The Influences of Soluble Phosphorus on Hydration Process and Mechanical Properties of Hemihydrate Gypsum under Deep Retarding Condition
Abstract
:1. Introduction
2. Materials and Methods
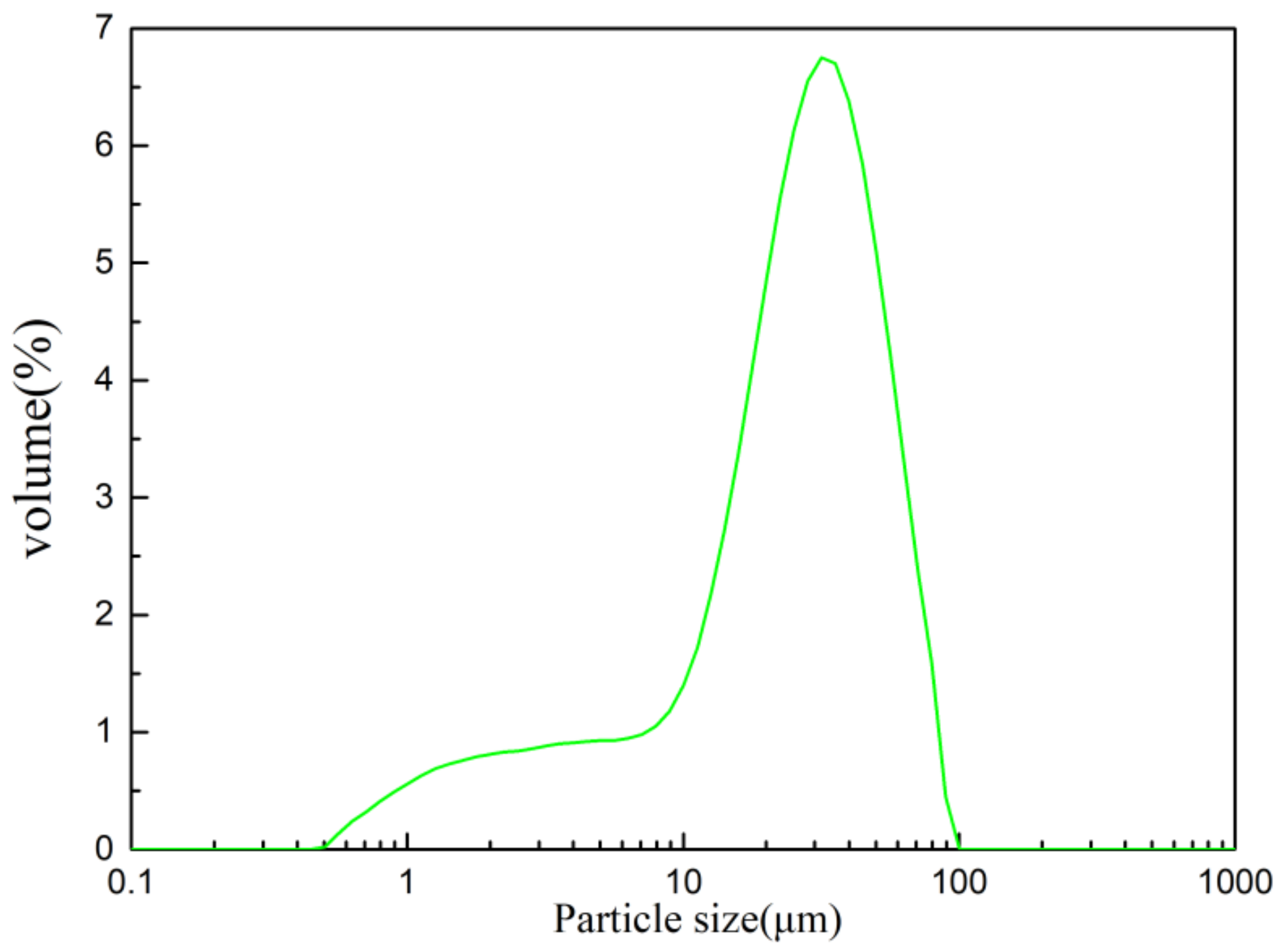
2.1. Raw Materials
2.2. Methods
Sample Preparation
2.3. Measurements
2.3.1. Determination of the Setting Time
2.3.2. Determination of the Mechanical Strength
2.4. Characterization
3. Results
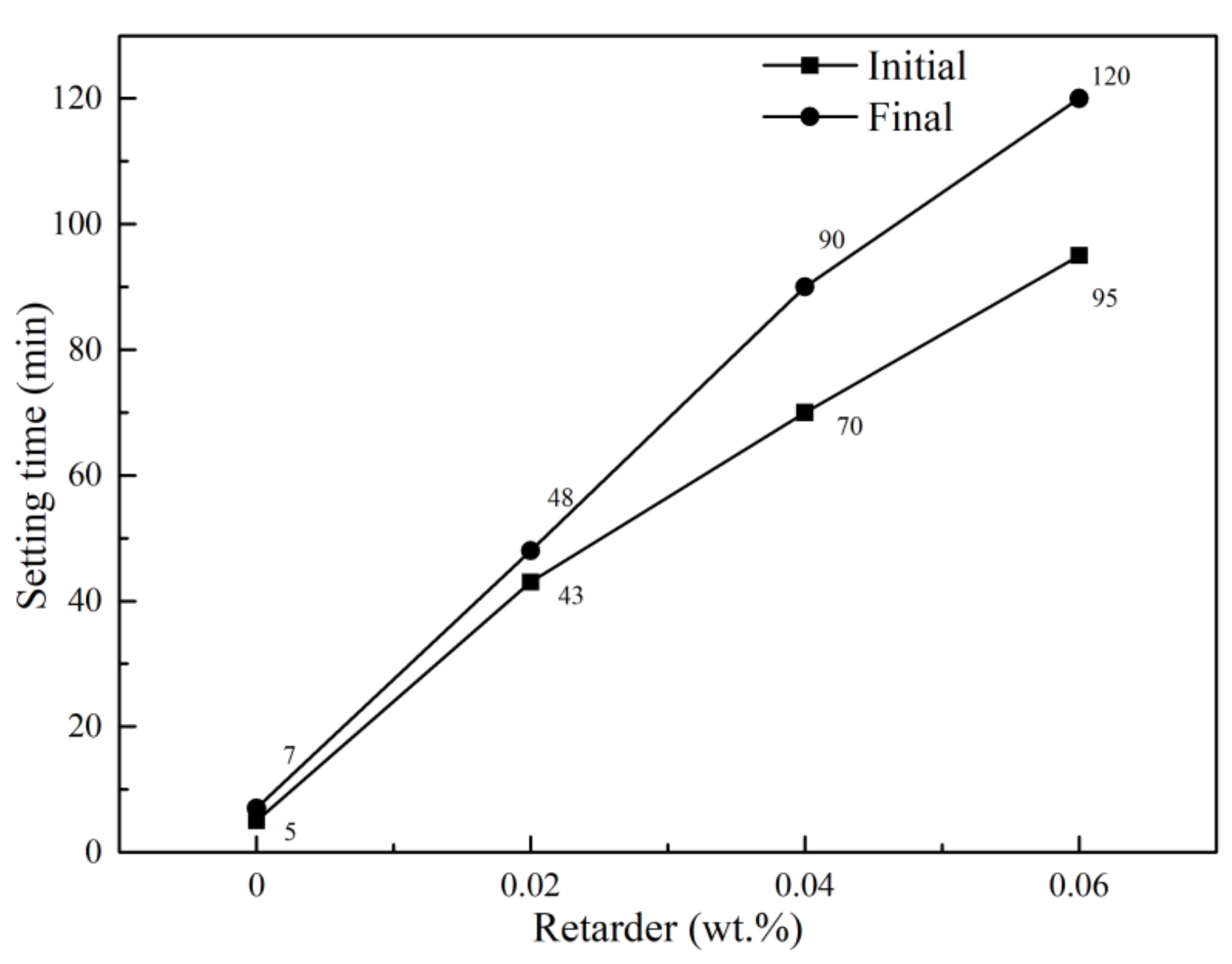
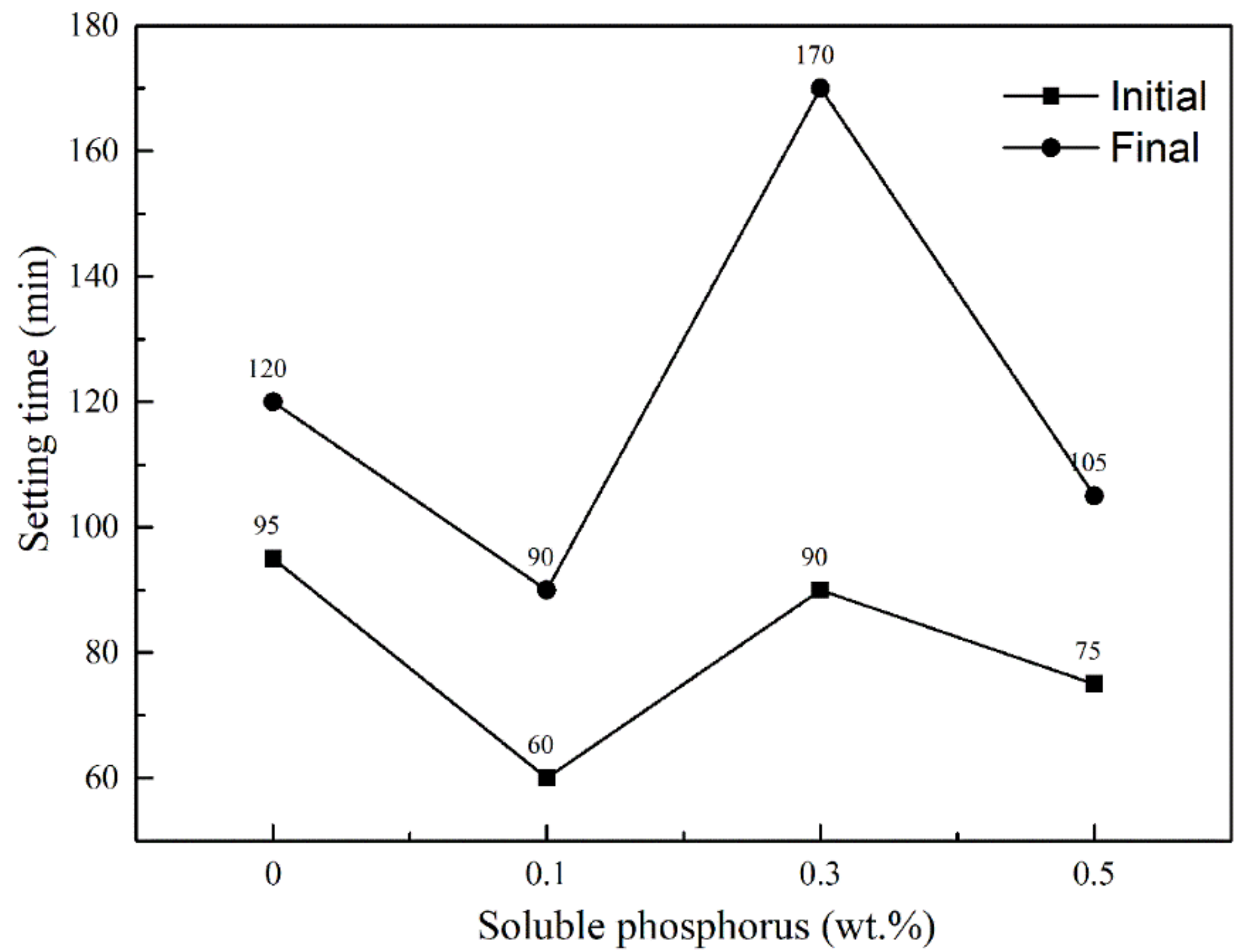
3.1. Influence of Soluble Phosphorus on Setting Time of the Calcined Gypsum
3.2. Influence of Soluble Phosphorus on Mechanical Strength of the Calcined Gypsum
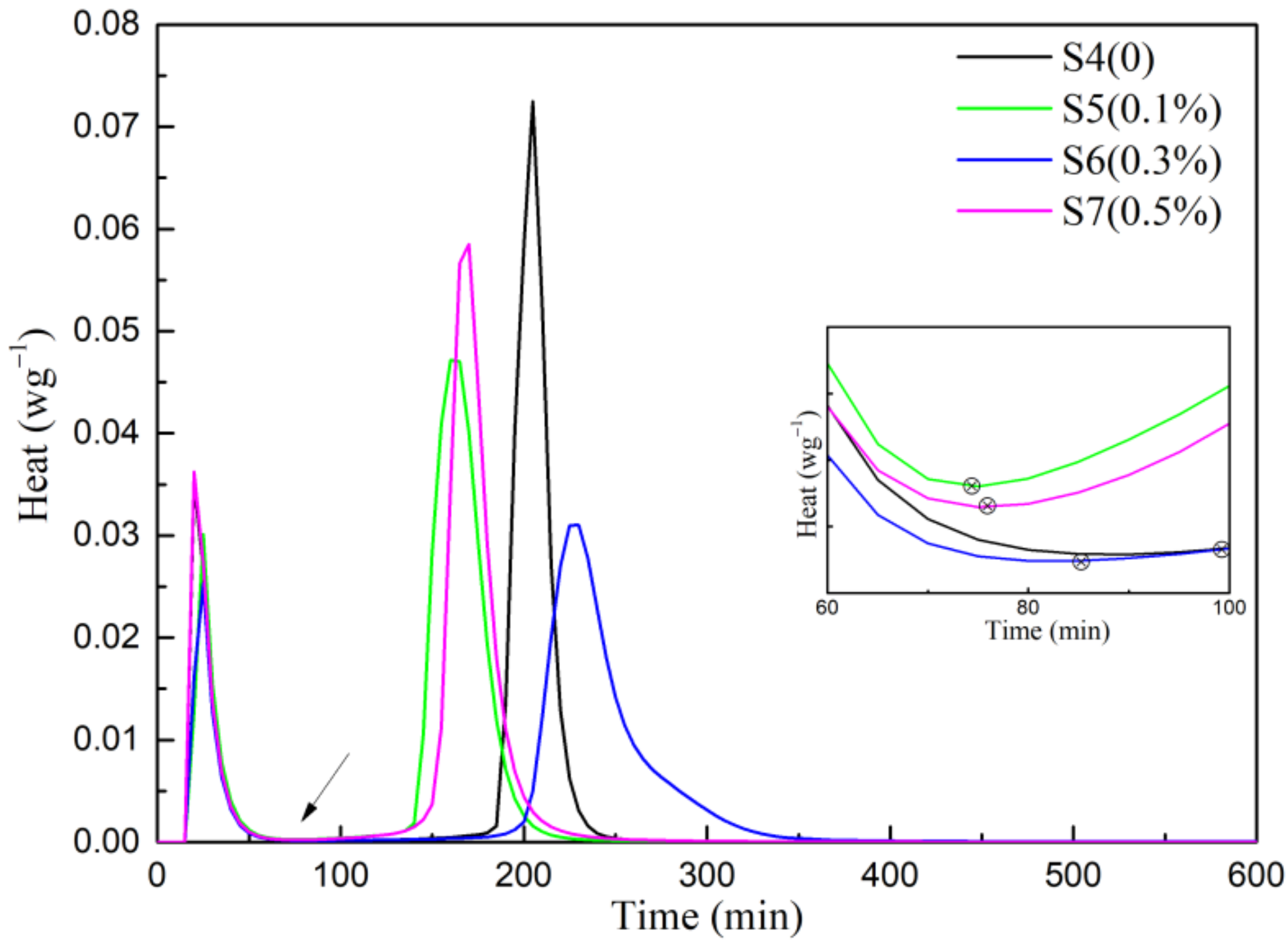
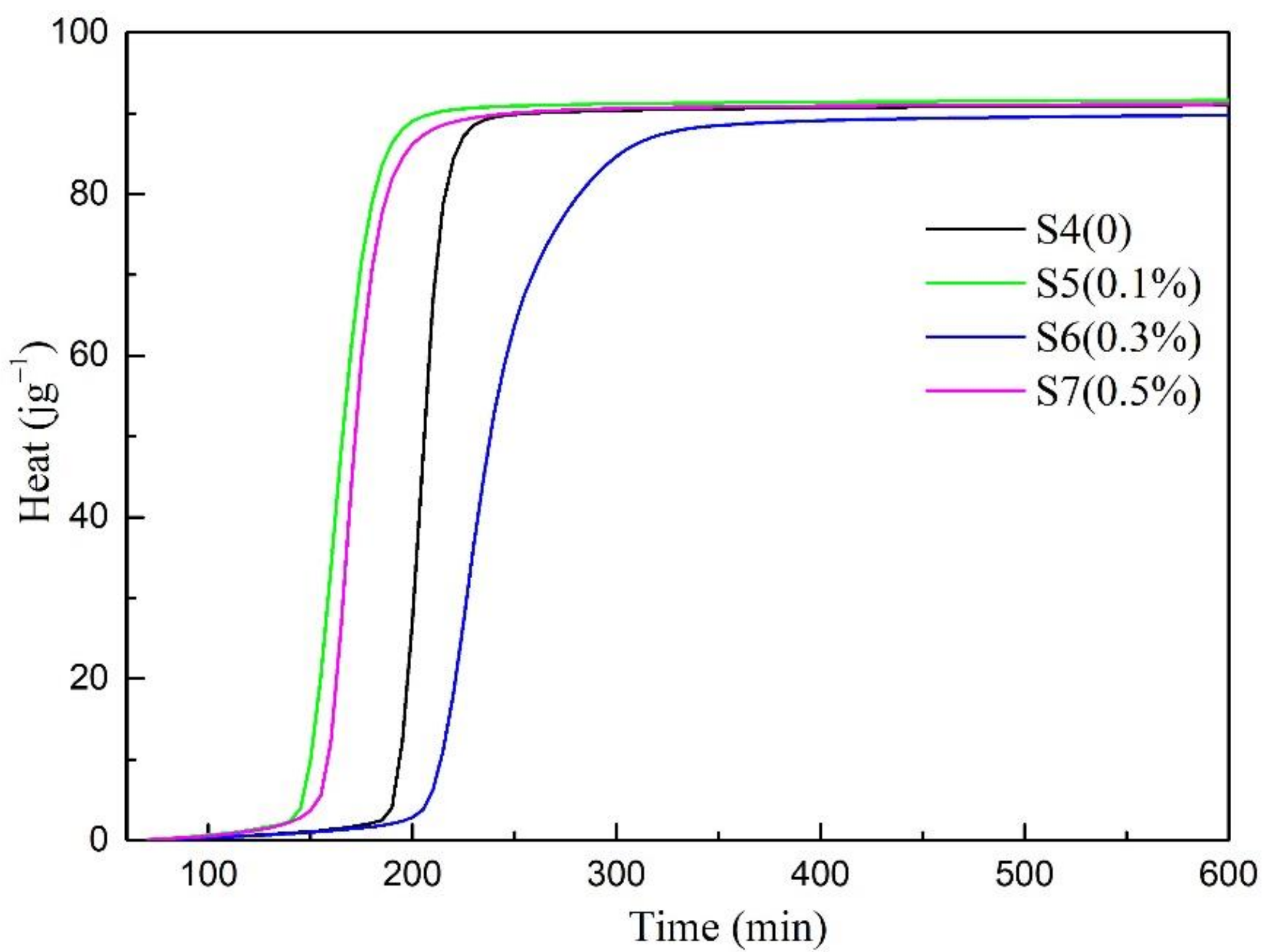
3.3. Hydration Kinetics
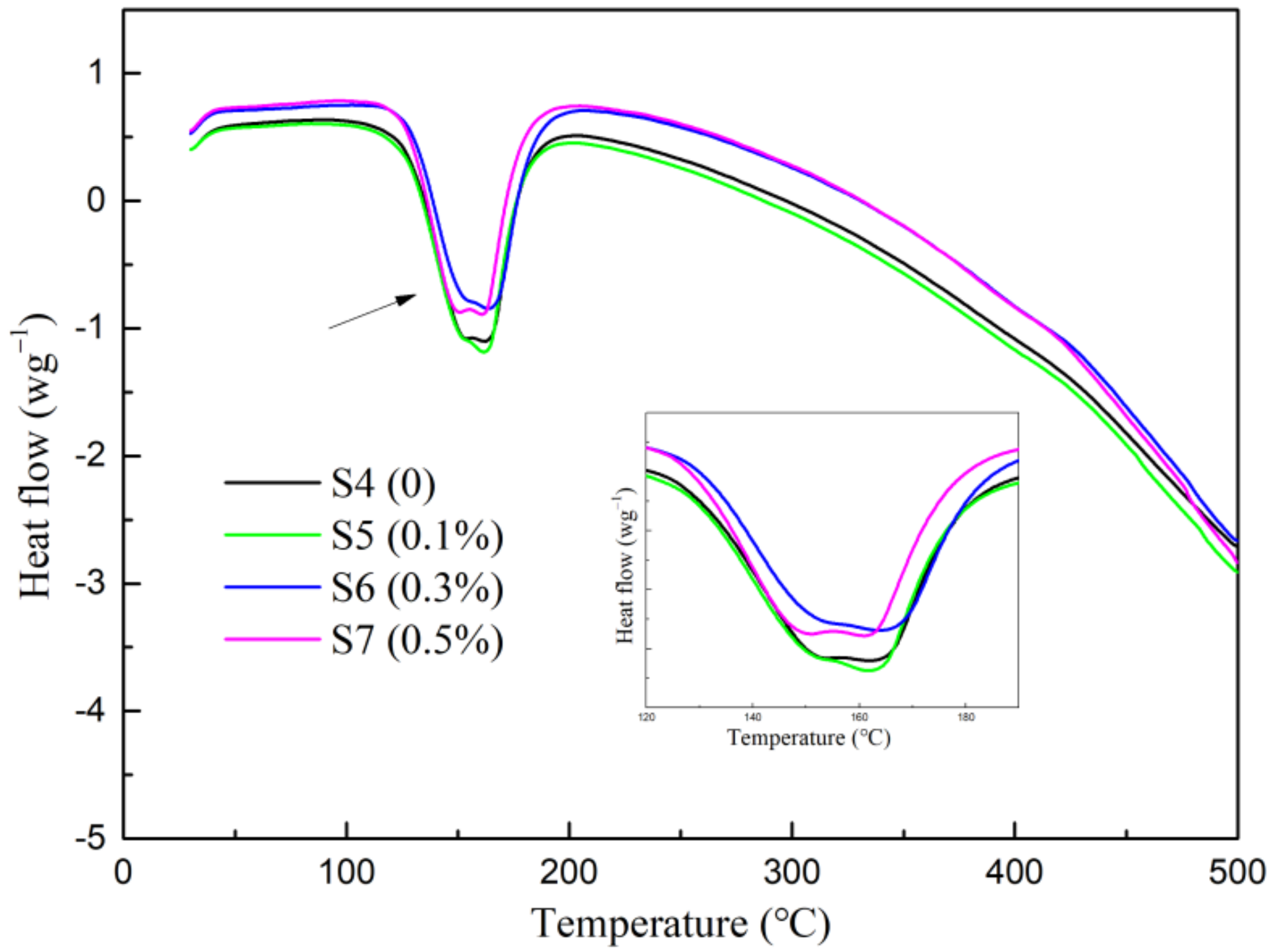
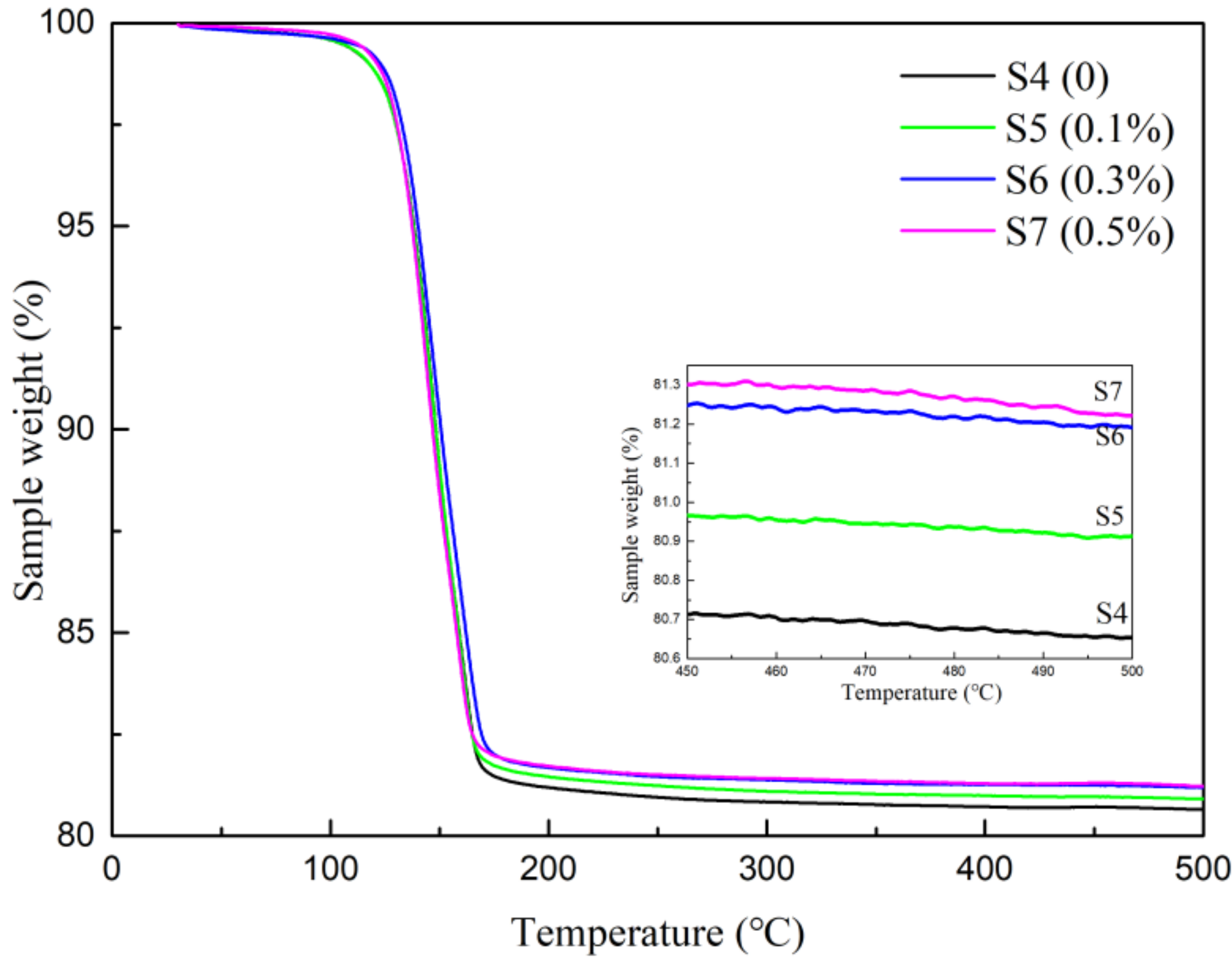
3.4. DSC/TG Analysis
3.5. XRD Analysis
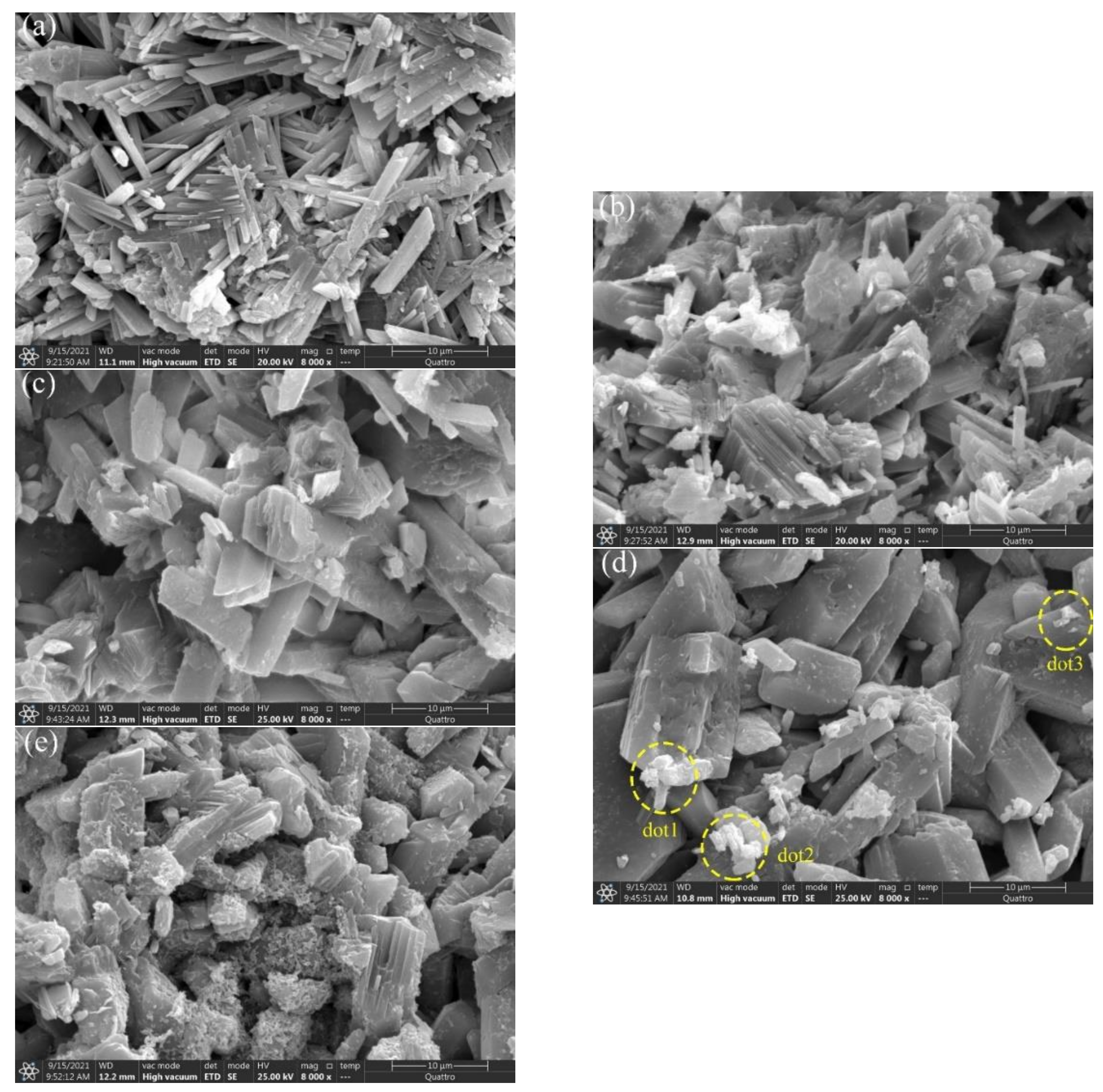
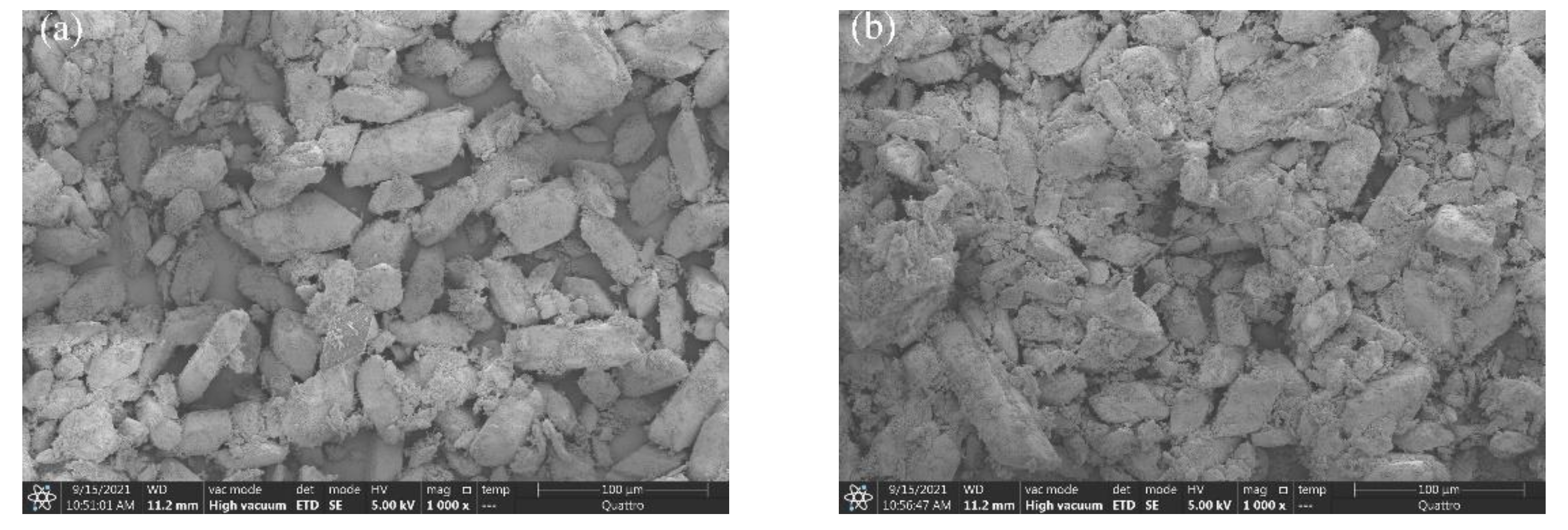
3.6. SEM Crystal Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuzmanovic, P.; Todorovic, N.; Mrda, D.; Forkapic, S.; Petrovic, L.F.; Miljevic, B.; Hansman, J.; Knezevic, J. The possibility of the phosphogypsum use in the production of brick: Radiological and structural characterization. J. Hazard. Mater. 2021, 413, 125343. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Bakshi, P.; Pappu, A.; Gupta, M.K. A review on calcium-rich industrial wastes: A sustainable source of raw materials in India for civil infrastructure—Opportunities and challenges to bond circular economy. J. Mater. Cycles Waste Manag. 2021, 24, 49–62. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Cleaner Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Goncalves, F.; Rombke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef]
- Huang, Y.B.; Qian, J.S.; Kang, X.J.; Yu, J.C.; Fan, Y.R.; Dang, Y.D.; Zhang, W.S.; Wang, S.D. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, J.M.; Zhao, Y.S. Investigation on the hydration of hemihydrate phosphogypsum after post treatment. Constr. Build. Mater. 2019, 229, 116864. [Google Scholar] [CrossRef]
- Yang, M.; Qian, J.S.; Wang, Z.; Huang, Y.B. Effect of Impurities on the Working Performance of Phosphogypsum. Mater. Rev. 2007, 21, 104–106. [Google Scholar]
- Brouwers, H.J.H. Paste models for hydrating calcium sulfates, using the approach by Powers and Brownyard. Constr. Build. Mater. 2012, 36, 1044–1047. [Google Scholar] [CrossRef] [Green Version]
- Li, M. Study on Quality Infulencing Factors of Phosphogypsum and Its Utilization as Building Materials; Chongqing University: Chongqing, China, 2012. [Google Scholar]
- Singh, M. Processing of phosphogypsum for the manufacture of gypsum plaster. Res. Ind. 1982, 27, 167–169. [Google Scholar]
- Peng, J.H.; Peng, Z.H.; Zhang, J.X.; Wan, T.Z. Study on the form and distribution of water-soluble P2O5 in phosphogypsum and effective mechanism of properties. J. China Ceram. Soc. 2000, 28, 309–313. [Google Scholar]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Singh, M. Effect of phosphatic and fluoride impurities of phosphogypsum on the properties of selenite plaster. Cem. Concr. Res. 2003, 33, 1363–1369. [Google Scholar] [CrossRef]
- Singh, M. Role of phosphogypsum impurities on strength and microstructure of selenite plaster. Constr. Build. Mater. 2005, 19, 480–486. [Google Scholar] [CrossRef]
- Zhou, S.T.; Li, X.B.; Zhou, Y.N.; Min, C.D.; Shi, Y. Effect of phosphorus on the properties of phosphogypsum-based cemented backfill. J. Hazard. Mater. 2020, 399, 122993. [Google Scholar] [CrossRef]
- Kaziliunas, A.; Leskeviciene, V.; Vektaris, B.; Valancius, Z. The study of neutralization of the dihydrate phosphogypsum impurities. Ceram. Silik. 2006, 50, 178–184. [Google Scholar]
- Camarini, G.; Pinto, M.C.C.; de Moura, A.G.; Manzo, N.R. Effect of citric acid on properties of recycled gypsum plaster to building components. Constr. Build. Mater. 2016, 124, 383–390. [Google Scholar] [CrossRef]
- Akyol, E.; Oner, M.; Barouda, E.; Demadis, K.D. Systematic Structural Determinants of the Effects of Tetraphosphonates on Gypsum Crystallization. Cryst. Growth Des. 2009, 9, 5145–5154. [Google Scholar] [CrossRef]
- Heijnen, W.M.M.; Hartman, P. Structural morphology of gypsum (CaSO4·2H2O), brushite (CaHPO4·2H2O) and pharmacolite (CaHAsO4·2H2O). J. Cryst. Growth 1991, 108, 290–300. [Google Scholar] [CrossRef]
- El-Shall, H.; Rashad, M.M.; Abdel-Aal, E.A. Effect of phosphonate additive on crystallization of gypsum in phosphoric and sulfuric acid medium. Cryst. Res. Technol. 2002, 37, 1264–1273. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yang, J.S.; Cao, X.Y. Effects of several retarders on setting time and strength of building gypsum. Constr. Build. Mater. 2020, 240, 117927. [Google Scholar] [CrossRef]
- Ziegenheim, S.; Szabados, M.; Konya, Z.; Kukovecz, A.; Palinko, I.; Sipos, P. Manipulating the crystallization kinetics and morphology of gypsum, CaSO4·2H2O via addition of citrate at high levels of supersaturation and the effect of high salinity. Polyhedron 2021, 204, 115253. [Google Scholar] [CrossRef]
- Peng, J.H. Research on the Effect Mechanism of Water-Reducing Agent and Retarder in Building Gypsum; Chongqing University: Chongqing, China, 2004. [Google Scholar]
- Ding, X.L.; Shan, Z.H.; Long, Z.Z.; Chen, Z.J. Utilization of collagen protein extracted from chrome leather scraps as a set retarders in gypsum. Constr. Build. Mater. 2020, 237, 117584. [Google Scholar] [CrossRef]
- Moghadam, H.A.; Mirzaei, A. Comparing the effects of a retarder and accelerator on properties of gypsum building plaster. J. Build. Eng. 2020, 28, 101075. [Google Scholar] [CrossRef]
- Qu, J.D.; Peng, J.H.; Wu, L.; Zhang, J.X. Research situation and development tendency of admixtures for building gypsum. J. Mater. Sci. Eng. 2004, 22, 466–469. [Google Scholar]
- GB/T 17669.3–1999; Gypsum plasters—Determination of mechanical properties. Quality Supervision Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1999.
- GB/T 28627–2012; Gypsum plaster. Quality Supervision Inspection and Quarantine of the People’s Republic of China and Standardization Administration of the People’s Republic China: Beijing, China, 2012.
- GB/T 23456–2018; Phosphogypsum. Quality Supervision Inspection and Quarantine of the People’s Republic of China and Standardization Administration of the People’s Republic China: Beijing, China, 2018.
- GB/T 17669. 4–1999.; Gypsum plasters-Determination of physical properties of pure paste. Quality Supervision Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1999.
- Ding, Y.; Fang, Y.; Fang, H.; Zhang, Q.; Zhang, F.-J.; Chun, O.W. Study on the Retarding Mechanism and Strength Loss of Gypsum from Hydrolyzed Wheat Protein Retarder. J. Korean Ceram. Soc. 2015, 52, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.H.; Zhang, J.X.; Chen, M.F.; Wu, L.; Qu, J.D. Influence of macromolecule-type retarders on the hydration process of building gypsum and its retarding mechanism. J. China Ceram. Soc. 2008, 36, 896–900. [Google Scholar]
- Ding, X.L.; Wei, B.Q.; Deng, M.G.; Chen, H.; Shan, Z.H. Effect of protein peptides with different molecular weights on the setting and hydration process of gypsum. Constr. Build. Mater. 2022, 318, 126185. [Google Scholar] [CrossRef]
- Jia, R.Q.; Wang, Q.; Luo, T. Reuse of phosphogypsum as hemihydrate gypsum: The negative effect and content control of H3PO4. Conserv. Recy. 2021, 174, 105830. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Mater 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Rendel, P.M.; Gavrieli, I.; Wolff-Boenisch, D.; Ganor, J. Towards establishing a combined rate law of nucleation and crystal growth—The case study of gypsum precipitation. J. Cryst. Growth 2018, 485, 28–40. [Google Scholar] [CrossRef]
- Nilles, V.; Plank, J. Study of the retarding mechanism of linear sodium polyphosphates on alpha-calcium sulfate hemihydrate. Cem. Concr. Res. 2012, 42, 736–744. [Google Scholar] [CrossRef]
- Park, S.H.; Manzello, S.L.; Bentz, D.P.; Mizukami, T. Determining thermal properties of gypsum board at elevated temperatures. Fire Mater. 2010, 34, 237–250. [Google Scholar] [CrossRef]
- Romero-Hermida, M.I.; Borrero-Lopez, A.M.; Alejandre, F.J.; Flores-Ales, V.; Santos, A.; Franco, J.M.; Esquivias, L. Phosphogypsum waste lime as a promising substitute of commercial limes: A rheological approach. Cem. Concr. Compos. 2019, 95, 205–216. [Google Scholar] [CrossRef]
- Boisvert, J.P.; Domenech, M.; Foissy, A.; Persello, J.; Mutin, J.C. Hydration of calcium sulfate hemihydrate (CaSO4·1/2H2O) into gypsum (CaSO4·2H2O). The influence of the sodium poly(acrylate)/surface interaction and molecular weight. J. Cryst. Growth 2000, 220, 579–591. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, S.Y.; Jia, R.Q. An investigation on the anti-water properties of phosphorus building gypsum (PBG)-based mortar. J. Therm. Anal. Calorim. 2019, 136, 1575–1585. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Xia, J.P.; Han, Y.W.; Chen, Z.J.; Liu, C.L. Effect of retarder on properties of phosphate gypsum based building gypsum. Non-Met. Mines 2019, 42, 79–81. [Google Scholar]



| CaO | SO3 | SiO2 | Al2O3 | MgO | Fe2O3 | Na2O | LOI |
|---|---|---|---|---|---|---|---|
| 41.92 | 52.15 | 2.72 | 1.11 | 0.75 | 0.51 | 0.16 | 6.67 |
| Water Consumption of Normal Consistency (%) | Specific Gravity (g/cm3) | Setting Time (min) | Flexural Strength (MPa) | Compressive Strength (Mpa) | |||
|---|---|---|---|---|---|---|---|
| Initial | Final | 2h | 1d Dry | 2h | 1d Dry | ||
| 56 | 2.38 | 4.6 | 7.4 | 3.2 | 7.6 | 8.6 | 22.2 |
| Sample | Gypsum(wt.%) | W/G | Retarder(wt.%) | P2O5(wt.%) |
|---|---|---|---|---|
| S1 | 100 | 0.43 | 0 | 0 |
| S2 | 100 | 0.43 | 0.02 | 0 |
| S3 | 100 | 0.43 | 0.04 | 0 |
| S4 | 100 | 0.43 | 0.06 | 0 |
| S5 | 100 | 0.43 | 0.06 | 0.1 |
| S6 | 100 | 0.43 | 0.06 | 0.3 |
| S7 | 100 | 0.43 | 0.06 | 0.5 |
| Dot | Weight of P(%) |
|---|---|
| 1 | 4.87 |
| 2 | 4.00 |
| 3 | 3.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Zhang, M.; Zhao, M.; Peng, J.; Gao, K.; Huang, J.; Yi, W.; Zhu, C. The Influences of Soluble Phosphorus on Hydration Process and Mechanical Properties of Hemihydrate Gypsum under Deep Retarding Condition. Materials 2022, 15, 2680. https://doi.org/10.3390/ma15072680
Fan P, Zhang M, Zhao M, Peng J, Gao K, Huang J, Yi W, Zhu C. The Influences of Soluble Phosphorus on Hydration Process and Mechanical Properties of Hemihydrate Gypsum under Deep Retarding Condition. Materials. 2022; 15(7):2680. https://doi.org/10.3390/ma15072680
Chicago/Turabian StyleFan, Puyue, Mingtao Zhang, Min Zhao, Jiahui Peng, Kai Gao, Jing Huang, Wei Yi, and Cong Zhu. 2022. "The Influences of Soluble Phosphorus on Hydration Process and Mechanical Properties of Hemihydrate Gypsum under Deep Retarding Condition" Materials 15, no. 7: 2680. https://doi.org/10.3390/ma15072680
APA StyleFan, P., Zhang, M., Zhao, M., Peng, J., Gao, K., Huang, J., Yi, W., & Zhu, C. (2022). The Influences of Soluble Phosphorus on Hydration Process and Mechanical Properties of Hemihydrate Gypsum under Deep Retarding Condition. Materials, 15(7), 2680. https://doi.org/10.3390/ma15072680





