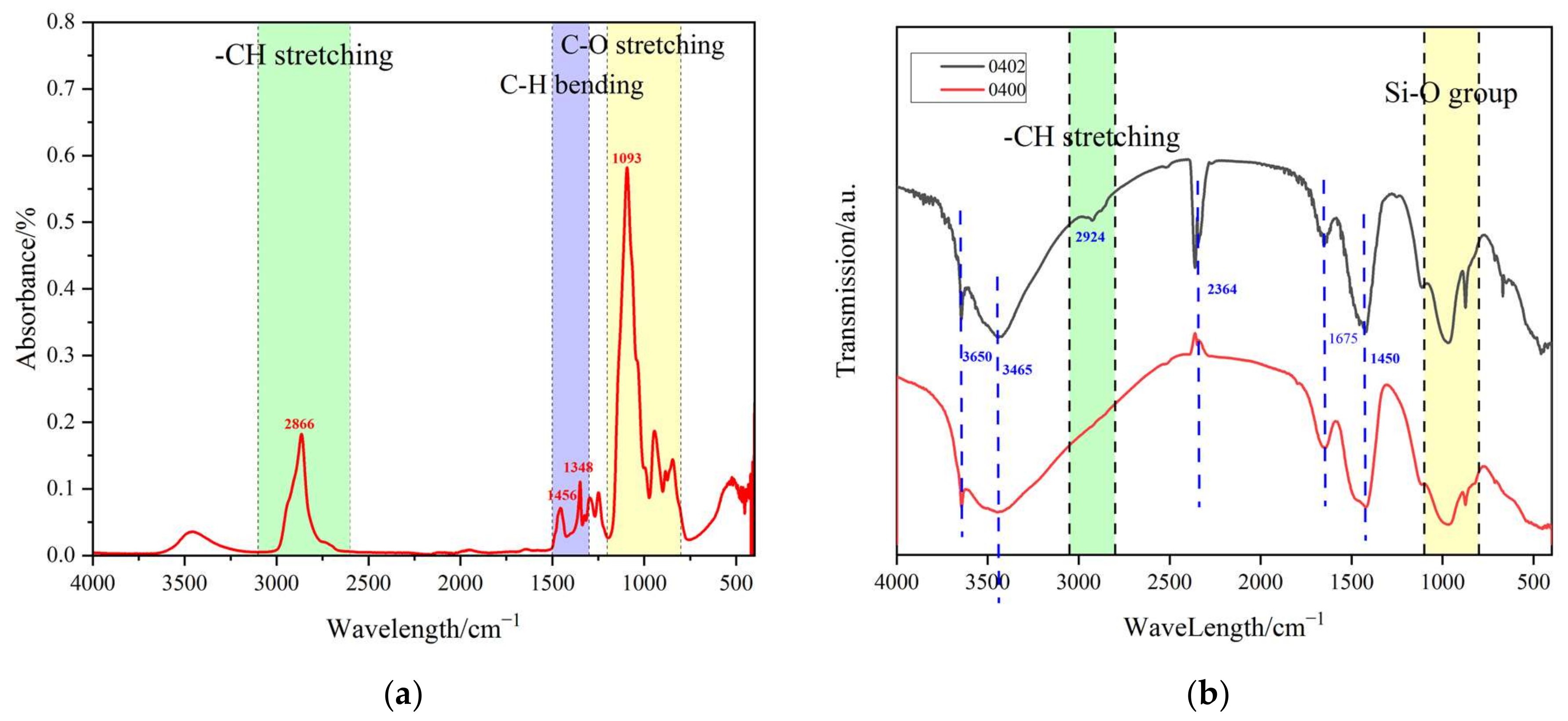
1. Introduction
According to CABEE (Professional Committee on Building Energy and Emissions) research, building energy consumption accounts for 51.3% of China’s total carbon emissions, making it a major priority to reduce building energy [
1]. Zero-energy building design is one of the most successful strategies for the sustainable growth of the construction industry, and the use of phase change materials in buildings is a critical component of this.
Phase change materials (PCMs) are a novel kind of green energy material that leverage the endothermic and exothermic effects of phase change to accomplish energy storage and autonomous release, as well as energy utilization [
2]. When applied to a building’s external wall, the heat absorbed at a high temperature limits the amount of heat transported to the outer wall through latent heat in the wall. When the temperature is dropped, the heat exchanger returns the stored heat to the environment, providing thermal insulation and minimizing interior temperature swings. Thus, human comfort will be greatly enhanced, and the rate of heating will be lowered if a fire arises, thus slowing the spread of the fire [
3,
4,
5]. PCMs are typically packed first to minimize the potential of leaking. Although these products have a high degree of cyclic stability, their thermal characteristics sometimes diverge from those of PCMs [
6,
7,
8]. However, the package has the potential to mitigate the effect of PCMs on the mechanical characteristics of the concrete. The strength of phase change concrete is dramatically reduced during the mixing of the PCMs, particularly when the PCM dose is increased. Additionally, high-density polyethylene (HDPE) is sometimes used to form paraffin. This approach does not alter the thermal characteristics, although its reliability is questionable [
9,
10]. The poor thermal conductivity of HDPE/PCM results in lower overall energy storage efficiency, so it is not now widely used.
Due to its more suitable phase transition point, at about 20~30 °C, and inexpensive cost, polyethylene glycol (PEG) is one of the most often used PCMs in the construction sector. PEG is often coupled with other porous materials to form stable phase change energy storage technologies. The polyethylene glycol/diatomite system has a maximum mass concentration of 58%, a phase transition temperature of 45~57 °C, and a phase transition enthalpy of 91.72~105.70 J/g [
11]. Similarly, polyethylene glycol/silica is a popular phase change energy storage material with a maximum mass concentration of 98% and a phase change enthalpy of up to 164.9 J/g [
12,
13]. However, these fixed PCMs have a low thermal conductivity, which is incompatible with the energy storage function. Currently, the most popular solution to the conductivity issue is to employ in situ copper to replace the shell materials or to augment the thermal conductivity of the device using graphite, graphene, or another high-thermal-conductivity material [
14,
15,
16]. However, the strength of concrete encapsulated with PEG has been found to be dramatically reduced [
17]. To maximize energy storage, researchers are also experimenting with directly incorporating phase change energy storage material into the wall interlayer or wall. For instance, PEG-600 was applied directly to a hollow aluminum tube as PCMs to create a phase change energy storage package. Then, it was reinforced with fiber and plywood to create a wallboard. As seen in
Figure 1, the wall panels were directly connected to form a basic cube structure. It was discovered that, by using phase change energy storage materials, the temperature fluctuation range in summer could be reduced by more than 20 °C, but the inside temperature may also be enhanced by around 6 °C [
18]. Similarly, a pipe filled with PCMs was utilized in the building of mass concrete to lower the temperature increase during the hydration phase of the concrete [
19,
20,
21]. As a result, several researchers have concluded that the direct application of PCMs to building walls or the construction sector is a worthwhile endeavor. Direct application of paraffin to concrete may increase its impermeability and several other properties of durability [
22].
PEG with a molecular weight of 400 (PEG-400) and PEG with a molecular weight of 600 (PEG-600) are significant self-curing agents in concrete. When the PEG-400 content is kept within 2.4%, it was discovered that the strength of the concrete may be raised by more than 40%, and the mass-loss rate may be lowered from 4.2% to 2.06% [
23]. Another study suggested that PEG-400 may boost strength and fluidity at concentrations ranging from 0% to 6% [
24]. PEG-600 can also be used as a self-curing agent in concrete. The compressive strength of concrete with PEG-600 at 28 days is nearly the same as concrete with PEG-400. PEG with a higher molecular weight can also play the role of a self-curing agent in concrete. However, it was found that, as the molecular weight increased, the optimum dosage decreased [
25]. In another study, it was found that the optimum dosage of PEG-600, as an internal curing agent, was about 1% with an increase of 23.94% in compressive strength, 20.28% in splitting tensile strength, and 60.98% in the modulus of elasticity over comparative specimens [
26]. The mechanism of concrete formation is described as follows: “from the concrete surface, the evaporation of moisture takes place continuously due to the variation in the chemical potential and the free energy space between the liquid and the vapor phase. At the time of mixing the polymers are added to the concrete mainly forming hydrogen bonds between the water molecules; this helps to reduce the chemical potential of the molecules due to reduction in the vapor pressure and in the rate of evaporation from the exterior surface” [
27]. Additionally, the nanostructure and the physical and chemical characteristics of C-S-H-PEG are discussed. It has also been found that PEG may alter the structure of C-S-H under specific circumstances, compromising the concrete’s durability [
28]. It is important to note that alcoholic hydroxyl groups have a stronger effect on the rate of cement hydration. For example, when the concentration of ethylene glycol is less than 0.3%, it may act as an accelerator of coagulation; however, when the concentration surpasses this threshold, ethylene glycol acts as a retarder. Glycerol and other compounds create comparable difficulties.
Most studies on concrete with PEG as a self-curing agent have focused on strength development and durability changes, while ignoring the thermal property changes brought about by PEG itself as a kind of PCM. Moreover, the research on phase change energy storage of PEG has ignored its excellent compatibility with concrete and encapsulation by various methods. As a self-curing agent, the amount of PEG added directly to the concrete is not more than 2%. In contrast to most available research, this study examined the maximum quantity of PEG-600 in the cement paste while also examining the development of the cement paste’s mechanical characteristics and porosity at PEG contents of 0% to 10%. It further examined the microstructure of PEG-600 that exists in cement paste at various doses and water–cement ratios, as well as its effect on energy storage, the cement hydration process, and the hydration products. Following the above investigation, this study concludes that PEG has significant research value as a kind of PCM that may be directly added to concrete and has only minor adverse impacts on the cement matrix.