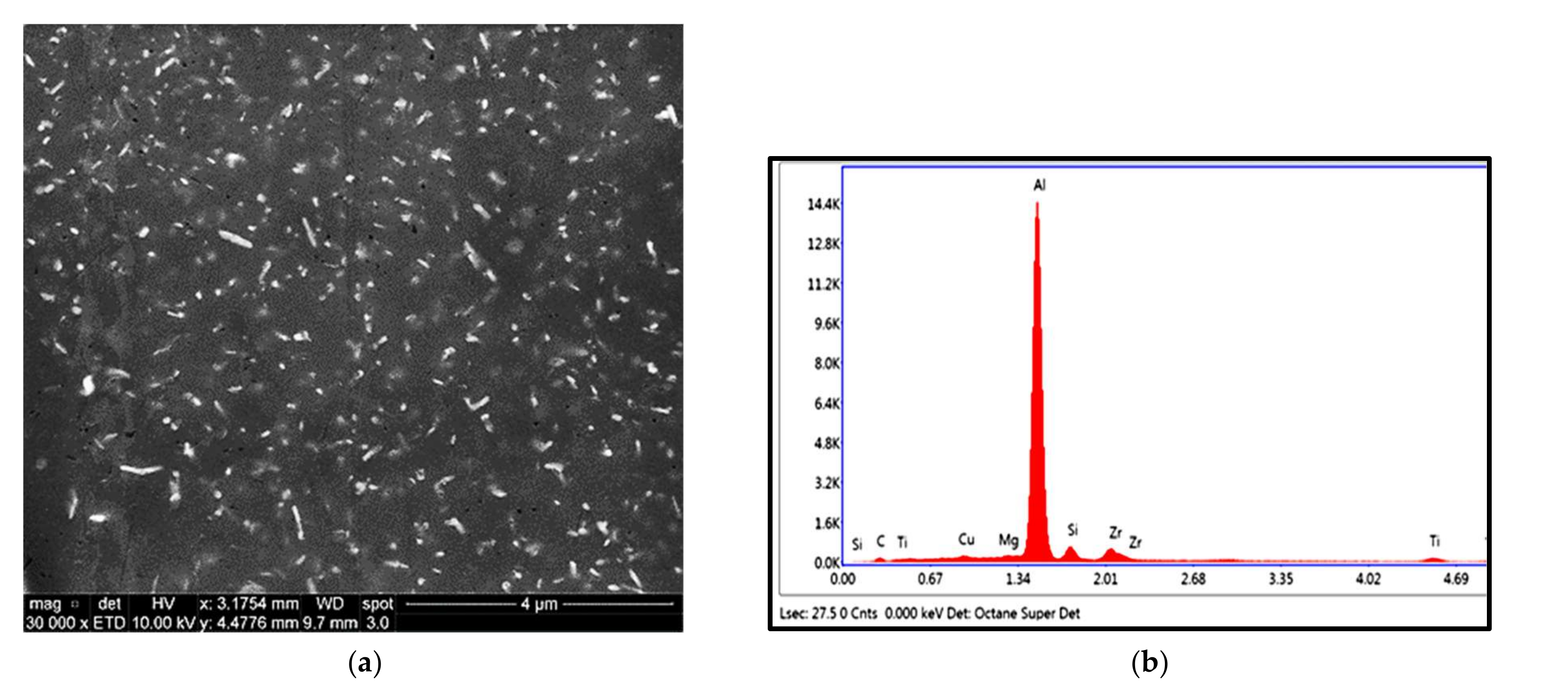
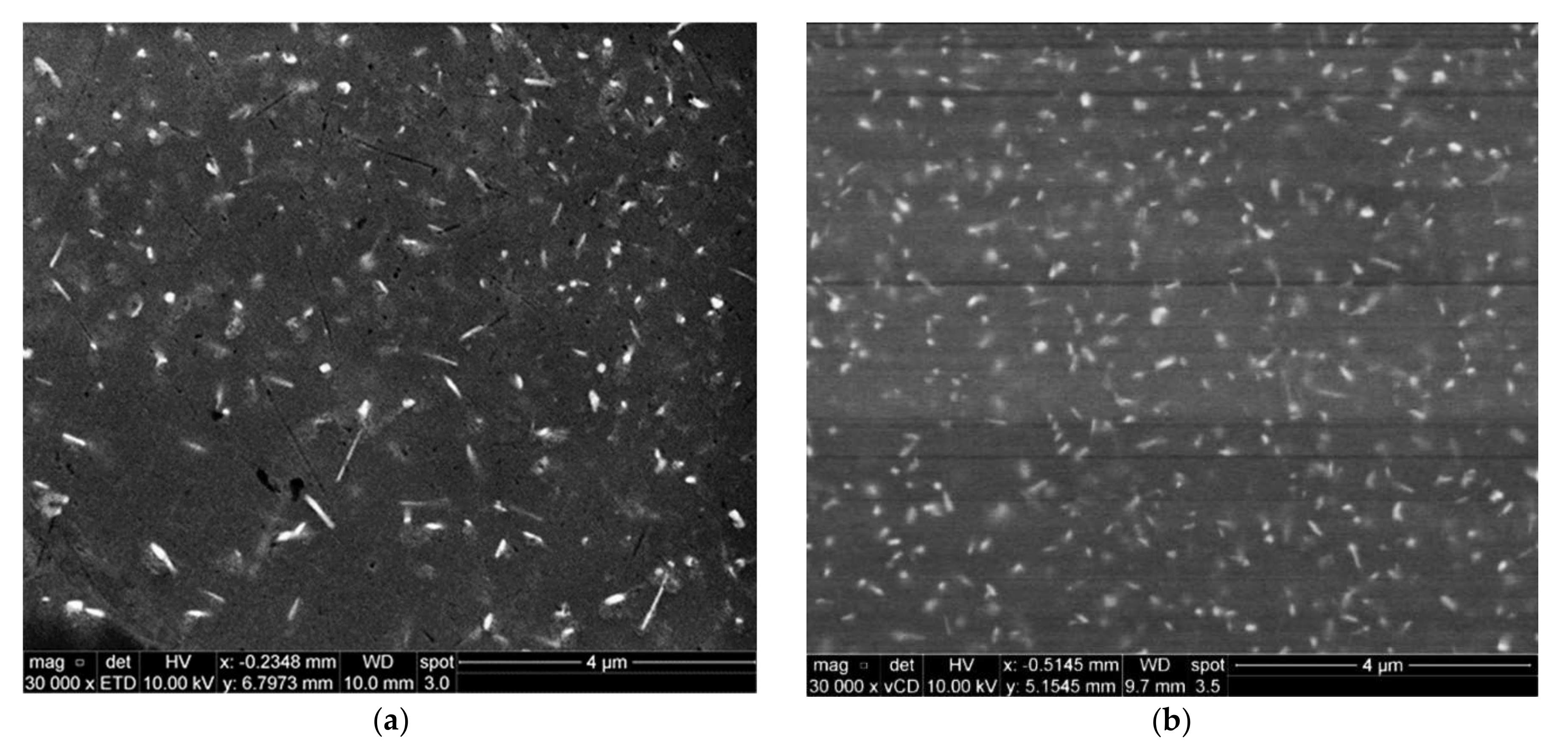
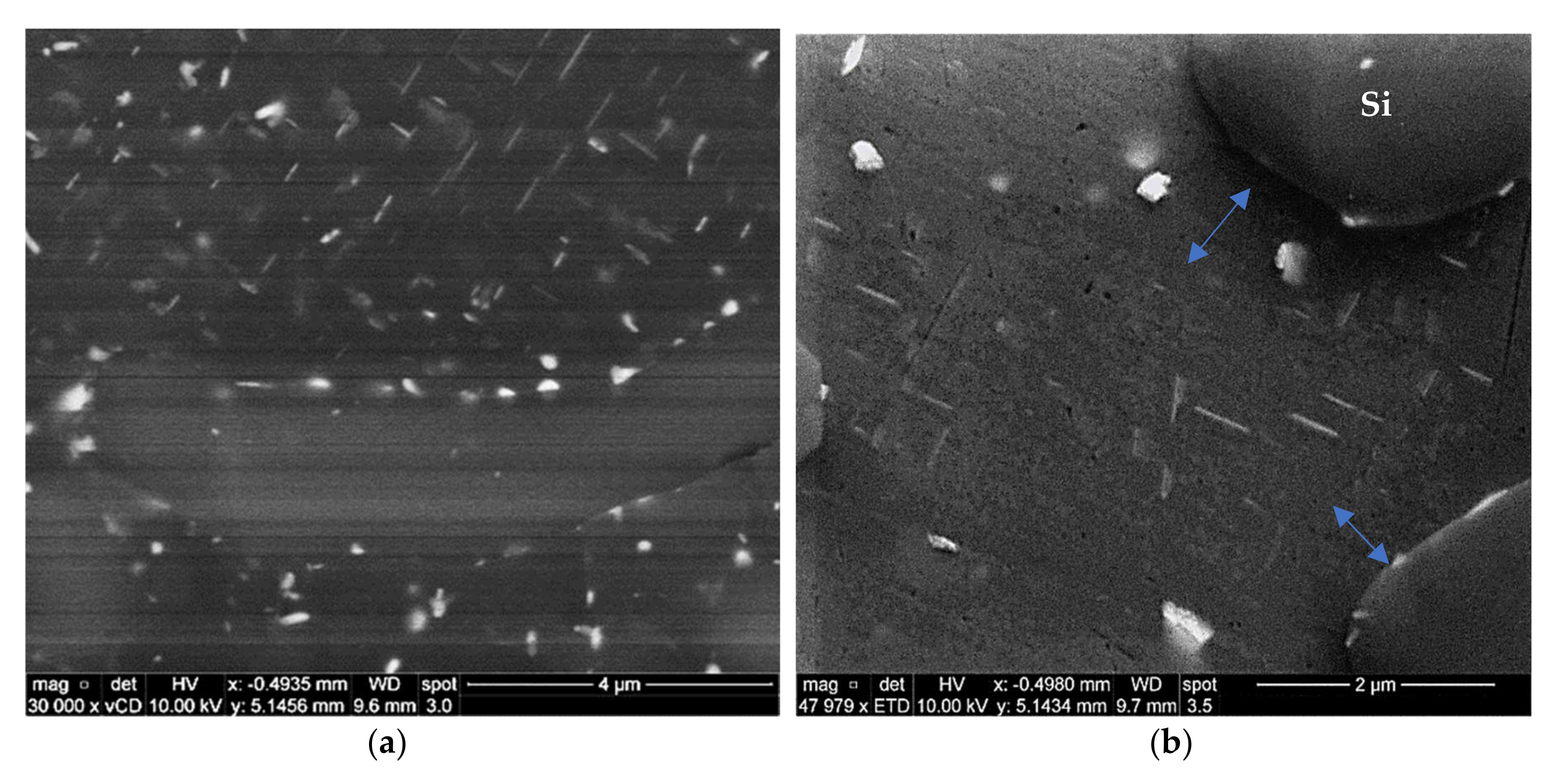
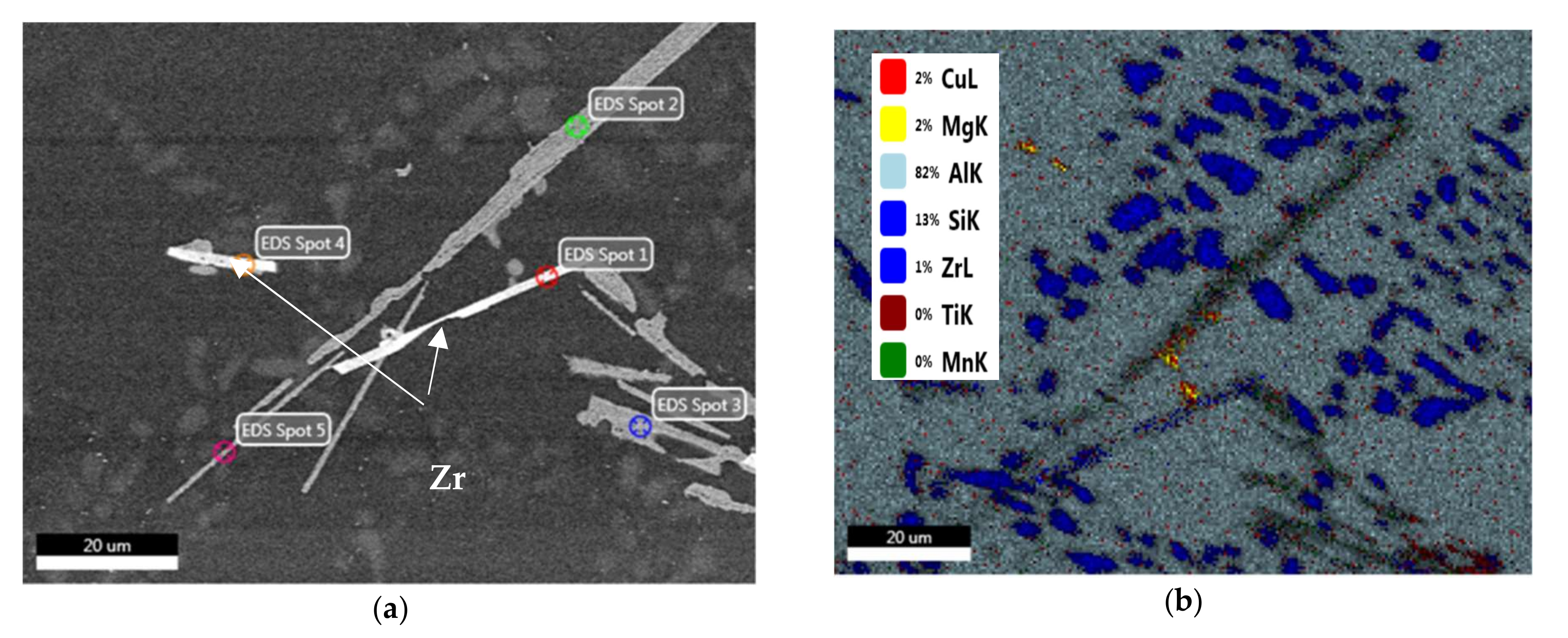
2.3. Advanced Microscopy Investigations
3. Results and Discussion
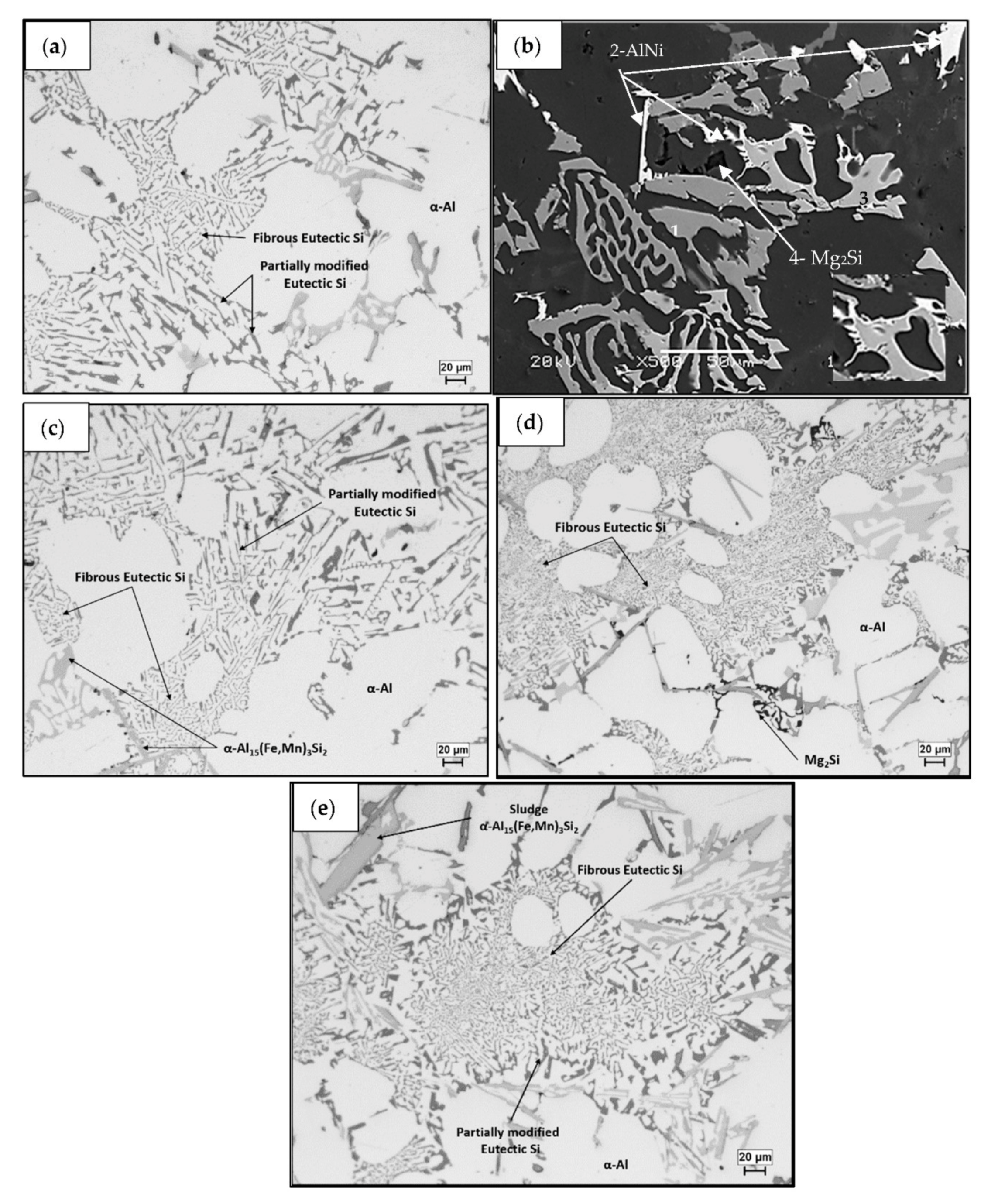
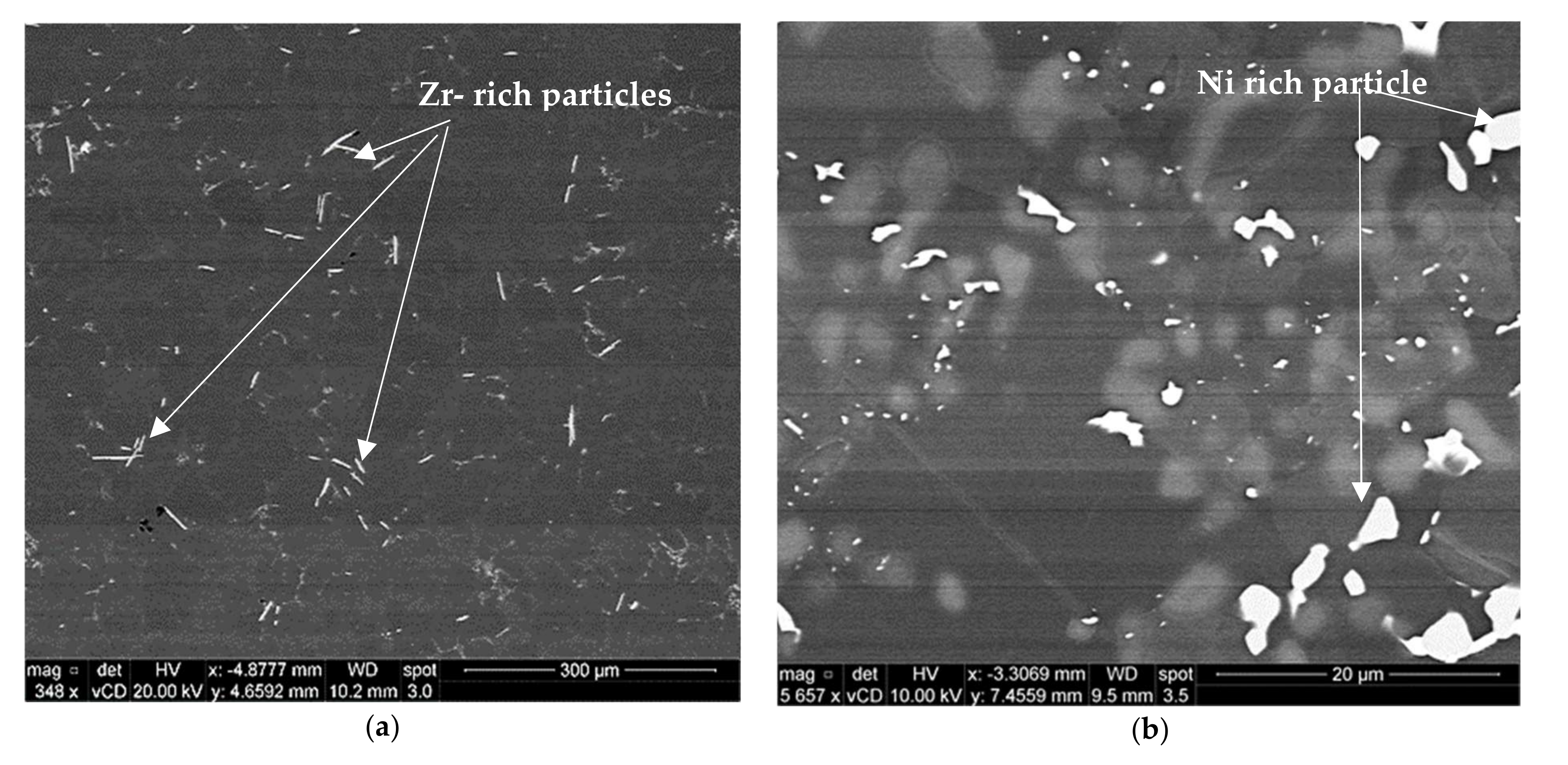
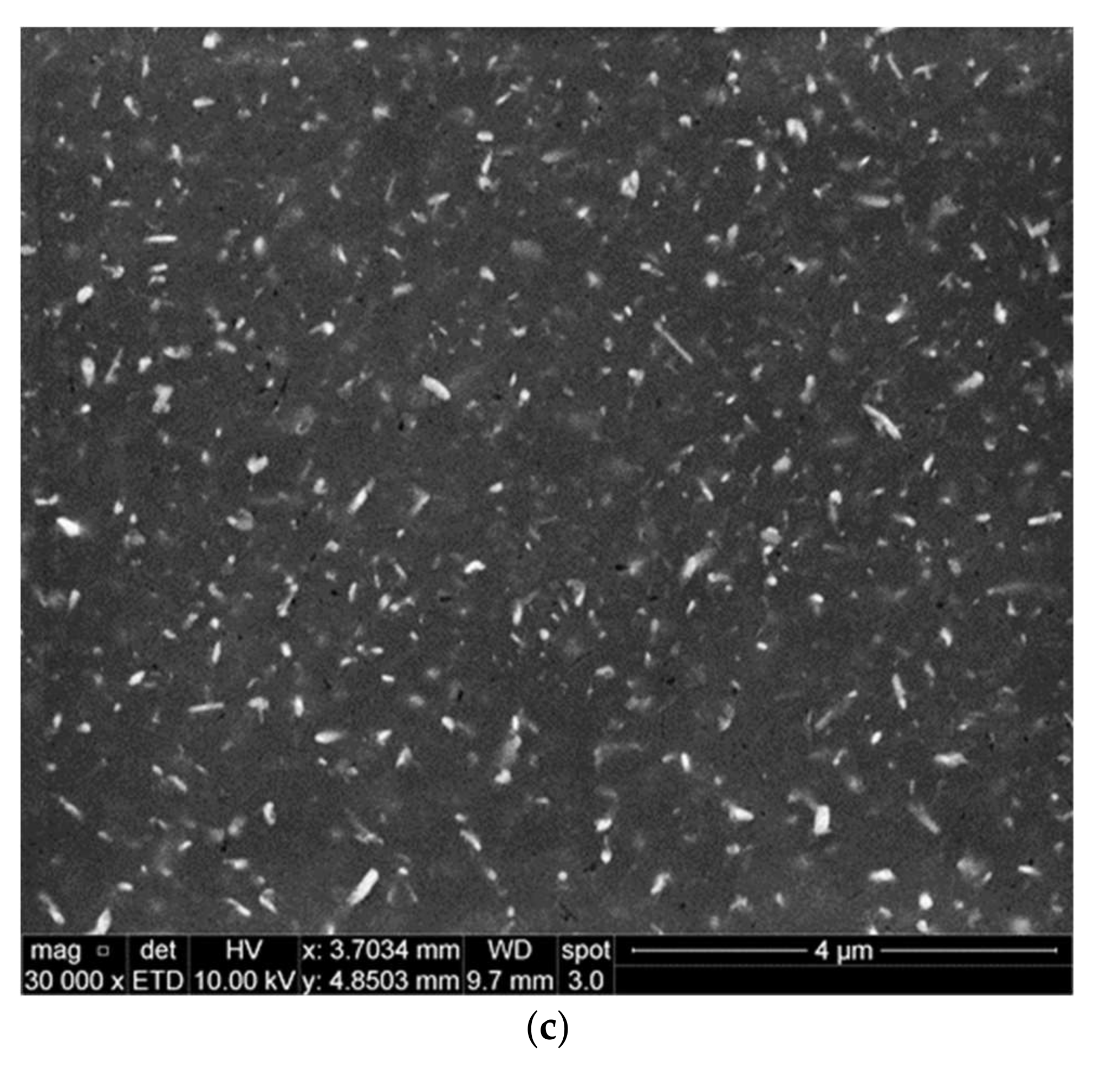
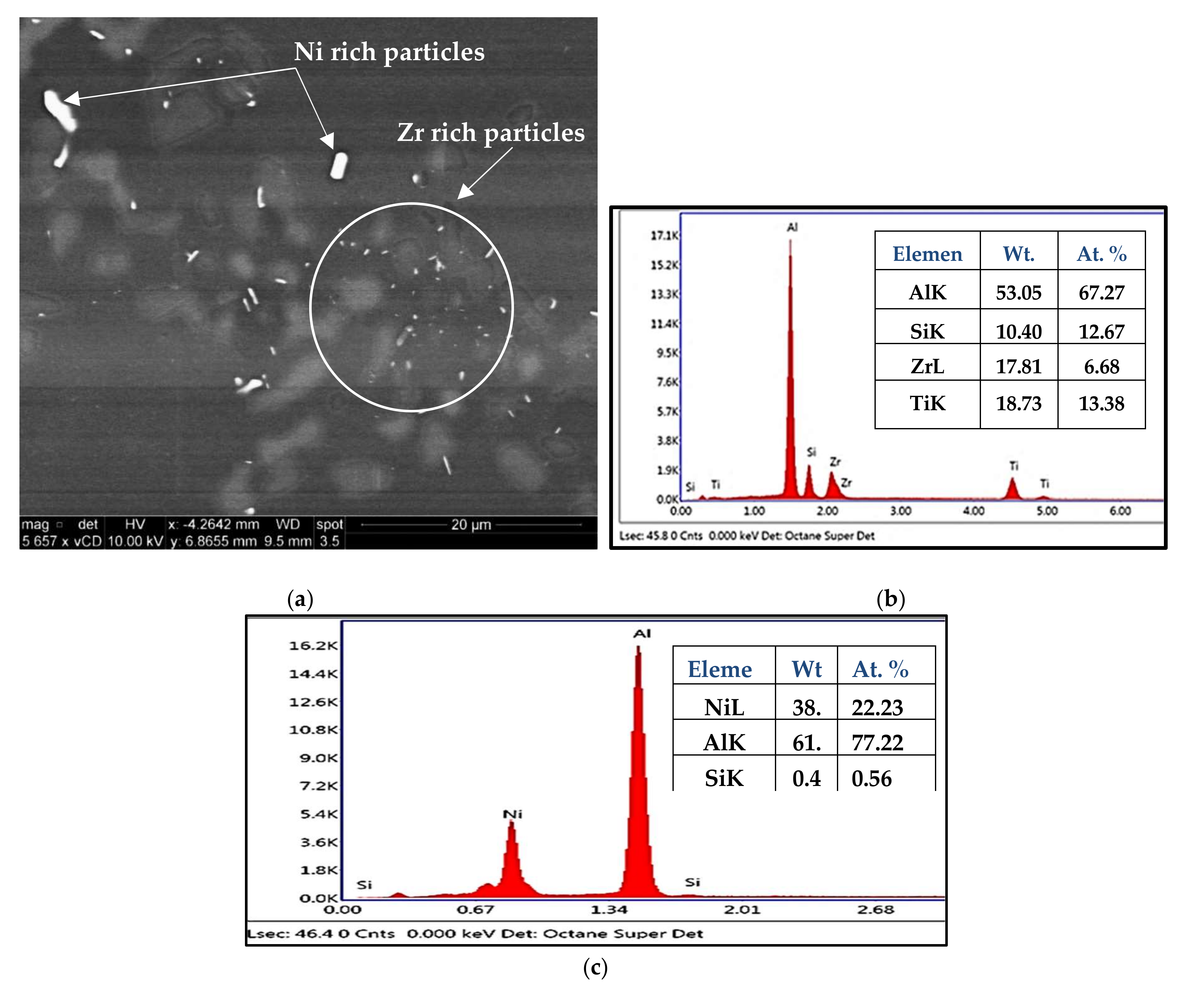
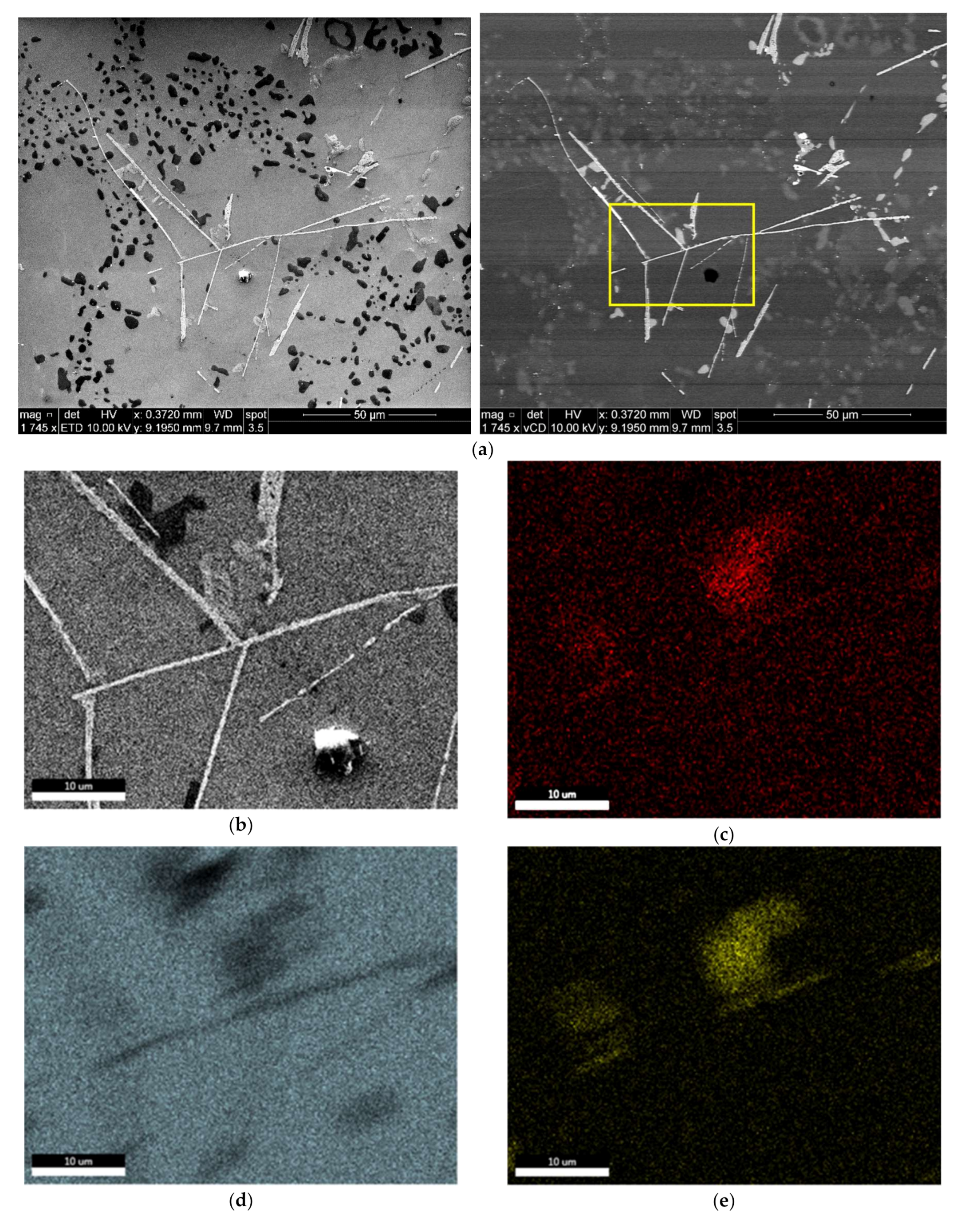
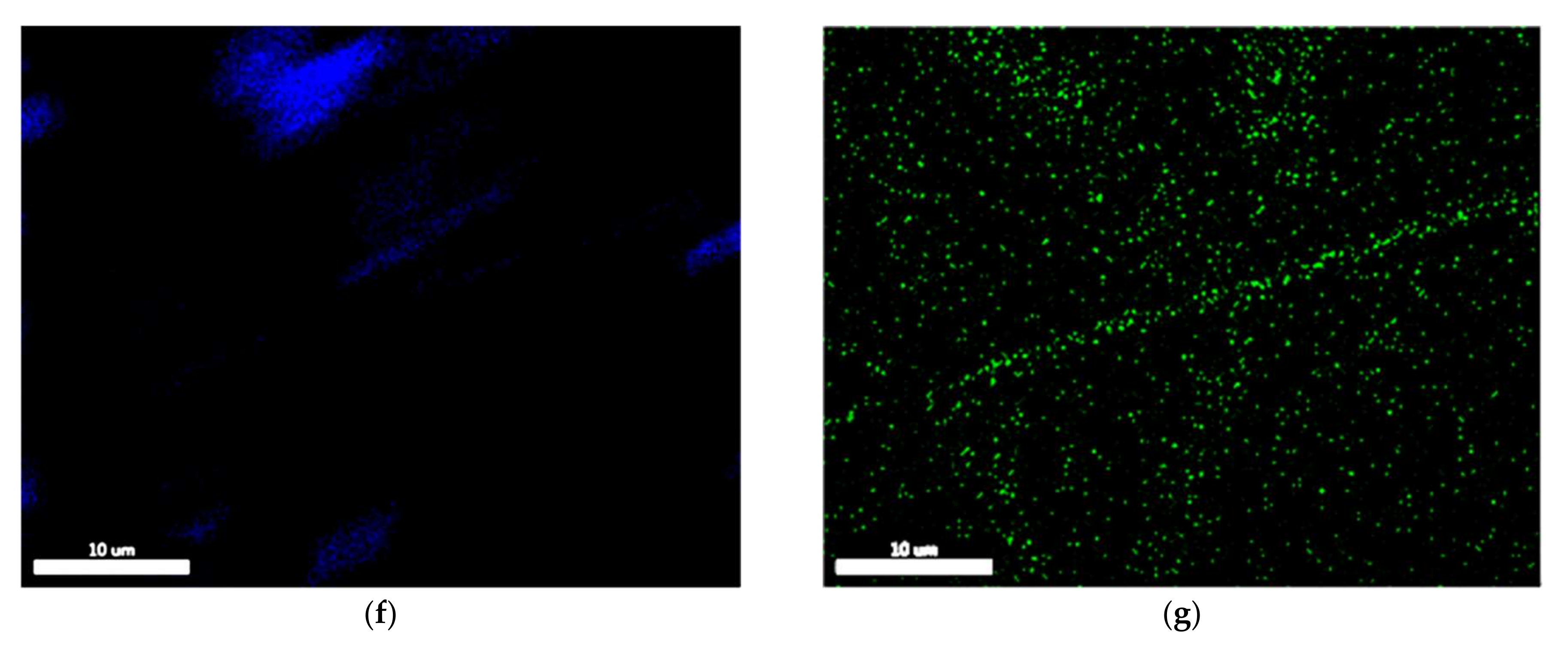
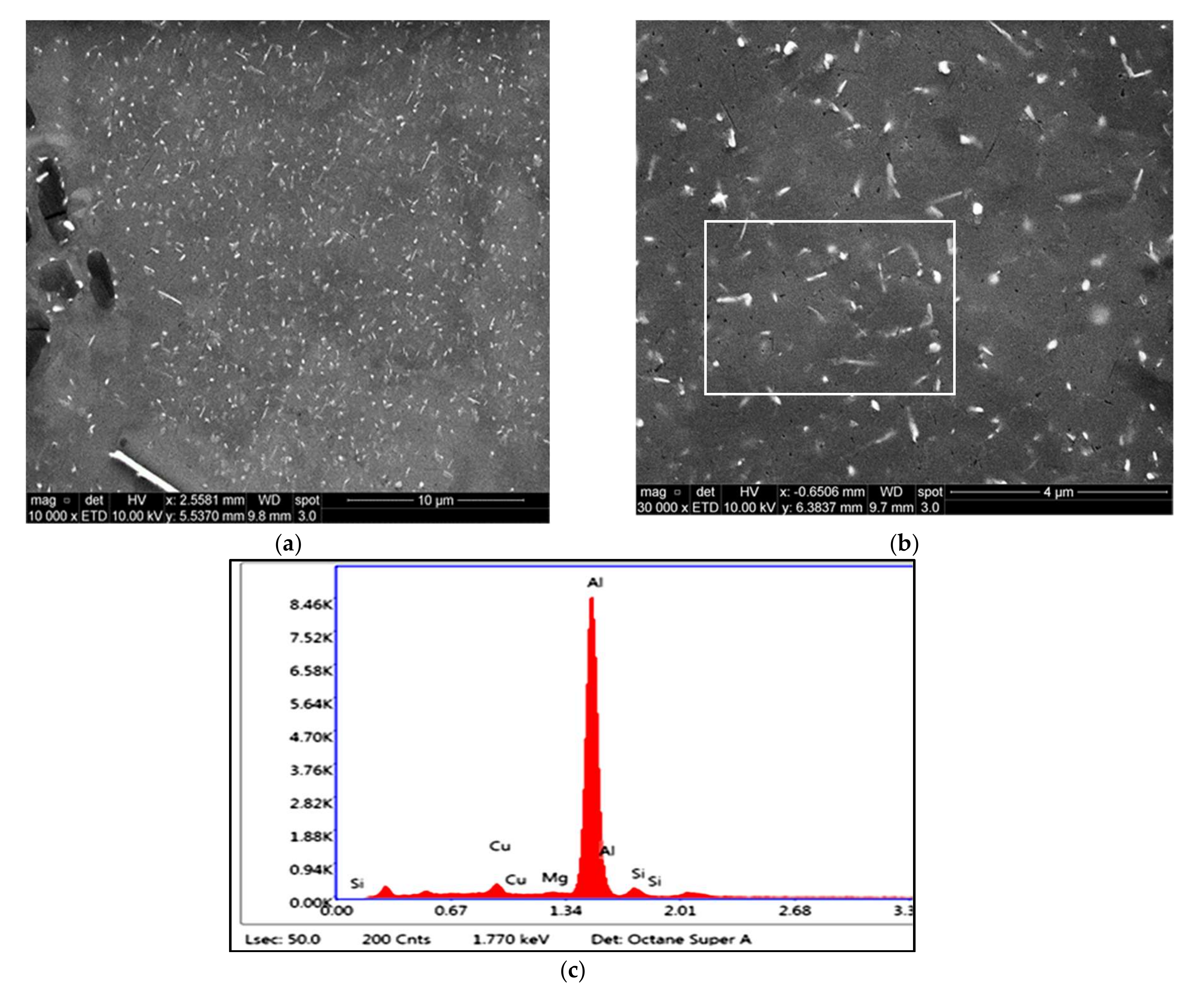
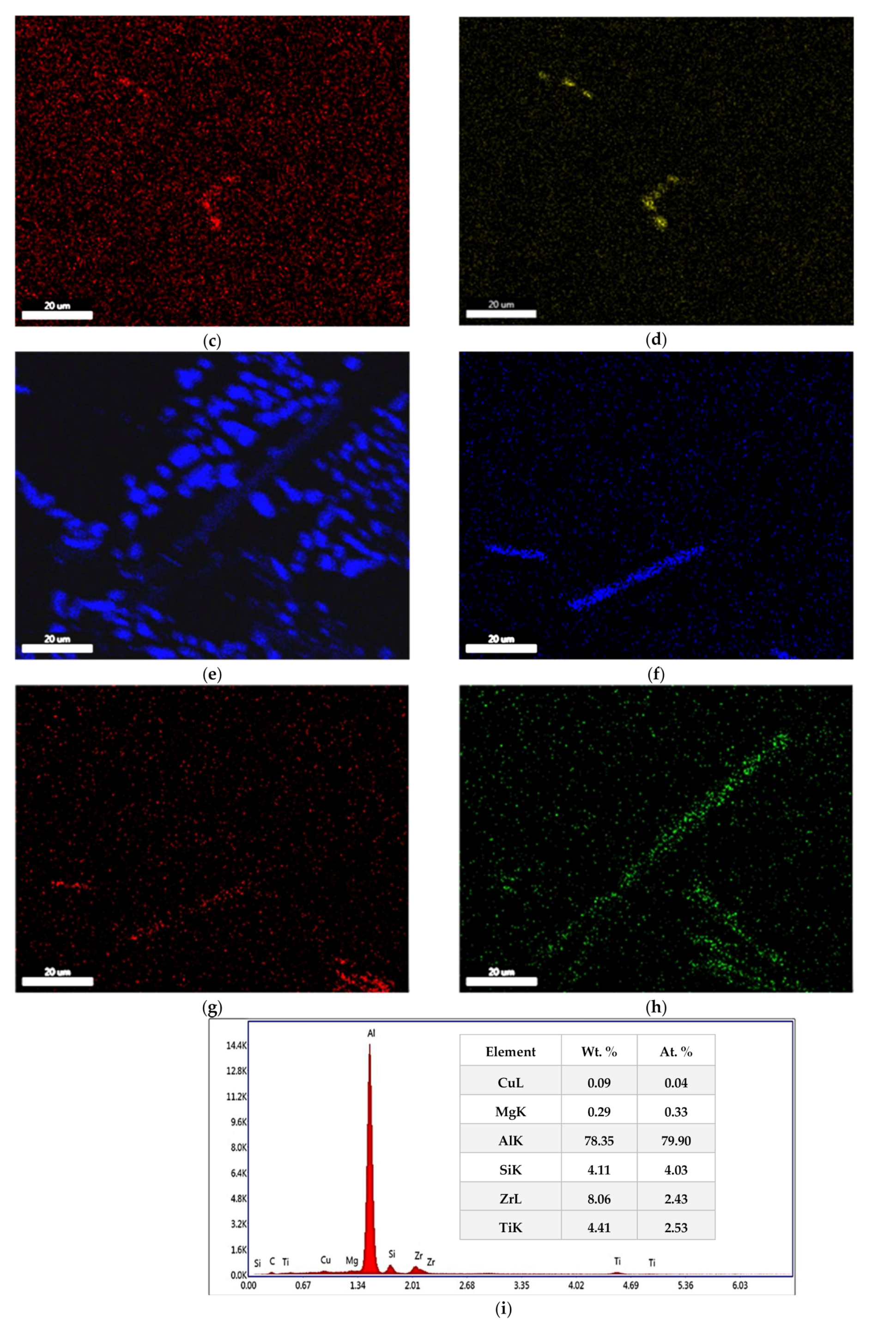
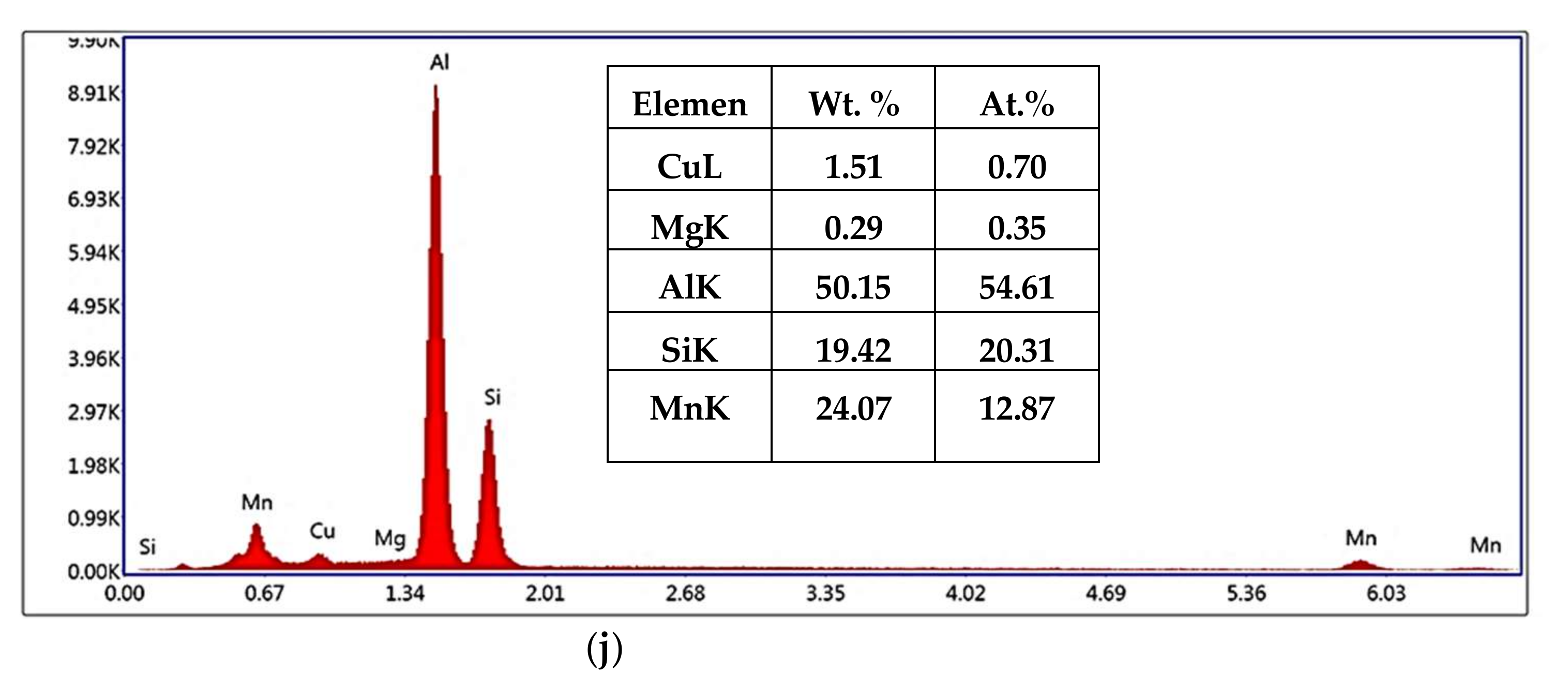
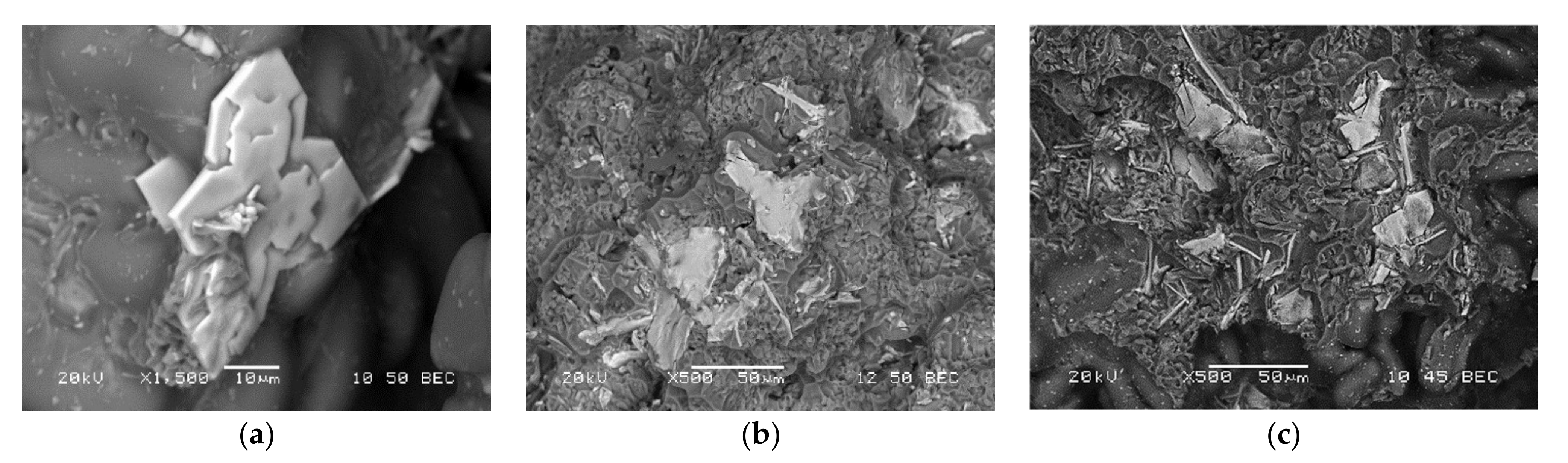
3.1. Microstructural Characterization
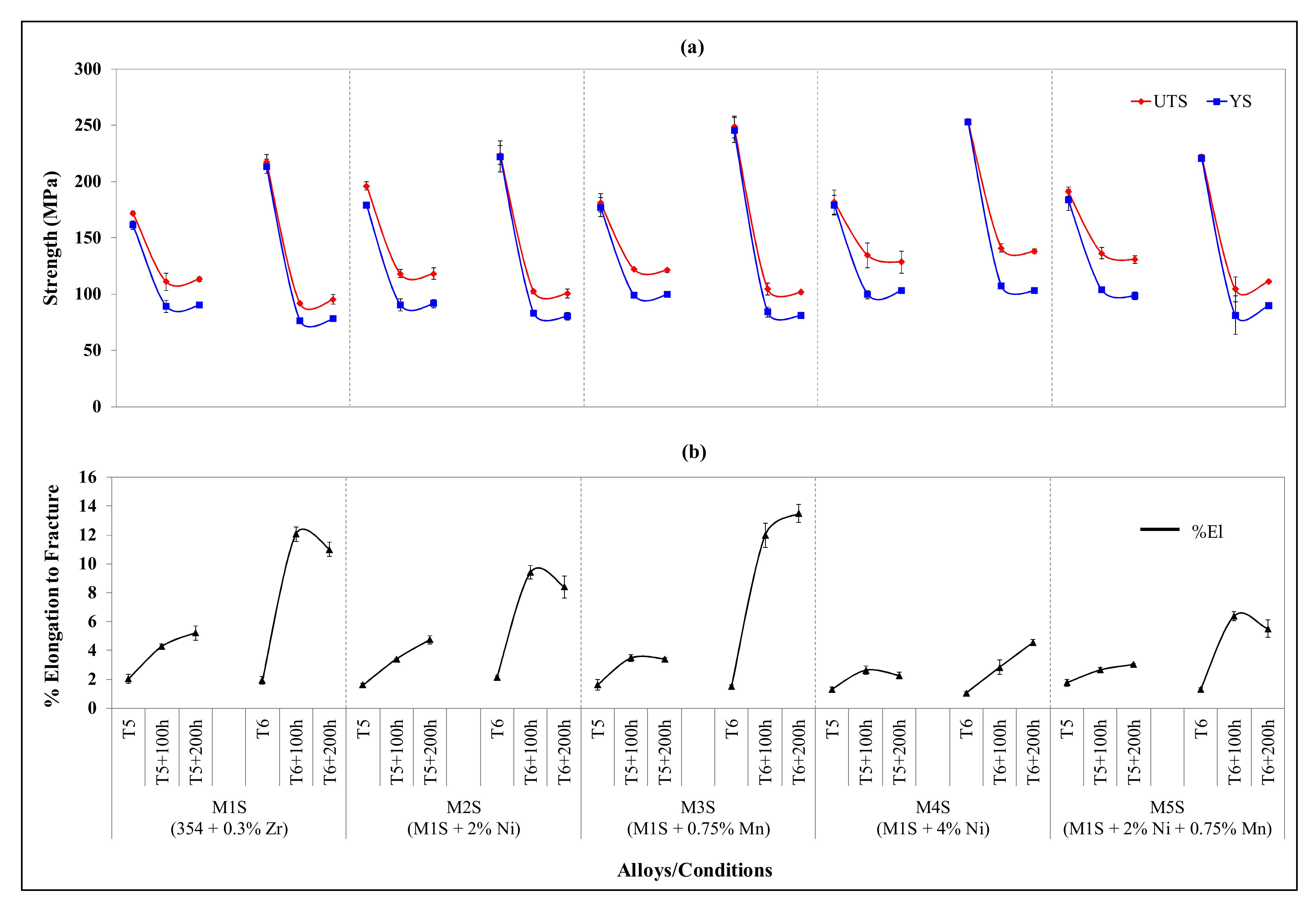
3.2. Ambient-Temperature Tensile Properties
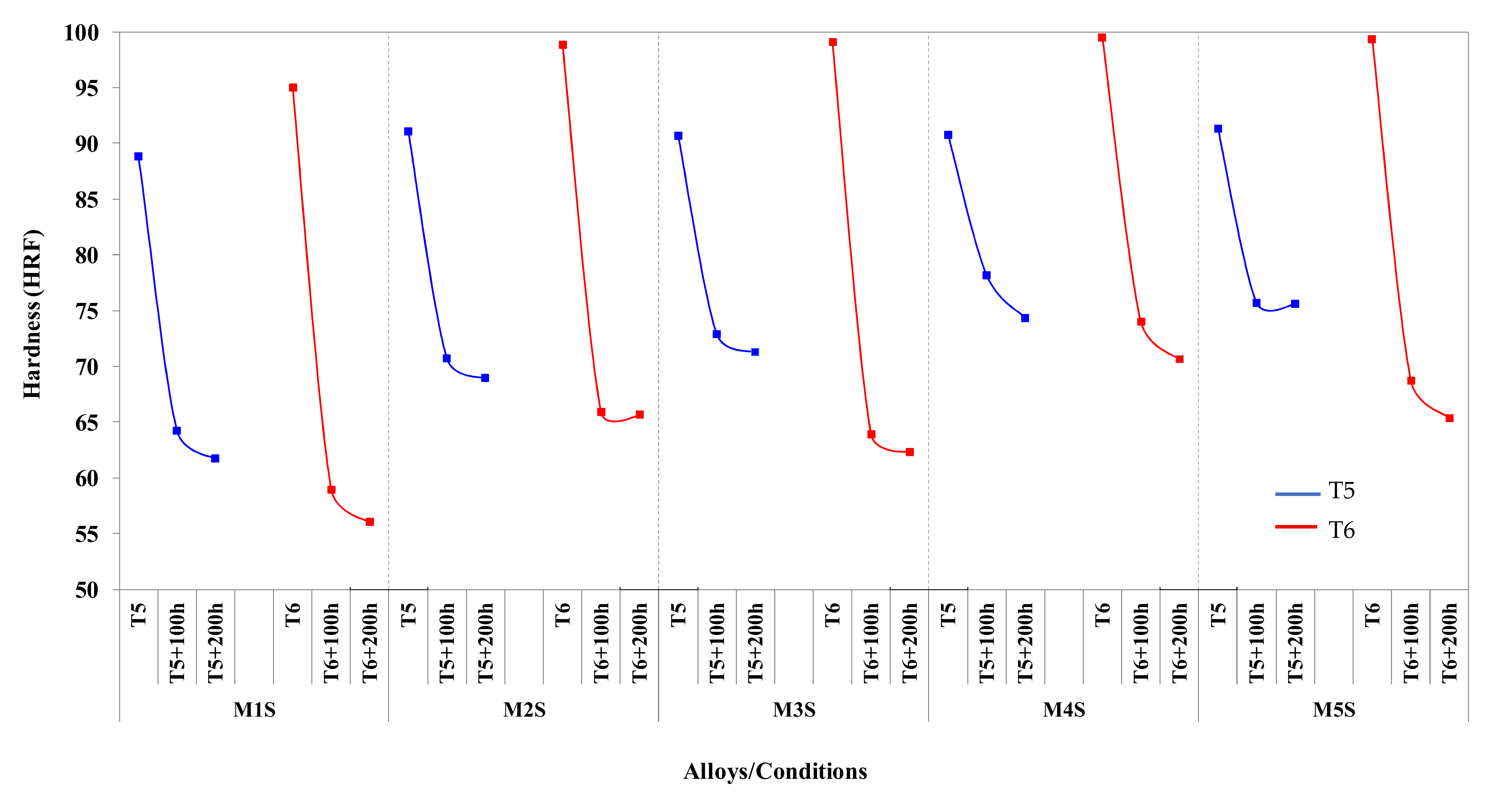
3.3. Hardness Values
3.4. Elevated-Temperature Tensile Properties
3.5. Precipitate Evolution during Thermal Stabilization
3.6. Fractography
4. Conclusions
- Coarsening of the strengthening precipitates following prolonged exposure at 250 °C has a deleterious effect on the tensile strength and hardness values, thereby resulting in a significant increase in the ductility values.
- The coarsening kinetics of the precipitates decay with time, due to the continuously increased distance between the precipitates with increase in the exposure time, causing deterioration in the mechanical performance after thermal exposure at 250 °C up to 100 h. Further thermal exposure up to 200 h does not reduce the strength and hardness values.
- The strength values (UTS and YS) obtained at room temperature for the stabilized T5-treated conditions are comparable to, and in most alloys exceed, those of the stabilized T6-treated conditions.
- In the case of elevated-temperature tensile testing, the T5 strength values in the stabilized conditions are always higher than those obtained for the stabilized T6 condition.
- Addition of 0.75 wt.% Mn is competitive with the addition of 2 and 4 wt.% Ni with respect to the elevated- and ambient-temperature strength values, respectively, with the Mn-containing alloy providing the advantage of higher ductility values.
- Precipitation of different strengthening/intermetallic phases identified from the TEM investigations include Al2Cu, Al-Ni-Cu, Al-Ni, Al-Si-Mn-Fe, Al-Ti-Zr phases, and fine Zr-rich and Al6Mn precipitates. The phases present and their evolution with the type and duration of the heat treatment determine the alloy properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
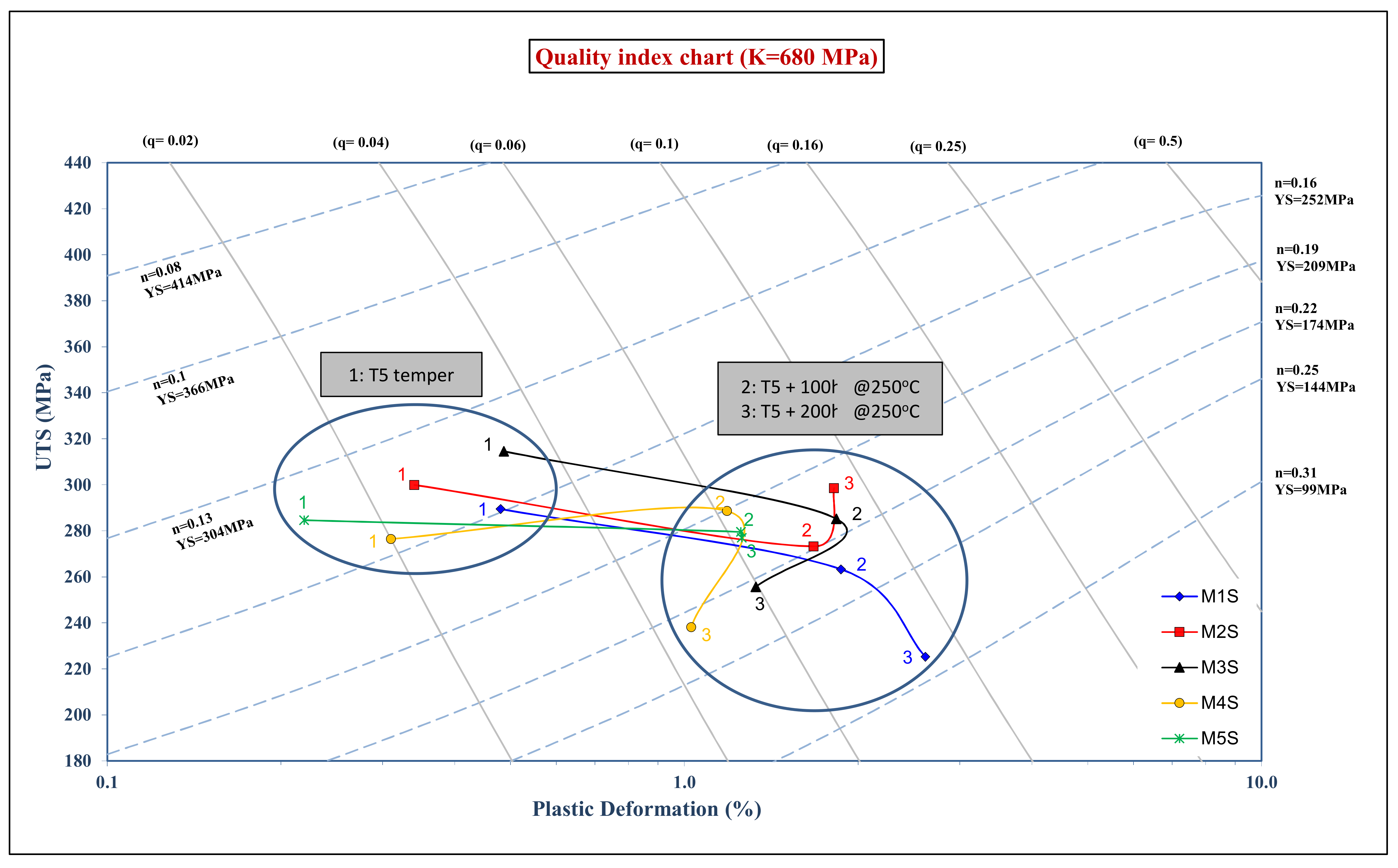
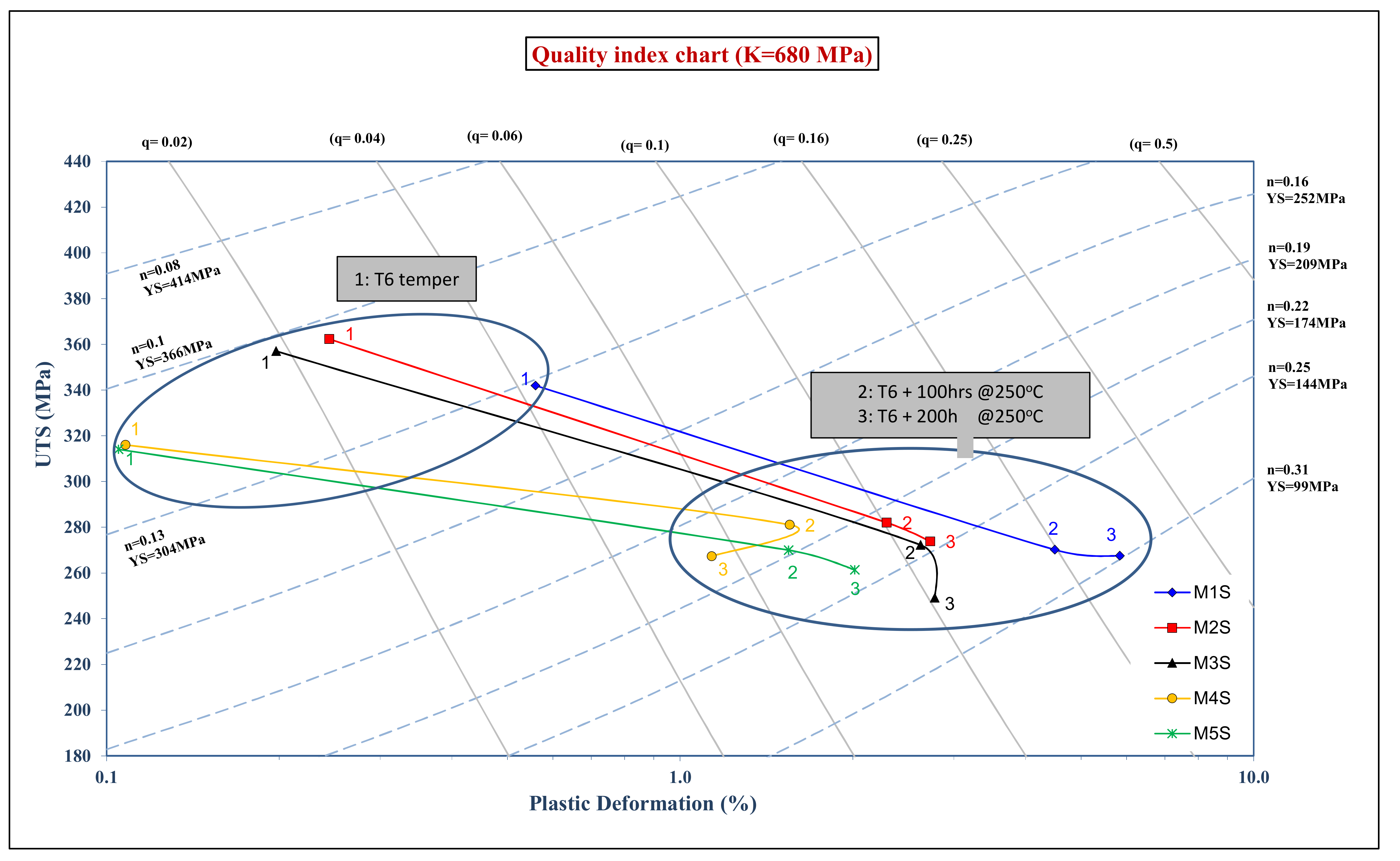
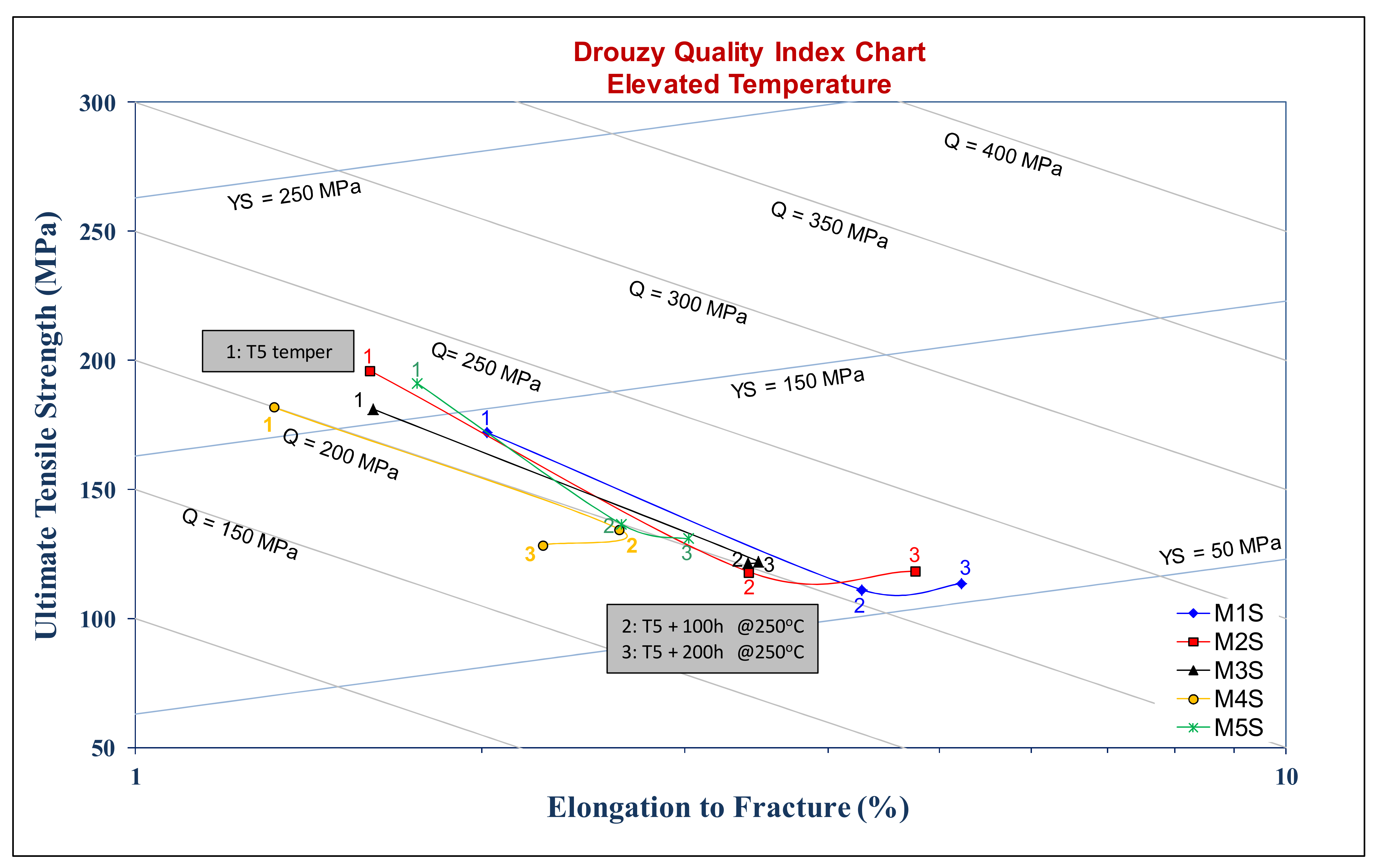
Appendix A. Quality Index
- 1-
- Quality Index at Ambient Temperature:
- 2-
- Quality Index at Elevated Temperature:

References
- Ducker Worldwide. The Past, Present and Future of Aluminum in North American Light Vehicles; Ducker: Troy, MI, USA, 2012. [Google Scholar]
- Lee, J.A.; Chen, P.-S. High Strength Aluminum Alloy for High Temperature Applications. Google Patents Patent Number EP1492894A1, 5 January 2005. [Google Scholar]
- Kliemt, C. Thermo-Mechanical Fatigue of Cast Aluminium Alloys for Engine Applications under Severe Conditions. Ph.D. Thesis, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, UK, 2012. [Google Scholar]
- Lee, J.A. Cast aluminum alloy for high temperature applications. In Proceedings of the 132nd TMS Annual Meeting & Exhibition, San Diego, CA, USA, 2–6 March 2003; Volume 1, pp. 145–151. [Google Scholar]
- Garza-Elizondo, G.H. Effect of Ni, Mn, Zr and Sc Additions on the Performance of Al-Si-Cu-Mg Alloys. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2016. [Google Scholar]
- Hernandez-Sandoval, J. Improving the Performance of 354 Type Alloy. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2010. [Google Scholar]
- Shaha, S.K. Development and Characterization of Cast Modified Al-Si-Cu-Mg Alloys for Heat Resistant Power Train Applications. Ph.D. Thesis, Ryerson University, Toronto, ON, Canada, 2015. [Google Scholar]
- Zappi, E. The Effect of Zr and V on the Structural and Mechanical Properties of the 2618 Al Alloy. Master’s Thesis, NTNU-Trondheim, Norwegian University of Science and Technology, Trondheim, Norway, 2014. [Google Scholar]
- Asghar, Z.; Requena, G.; Kubel, F. The role of Ni and Fe aluminides on the elevated temperature strength of an AlSi12 alloy. Mater. Sci. Eng. A 2010, 527, 5691–5698. [Google Scholar] [CrossRef]
- Knipling, K.E. Development of a Nanoscale Precipitation-Strengthened Creep-Resistant Aluminum Alloy Containing Trialuminide Precipitates. Ph.D. Thesis, Northwestern University, Evanston, IL, USA, 2006. [Google Scholar]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Monotonic and cyclic deformation behavior of the Al–Si–Cu–Mg cast alloy with micro-additions of Ti, V and Zr. Int. J. Fatigue 2015, 70, 383–394. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Static versus dynamic thermal exposure of transition elements-containing Al-Si-Cu-Mg cast alloy. Mater. Sci. Eng. A 2019, 739, 499–512. [Google Scholar] [CrossRef]
- Ceschini, L.; Morri, A.; Morri, A.; Rotundo, F.; Toschi, S. Heat treatment response and influence of overaging on mechanical properties of C355 cast aluminum alloy. Metall. Ital. 2014, 5, 11–17. [Google Scholar]
- Xia, F.; Li, J.P.; Guo, Y.C.; Yang, Z. Microstructure Evolution and Mechanical Properties of an Al-Si-Cu-Mg-Ni Aluminium Alloy after Thermal Exposure. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland. [CrossRef]
- Jabra, J.; Romios, M.; Lai, J.; Lee, E.; Setiawan, M.; Ogren, J.R.; Clark, R.; Oppenheim, T.; Es-Said, O.S.; Lee, E.W.; et al. The effect of thermal exposure on the mechanical properties of 2099-T6 die forgings, 2099-T83 extrusions, 7075-T7651 plate, 7085-T7452 die forgings, 7085-T7651 plate, and 2397-T87 plate aluminum alloys. J. Mater. Eng. Perform. 2006, 15, 601–607. [Google Scholar] [CrossRef]
- Kai, S.; Chen, J.-L.; Yin, Z.-M. TEM study on microstructures and properties of 7050 aluminum alloy during thermal exposure. Trans. Nonferrous Met. Soc. China 2009, 19, 1405–1409. [Google Scholar]
- Drouzy, M.; Jacob, S.; Richard, M. Interpretation of Tensile Results by Means of Quality Index and Probable Yield Strength—Application to Al-Si7 Mg Foundry Alloys. AFS Int. Cast Met. J. 1980, 5, 43–50. [Google Scholar]
- Cáceres, C.H. A rationale for the quality index of Al-Si-Mg casting alloys. Int. J. Cast Met. Res. 2000, 12, 385–391. [Google Scholar] [CrossRef]
- Jacob, S. Quality Index in Prediction of Properties of Aluminum Castings—A Review. AFS Trans. 2000, 208, 811–818. [Google Scholar]
- Gruzleski, J.E.; Closset, B.M. The Treatment of Liquid Aluminum-Silicon Alloys; American Foundrymen’s Society: Des Plaines, IL, USA, 1990. [Google Scholar]
- Ceschini, L.; Boromei, I.; Morri, A.; Seifeddine, S.; Svensson, I.L. Microstructure, tensile and fatigue properties of the Al–10% Si–2% Cu alloy with different Fe and Mn content cast under controlled conditions. J. Mater. Process. Technol. 2009, 209, 5669–5679. [Google Scholar] [CrossRef]
- Cáceres, C.H. A phenomenological approach to the Quality Index of Al-Si-Mg casting alloys. Int. J. Cast Met. Res. 2000, 12, 367–375. [Google Scholar] [CrossRef]
- Cáceres, C.H. Microstructure design and heat treatment selection for casting alloys using the quality index. J. Mater. Eng. Perform. 2000, 9, 215–221. [Google Scholar] [CrossRef]
- Cáceres, C.H. Particle Crack Damage and Quality Index of Al-Si-Mg Casting Alloys. AFS Trans. 2001, 108, 709–712. [Google Scholar]
- Cáceres, C.H.; Barresi, J. Selection of temper and Mg content to optimise the quality index of Al-7Si-Mg casting alloys. Int. J. Cast Met. Res. 2000, 12, 377–384. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Sokolowski, J.H.; Gallo, P. Effect of ageing and Mg content on the Quality Index of two Al-1%Cu-4.5%Si-Mg alloys. Mater. Sci. Eng. A 1999, 271, 53–61. [Google Scholar] [CrossRef]
- Caceres, C.H.; Makhlouf, M.; Apelian, D.; Wang, L. Quality index chart for different alloys and temperatures: A case study on aluminium die-casting alloys. J. Light Met. 2001, 1, 51–59. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Din, T.; Rashid, A.K.M.B.; Campbell, J. Effect of aging on quality index of an Al–Cu casting alloy. Mater. Sci. Technol. 1999, 15, 711–716. [Google Scholar] [CrossRef]
- Caceres, C.H.; Wang, L.; Apelian, D.; Makhlouf, M. Alloy selection for die castings using the quality index. AFS Trans. 1999, 107, 239–247. [Google Scholar]
- Cáceres, C.; Svensson, I.L.; Taylor, J.A. Strength-Ductiliy Behaviour of Al-Si-Cu-Mg Casting Alloys in T6 Temper. Int. J. Cast Met. Res. 2003, 15, 531–543. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Qiang, J.; Dong, C. Bulk metallic glasses in the Zr-Al-Ni-Cu system. Acta Mater. 2003, 51, 1899–1907. [Google Scholar] [CrossRef]
- Garza-Elizondo, G.H.; Samuel, A.M.; Valtierra, S.; Samuel, F.H. Phase precipitation in transition metal-containing 354-type alloys. Int. J. Mater. Res. 2017, 108, 108–125. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Elgallad, E.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Melting and solidification characteristics of Zr-, Ni-, and Mn-containing 354-type Al-Si-Cu-Mg cast alloys. Philos. Mag. 2019, 99, 1633–1655. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the influences of alloying elements on the microstructure and mechanical properties of aluminum alloys and aluminum alloy composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Hanafee, J. Effect of Nickel on Hot Hardness of Aluminum-Silicon Alloys. Mod. Cast. 1963, 44, 514–520. [Google Scholar]
- Mbuya, T.; Odera, B.; Ng’ang’a, S. Influence of iron on castability and properties of aluminium silicon alloys: Literature review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Lu, L.; Dahle, A. Iron-rich intermetallic phases and their role in casting defect formation in hypoeutectic Al-Si alloys. Metall. Mater. Trans. A 2005, 36, 819–835. [Google Scholar]
- Elsharkawi, E. Effects of Metallurgical Parameters on the Decomposition of AlFeMgSi Phase in Al-Si-Mg Alloys and Its Influence on the Mechanical Properties. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2011. [Google Scholar]
- Nam, S.W.; Lee, D.H. The effect of Mn on the mechanical behavior of Al alloys. Met. Mater. Int. 2000, 6, 13–16. [Google Scholar] [CrossRef]
- Park, D.S.; Nam, S.W. Effects of manganese dispersoid on the mechanical properties in Al-Zn-Mg alloys. J. Mater. Sci. 1995, 30, 1313–1320. [Google Scholar] [CrossRef]
- Prasad, P. Characterization of New, Cast, High Temperature Aluminum Alloys for Diesel Engine Applications. Ph.D. Dissertation, University of Cincinnati, Cincinnati, OH, USA, 2006. [Google Scholar]
- Lee, D.; Park, J.; Nam, S. Enhancement of mechanical properties of Al–Mg–Si alloys by means of manganese dispersoids. Mater. Sci. Technol. 1999, 15, 450–455. [Google Scholar] [CrossRef]
- Tavitas-Medrano, F.J.; Gruzleski, J.E.; Samuel, F.H.; Valtierra, S.; Doty, H.W. Effect of Mg and Sr-modification on the mechanical properties of 319-type aluminum cast alloys subjected to artificial aging. Mater. Sci. Eng. A 2008, 480, 356–364. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of additives on the microstructure and tensile properties of Al-Si alloys. J. Mater. Res. Technol. 2019, 8, 2255–2268. [Google Scholar] [CrossRef]
- Hernandez-Sandoval, J.; Zedan, Y.; Garza-Elizondo, G.H.; Abdelaziz, M.H.; Songmene, V.; Samuel, F.H. Effect of Minor Addition of Ni and Zr on the High-Temperature Performance of Al–Si–Cu–Mg Cast Alloys. Int. J. Met. 2021, 1–17. [Google Scholar] [CrossRef]





| Chemical Analysis (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alloy Description | Elements | ||||||||||
| Type | Code | Si | Cu | Mg | Fe | Ti | Zr | Ni | Mn | Sr | Al |
| 354 | M1S | 8.5 | 1.76 | 0.55 | 0.12 | 0.2 | 0.32 | <0.1 | 0.01 | 0.02 | Bal. |
| M2S | 8.4 | 1.7 | 0.60 | 0.14 | 0.21 | 0.33 | 1.9 | 0.01 | 0.02 | Bal. | |
| M3S | 8.6 | 1.8 | 0.55 | 0.11 | 0.20 | 0.33 | <0.1 | 0.75 | 0.02 | Bal. | |
| M4S | 8.6 | 1.8 | 0.67 | 0.12 | 0.22 | 0.29 | 4.0 | 0.01 | 0.02 | Bal. | |
| M5S | 8.6 | 1.8 | 0.60 | 0.15 | 0.20 | 0.29 | 1.9 | 0.76 | 0.02 | Bal. | |
| Heat Treatment Procedures and Parameters | |||
|---|---|---|---|
| Heat Treatment | Solution Treatment | Quenching | Aging |
| T5 | NA | NA | 8 h @ 180 °C |
| T6 | 5 h @ 495 °C | Warm water (60 °C) | 8 h @ 180 °C |
| Volume Fraction (%) | Alloy Code | |||||
|---|---|---|---|---|---|---|
| M1S | M2S | M3S | M4S | M5S | ||
| SHT | Average | 1.11 | 5.54 | 3.64 | 9.60 | 7.68 |
| SD | 0.28 | 0.61 | 0.16 | 0.65 | 0.52 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Songmene, V.; Samuel, F.H. Mechanical Performance and Precipitation Behavior in Al-Si-Cu-Mg Cast Alloys: Effect of Prolonged Thermal Exposure. Materials 2022, 15, 2830. https://doi.org/10.3390/ma15082830
Abdelaziz MH, Samuel AM, Doty HW, Songmene V, Samuel FH. Mechanical Performance and Precipitation Behavior in Al-Si-Cu-Mg Cast Alloys: Effect of Prolonged Thermal Exposure. Materials. 2022; 15(8):2830. https://doi.org/10.3390/ma15082830
Chicago/Turabian StyleAbdelaziz, Mohamed H., Agnes M. Samuel, Herbert W. Doty, Victor Songmene, and Fawzy H. Samuel. 2022. "Mechanical Performance and Precipitation Behavior in Al-Si-Cu-Mg Cast Alloys: Effect of Prolonged Thermal Exposure" Materials 15, no. 8: 2830. https://doi.org/10.3390/ma15082830
APA StyleAbdelaziz, M. H., Samuel, A. M., Doty, H. W., Songmene, V., & Samuel, F. H. (2022). Mechanical Performance and Precipitation Behavior in Al-Si-Cu-Mg Cast Alloys: Effect of Prolonged Thermal Exposure. Materials, 15(8), 2830. https://doi.org/10.3390/ma15082830








