Low-Temperature, Efficient Synthesis of Highly Crystalline Urchin-like Tantalum Diboride Nanoflowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experiment Procedure
2.3. Characterization and Testing
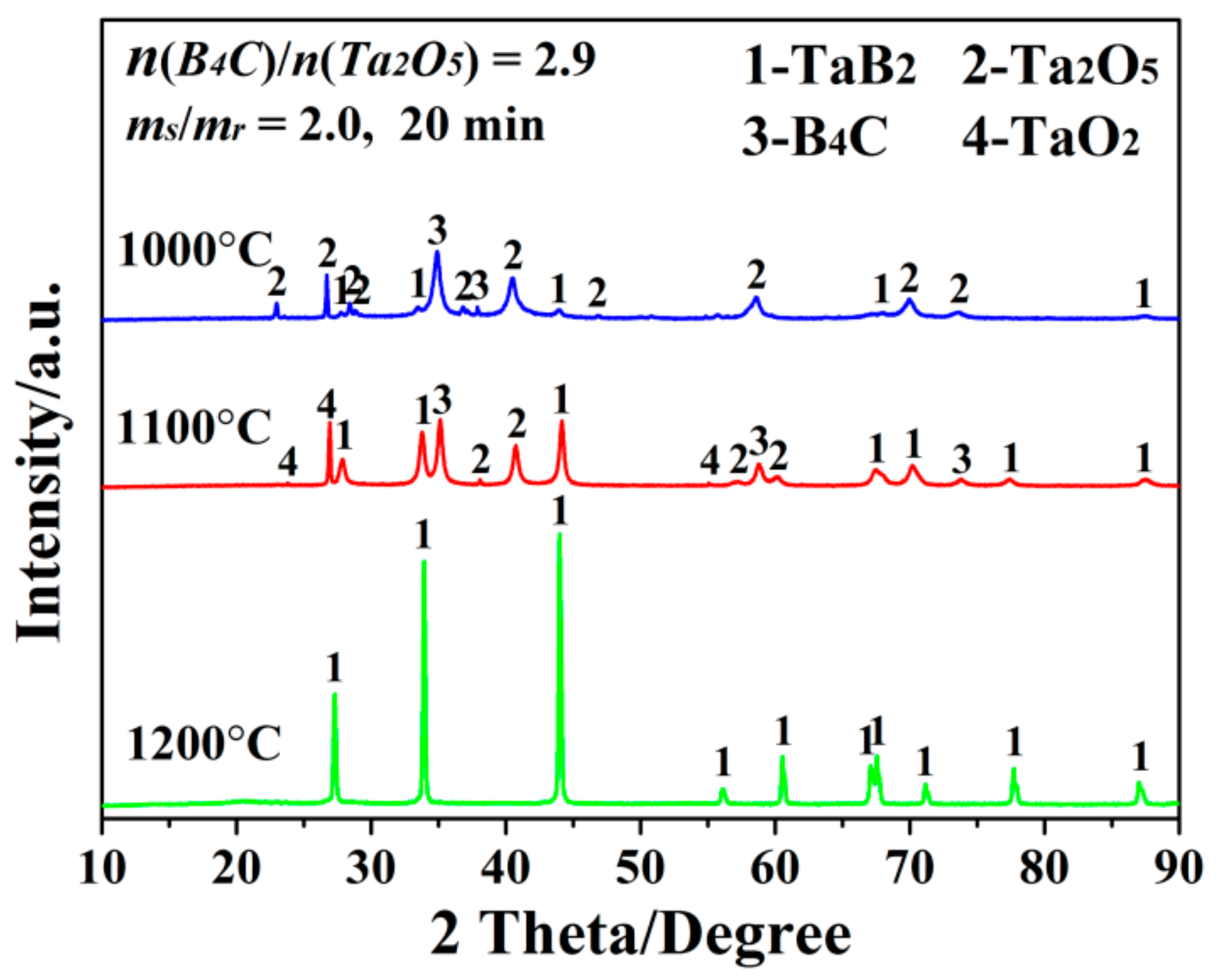
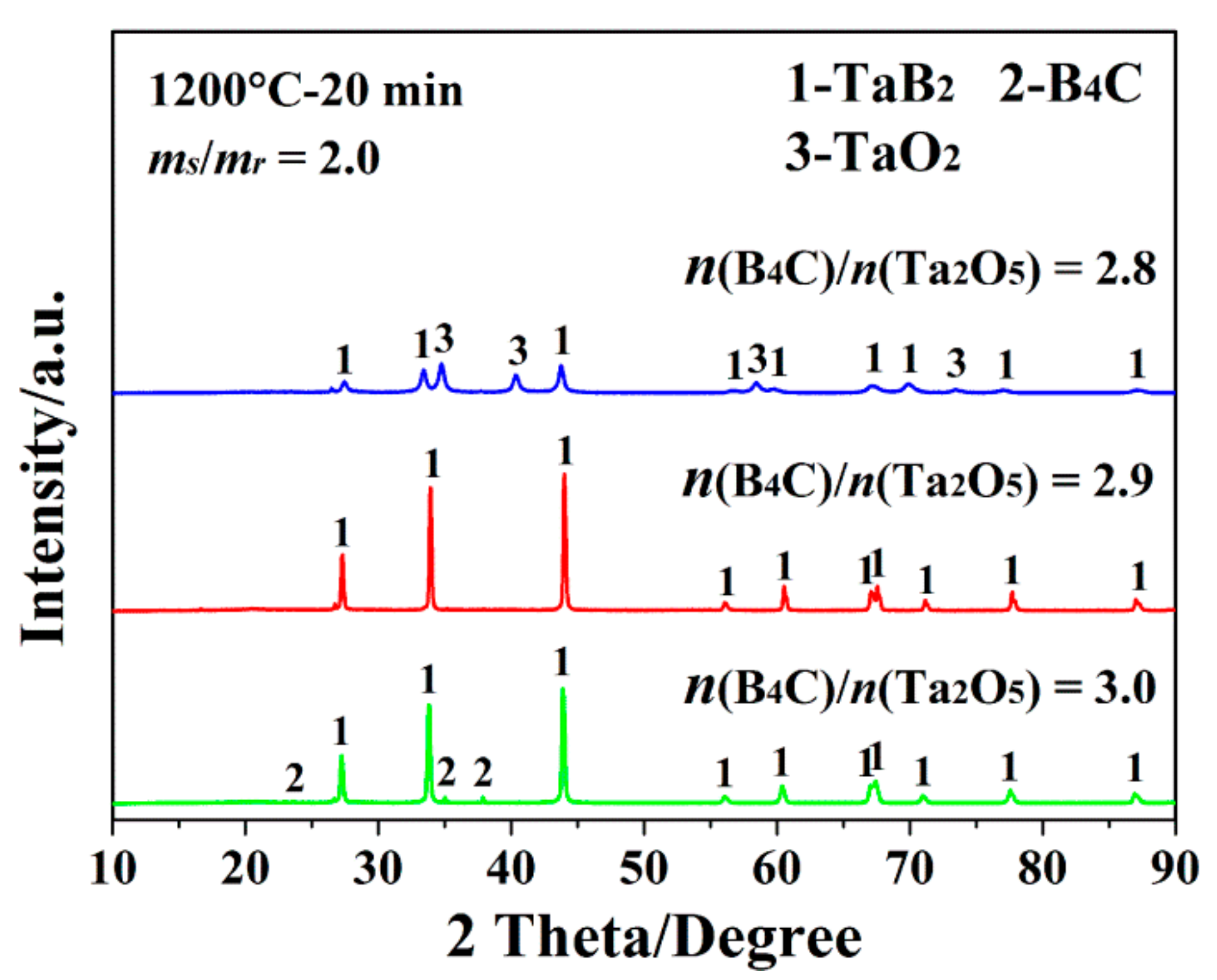
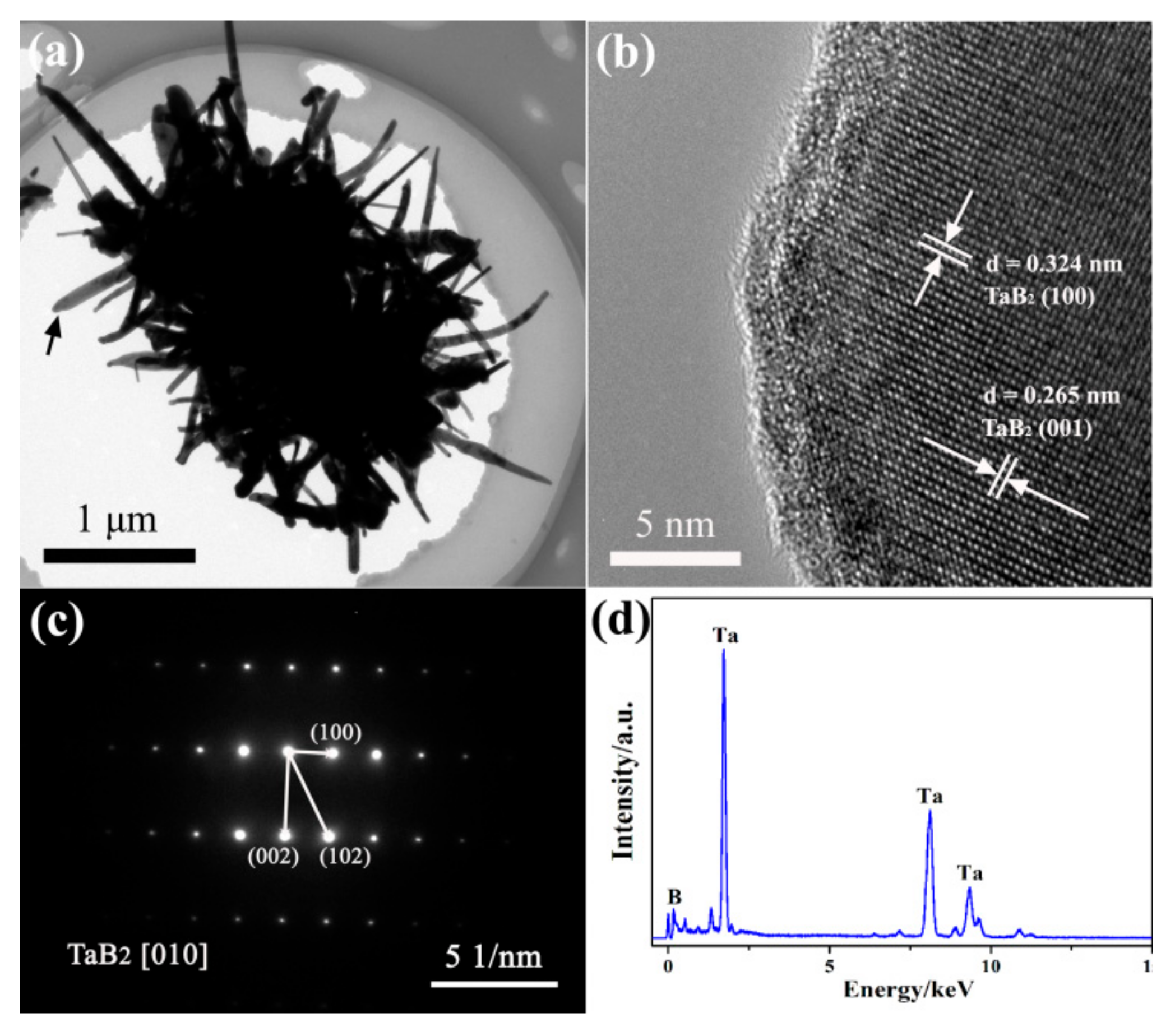
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on ultra-high temperature boride ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Monteverde, F. Resistance to thermal shock and to oxidation of metal diborides-SiC ceramics for aerospace application. J. Am. Ceram. Soc. 2007, 90, 1330–1338. [Google Scholar] [CrossRef]
- Zimmermann, W.J.; Hilmas, G.E.; Fahrenholtz, W.G. Thermal shock resistance of ZrB2 and ZrB2-30% SiC. Mater. Chem. Phys. 2008, 112, 140–145. [Google Scholar] [CrossRef]
- Paula, A.; Venugopal, S.; Binner, J.G.P.; Vaidhyanathan, B.; Heaton, A.C.J.; Brown, P.M. UHTC-carbon fibre composites: Preparation, xxyacetylene torch testing and characterisation. J. Eur. Ceram. Soc. 2013, 33, 423–432. [Google Scholar] [CrossRef]
- Zhan, G.D.; Kuntz, J.D.; Wang, J.L.; Mukherjee, A.K. Single-wall carbon nanotubes as attractive toughening agents in alumina-based nanocomposites. Nature Mater. 2003, 2, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in graphene ceramic composites. ACS Nano 2011, 5, 3182–3190. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zou, J.; Ni, D.W.; Liu, H.T.; Kan, Y.M. Boride ceramics: Densification, microstructure tailoring and properties improvement. J. Inorg. Mater. 2012, 27, 225–233. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.H.; Huang, L.; Tian, L.; Wang, S.; Zhang, G.; Li, J.Y.; Liang, F.; Zhang, H.J.; Jia, Q.L.; et al. One-step synthesis of dandelion-like lanthanum titanate nanostructures for enhanced photocatalytic performance. NPG Asia Mater. 2020, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, J.H.; Zeng, Y.; Zhang, H.J.; Li, Z. Low-temperature high-efficiency preparation of TiB2 micro-platelets via boro/carbothermal reduction in microwave heated molten salt. Materials 2019, 12, 2555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.W. High temperature ceramic materials. Materials 2021, 14, 2031. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Tian, L.; Huang, Z.; Liang, F.; Guan, K.K.; Jia, Q.L.; Zhang, H.J.; Zhang, S.W. Molten salt synthesis of carbon-doped boron nitride nanosheets with enhanced adsorption performance. Nanotechnology 2020, 31, 505606. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.M.; Zeng, L.Y.; Su, G.K.; Li, H.; Lin, H.T.; Wu, S.H. Synthesis of TaB2 powders by borothermal reduction. J. Am. Ceram. Soc. 2017, 100, 2368–2372. [Google Scholar] [CrossRef]
- Guo, W.M.; Zhang, G.J. Reaction processes and characterization of ZrB2 powder prepared by boro/carbothermal reduction of ZrO2 in vacuum. J. Am. Ceram. Soc. 2009, 92, 264–267. [Google Scholar] [CrossRef]
- Zhang, X.H.; Hilmas, G.E.; Fahrenholtz, W.G. Synthesis, densification, and mechanical properties of TaB2. Mater. Lett. 2008, 62, 4251–4253. [Google Scholar] [CrossRef]
- Sondhi, A.; Morandi, C.; Reidy, R.F.; Scharf, T.W. Theoretical and experimental investigations on the mechanism of carbothermal reduction of zirconia. Ceram. Int. 2013, 39, 4489–4497. [Google Scholar] [CrossRef]
- Ren, X.R.; Feng, P.Z.; Guo, L.T.; Tao, X.Y.; Li, Z.Y.; Shi, H.L.; Zhang, X.X.; Wang, H. Synthesis of ultra-fine TaB2 nano powders by liquid phase method. J. Am. Ceram. Soc. 2017, 100, 5358–5362. [Google Scholar] [CrossRef]
- Wei, T.T.; Liu, Z.T.; Ren, D.L.; Deng, X.G.; Deng, Q.H.; Huang, Q.; Ran, S.L. Low temperature synthesis of TaB2 nanorods by molten-salt assisted borothermal reduction. J. Am. Ceram. Soc. 2018, 101, 45–49. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Chem. Soc. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and modification of carbon nanomaterials utilizing microwave heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef]
- Liu, J.H.; Huang, Z.; Huo, C.G.; Li, F.L.; Zhang, H.J. Low-temperature rapid synthesis of rod-like ZrB2 powders by molten-salt and microwave co-assisted carbothermal reduction. J. Am. Ceram. Soc. 2016, 99, 2895–2898. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, J.H.; Liang, F.; Xu, H.Y.; Zhang, H.J.; Zhang, S.W. Highly-efficient preparation of anisotropic ZrB2-SiC powders and dense ceramics with outstanding mechanical properties. J. Am. Ceram. Soc. 2019, 102, 2426–2439. [Google Scholar]
- Shah, L.A. Low-temperature molten salt synthesis and characterization of nanowire-like TaB2 powder. JOM 2021, 73, 1023–1029. [Google Scholar] [CrossRef]
- Kuang, X.L.; Liu, T.M.; Zhang, Y.Y.; Wang, W.X.; Yang, M.P.; Zeng, W.; Hussain, S.; Peng, X.H. Urchin-like SnO2 nanoflowers via hydrothermal synthesis and their gas sensing properties. Mater. Lett. 2015, 161, 153–156. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Eid, A.I.; Selim, M.M.; EI-Safty, S.A. Portable sensitive and selective biosensing assay of dopamine in live cells using dual phosphorus and nitrogen doped carbon urchin-like structure. Chem. Eng. J. 2022, 430, 132818. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zeng, D.Q.; Zhang, K.; Lu, A.L.; Wang, L.S.; Peng, D.L. Au-ZnO hybrid nanoflowers, nanomultipods and nanopyramids: One-pot reaction synthesis and photocatalytic properties. Nanoscale 2014, 6, 874–881. [Google Scholar] [CrossRef] [PubMed]


| Sample No. | Molar Ratio | Heating Mode | Temperature (°C) | Dwelling Time (min) | Salt Medium | |
|---|---|---|---|---|---|---|
| Ta2O5 | B4C | |||||
| MSMBC-1 | 1.0 | 2.9 | MWH a | 1000 | 20 | NaCl/KCl |
| MSMBC-2 | 1.0 | 2.9 | MWH | 1100 | 20 | NaCl/KCl |
| MSMBC-3 | 1.0 | 2.9 | MWH | 1200 | 20 | NaCl/KCl |
| MSMBC-4 | 1.0 | 2.8 | MWH | 1200 | 20 | NaCl/KCl |
| MSMBC-5 | 1.0 | 3.0 | MWH | 1200 | 20 | NaCl/KCl |
| MSMBC-6 | 1.0 | 2.9 | MWH | 1200 | 20 | — |
| MSMBC-7 | 1.0 | 2.9 | CH b | 1200 | 20 | NaCl/KCl |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Liu, J.; Ye, P.; Zhang, H.; Zhang, S. Low-Temperature, Efficient Synthesis of Highly Crystalline Urchin-like Tantalum Diboride Nanoflowers. Materials 2022, 15, 2799. https://doi.org/10.3390/ma15082799
Liu D, Liu J, Ye P, Zhang H, Zhang S. Low-Temperature, Efficient Synthesis of Highly Crystalline Urchin-like Tantalum Diboride Nanoflowers. Materials. 2022; 15(8):2799. https://doi.org/10.3390/ma15082799
Chicago/Turabian StyleLiu, Delei, Jianghao Liu, Peikan Ye, Haijun Zhang, and Shaowei Zhang. 2022. "Low-Temperature, Efficient Synthesis of Highly Crystalline Urchin-like Tantalum Diboride Nanoflowers" Materials 15, no. 8: 2799. https://doi.org/10.3390/ma15082799
APA StyleLiu, D., Liu, J., Ye, P., Zhang, H., & Zhang, S. (2022). Low-Temperature, Efficient Synthesis of Highly Crystalline Urchin-like Tantalum Diboride Nanoflowers. Materials, 15(8), 2799. https://doi.org/10.3390/ma15082799







