The Influence of the Acceleration Admixture Type and Composition of Cement on Hydration Heat and Setting Time of Slag Blended Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cement
2.2. Accelerators
- A modern set and hardening acceleration admixture (symbol S) is available on the market. It contains crystal seeds in the form of C-S-H nanoparticles.
- Traditional accelerating admixture—calcium nitrate (symbol C). The 20% solution of calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) was added to reach the amount of 2% calcium nitrate per cement mass.
- The 20% sodium hydroxide solution (NaOH; symbol N) was added to reach the amount of 5% sodium hydroxide per cement mass.
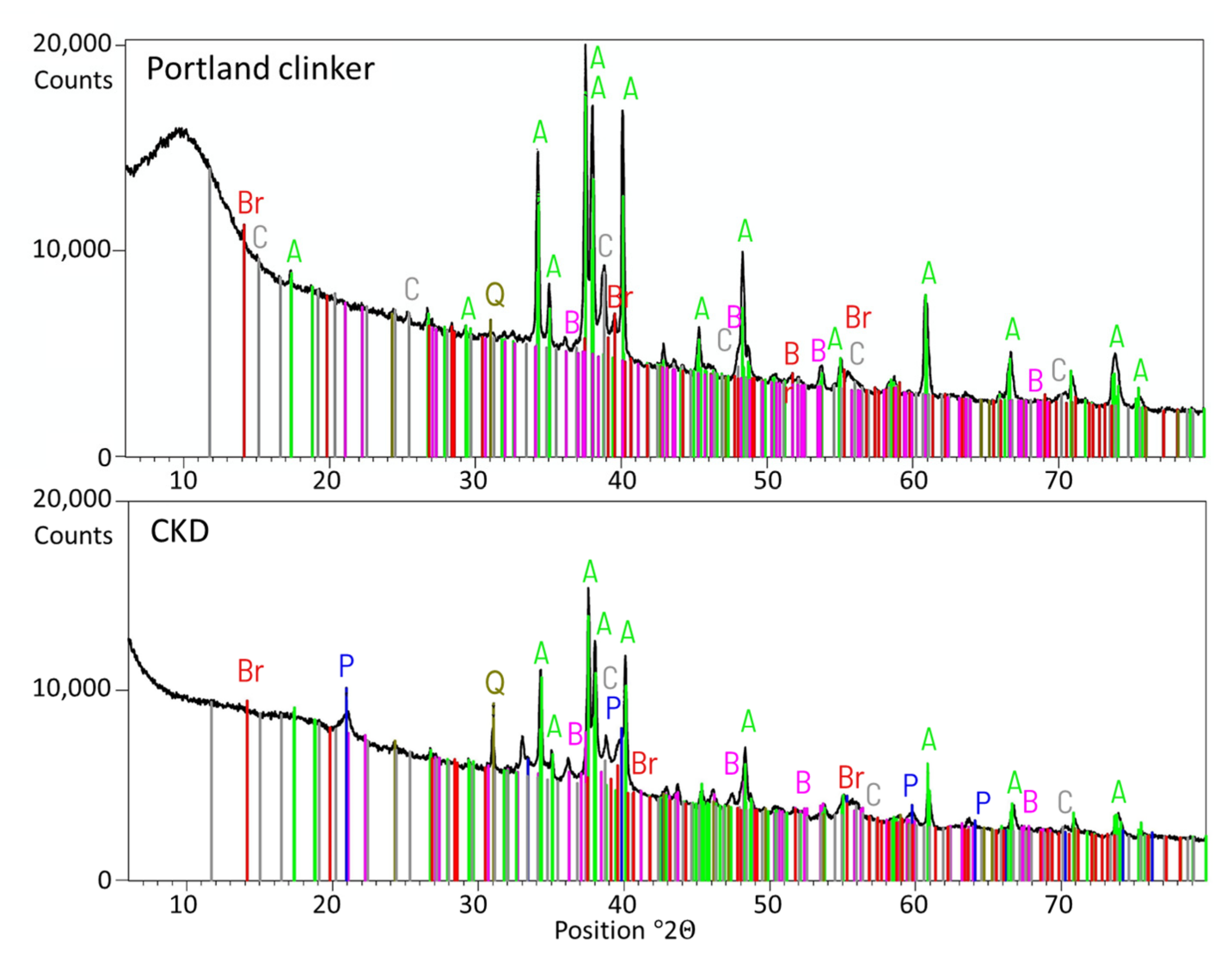
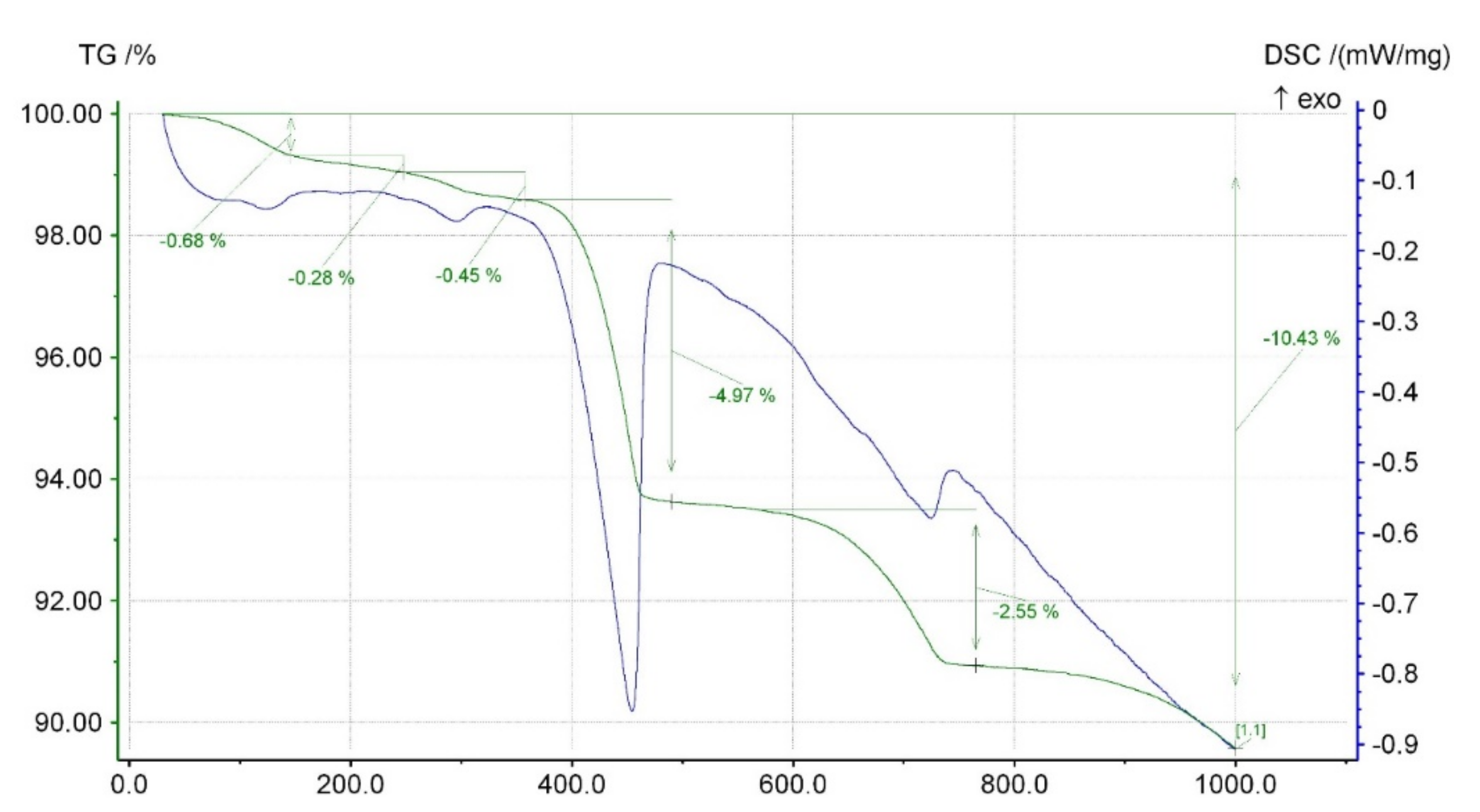
- Cement kiln dust (CKD) (symbol D) was obtained from one of the polish cement plants. CKD was introduced as an ingredient in cement in the amount of 10% of the total mass of cement. The chemical composition of CKD is given in Table 5. CKD XRD and TG-DCS analyses are presented in Figure 4 and Figure 5. The resemblance of the chemical composition described before is visible.
2.3. Mixtures
2.4. Methods
3. Results
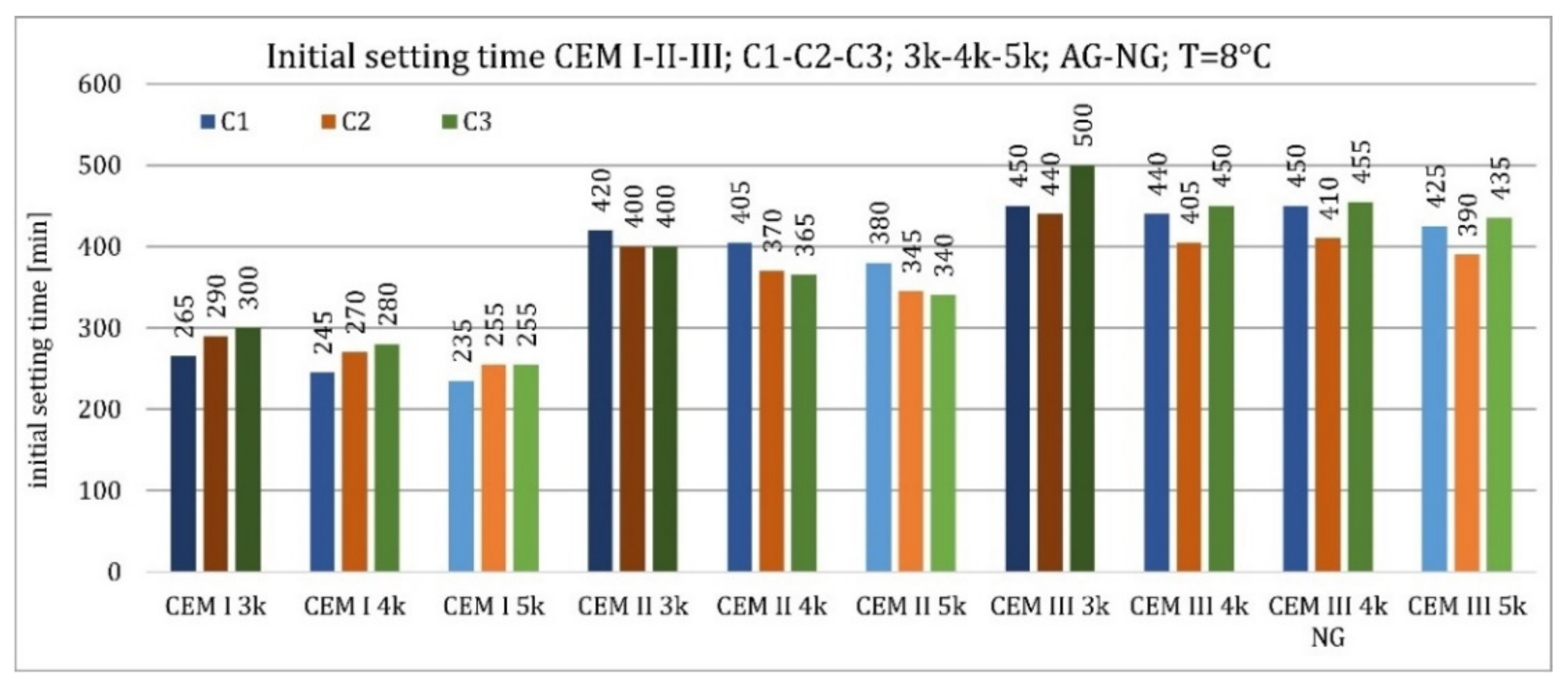
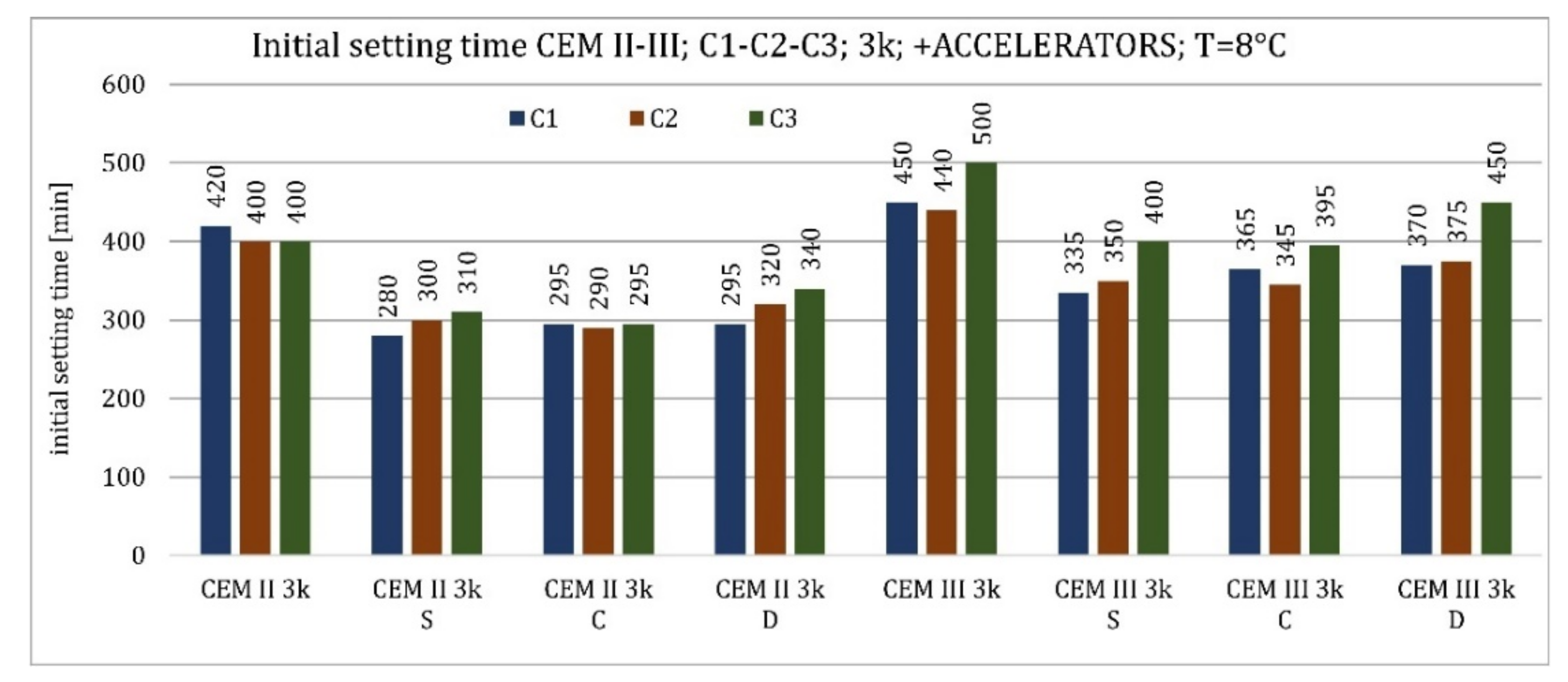
3.1. Initial Setting Time
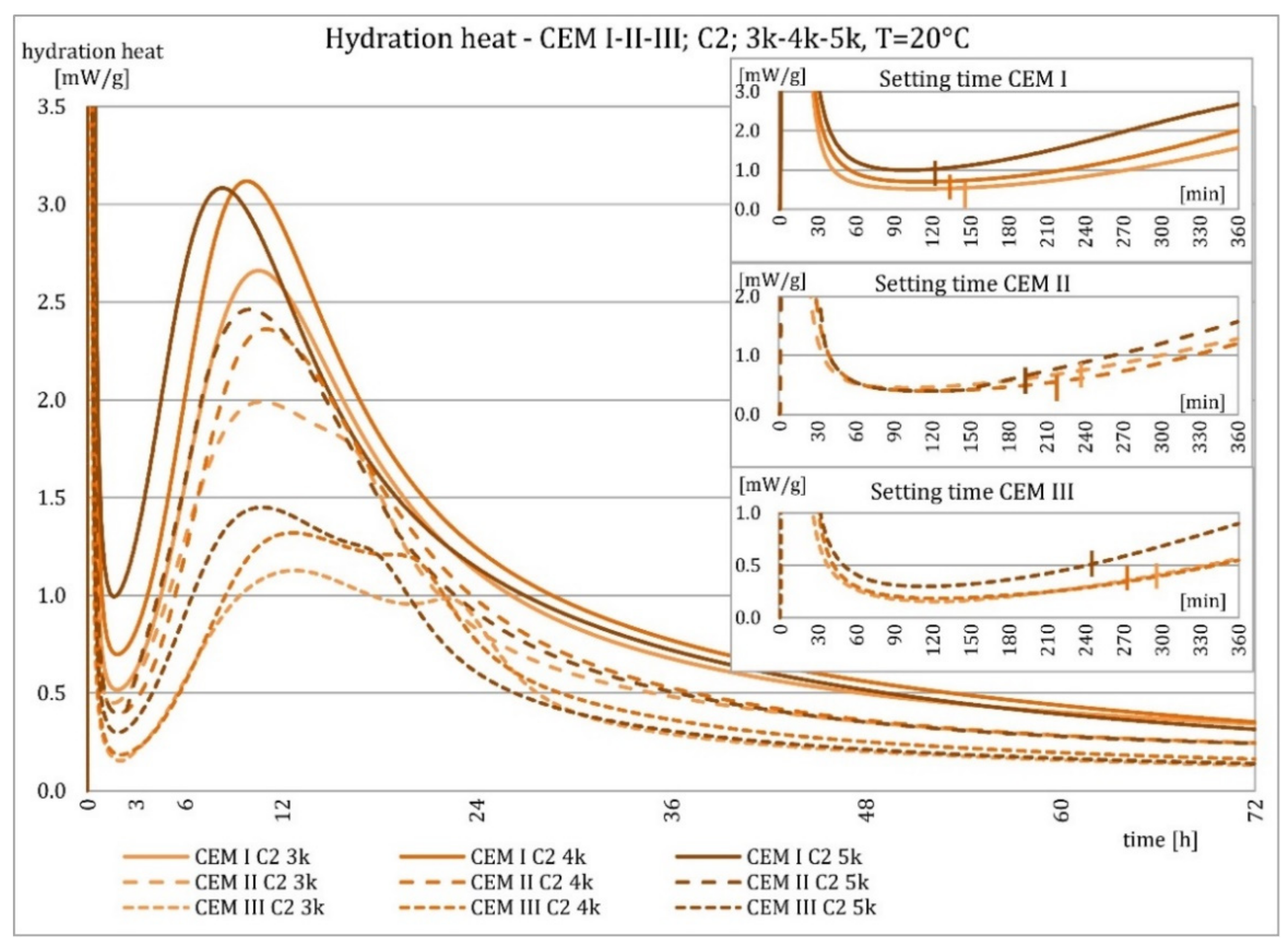
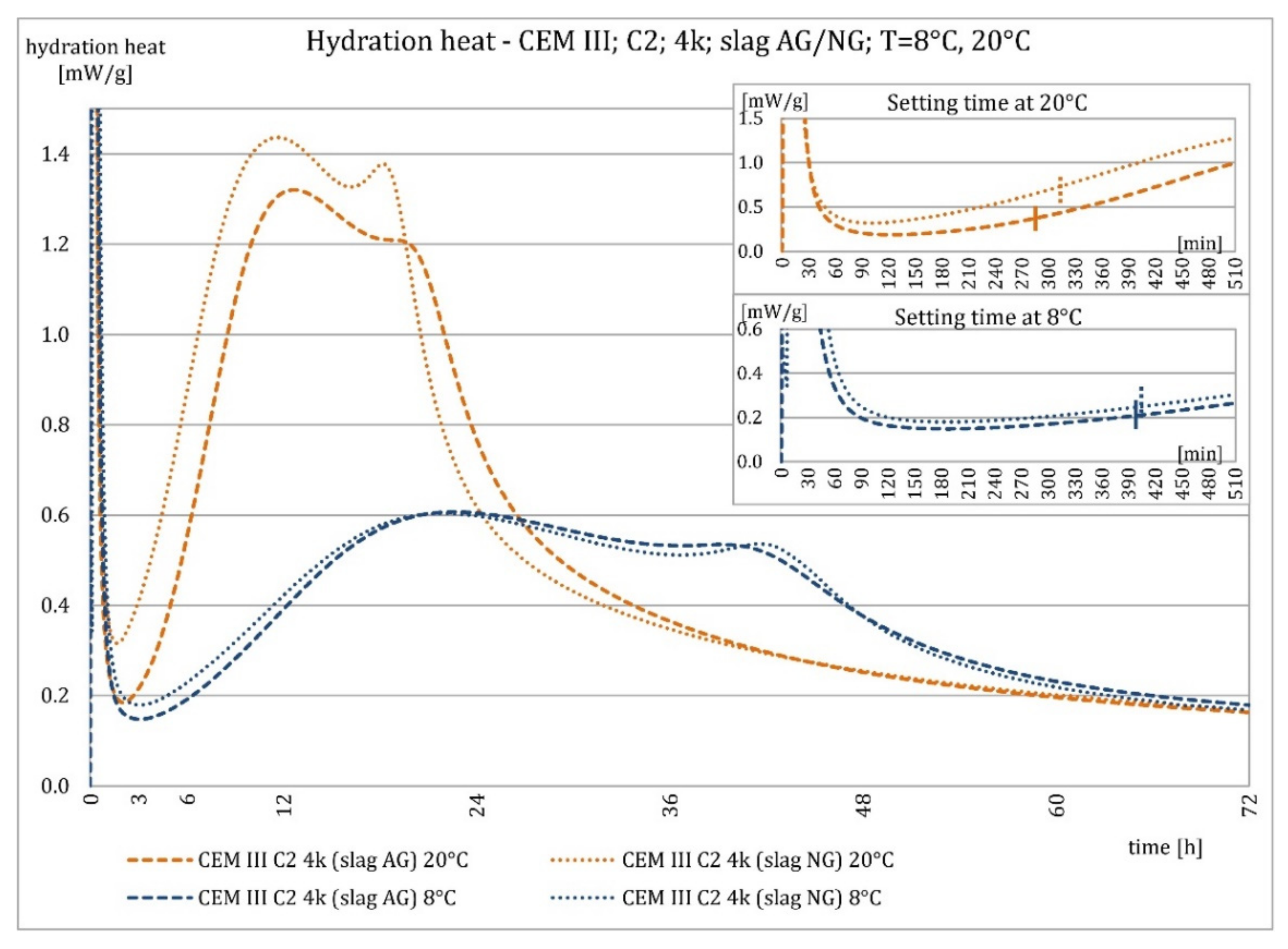
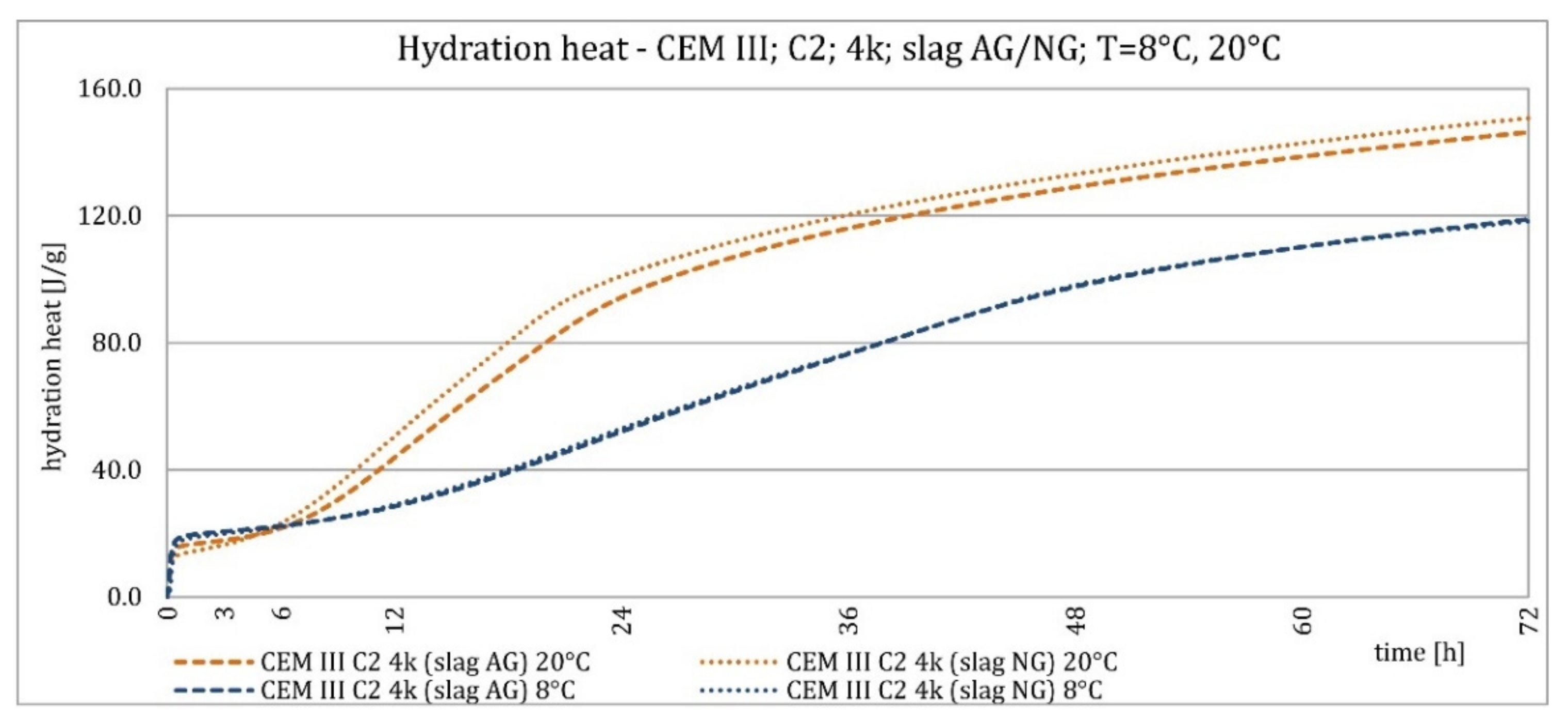
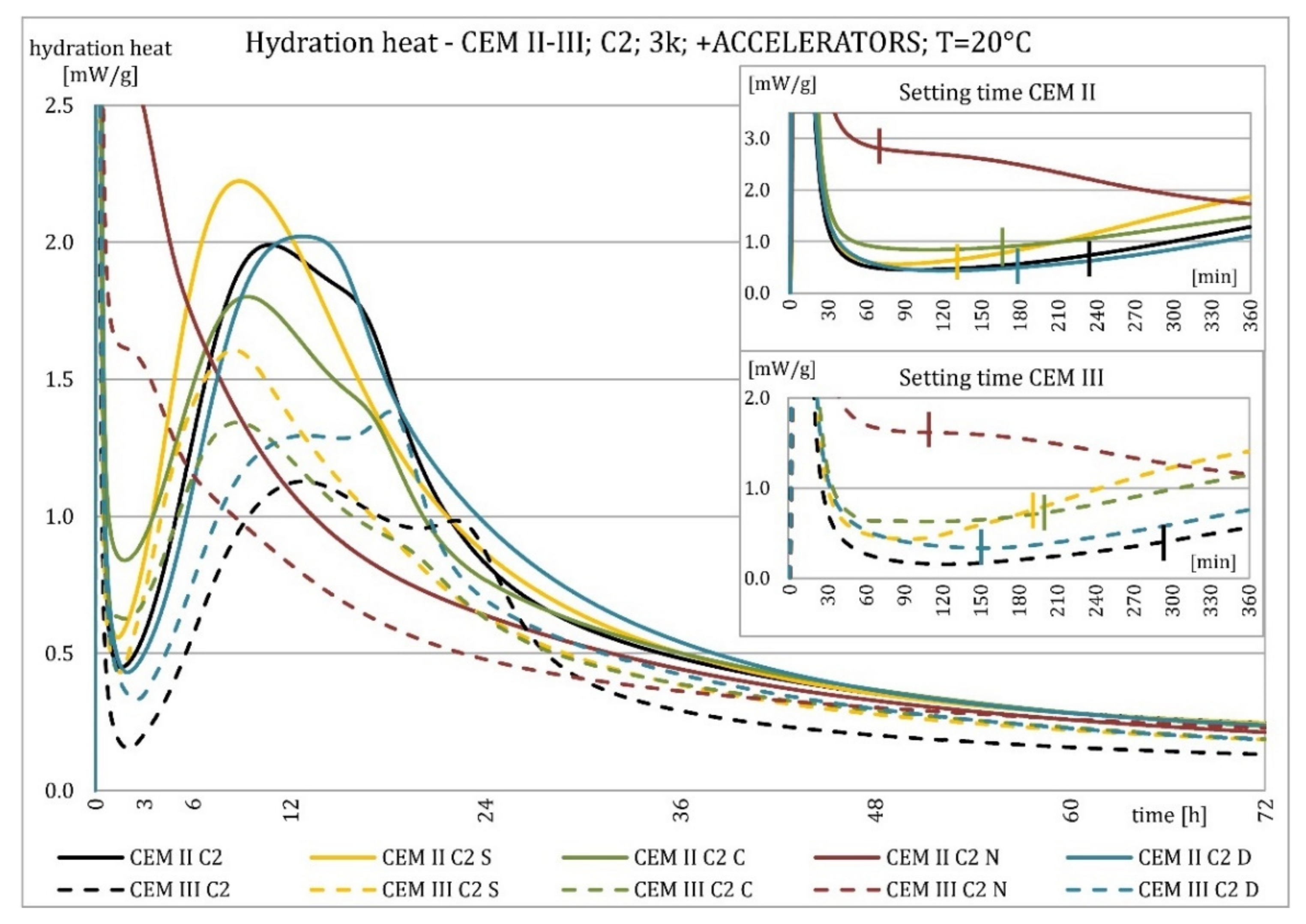
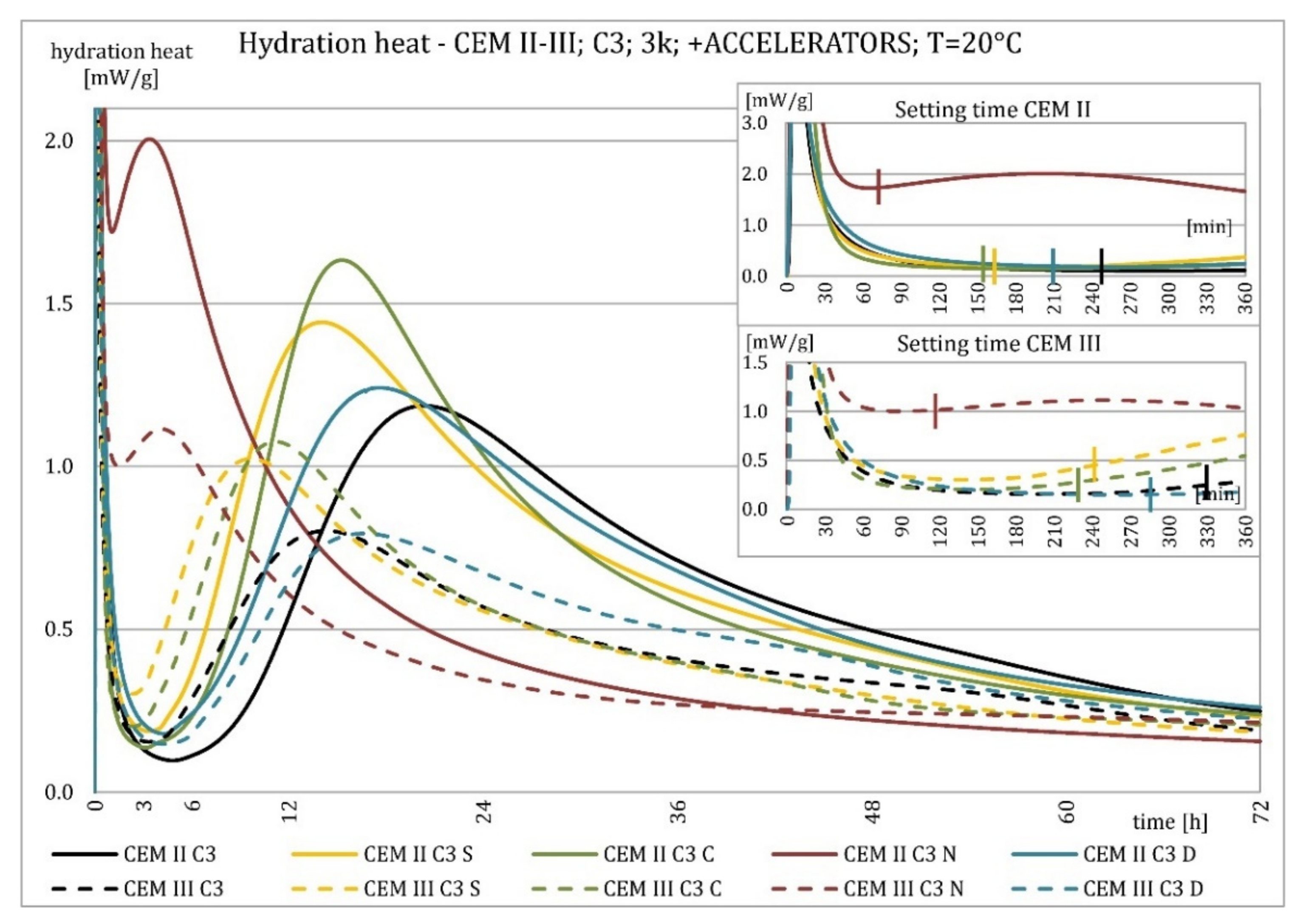
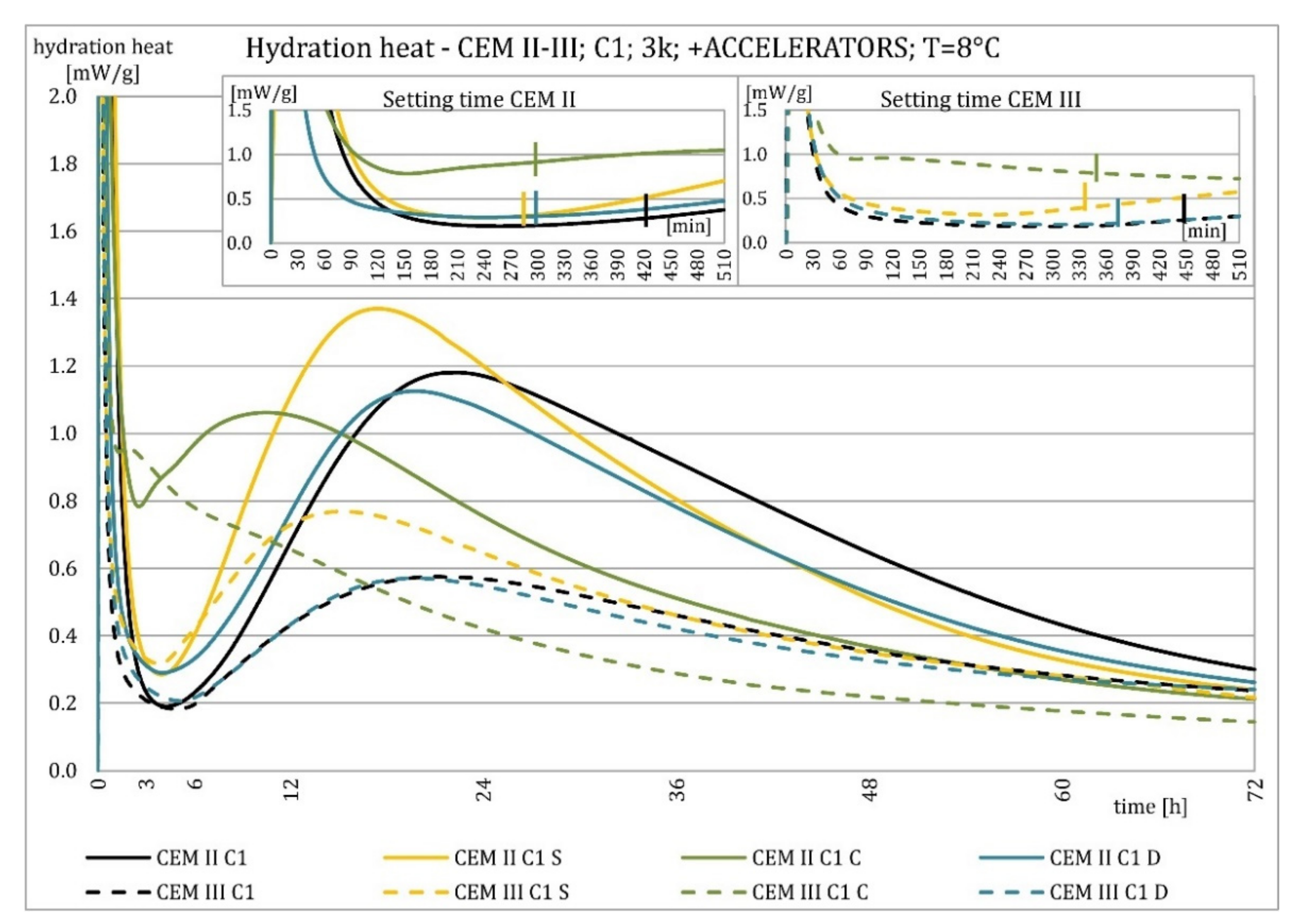
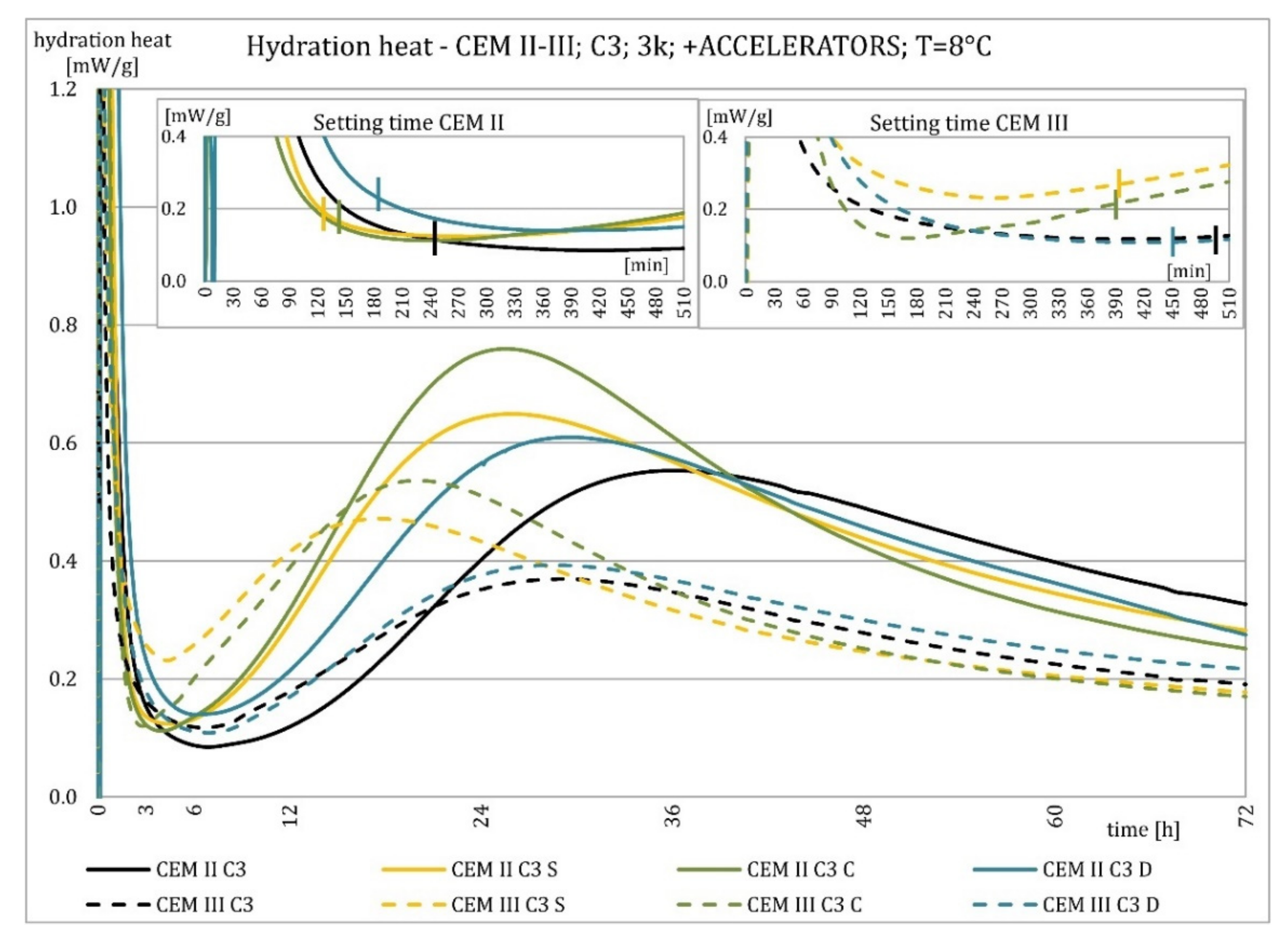
3.2. Hydration Heat Evolution
3.3. Summary
4. Discussion
4.1. Initial Setting Time
4.2. Hydration Heat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mailvaganam, N.P.; Rixom, M.; Manson, D.P.; Gonzales, C. Chemical Admixtures for Concrete, 3rd ed.; Routledge: Abingdon, UK, 1999. [Google Scholar]
- Neville, A.M. Properties of Concrete; Pearson Education Limited: London, UK, 2011; ISBN 9780273755807. [Google Scholar]
- Concrete Admixtures Handbook. Properties, Science and Technology; Ramachandran, V.S., Ed.; Noyes Publications: Park Ridge, IL, USA, 1995. [Google Scholar]
- Lee, T.; Lee, J.; Kim, Y. Effects of admixtures and accelerators on the development of concrete strength for horizontal form removal upon curing at 10 °C. Constr. Build. Mater. 2020, 237, 117652. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, S.; Chen, E.; Li, W. A review on corrosion detection and protection of existing reinforced concrete (RC) structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Justnes, H. Calcium nitrate as multifunctional concrete admixture. Concr. Plant Int. 2014, 3, 60–64. [Google Scholar]
- Reddy, P.N.; Naqash, J.A. Development of high early strength in concrete incorporating alccofine and non-chloride accelerator. SN Appl. Sci. 2019, 1, 755. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Luo, B.; Luo, Z.; Huang, H.; Li, J.; Wang, D. Effect of accelerators on the workability, strength, and microstructure of ultra-high-performance concrete. Materials 2021, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, A.; Choi, H.; Inoue, M.; Kim, J.; Lim, M.; Sudoh, Y. Effect of a nitrite/nitrate-based accelerator on the strength development and hydrate formation in cold-weather cementitious materials. Materials 2021, 14, 1006. [Google Scholar] [CrossRef]
- Choi, H.; Inoue, M.; Choi, H.; Kim, J.; Sudoh, Y.; Kwon, S.; Lee, B.; Yoneyama, A. Physicochemical study on the strength development characteristics of cold weather concrete using a nitrite-nitrate based accelerator. Materials 2019, 12, 2706. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Guo, H.; Tan, H.; He, X.; Zheng, Z.; Su, Y.; Yang, J. An accelerator prepared from waste concrete recycled powder and its effect on hydration of cement-based materials. Constr. Build. Mater. 2021, 296, 123767. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Li, H. Calcium-silicate-hydrate seeds as an accelerator for saving energy in cold weather concreting. Constr. Build. Mater. 2020, 264, 120191. [Google Scholar] [CrossRef]
- Yang, H.; Yan, Y.; Hu, Z. The preparation of nano calcium carbonate and calcium silicate hardening accelerator from marble waste by nitric acid treatment and study of early strength effect of calcium silicate on C30 concrete. J. Build. Eng. 2020, 32, 101507. [Google Scholar] [CrossRef]
- Das, S.; Ray, S.; Sarkar, S. Early strength development in concrete using preformed CSH nano crystals. Constr. Build. Mater. 2020, 233, 117214. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.; He, X.; Su, Y.; Tan, H.; Strnadel, B. Nano-carbide slag seed as a new type accelerator for Portland cement. Mater. Lett. 2020, 278, 128464. [Google Scholar] [CrossRef]
- Miah, J.; Patoary, M.H.; Paul, S.C.; Babafemi, A.J.; Panda, B. Enhancement of mechanical properties and porosity of concrete using steel slag coarse aggregate. Materials 2020, 13, 2865. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Gołaszewska, M.; Giergiczny, Z. Study of the properties of blended cements containing various types of slag cements and limestone powder. Materials 2021, 14, 6072. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Xu, Z.; Li, S.; Luo, X.; Chen, G. Long-term hydration and microstructure evolution of blended cement containing ground granulated blast furnace slag and waste clay brick. Cem. Concr. Compos. 2021, 118, 103982. [Google Scholar] [CrossRef]
- Klemczak, B.; Batog, M. Heat of hydration of low-clinker cements: Part I. Semi-adiabatic and isothermal tests at different temperature. J. Therm. Anal. Calorim. 2016, 123, 1351–1360. [Google Scholar] [CrossRef]
- Park, K.-B.; Wang, Y.-S.; Wang, X.-Y. Property analysis of slag composite concrete using a kinetic-thermodynamic hydration model. Appl. Sci. 2021, 11, 7191. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Su, Y.; Tan, H.; Yang, J.; Lan, M.; Ma, M.; Strnadel, B. Self-hydration characteristics of ground granulated blast-furnace slag (GGBFS) by wet-grinding treatment. Constr. Build. Mater. 2018, 167, 96–105. [Google Scholar] [CrossRef]
- Hussain, F.; Kaur, I.; Hussain, A. Reviewing the influence of GGBFS on concrete properties. Mater. Today Proc. 2020, 32, 997–1004. [Google Scholar] [CrossRef]
- Choi, S.; Pyo, S. Fresh and Hardened Properties of Portland Cement-Slag Concrete Activated Using the By-Product of the Liquid Crystal Display Manufacturing Process. Materials 2020, 13, 4354. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.M.; Rahman, R.; Phing, C.Y.W.; Chie, A.W.M.; Bin Bakri, M.K. The curing times effect on the strength of ground granulated blast furnace slag (GGBFS) mortar. Constr. Build. Mater. 2020, 260, 120622. [Google Scholar] [CrossRef]
- Norrarat, P.; Tangchirapat, W.; Jaturapitakkul, C. Evaluation of heat evolution of pastes containing high volume of ground river sand and ground granulated blast furnace slag. Mater. Sci. 2017, 23, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Mehdipour, I.; Khayat, K.H. Effect of particle-size distribution and specific surface area of different binder systems on packing density and flow characteristics of cement paste. Cem. Concr. Compos. 2017, 78, 120–131. [Google Scholar] [CrossRef]
- Ehikhuenmen, S.O.; Igba, U.T.; Balogun, O.O.; Oyebisi, S.O. The influence of cement fineness on the structural characteristics of normal concrete. IOP Conf. Ser. Mater. Sci. Eng. 2019, 640, 012043. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, T.; Li, H.; Wu, S. Exploring the Influence Factors of Early Hydration of Ultrafine Cement. Materials 2021, 14, 5677. [Google Scholar] [CrossRef]
- Newman, J.; Choo, B.S. Advanced Concrete Technology; Elsevier LTD: Oxford, UK, 2003. [Google Scholar]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Hesse, C.; Goetz-Neunhoeffer, F.; Neubauer, J. A new approach in quantitative in-situ XRD of cement pastes: Correlation of heat flow curves with early hydration reactions. Cem. Concr. Res. 2011, 41, 123–128. [Google Scholar] [CrossRef]
- Shariq, M.; Prasad, J.; Abbas, H. Creep and Drying Shrinkage of Concrete Containing {GGBFS}. Cem. Concr. Compos. 2016, 68, 35–45. [Google Scholar] [CrossRef]
- Yousuf, F.; Xiaosheng, W. Early strength development and hydration of cement pastes at different temperatures or with superplasticiser characterised by electrical resistivity. Case Stud. Constr. Mater. 2022, 16, e00911. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, S.K.; Singh, N. Heat evolution during the hydration of Portland cement in the presence of fly ash, calcium hydroxide and super plasticizer. Thermochim. Acta 2012, 548, 27–32. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Pu, S.; Zhang, Z. Hydration heat and kinetics of composite binder containing blast furnace ferronickel slag at different temperatures. Thermochim. Acta 2021, 702, 178985. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, Z. Hydration mechanism of composite binders containing blast furnace ferronickel slag at different curing temperatures. J. Therm. Anal. 2018, 131, 2291–2301. [Google Scholar] [CrossRef]
- Wang, D.-H.; Yao, X.; Yang, T.; Xiang, W.-R.; Feng, Y.-T.; Chen, Y. Controlling the early-age hydration heat release of cement paste for deep-water oil well cementing: A new composite designing approach. Constr. Build. Mater. 2021, 285, 122949. [Google Scholar] [CrossRef]
- Pedrosa, H.C.; Reales, O.M.; Reis, V.D.; Paiva, M.D.D.; Fairbairn, E.M.R. Hydration of Portland cement accelerated by C-S-H seeds at different temperatures. Cem. Concr. Res. 2020, 129, 105978. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Xu, K.; Monteiro, P.J. Fibrillar calcium silicate hydrate seeds from hydrated tricalcium silicate lower cement demand. Cem. Concr. Res. 2020, 137, 106195. [Google Scholar] [CrossRef]
- Zhao, W.; Ji, X.; Jiang, Y.; Pan, T. Effect of C-S-H nucleating agent on cement hydration. Appl. Sci. 2021, 11, 6638. [Google Scholar] [CrossRef]
- Zou, F.; Hu, C.; Wang, F.; Ruan, Y.; Hu, S. Enhancement of early-age strength of the high content fly ash blended cement paste by sodium sulfate and C–S–H seeds towards a greener binder. J. Clean. Prod. 2020, 244, 118566. [Google Scholar] [CrossRef]
- Szostak, B.; Golewski, G.L. Improvement of strength parameters of cement matrix with the addition of siliceous fly ash by using nanometric C-S-H seeds. Energies 2020, 13, 6734. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Illikainen, M. Improved strength development and frost resistance of Portland cement ground-granulated blast furnace slag binary binder cured at 0 °C with the addition of calcium silicate hydrate seeds. J. Build. Eng. 2021, 48, 103904. [Google Scholar] [CrossRef]
- Land, G.; Stephan, D. The effect of synthesis conditions on the efficiency of C-S-H seeds to accelerate cement hydration. Cem. Concr. Compos. 2018, 87, 73–78. [Google Scholar] [CrossRef]
- Kičaitė, A.; Pundienė, I.; Skripkiūnas, G. The influence of calcium nitrate on setting and hardening rate of Portland cement concrete at different temperatures. IOP Conf. Series: Mater. Sci. Eng. 2017, 251, 12017. [Google Scholar] [CrossRef]
- Kicaite, A. The effect of calcium nitrate on the properties of portland cement pastes and concrete hardening at low temperatures. Ceram. Silik. 2020, 64, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Siddique, R. Utilization of cement kiln dust (CKD) in cement mortar and concrete—an overview. Resour. Conserv. Recycl. 2006, 48, 315–338. [Google Scholar] [CrossRef]
- Siddique, R. Cement kiln dust. In Waste Materials and By-Products in Concrete; Springer: Berlin/Heidelberg, Germany, 2008; pp. 351–380. [Google Scholar]
- Stryczek, S.; Gonet, A.; Czapik, P. Kształtowanie Właściwości Technologicznych Zaczynów Uszczelniających Za Pomocą Pyłów Cementowych. Wiert. Naft. Gaz. 2009, 26, 345–354. [Google Scholar]
- Kalina, L.; Bílek, V., Jr.; Kiripolský, T.; Novotný, R.; Másilko, J. Cement kiln by-pass dust: An effective alkaline activator for pozzolanic materials. Materials 2018, 11, 1770. [Google Scholar] [CrossRef] [Green Version]
- Colangelo, F.; Cioffi, R. Use of cement kiln dust, blast furnace slag and marble sludge in the manufacture of sustainable artificial aggregates by means of cold bonding pelletization. Materials 2013, 6, 3139–3159. [Google Scholar] [CrossRef] [Green Version]
- Lachemi, M.; Hossain, K.M.A.; Shehata, M.; Thaha, W. Controlled low strength materials incorporating cement kiln dust from various sources. Cem. Concr. Compos. 2008, 30, 381–392. [Google Scholar] [CrossRef]
- Hassan, I.H.; Abdul-Kareem, O.M.; Shihab, A.Y. Utilization of Cement Kiln Dust (CKD) as a partial replacement of cement in mortar and concrete. Coll. Eng. Univ. Mosul 2013, 21, 72–87. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Shah, S.P. Hydration and properties of novel blended cements based on cement kiln dust and blast furnace slag. Cem. Concr. Res. 2003, 33, 1269–1276. [Google Scholar] [CrossRef]
- Elhelloty, A.E.; Nooman, M.T.; Abdelwahab, R.K.; Abdullah, A. Utilization of Cement Kiln Dust (CKD) with Silica Fume (SF) as a partial replacement of cement in concrete production. J. Miner. Mater. Charact. Eng. 2019, 7, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Dave, N.; Misra, A.K.; Srivastava, A.; Kaushik, S.K. Experimental analysis of strength and durability properties of quaternary cement binder and mortar. Constr. Build. Mater. 2016, 107, 117–124. [Google Scholar] [CrossRef]
- Alharthi, Y.M.; Elamary, A.S.; Abo-El-Wafa, W. Performance of plain concrete and cement blocks with cement partially replaced by cement kiln dust. Materials 2021, 14, 5647. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, K.; Saravana, K.R.M. Effect of sodium hydroxide concentration and alkaline ratio on the compressive strength of slag based geopolymer concrete. Int. J. Chem. Technol. Res. 2014, 6, 2446–2450. [Google Scholar]
- Hashim, A.N.; Hussin, K.; Begum, N.; Abdullah, M.M.A.B.; Razak, K.A.; Ekaputri, J. Effect of Sodium Hydroxide (NaOH) concentration on compressive strength of Alkali-Activated Slag (AAS) mortars. Appl. Mech. Mater. 2015, 754-755, 300–304. [Google Scholar] [CrossRef]
- Mohamed, O.A. A review of durability and strength characteristics of alkali-activated slag concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, B. Hydration and properties of slag cement activated by alkali and sulfate. J. Mater. Civ. Eng. 2017, 29, 04017091. [Google Scholar] [CrossRef]
- Pizoń, J.; Łaźniewska-Piekarczyk, B. Microstructure of CEM II/B-S pastes modified with set accelerating admixtures. Materials 2021, 14, 6300. [Google Scholar] [CrossRef]
- George, S. Flexural behaviour of activated fly ash concrete. Int. J. Eng. Sci. Technol. 2011, 3, 7633–7642. [Google Scholar]
- Phoo-Ngernkham, T.; Hanjitsuwan, S.; Damrongwiriyanupap, N.; Chindaprasirt, P. Effect of sodium hydroxide and sodium silicate solutions on strengths of alkali activated high calcium fly ash containing Portland cement. KSCE J. Civ. Eng. 2017, 21, 2202–2210. [Google Scholar] [CrossRef]
- Pizon, J.; Lazniewska-Piekarczyk, B. Efficiency assessment of accelerating admixtures and cement kiln dust with cooperation with different phase composition slag blended cements. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; IOP Publishing: Bristol, UK, 2019; Volume 603, p. 032088. [Google Scholar]
- Pizon, J.; Lazniewska-Piekarczyk, B. Microstructure of high C3A portland slag cement pastes, modified with accelerating admixtures for concrete. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; IOP Publishing: Bristol, UK, 2019; Volume 603, p. 032089. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997; pp. 271–272. [Google Scholar]
- Gu, X.; Tan, H.; He, X.; Smirnova, O.; Zhang, J.; Luo, Z. Utilization of carbide slag by wet grinding as an accelerator in calcium sulfoaluminate cement. Materials 2020, 13, 4526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ye, H.; Yuan, Q.; Li, B.; Li, Y.; Zhou, D. Factors influencing the hydration, dimensional stability, and strength development of the OPC-CSA-anhydrite ternary system. Materials 2021, 14, 7001. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Khan, M.I. (Eds.) Supplementary Cementing Materials; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Myrdal, R. Accelerating Admixtures for Concrete; SINTEF Report N SBF BK A07025; SINTEF Industri: Trondheim, Norway, 2007. [Google Scholar]
- Benzer, H.; Aydogan, N.; Dundar, H.; Lynch, A. Reducing The Energy Required in Grinding Clinker To Cement: Some Case Studies. Available online: https://www.scribd.com/doc/234610255/Reducing-the-Energy-Required-in-Grinding-Clinker-to-Cement-Benzer-Aydogan-Dundar-and-Lynch (accessed on 11 February 2022).




















| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Cl− | Na2O | K2O | Ign. Loss. | Blaine’s Specific Surface Area |
|---|---|---|---|---|---|---|---|---|---|---|
| 37.35 | 7.30 | 1.22 | 43.90 | 5.73 | 0.62 | 0.03 | 0.55 | 0.56 | 0.17 | Symbol NG: 3200 cm2/g Symbol AG: 3870 cm2/g |
| Symbol | C2S | C3S | C3A | C4AF |
|---|---|---|---|---|
| C1 | 10.9 | 64.4 | 13.5 | 7.3 |
| C2 | 12.4 | 68.7 | 4.0 | 12.1 |
| C3 | 18.3 | 58.0 | 2.4 | 17.5 |
| Sym. | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Cl− | Na2O | K2O | CaOfree | Ignition Losses | Blaine’s Specific Surface Area |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 20.25 | 6.83 | 3.23 | 65.66 | 1.39 | 0.69 | 0.01 | 0.15 | 1.02 | 2.80 | 0.15 | 3000; 4000; 5000 cm2/g |
| C2 | 21.25 | 5.00 | 3.40 | 64.81 | 2.09 | 0.55 | 0.03 | 0.11 | 1.03 | 1.48 | 0.35 | 3000; 4000; 5000 cm2/g |
| C3 | 21.62 | 4.58 | 5.75 | 65.38 | 0.79 | 0.27 | 0.01 | 0.30 | 0.25 | 0.95 | 0.25 | 3000; 4000; 5000 cm2/g |
| SiO2 | CaO | MgO | SO3 | Na2O | Ignition Losses |
|---|---|---|---|---|---|
| 0.61 | 40.16 | 0.40 | 54.83 | 0.02 | 2.71 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Cl− | Na2O | K2O | P2O5 | TiO2 | Mn2O3 | SrO | ZnO | Ignition Losses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18.16 | 4.55 | 2.03 | 57.13 | 1.26 | 1.95 | 1.44 | 0.12 | 2.38 | 0.12 | 0.22 | 0.06 | 0.10 | 0.24 | 11.70 |
| Cement Type | Portland Clinker | GGBFS | Anhydrite | Water | CKD | Admixture | |||
|---|---|---|---|---|---|---|---|---|---|
| type | [g] | [g] | [g] | [g] | type | [% m.c.] | [g] | ||
| CEM I | C1 | 439.1 | - | 10.9 | Amount of water depends on cement type and fineness, accelerator used and temperature during the test. Results given in Table 7. | - | - | - | - |
| C2 | 438.0 | - | 12.0 | - | - | - | - | ||
| C3 | 435.7 | - | 14.3 | - | - | - | - | ||
| CEM II | C1 | 285.3 | 153.6 | 11.07 | - | - | - | - | |
| S | 4.0 | 18.0 | |||||||
| C | 2.0 | 9.0 | |||||||
| N | 5.0 | 22.5 | |||||||
| 256.8 | 138.2 | 10.0 | 45.0 | D | - | - | |||
| C2 | 284.8 | 153.4 | 11.8 | - | - | - | - | ||
| S | 4.0 | 18.0 | |||||||
| C | 2.0 | 9.0 | |||||||
| N | 5.0 | 22.5 | |||||||
| 256.3 | 138.0 | 10.7 | 45.0 | D | - | - | |||
| C3 | 283.9 | 152.9 | 13.3 | - | - | - | |||
| S | 4.0 | 18.0 | |||||||
| C | 2.0 | 9.0 | |||||||
| N | 5.0 | 22.5 | |||||||
| 255.5 | 137.6 | 12.0 | 45.0 | D | - | - | |||
| CEM III | C1 | 153.6 | 285.2 | 11.3 | - | - | - | - | |
| S | 4.0 | 18.0 | |||||||
| C | 2.0 | 9.0 | |||||||
| N | 5.0 | 22.5 | |||||||
| 138.2 | 256.7 | 10.1 | 45.0 | D | - | - | |||
| C2 | 153.4 | 284.9 | 11.7 | - | - | - | - | ||
| S | 4.0 | 18.0 | |||||||
| C | 2.0 | 9.0 | |||||||
| N | 2.0 | 22.5 | |||||||
| 138.1 | 256.4 | 10.5 | 45.0 | D | - | - | |||
| C3 | 153.1 | 284.4 | 12.5 | - | - | - | |||
| S | 4.0 | 18.0 | |||||||
| C | 2.0 | 9.0 | |||||||
| N | 5.0 | 22.5 | |||||||
| 137.8 | 256.0 | 11.2 | 45.0 | D | - | - | |||
| Water Amount [g] | ||||||
| Clinker | C1 | C2 | C3 | C1 | C2 | C3 |
| Temperature | 20 °C | 8 °C | ||||
| Symbol of cement | ||||||
| CEM I C * 3k ** | 193 | 147 | 120 | 188 | 139 | 112 |
| CEM I Cx4k | 197 | 168 | 121 | 186 | 160 | 115 |
| CEM I Cx5k | 203 | 172 | 129 | 191 | 162 | 119 |
| CEM II Cx3k | 195 | 134 | 116 | 190 | 124 | 111 |
| CEM II Cx4k | 197 | 138 | 118 | 189 | 128 | 106 |
| CEM II Cx5k | 198 | 148 | 124 | 192 | 136 | 113 |
| CEM III C× 3k | 135 | 135 | 122 | 128 | 129 | 113 |
| CEM III Cx 4k | 136 | 137 | 122 | 124 | 131 | 117 |
| CEM III Cx 4k NG *** | 135 | 135 | 122 | 122 | 128 | 117 |
| CEM III Cx 5k | 146 | 143 | 124 | 137 | 132 | 117 |
| CEM II Cx 3k S **** | 196 | 135 | 110 | 190 | 125 | 103 |
| CEM II Cx 3k C | 152 | 135 | 125 | 146 | 126 | 116 |
| CEM II Cx 3k N | 181 | 167 | 142 | not tested at 8 °C | ||
| CEM II Cx 3k D | 146 | 133 | 119 | 140 | 125 | 110 |
| CEM III Cx 3k S | 129 | 123 | 114 | 119 | 116 | 106 |
| CEM III Cx 3k C | 135 | 130 | 128 | 126 | 123 | 120 |
| CEM III Cx 3k N | 158 | 145 | 144 | not tested at 8 °C | ||
| CEM III Cx 3k D | 133 | 130 | 123 | 123 | 118 | 117 |
| Temperature | Clinker | Cement | Accelerator | Influence On | ||||
|---|---|---|---|---|---|---|---|---|
| Initial Setting Time | Dormant Period Length | Maximum Value in Post-Dormant Period | Time of Maximum Occurrence | Heat after 72 h | ||||
| 20 °C | C1 | II/B-S | S | - | - | + | ← | + |
| C | - | - | - | ← | 0 | |||
| N | - | - | + | ← | - | |||
| D | - | - | + | ← | 0 | |||
| III/A | S | - | - | + | ← | + | ||
| C | - | - | 0 | ← | - | |||
| N | - | - | + | ← | - | |||
| D | - | 0 | 0 | 0 | - | |||
| C2 | II/B-S | S | - | - | + | ← | + | |
| C | - | - | - | 0 | 0 | |||
| N | - | - | + | ← | + | |||
| D | - | 0 | 0 | 0 | 0 | |||
| III/A | S | - | - | + | ← | + | ||
| C | - | - | + | ← | + | |||
| N | - | - | + | ← | + | |||
| D | - | 0 | + | 0 | + | |||
| C3 | II/B-S | S | - | - | + | ← | + | |
| C | - | - | + | ← | + | |||
| N | - | - | + | ← | - | |||
| D | - | - | 0 | ← | + | |||
| III/A | S | - | - | + | ← | + | ||
| C | - | - | + | ← | + | |||
| N | - | - | + | ← | - | |||
| D | - | 0 | 0 | 0 | + | |||
| 8 °C | C1 | II/B-S | S | - | - | + | ← | 0 |
| C | - | - | 0 | ← | - | |||
| D | - | 0 | 0 | 0 | - | |||
| III/A | S | - | - | + | ← | + | ||
| C | - | - | + | ← | 0 | |||
| D | - | 0 | 0 | 0 | 0 | |||
| C2 | II/B-S | S | - | - | + | ← | + | |
| C | - | - | 0 | ← | - | |||
| D | - | 0 | 0 | 0 | 0 | |||
| III/A | S | - | - | + | ← | + | ||
| C | - | - | + | ← | 0 | |||
| D | - | 0 | + | ← | 0 | |||
| C3 | II/B-S | S | - | - | + | ← | + | |
| C | - | - | + | ← | + | |||
| D | - | 0 | + | ← | + | |||
| III/A | S | - | - | + | ← | + | ||
| C | - | - | + | ← | + | |||
| D | - | 0 | 0 | 0 | + | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizoń, J.; Łaźniewska-Piekarczyk, B.; Miera, P. The Influence of the Acceleration Admixture Type and Composition of Cement on Hydration Heat and Setting Time of Slag Blended Cement. Materials 2022, 15, 2797. https://doi.org/10.3390/ma15082797
Pizoń J, Łaźniewska-Piekarczyk B, Miera P. The Influence of the Acceleration Admixture Type and Composition of Cement on Hydration Heat and Setting Time of Slag Blended Cement. Materials. 2022; 15(8):2797. https://doi.org/10.3390/ma15082797
Chicago/Turabian StylePizoń, Jan, Beata Łaźniewska-Piekarczyk, and Patrycja Miera. 2022. "The Influence of the Acceleration Admixture Type and Composition of Cement on Hydration Heat and Setting Time of Slag Blended Cement" Materials 15, no. 8: 2797. https://doi.org/10.3390/ma15082797
APA StylePizoń, J., Łaźniewska-Piekarczyk, B., & Miera, P. (2022). The Influence of the Acceleration Admixture Type and Composition of Cement on Hydration Heat and Setting Time of Slag Blended Cement. Materials, 15(8), 2797. https://doi.org/10.3390/ma15082797








