Wettability, Corrosion Resistance, and Osteoblast Response to Reduced Graphene Oxide on CoCr Functionalized with Hyaluronic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Reduction of Graphene Oxide
2.3. Functionalization with Hyaluronic Acid of Electrochemically Reduced Graphene Oxide on CoCr
2.4. Contact Angle Measurement
Statistical Analysis
2.5. Electrochemical Behavior of Functionalized CoCr Surfaces
2.6. Osteoblast Cell Cultures Assays
2.6.1. Measurement of Mitochondrial Activity
2.6.2. Measurement of Lactate Dehydrogenase Activity
2.6.3. Immunofluorescence Detection of Vimentin and ICAM-1 in MC3T3-E1 Osteoblast Cell Cultures by Confocal Microscopy
2.6.4. Statistical Analysis of the WST-1 and LDH Assays
3. Results and Discussion
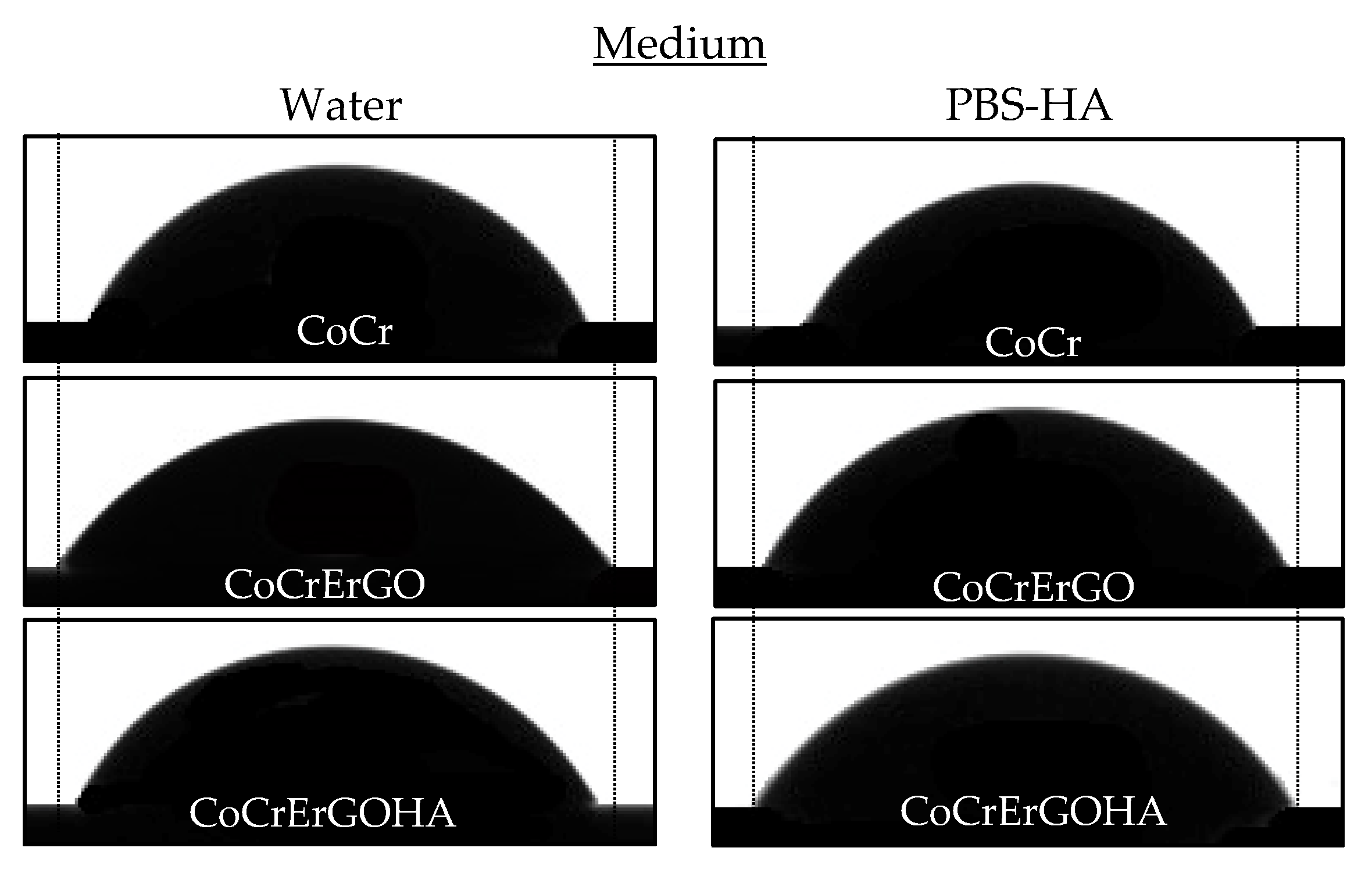
3.1. Wettability of Functionalized CoCr Surfaces
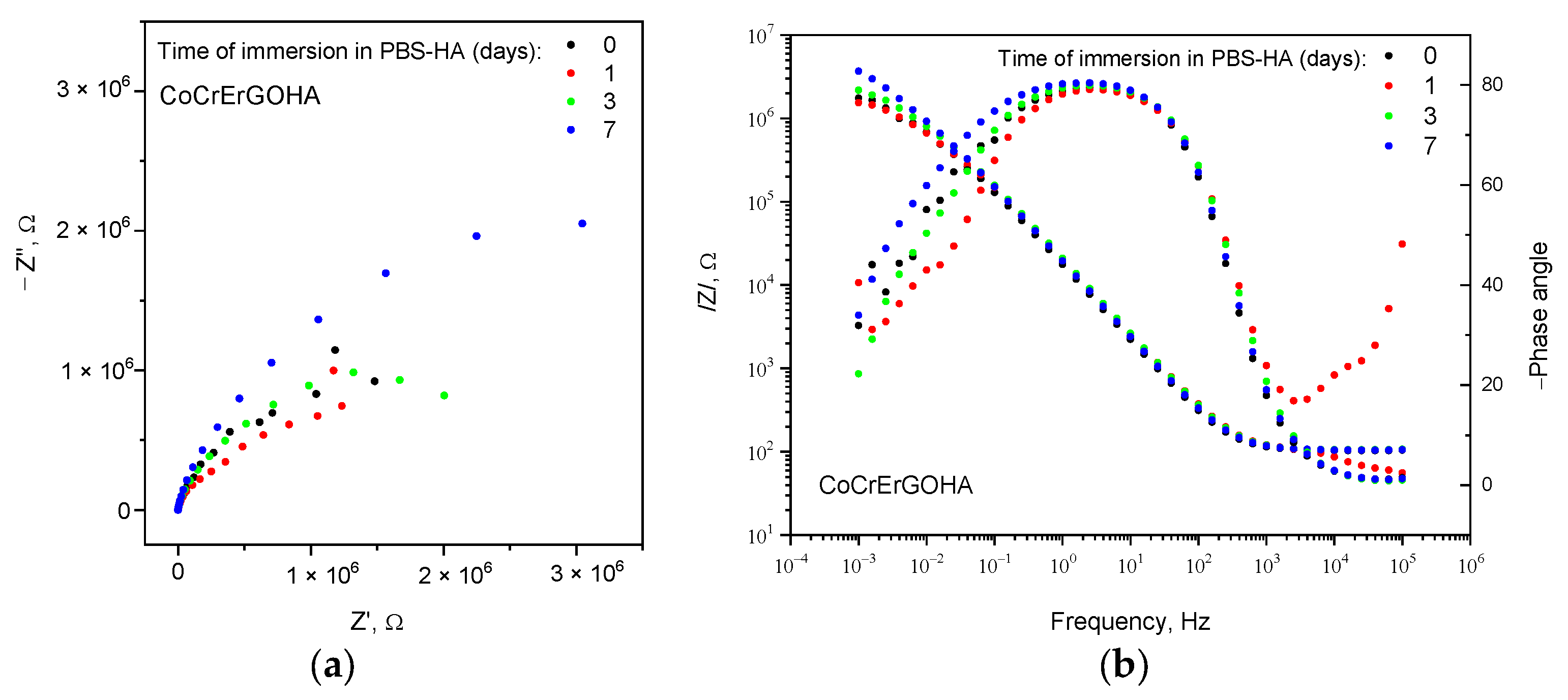
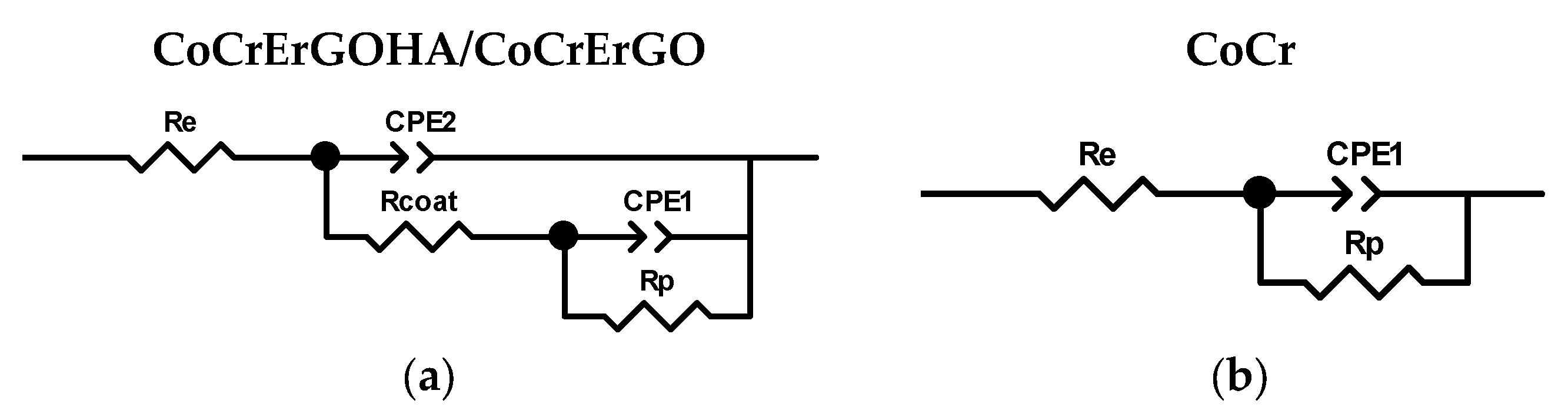
3.2. Corrosion Resistance of Functionalized CoCr Surfaces in PBS-HA Medium
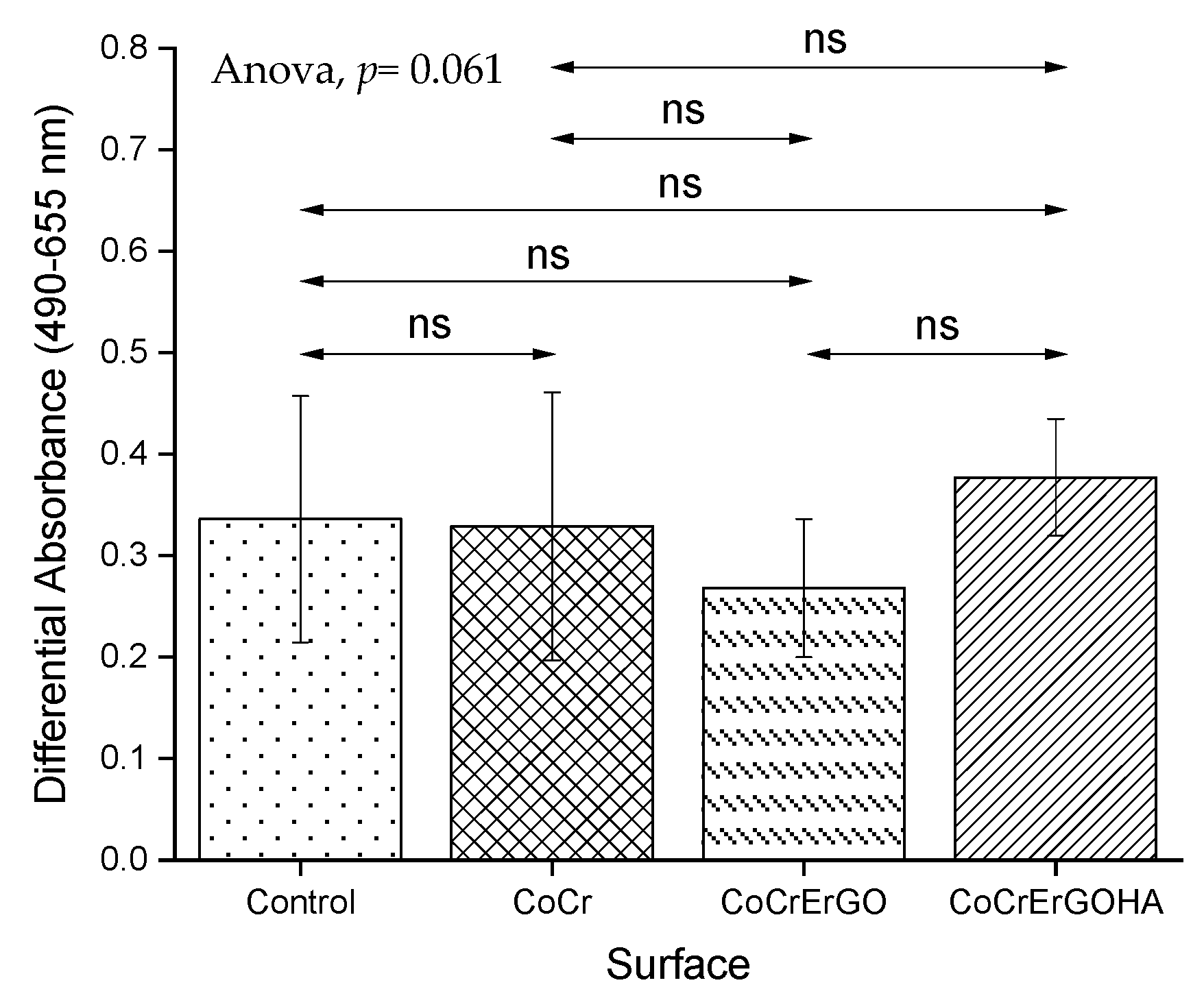
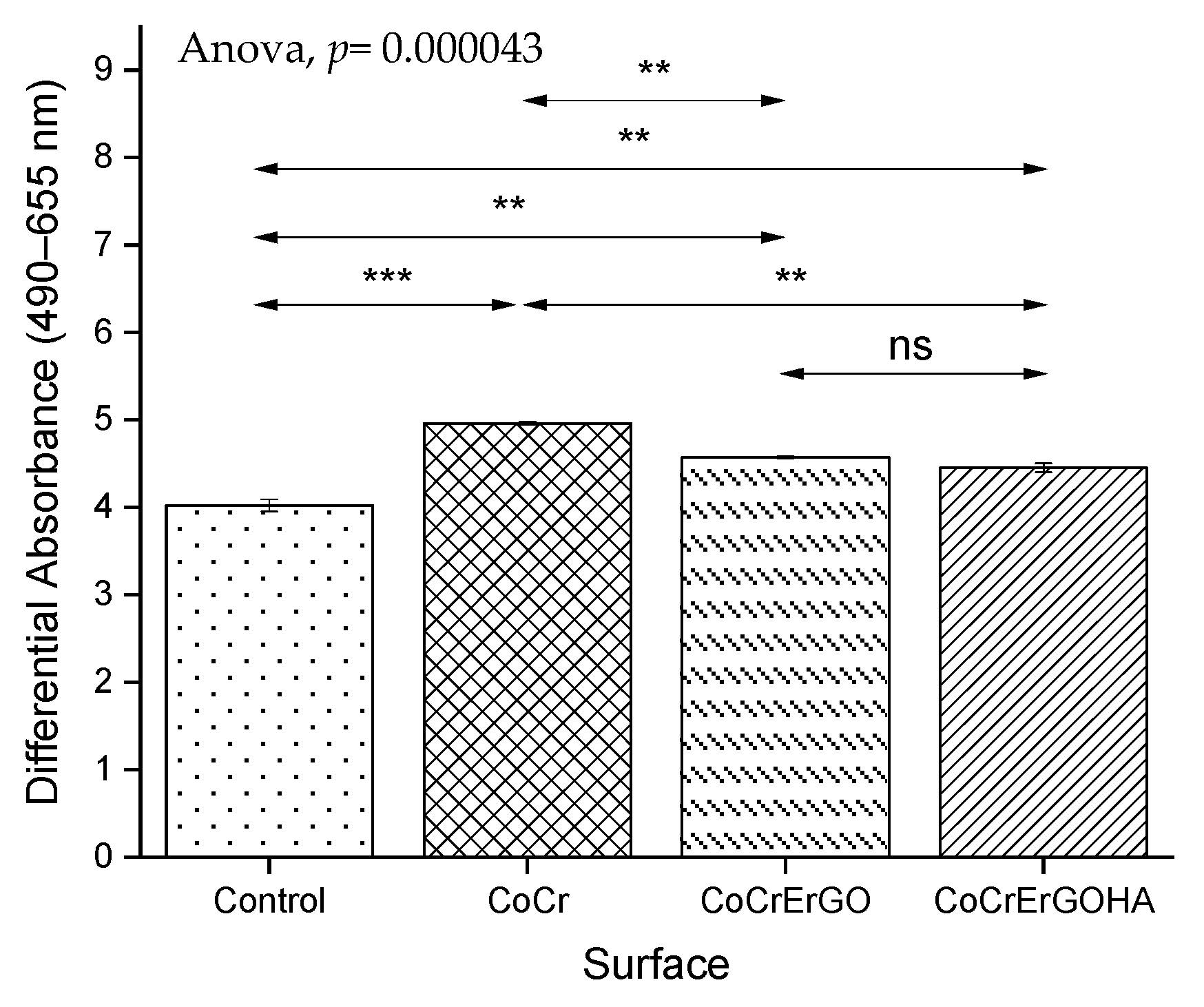
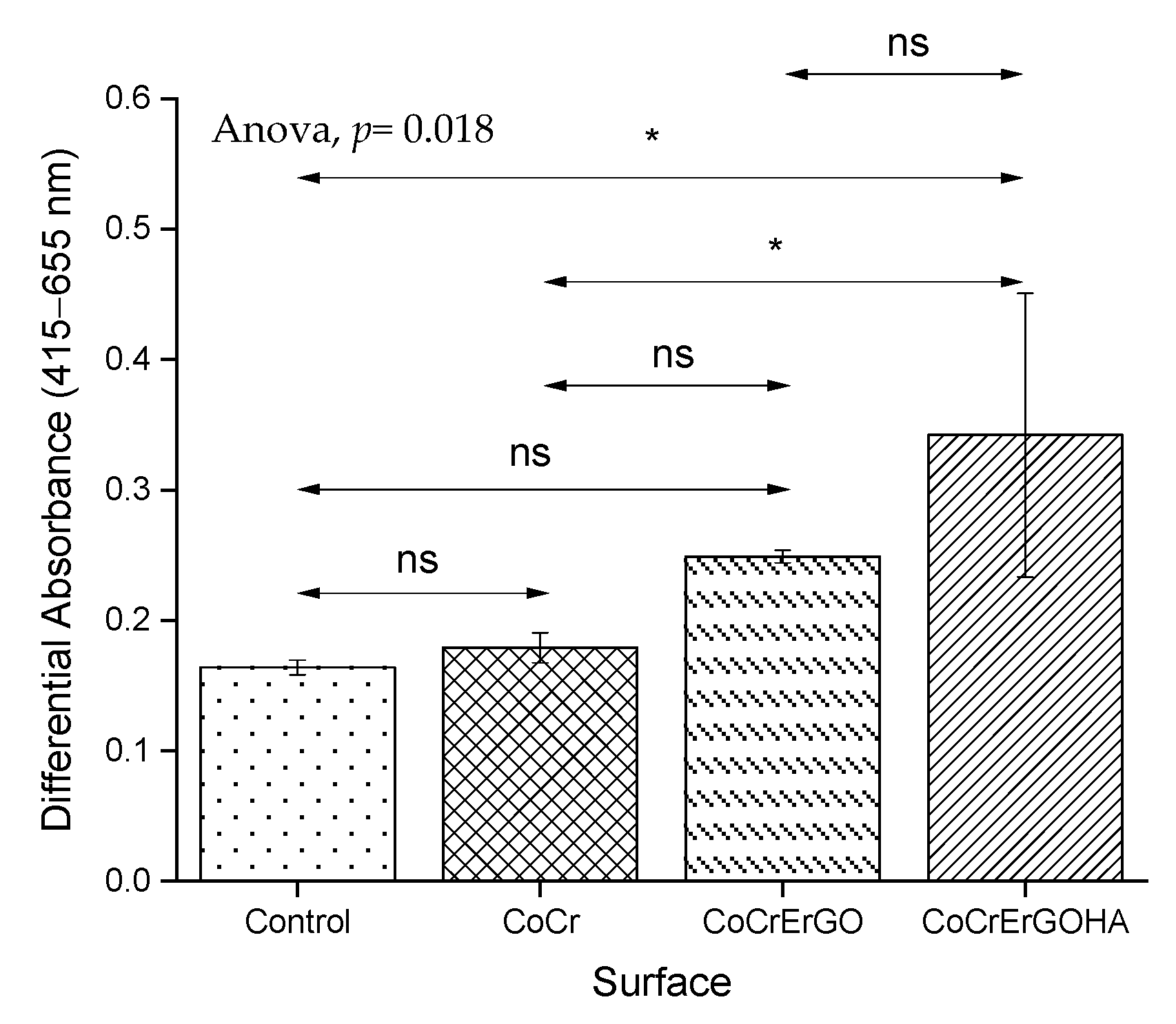
3.3. Measurement of LDH Activity in Supernatants and Cell Cultures and of MC3T3-E1 Osteoblasts Exposed to Functionalized CoCr Surfaces
3.4. MC3T3-E1 Osteoblasts Biocompatibility of Functionalized CoCr Surfaces
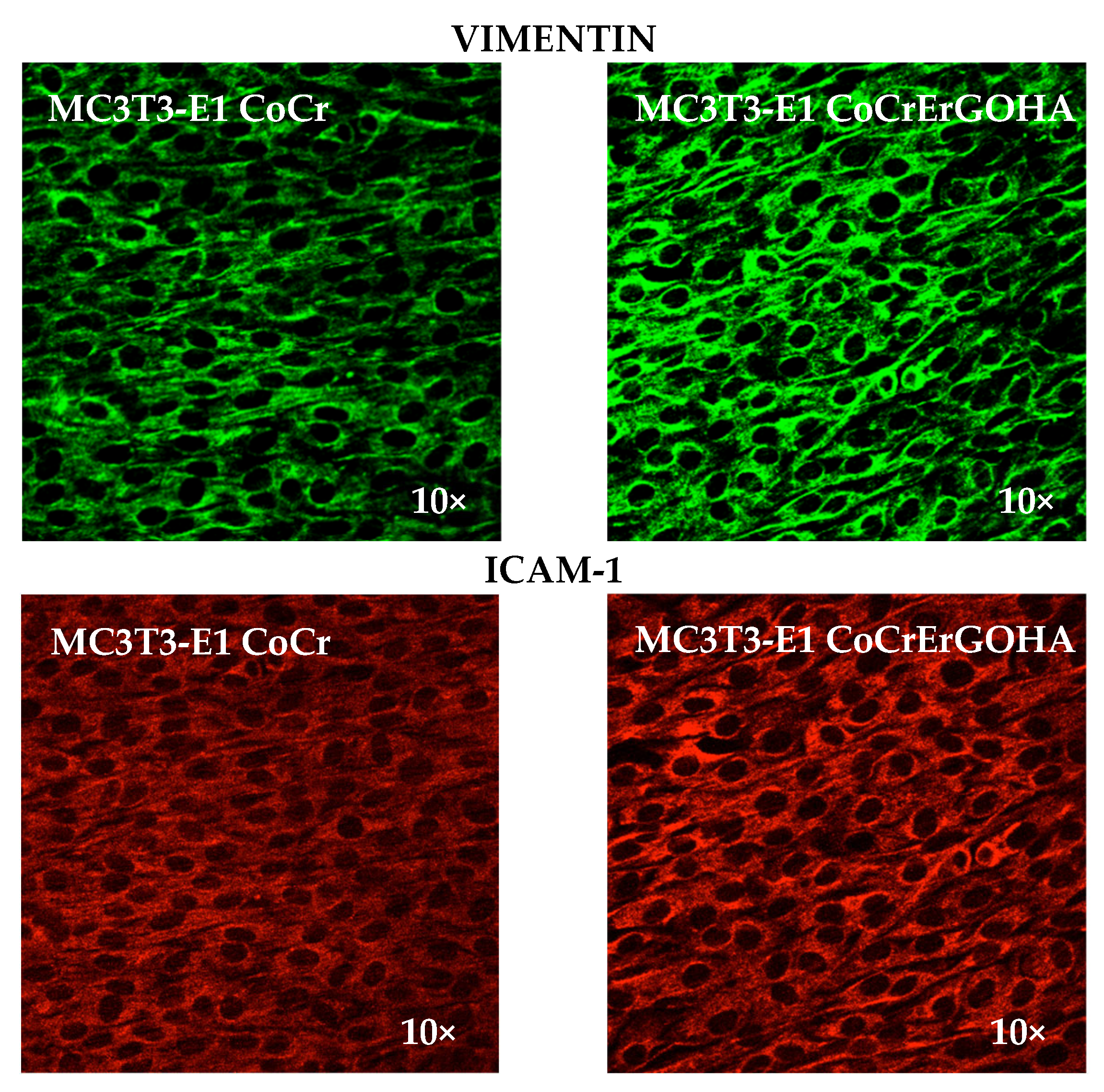
3.5. Immunodetection of Vimentin and ICAM-1 in MC3T3-E1 Osteoblast Cell Cultures Exposed to CoCrErGOHA
4. Conclusions
- The highest hydrophilicity occurs on the ErGOHA functionalized CoCr surface in the PBS-HA medium.
- The corrosion behavior of CoCrErGOHA and CoCrErGO is similar from the point of view of mechanism and resistance in the PBS-HA medium.
- High expressions of vimentin and ICAM-1 upon ErGOHA functionalization favor osteoblast adhesion. The increase in ICAM-1 expression together with the better cell adhesion observed in the presence of ErGOHA can be related to the increase in wettability, favoring the adhesion of cells to the surface, which is desirable in the case of implanted materials used for bone repair.
- Functionalization with HA through ErGO layers improves the applicability of CoCr in artificial prostheses, due to the increased wettability and cell adhesion, maintaining corrosion resistance in a physiological medium containing HA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damm, P.; Dymke, J.; Ackermann, R.; Bender, A.; Graichen, F.; Halder, A.; Beier, A.; Bergmann, G. Friction in Total Hip Joint Prosthesis Measured In Vivo during Walking. PLoS ONE 2013, 8, e78373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzucco, D.; Spector, M. The John Charnley Award Paper. The Role of Joint Fluid in the Tribology of Total Joint Arthroplasty. Clin. Orthop. Relat. Res. 2004, 429, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.-T.; Nam, J.-S.; Seo, E.-M.; Patro, B.P.; Chang, J.-D.; Park, S.; Lee, S.-S. Tribological Property of the Cobalt-Chromium Femoral Head with Different Regions of Wear in Total Hip Arthroplasty. Proc. Inst. Mech. Eng. Med. 2010, 224, 541–549. [Google Scholar] [CrossRef]
- Linn, F.C. Lubrication of Animal Joints: II The Mechanism. J. Biomech. 1968, 1, 193–205. [Google Scholar] [CrossRef]
- Katta, J.; Jin, Z.; Ingham, E.; Fisher, J. Biotribology of Articular Cartilage—A Review of the Recent Advances. Med. Eng. Phys. 2008, 30, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Naito, M.; Moriyama, S. Highly Viscous Sodium Hyaluronate and Joint Lubrication. Int. Orthop. 2002, 26, 116. [Google Scholar] [CrossRef] [Green Version]
- Higaki, H.; Murakami, T.; Nakanishi, Y.; Miura, H.; Mawatari, T.; Iwamoto, Y. The Lubricating Ability of Biomembrane Models with Dipalmitoyl Phosphatidylcholine and Gamma-Globulin. Proc. Inst. Mech. Eng. Med. 1998, 212, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pincus, M.; Mcpherson, R. Henry’s Clinical Diagnosis and Management by Laboratory Methods; Elsevier Inc.: Philadelphia, PA, USA, 2006. [Google Scholar]
- Klein, J. Molecular Mechanisms of Synovial Joint Lubrication. Proc. Inst. Mech.Eng. Part J J. Eng. Tribol. 2006, 220, 691–710. [Google Scholar] [CrossRef]
- Sawae, Y.; Murakami, T.; Chen, J. Effect of Synovia Constituents on Friction and Wear of Ultra-High Molecular Weight Polyethylene Sliding against Prosthetic Joint Materials. Wear 1998, 216, 213–219. [Google Scholar] [CrossRef]
- Bell, C.J.; Ingham, E.; Fisher, J. Influence of Hyaluronic Acid on the Time-Dependent Friction Response of Articular Cartilage under Different Conditions. Proc. Inst. Mech. Eng. Med. 2006, 220, 23–31. [Google Scholar] [CrossRef]
- Chang, D.P.; Abu-Lail, N.I.; Guilak, F.; Jay, G.D.; Zauscher, S. Conformational Mechanics, Adsorption, and Normal Force Interactions of Lubricin and Hyaluronic Acid on Model Surfaces. Langmuir 2008, 24, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Duong, C.-T.; Chang, H.-G.; Sharma, A.R.; Thompson, M.S.; Park, S.; Kwak, B.-C.; Kim, T.-Y.; Lee, S.-S.; Park, S. Role of Hyaluronic Acid and Phospholipid in the Lubrication of a Cobalt–Chromium Head for Total Hip Arthroplasty. Biointerphases 2014, 9, 031007. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.T.; Nagelli, C.; Pourzal, R.; Fischer, A.; Laurent, M.P.; Jacobs, J.J.; Wimmer, M.A. Tribolayer Formation in a Metal-on-Metal (MoM) Hip Joint: An Electrochemical Investigation. J. Mech. Behav. Biomed. Mater. 2014, 29, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Pourzal, R.; Wimmer, M.A.; Jacobs, J.J.; Fischer, A.; Marks, L.D. Graphitic Tribological Layers in Metal-on-Metal Hip Replacements. Science 2011, 334, 1687–1690. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lee, S.; McNear, K.L.; Chung, T.F.; Lee, S.; Lee, K.; Crist, S.A.; Ratliff, T.L.; Zhong, Z.; Chen, Y.P.; et al. Use of Graphene as Protection Film in Biological Environments. Sci. Rep. 2014, 4, 4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Argumánez, A.; Llorente, I.; Caballero-Calero, O.; González, Z.; Menéndez, R.; Escudero, M.L.; García-Alonso, M.C. Electrochemical Reduction of Graphene Oxide on Biomedical Grade CoCr Alloy. Appl. Surf. Sci. 2019, 465, 1028–1036. [Google Scholar] [CrossRef]
- Dadsetan, M.; Pumberger, M.; Casper, M.E.; Shogren, K.; Giuliani, M.; Ruesink, T.; Hefferan, T.E.; Currier, B.L.; Yaszemski, M.J. The Effects of Fixed Electrical Charge on Chondrocyte Behavior. Acta Biomater. 2011, 7, 2080–2090. [Google Scholar] [CrossRef] [Green Version]
- Makohliso, S.A.; Valentini, R.F.; Aebischer, P. Magnitude and Polarity of a Fluoroethylene Propylene Electret Substrate Charge Influences Neurite Outgrowth in Vitro. J. Biomed. Mater. Res. 1993, 27, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Al-Arjan, W.S.; Aslam Khan, M.U.; Nazir, S.; Abd Razak, S.I.; Abdul Kadir, M.R. Development of Arabinoxylan-Reinforced Apple Pectin/Graphene Oxide/Nano-Hydroxyapatite Based Nanocomposite Scaffolds with Controlled Release of Drug for Bone Tissue Engineering: In-Vitro Evaluation of Biocompatibility and Cytotoxicity against MC3T3-E1. Coatings 2020, 10, 1120. [Google Scholar] [CrossRef]
- Chico, B.; Pérez-Maceda, B.T.; San José, S.; Escudero, M.L.; García-Alonso, M.C.; Lozano, R.M. Corrosion Behaviour and J774A.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade CoCr. Metals 2021, 11, 1078. [Google Scholar] [CrossRef]
- Mazzucco, D.; Scott, R.; Spector, M. Composition of Joint Fluid in Patients Undergoing Total Knee Replacement and Revision Arthroplasty: Correlation with Flow Properties. Biomaterials 2004, 25, 4433–4445. [Google Scholar] [CrossRef] [PubMed]
- Perez-Maceda, B.; López-Fernández, M.; Díaz, I.; Kavanaugh, A.; Billi, F.; Escudero, M.; García-Alonso, M.; Lozano, R. Macrophage Biocompatibility of CoCr Wear Particles Produced under Polarization in Hyaluronic Acid Aqueous Solution. Materials 2018, 11, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for Modifying Current Cytotoxicity Testing Standards for Biodegradable Magnesium-Based Materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Lozano, R.M.; Pérez-Maceda, B.T.; Carboneras, M.; Onofre-Bustamante, E.; García-Alonso, M.C.; Escudero, M.L. Response of MC3T3-E1 osteoblasts, L929 fibroblasts, and J774 macrophages to fluoride surface-modified AZ31 magnesium alloy. J. Biomed. Mater. Res. Part A 2013, 101, 2753–2762. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Pu, J.; Mo, Y.; Wan, S.; Wang, L. Fabrication of Novel Graphene-Fullerene Hybrid Lubricating Films Based on Self-Assembly for MEMS Applications. Chem. Commun. 2014, 50, 469–471. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.; Jang, L.K.; Sunwoo, S.H.; Kim, T.; Cho, S.-W.; Lee, J.Y. Electrochemical Deposition of Dopamine–Hyaluronic Acid Conjugates for Anti-Biofouling Bioelectrodes. J. Mater. Chem. B 2017, 5, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, F.; Lozano Puerto, R.M.; Pérez-Maceda, B.; Grillo, C.A.; Fernández Lorenzo de Mele, M. Time-Lapse Evaluation of Interactions Between Biodegradable Mg Particles and Cells. Microsc. Microanal. 2016, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- LeBert, D.; Squirrell, J.M.; Freisinger, C.; Rindy, J.; Golenberg, N.; Frecentese, G.; Gibson, A.; Eliceiri, K.W.; Huttenlocher, A. Damage-Induced Reactive Oxygen Species Regulate Vimentin and Dynamic Collagen-Based Projections to Mediate Wound Repair. eLife 2018, 7, e30703. [Google Scholar] [CrossRef] [Green Version]
- Matveeva, E.A.; Chernoivanenko, I.S.; Minin, A.A. Vimentin Intermediate Filaments Protect Mitochondria from Oxidative Stress. Biochem. Moscow Suppl. Ser. A 2010, 4, 321–331. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Love, N.R.; Chen, Y.; Ishibashi, S.; Kritsiligkou, P.; Lea, R.; Koh, Y.; Gallop, J.L.; Dorey, K.; Amaya, E. Amputation-Induced Reactive Oxygen Species Are Required for Successful Xenopus Tadpole Tail Regeneration. Nat. Cell Biol. 2013, 15, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and Reversing Chronic Wounds in Diabetic Mice by Manipulating Wound Redox Parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [Green Version]
- Danielsson, F.; Peterson, M.K.; Caldeira Araújo, H.; Lautenschläger, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Morimoto, I.; Nakano, Y.; Okada, Y.; Hirota, S.; Nomura, S.; Nakamura, T.; Eto, S. Osteoblasts Are Regulated by the Cellular Adhesion through ICAM-1 and VCAM-1. J. Bone Miner. Res. 1995, 10, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast Adhesion on Biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning Surface Properties of Bone Biomaterials to Manipulate Osteoblastic Cell Adhesion and the Signaling Pathways for the Enhancement of Early Osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]

| Total | Medium | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | PBS-HA | ||||||||
| Surface | N | Mean | SD | N | Mean | SD | N | Mean | SD |
| CoCr | 152 | 69.4 | 3.4 | 72 | 71.4 | 2.0 | 80 | 67.5 | 3.4 |
| CoCrErGO | 144 | 64.5 | 6.4 | 68 | 63.5 | 5.0 | 76 | 65.5 | 7.3 |
| CoCrErGOHA | 178 | 61.5 | 10.7 | 76 | 65.3 | 6.6 | 102 | 58.6 | 12.2 |
| Total | 474 | 64.9 | 8.4 | 216 | 66.8 | 6.0 | 258 | 63.4 | 9.7 |
| Surface | Time, d | Re ± Error %, Ω | Rcoat ± Error %, MΩ | CPE2 ± Error %, μsn/Ω | n2 ± Error % | Rp ± Error %, MΩ | CPE1 ± Error %, μsn/Ω | n1 ± Error % | Chi2 |
|---|---|---|---|---|---|---|---|---|---|
| CoCrERGOHA | 0 | 103.6 ± 0.5 | 0.4 ± 29.8 | 10.7 ± 1.1 | 0.90 ± 0.2 | 3.94 ± 41.9 | 3.87 ± 32.7 | 0.47 ± 18.5 | 0.0003 |
| 1 | 101.9 ± 2.1 | 0.6 ± 13.5 | 9.30 ± 2.0 | 0.89 ± 0.5 | 1.38 ± 21.7 | 17.1 ± 49.8 | 0.78 ± 14.9 | 0.0021 | |
| 3 | 105.3 ± 0.3 | 0.9 ± 3.4 | 9.11 ± 0.4 | 0.90 ± 0.1 | 1.74 ± 3.9 | 12.5 ± 12.0 | 0.79 ± 3.1 | 0.0001 | |
| 7 | 105.1 ± 0.4 | 1.6 ± 6.1 | 9.79 ± 0.5 | 0.90 ± 0.1 | 3.61 ± 5.9 | 7.74 ± 17.8 | 0.81 ± 4.5 | 0.0002 | |
| CoCrErGO | 3 | 88.2 ± 0.8 | 0.5 ± 9.7 | 9.89 ± 1.2 | 0.89 ± 0.3 | 0.71 ± 10.8 | 14.9 ± 33.6 | 0.75 ± 9.2 | 0.0007 |
| CoCr | 3 | 107.8 ± 0.7 | - | - | - | 5.12 ± 1.6 | 7.15 ± 0.5 | 0.92 ± 0.1 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chico, B.; Pérez-Maceda, B.T.; San-José, S.; Escudero, M.L.; García-Alonso, M.C.; Lozano, R.M. Wettability, Corrosion Resistance, and Osteoblast Response to Reduced Graphene Oxide on CoCr Functionalized with Hyaluronic Acid. Materials 2022, 15, 2693. https://doi.org/10.3390/ma15072693
Chico B, Pérez-Maceda BT, San-José S, Escudero ML, García-Alonso MC, Lozano RM. Wettability, Corrosion Resistance, and Osteoblast Response to Reduced Graphene Oxide on CoCr Functionalized with Hyaluronic Acid. Materials. 2022; 15(7):2693. https://doi.org/10.3390/ma15072693
Chicago/Turabian StyleChico, Belén, Blanca Teresa Pérez-Maceda, Sara San-José, María Lorenza Escudero, María Cristina García-Alonso, and Rosa María Lozano. 2022. "Wettability, Corrosion Resistance, and Osteoblast Response to Reduced Graphene Oxide on CoCr Functionalized with Hyaluronic Acid" Materials 15, no. 7: 2693. https://doi.org/10.3390/ma15072693
APA StyleChico, B., Pérez-Maceda, B. T., San-José, S., Escudero, M. L., García-Alonso, M. C., & Lozano, R. M. (2022). Wettability, Corrosion Resistance, and Osteoblast Response to Reduced Graphene Oxide on CoCr Functionalized with Hyaluronic Acid. Materials, 15(7), 2693. https://doi.org/10.3390/ma15072693






