Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries
Abstract
:1. Introduction
2. Characterization and Synthetic Methods of TMD
3. TMDs as ZIBs Cathode
3.1. MoS2 and Modification
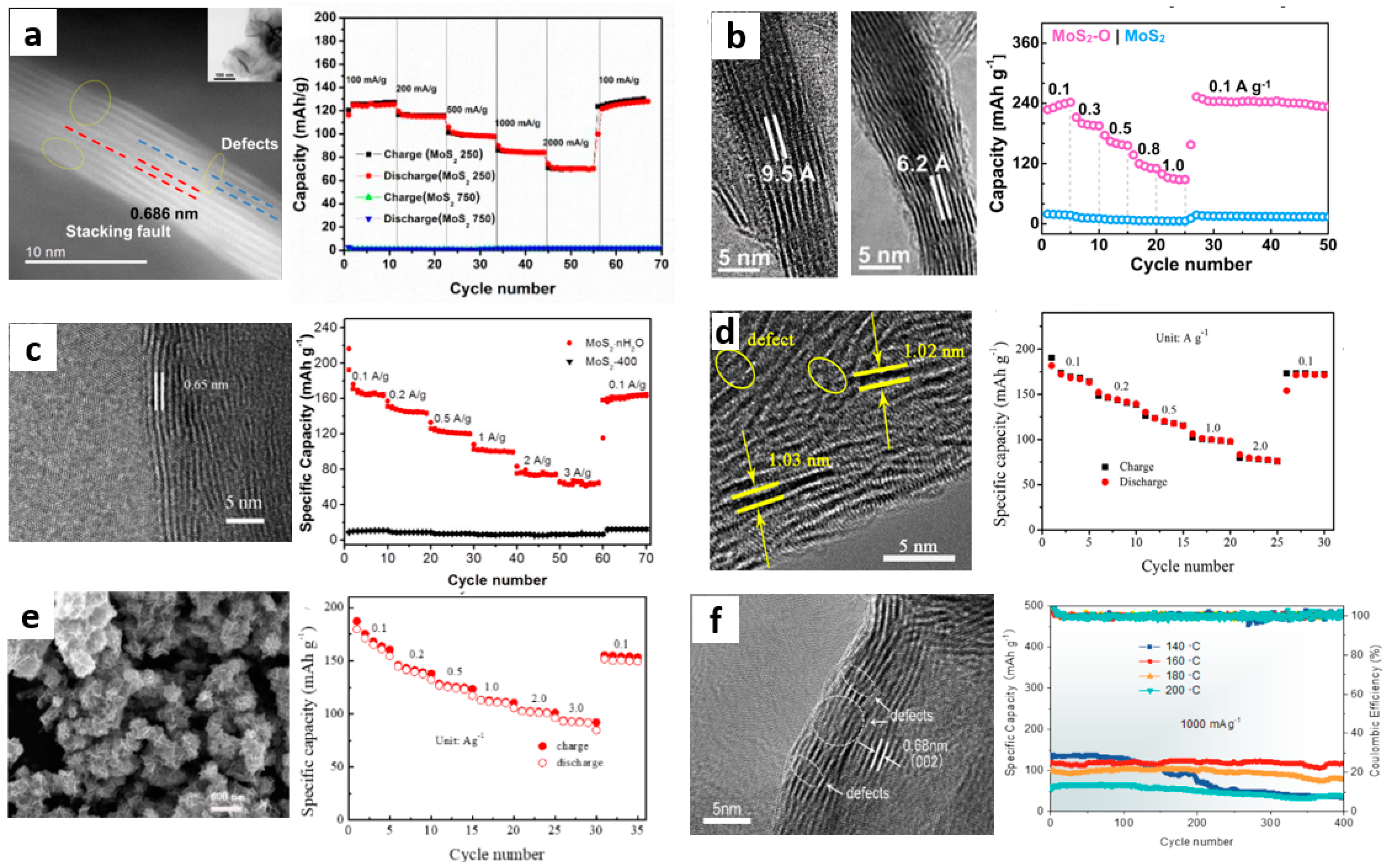
3.1.1. Defect Engineering
| Cathode: | MoS2−x + 0.36Zn2+ + 0.72e− → Zn0.36MoS2−x |
| Anode: | Zn2+ + 2e− → Zn |
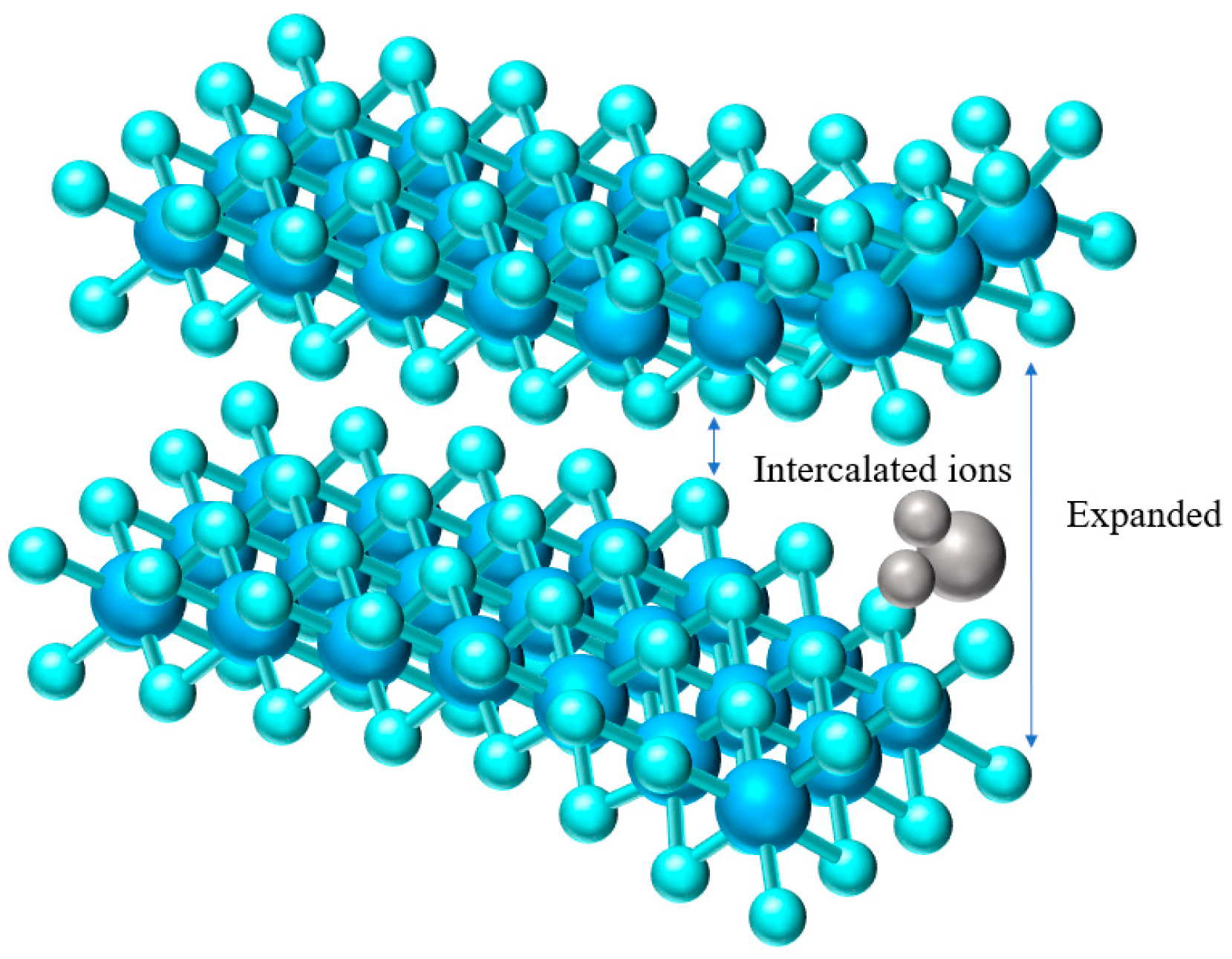
3.1.2. Interlayer Engineering
| Cathode: | xZn2+ + 2xe−+ MoS2 → ZnxMoS2 |
| Anode: | xZn2+ + 2xe− → xZn |
3.1.3. Hybridization
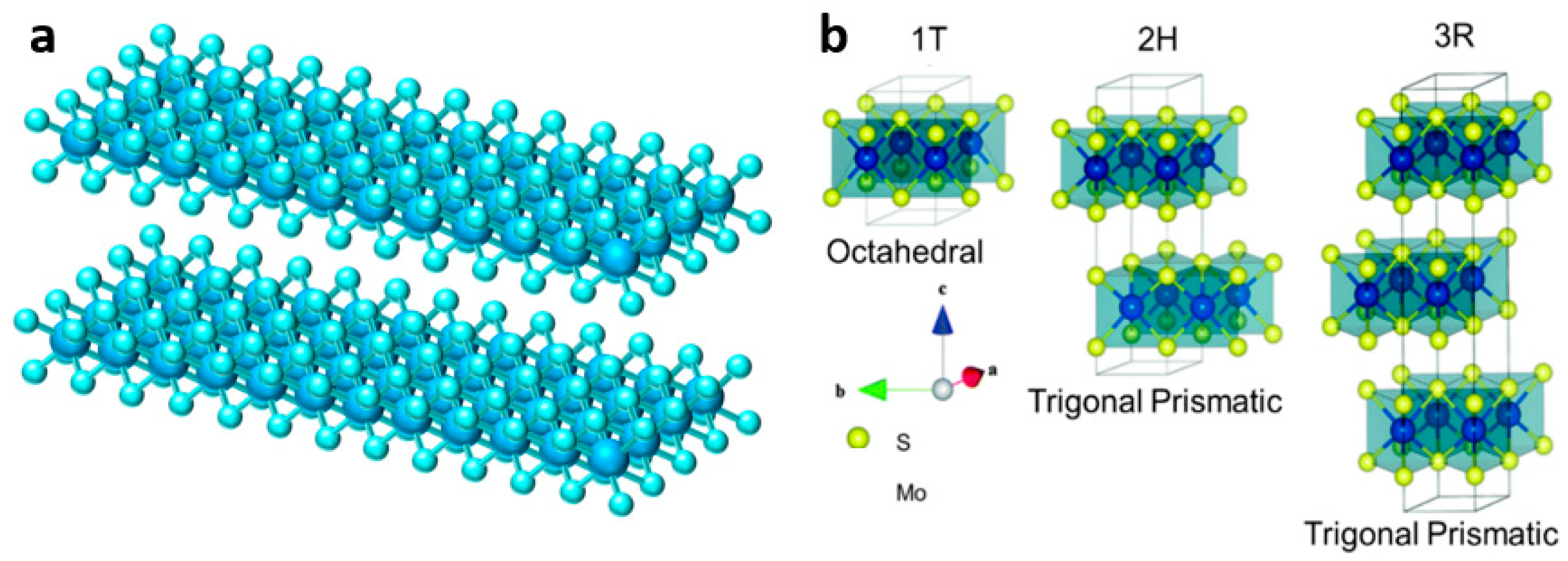
3.1.4. Phase Engineering
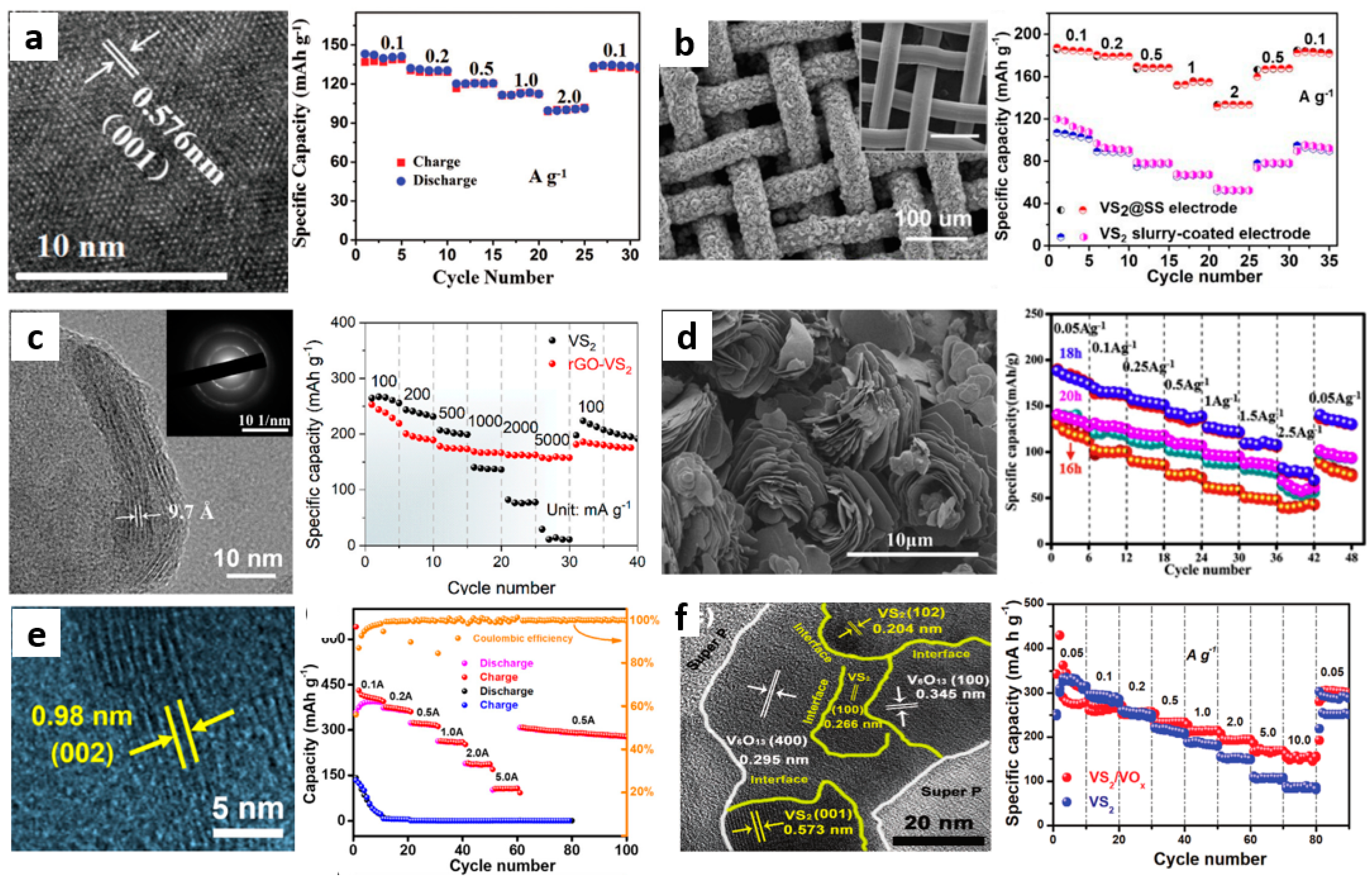
3.2. VS2 and Modification
Zn0.09VS2 + 0.14Zn2+ + 0.28e− → Zn0.23VS2
3.2.1. Hybridization
3.2.2. In Suit Electrochemical Oxidation
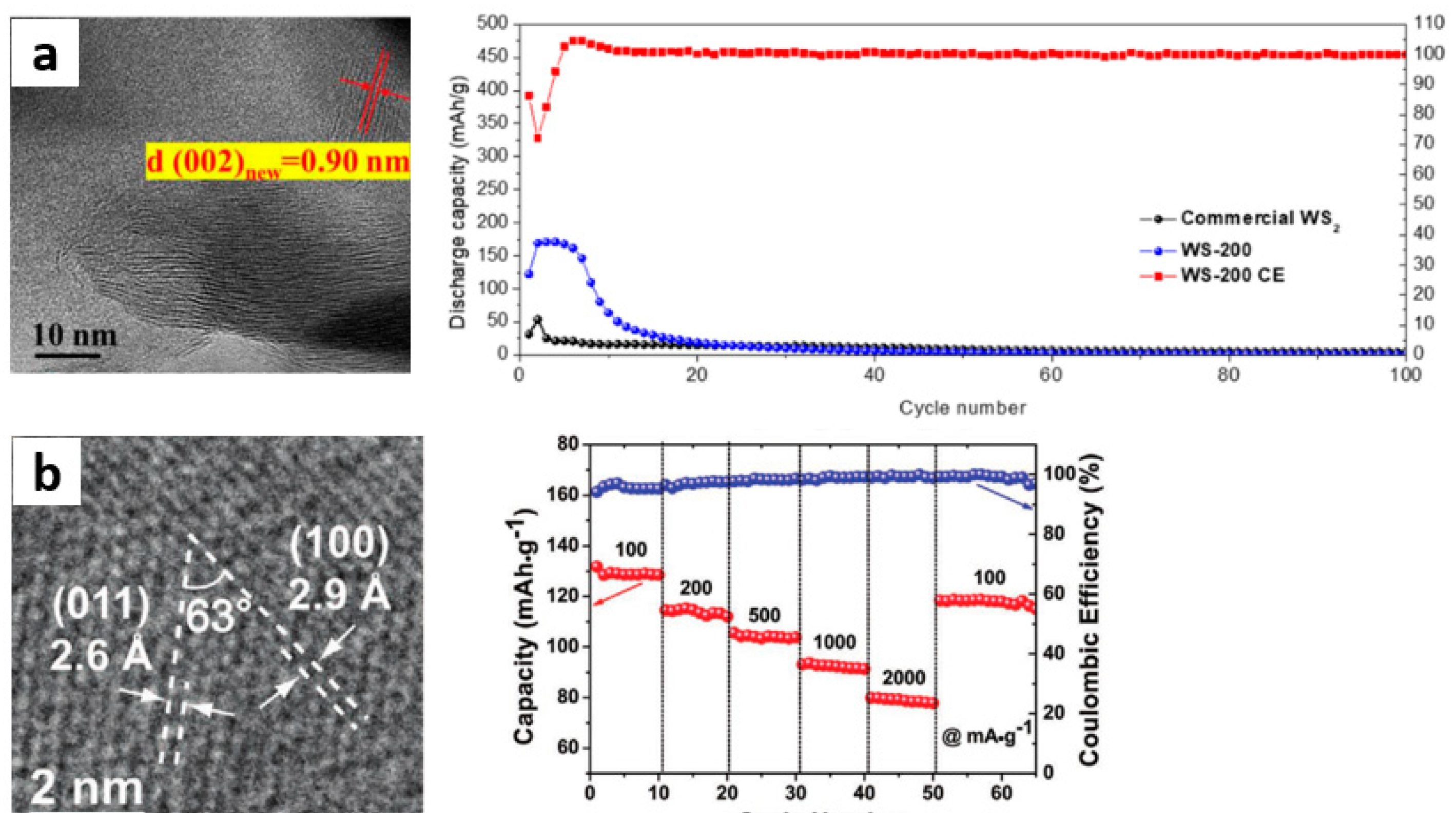
3.3. WS2 and Modification
3.4. Other Materials
| Cathode: | VSe2 + 0.23Zn2+ + 0.46e− → Zn0.23VSe2 |
| Zn0.23VSe2 + 0.17Zn2+ + 0.34e− → Zn0.4VSe2 |
| Anode: | 0.4Zn2+ + 0.8e− → 0.4Zn |
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2021, 452, 214297. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Xiao, X.; Pang, H. Development and application of carbon fiber in batteries. Chem. Eng. J. 2020, 384, 123294. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Zhang, Y.; Li, Y.; Sun, R.; Wang, Z.; Wang, H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2020, 35, 19. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Li, Y.; Zheng, M.; Pang, H. Applications of MxSey (M = Fe, Co, Ni) and their composites in electrochemical energy storage and conversion. Nano-Micro Lett. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301. [Google Scholar] [CrossRef]
- Hua, Y.; Li, X.; Chen, C.; Pang, H. Cobalt Based metal-organic frameworks and their derivatives for electrochemical energy conversion and storage. Chem. Eng. J. 2019, 370, 37. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, X.; Pang, H. Transition metal (Fe, Co, Ni) fluoride-based materials for electrochemical energy storage. Nanoscale Horiz. 2019, 4, 99. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Zhao, Y.; Wang, Y.; Wang, Z.; Zhang, W.; Ren, F. Facile synthesis of two-dimensional porous MgCo2O4 nanosheets as anode for lithium-ion batteries. Appl. Sci. 2017, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; Wu, D.; Dong, Y.; Liao, J.; Foster, C.W.; O’Dwyer, C.; Feng, Y.; Liu, C.; Ma, J. Cobalt-based electrode materials for sodium-ion batteries. Chem. Eng. J. 2019, 370, 185. [Google Scholar] [CrossRef]
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon nanotube-based materials for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 17204. [Google Scholar] [CrossRef]
- Sharma, L.; Gond, R.; Senthilkumar, B.; Roy, A.; Barpanda, P. Fluorophosphates as efficient bifunctional electrocatalysts for metal air batteries. ACS Catal. 2020, 10, 43. [Google Scholar] [CrossRef]
- Ru, Y.; Zheng, S.; Xue, H.; Pang, H. Potassium cobalt hexacyanoferrate nanocubic assemblies for high performance aqueous aluminum ion batteries. Chem. Eng. J. 2020, 382, 122853. [Google Scholar] [CrossRef]
- Wrogemann, J.M.; Künne, S.; Heckmann, A.; Rodríguez-Pérez, I.A.; Siozios, V.; Yan, B.; Li, J.; Winter, M.; Beltrop, K.; Placke, T. Development of safe and sustainable dual ion batteries through hybrid aqueous/nonaqueous electrolytes. Adv. Energy Mater. 2020, 10, 1902709. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410. [Google Scholar] [CrossRef]
- Kasiri, G.; Glenneberg, J.; Hashemi, A.B.; Kun, R.; Mantia, F.L. Mixed copper-zinc hexacya-noferrates as cathode materials for aqueous zinc-ion batteries. Energy Storage Mater. 2019, 19, 360. [Google Scholar] [CrossRef]
- Jin, T.; Ji, X.; Wang, P.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S.; et al. High energy aqueous sodium-ion batteries. Angew. Chem. Int. Ed. 2021, 60, 11943–11948. [Google Scholar] [CrossRef]
- Zhu, K.; Li, Z.; Jin, T.; Jiao, L. Low defects potassium cobalt hexacyanoferrate as a superior cathode for aqueous potassium ion batteries. J. Mater. Chem. A. 2020, 8, 21103–21109. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, S.; Yu, X.; He, W.; Zhang, S.; Tian, Y.; Xing, M.; Fu, Y.; Luo, X. Realizing reversible storage of trivalent aluminum ions using VOPO4·2H2O nanosheets as cathode material in aqueous aluminum metal batteries. J. Alloy. Compd. 2021, 885, 161008. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, J.; Ni, M.; Guo, Q.; Zan, F.; Xia, H. Superior performance of calcium birnessite by electrochemical conversion as cathode for aqueous calcium ion battery. Mater. Res. Bull. 2021, 144, 111475. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent advances in aqueous zinc ion batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.; Myung, S. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Fang, G.; Zhang, C.; Zhang, H.; Guo, X.; Cao, X.; Zhou, J.; Pan, A.; Liang, S. Electrochemical activation of manganese based cathode in aqueous Zinc-Ion electrolyte. Adv. Funct. Mater. 2020, 30, 2002711. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Ji, D.; Zhao, K.; Wang, S.; Cheng, F. Vanadium-based cathodes for aqueous zinc-ion batteries: From crystal structures, diffusion channels to storage mechanisms. J. Mater. Chem. A 2021, 9, 5258–5275. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, F.; Chen, M.; Shao, T.; Li, Z.; Xu, Q.; Cheng, D.; Liu, H.; Xia, Y. Cubic manganese potassium hexacyanoferrate regulated by controlling of the water and fefects as a high capacity and stable cathode material for rechargeable aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 26924–26935. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and transition metal dichalcogenides as functional zinc-ion battery cathode: A perspective. Small Methods 2020, 5, 2000815. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ciucci, F.; Kim, J.-K. Molybdenum disulfide based nanomaterials for rechargeable batteries. Chem. Eur. J. 2020, 26, 6296–6319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sui, J.; Li, F.; Fang, H.; Wang, H.; Li, J.; Cai, W.; Cao, G. Lamellar MoSe2 nanosheets embedded with MoO2 nanoparticles: Novel hybrid nanostructures promoted excellent performances for lithium ion batteries. Nanoscale 2016, 8, 17902–17910. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Z.; Wang, W.; Bai, X. Atomic mechanism of dynamic electrochemical lithiation processes of MoS2 nanosheets. J. Am. Chem. Soc. 2014, 136, 6693–6697. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190. [Google Scholar] [CrossRef]
- Kuc, A.; Heine, T. The electronic structure calculations of two-dimensional transition-metal dichalcogenides in the presence of external electric and magnetic fields. Chem. Soc. Rev. 2015, 44, 2603. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Hafez, A.M.; Cao, D.; Mukhopadhyay, A.; Ma, Y.; Zhu, H. Metallic MoS2 for high performance energy storage and energy conversion. Small 2018, 14, 1800640. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Wu, M.; Chao, Y.; He, P.; Ma, J. Porous bowl-shaped VS2 nanosheets/graphene composite for high rate lithium-ion storage. J. Energy Chem. 2020, 43, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Jing, P.; Lu, H.; Yang, W.; Cao, Y. Interlayer-expanded and binder-free VS2 nanosheets assemblies for enhanced Mg2+ and Li+/Mg2+ hybrid ion storage. Electrochim. Acta 2020, 330, 135263–135272. [Google Scholar] [CrossRef]
- Zhu, J.; Jian, T.; Wu, Y.; Ma, W.; Lu, Y.; Sun, L.; Meng, F.; Wang, B.; Cai, F.; Gao, J.; et al. A highly stable aqueous Zn/VS2 battery based on an intercalation reaction. Appl. Surf. Sci. 2021, 544, 148882. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, B.K.; Lee, I.H.; Kim, M.J.; Chun, S.; Jozwiak, C.; Bostwick, A.; Rotenberg, E.; Chang, Y.J. Electronic structure and charge-density wave transition in monolayer VS2. Curr. Appl. Phys. 2021, 30, 8–13. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. A VS2@N-doped carbon hybrid with strong interfacial interaction for high-performance rechargeable aqueous Zn-ion batteries. J. Mater. Chem. C 2021, 9, 6308–6315. [Google Scholar] [CrossRef]
- Xu, W.; Sun, C.; Zhao, K.; Cheng, X.; Rawal, S.; Xu, Y.; Wang, Y. Defect engineering activating (boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019, 16, 527–534. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-Ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous Zinc-Ion storage in MoS2 by tuning the intercalation energy. Nano Lett. 2019, 19, 3199. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Wang, R.; Li, H.; Yan, J.; Jin, Q.; Feng, P.; Wang, K.; Jiang, K. Crystal water assisting MoS2 nanoflowers for reversible zinc storage. J. Alloy. Compd. 2021, 872, 159599. [Google Scholar] [CrossRef]
- Huang, M.; Mai, Y.; Zhao, L.; Liang, X.; Fang, Z.; Jie, X. Tuning the kinetics of zinc ion in MoS2 by polyaniline intercalation. Electrochim. Acta 2021, 388, 138624. [Google Scholar] [CrossRef]
- Huang, M.; Mai, Y.; Zhao, L.; Liang, X.; Fang, Z.; Jie, X. Hierarchical MoS2@CNTs hybrid as a long life and high rate cathode for aqueous rechargeable Zn-Ion batteries. ChemElectroChem 2020, 7, 4218–4223. [Google Scholar] [CrossRef]
- Liu, J.; Xu, P.; Liang, J.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Boosting aqueous zinc-ion storage in MoS2 via controllable phase. Chem. Eng. J. 2020, 389, 124405. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 nanosheet based aqueous zn ion battery cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L.; et al. Binder-free hierarchical VS2 electrodes for high-performance aqueous zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, Q.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. VS2 nanosheets vertically grown on graphene as high-performance cathodes for aqueous zinc-ion batteries. J. Power Sources 2021, 477, 228652. [Google Scholar] [CrossRef]
- Pu, X.; Song, T.; Tang, L.; Tao, Y.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Rose-like vanadium disulfide coated by hydrophilic hydroxyvanadium oxide with improved electrochemical performance as cathode material for aqueous zinc-ion batteries. J. Power Sources 2019, 437, 226917. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Ben, H.; Zhao, M.; Luo, J.; Chen, D.; Lu, Z.; Wang, L.; Liu, C. Boosting the zinc ion storage capacity and cycling stability of interlayer-expanded vanadium disulfide through in-situ electrochemical oxidation strategy. J. Colloid Interface Sci. 2022, 607, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wei, Z.; Zhang, X.; Zeng, Y.; Wang, C.; Chen, G.; Shen, Z.X.; Du, F. Boosting Zn2+ and NH4+ storage in aqueous media via in-situ electrochemical induced VS2/VOx heterostructures. Adv. Funct. Mater. 2021, 31, 2008743. [Google Scholar] [CrossRef]
- Tang, B.; Tian, N.; Jiang, J.; Li, Y.; Yang, J.; Zhu, Q. Investigation of zinc storage capacity of WS2 nanosheets for rechargeable aqueous zn-ion batteries. J. Alloy. Compd. 2022, 894, 162391. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, C.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Gu, Q.; Sun, Z.; Hu, L. Ultrathin VSe2 nanosheets with fast ion diffusion and robust structural stability for rechargeable zinc-ion battery cathode. Small 2020, 16, 2000698. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hao, J.; Xu, C.; Mo, J.; Dong, L.; Jiang, F.; Wu, J.; Kang, Z.; Jiang, B.; Kang, F. Investigation of zinc ion storage of transition metal oxides, sulfides, and borides in zinc ion battery systems. Chem. Commun. 2017, 53, 6872–6874. [Google Scholar] [CrossRef]
- Chee, S.S.; Lee, W.J.; Jo, Y.R.; Cho, M.K.; Chun, D.; Baik, H.; Kim, B.J.; Yoon, M.H.; Lee, K.; Ham, M.H. Atomic vacancy control and elemental substitution in a monolayer molybdenum disulfide for high performance optoelectronic device arrays. Adv. Funct. Mater. 2020, 30, 1908147. [Google Scholar] [CrossRef]
- Shu, H.; Zhou, D.; Li, F.; Cao, D.; Chen, X. Defect engineering in MoSe2 for the hydrogen evolution reaction: From point defects to edges. ACS Appl. Mater. Interfaces 2017, 9, 42688–42698. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef]
- Shao, Y.; Fang, Y.; Li, T.; Wang, Q.; Dong, Q.; Deng, Y.; Yuan, Y.; Wei, H.; Wang, M.; Gruverman, A.; et al. Grain boundary dominated ion migration in polycrystalline organic–inorganic halide perovskite films. Energy Environ. Sci. 2016, 9, 1752–1759. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ren, H.; Duan, X.; Shakir, I.; Huang, Y.; Duan, X. Layered intercalation materials. Adv. Mater. 2021, 33, 2004557. [Google Scholar] [CrossRef]
- Cataldo, S.; Salice, P.; Menna, E.; Pignataro, B. Carbon nanotubes and organic solar cells. Energy Environ. Sci. 2012, 5, 5919–5940. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C. 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.T.; Zhou, W.; Loh, K.P. Phase restructuring in transition metal dichalcogenides for highly stable energy storage. ACS Nano 2016, 10, 9208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Xu, P.; Li, Y.C.; Zhou, D.; Sun, Y.; Nguyen, M.A.; Terrones, M.; Mallouk, T.E. Controlled exfoliation of MoS2 crystals into trilayer nanosheets. J. Am. Chem. Soc. 2016, 138, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jiang, D. Mechanism of hydrogen evolution reaction on 1T-MoS2 from first principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent development of metallic (1T) phase of Molybdenum disulfide for energy conversion and storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Zhou, S.; Li, H.; Wong, H.; Luo, Z.; Gan, L.; Zhai, T. Strategies on phase control in transition metal dichalcogenides. Adv. Funct. Mater. 2018, 28, 1802473. [Google Scholar] [CrossRef]
- Tan, C.; Luo, Z.; Chaturvedi, A.; Cai, Y.; Du, Y.; Gong, Y.; Huang, Y.; Lai, Z.; Zhang, X.; Zheng, L.; et al. Preparation of high percentage 1T-Phase transition metal dichalcogenide nanodots for electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, Z.; Cabrera, C.R.; Chen, Z. Metallic VS2 Monolayer: A promising 2D anode material for lithium ion batteries. J. Phys. Chem. C 2013, 117, 25409. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Keyu, X.; Kai, Y.; Xin, L.; Wei, L.; Chao, S.; Chenglu, L.; Robert, V.; Pulickel, A.; Bingqing, W. Superior potassium ion storage via vertical MoS2 “Nano-Rose” with expanded interlayers on graphene. Small 2017, 13, 1701471. [Google Scholar]
- Xiang, T.; Fang, Q.; Xie, H.; Wu, C.; Wang, C.; Zhou, Y.; Liu, D.; Chen, S.; Khalil, A.; Tao, S.; et al. Vertical 1T-MoS2 nanosheets with expanded interlayer spacing edged on a graphene frame for high rate lithium-ion batteries. Nanoscale 2017, 9, 6975–6983. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, F.; Li, W.; Zhang, X.; Lee, C.-S.; Wang, W.; Tang, Y. Porous tremella-like MoS2/polyaniline hybrid composite with enhanced performance for lithium-ion battery anodes. Electrochim. Acta 2015, 167, 132. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.M. Design strategies for development of TMD-Based heterostructures in electrochemical energy systems. Matter 2020, 2, 526. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 nanoparticle/MoS2 nanosheet composites with superior performances for lithium ion batteries. Small 2014, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, B.; Wang, F.; Yang, J.; Wu, F.; Ning, Y.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Anodic oxidation strategy toward structure-optimized V2O3 cathode via electrolyte regulation for Zn-Ion storage. ACS Nano 2020, 14, 7328–7337. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Gao, H.; Zhao, K.; Zheng, H.; Zhang, H.; Han, L.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. In-situ electrochemical conversion of vanadium dioxide for enhanced zinc-ion storage with large voltage range. J. Power Sources 2021, 487, 229369. [Google Scholar] [CrossRef]
- Ding, J.; Du, Z.; Li, B.; Wang, L.; Wang, S.; Gong, Y.; Yang, S. Unlocking the potential of disordered rocksalts for aqueous zinc-ion batteries. Adv. Mater. 2019, 31, 1904369. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Ge, Y.; Clemente, R.; Dong, P.; Wang, L.; Shen, J.; Ye, M.; Ajayan, P.M. An in situ electrochemical oxidation strategy for formation of nanogrid-shaped V3O7·H2O with enhanced zinc storage properties. J. Mater. Chem. A 2019, 7, 25262. [Google Scholar] [CrossRef]
- Georgiou, T.; Jalil, R.; Belle, B.D.; Britnell, L.; Gorbachev, R.V.; Morozov, S.V.; Kim, Y.-J.; Gholinia, A.; Haigh, S.J.; Makarovsky, O.; et al. Vertical field-effect transistor based on graphene–WS2 heterostructures for flexible and transparent electronics. Nat. Nanotechnol. 2013, 8, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Lezama, I.G.; Berger, H.; Morpurgo, A.F. Quantitative determination of the band gap of WS2 with ambipolar ionic liquid–gated transistors. Nano Lett. 2012, 12, 5218–5223. [Google Scholar] [CrossRef] [Green Version]
- Debela, T.T.; Lim, Y.R.; Seo, H.W.; Kwon, I.S.; Kwak, I.H.; Park, J.; Cho, W.I.; Kang, H.S. Two-Dimensional WS2@Nitrogen-Doped graphite for high-performance lithium ion batteries: Experiments and molecular dynamics simulations. ACS Appl. Mater. Interfaces 2018, 10, 37928–37936. [Google Scholar] [CrossRef] [PubMed]
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Xiao, Z.; Zhou, Y.; Chen, H.; Khalil, A.; Xiang, T.; Xu, J.; Chu, W.; Wu, X.; et al. Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions: The correlation between structure and electrical/optical properties. Adv. Mater. 2015, 27, 4837–4844. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.T.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.F.; Mallouk, T.E.; Terrones, M. Transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 56. [Google Scholar] [CrossRef]
- Ma, Y.D.; Dai, Y.; Guo, M.; Niu, C.W.; Zhu, Y.T.; Huang, B.B. Evidence of the existence of magnetism in pristine VX2 Monolayers (X = S, Se) and their strain-induced tunable magnetic properties. ACS Nano 2012, 6, 1695. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, P.; Li, X.; Wu, C.; Guo, Y.; Zhao, J.; Wu, X.; Xie, Y. Ultrathin Nanosheets of Vanadium Diselenide: A Metallic Two-Dimensional Material with Ferromagnetic Charge-Density-Wave Behavior. Angew. Chem. Int. Ed. 2013, 52, 10477. [Google Scholar] [CrossRef]
- Yang, J.R.F.; Lv, F.; Zhou, J.H.; Lin, C.F.; Wang, K.; Zhang, Y.L.; Yang, Y.; Wang, W.; Li, J.B.; Guo, S.J. Metallic graphene-like VSe2 ultrathin nanosheets: Superior potassium-ion storage and their working mechanism. Adv. Mater. 2018, 30, 1800036. [Google Scholar] [CrossRef]
- Zhang, R.X.; Pan, C.S.; Nuzzo, R.G.; Gewirth, A.A. CoS2 as a sulfur redox-active cathode material for high-capacity nonaqueous Zn batteries. J. Phys. Chem. C 2019, 123, 8740. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.L.; Cheng, S.J.; Jiang, K. An ultrastable presodiated titanium disulfide anode for aqueous “Rocking-Chair” zinc ion battery. Adv. Energy Mater. 2019, 9, 1900993. [Google Scholar] [CrossRef]




| Products | Method | Precursors | Temperature | Duration | Ref |
|---|---|---|---|---|---|
| MoS2−x | Hydrothermal | (NH4)6Mo7O24·4H2O, TAA | 200 °C | 18 h | [38] |
| E- MoS2 | Hydrothermal | Na2MoO4, CS(NH2)2, carbon cloth, glucose, HCl | 190 °C | 24 h | [39] |
| MoS2-O | Hydrothermal | (NH4)6Mo7O24·4H2O, thiourea | 180 °C | 24 h | [40] |
| MoS2·nH2O | Hydrothermal | (NH4)6Mo7O24·4H2O, thiourea | 170 °C | 24 h | [41] |
| MoS2/PANI | Solvothermal | Na2MoO4, thiourea, C17H33CO2Na, ethanol, OA, HCl | 180 °C | 24 h | [42] |
| MoS2@CNTs | Hydrothermal | Na2MoO4·2H2O, thiourea, CNTs, glucose | 200 °C | 24 h | [43] |
| MoS2-160 | Hydrothermal | (NH4)6Mo7O24·4H2O, thiourea | 160 °C | 24 h | [44] |
| VS2 | Hydrothermal | NH4VO3, TAA, NH3·H2O | 180 °C | 20 h | [45] |
| VS2@SS | Hydrothermal | NH4VO3, TAA, NH3·H2O, stainless steel mesh | 180 °C | 10 h | [46] |
| rGO-VS2 | Solvothermal | VO(acac)2, cysteine, GO, NMP | 200 °C | 8 h | [47] |
| VS2@VOOH | Hydrothermal | V2O5, TAA, NH3·H2O | 180 °C | 18 h | [48] |
| VS2·NH3 | Solvothermal | VO(acac)2, TAA, NMP | 200 °C | 24 h | [49] |
| VS2/VOx | Solvothermal | Na3VO4·12H2O, TAA, ethylene | 180 °C | 20 h | [50] |
| 1T-WS2 | Solvothermal | WCl6, TAA, DMF | 200 °C | 24 h | [51] |
| VSe2 | Chemical Liquid Phase Synthesis | VO(acac)2, Se powder, OAm | 330 °C | 5 h | [52] |
| Cathode Material | Electrolyte | Voltage | Capacity [mAh•g−1] | Cycle Stability | Ref |
|---|---|---|---|---|---|
| MoS2-O | 3M Zn (CF3SO3)2 | 0.2–1.4V | 232 at 0.1 A g−1 | 68% after 2000 cycles at 1.0 A g−1 | [40] |
| E- MoS2 | 2M Zn (CF3SO3)2 | 0.3–1.5V | 202.6 at 0.1 A g−1 | 98.6% after 600 cycles at 1.0 A g−1 | [39] |
| MoS2/PANI | 3M Zn (CF3SO3)2 | 0.2–1.3V | 181.6 at 0.1 A g−1 | 86% after 1000 cycles at 1.0 A g−1 | [42] |
| MoS2@CNTs | 3M Zn (CF3SO3)2 | 0.3–1.2V | 180 at 0.1 A g−1 | 80.1% after 500 cycles at 1.0 A g−1 | [43] |
| MoS2-160 | 3M Zn (CF3SO3)2 | 0.25–1.25V | 168 at 0.1 A g−1 | 98.1% after 400 cycles at 1.0 A g−1 | [44] |
| MoS2·nH2O | 3M Zn (CF3SO3)2 | 0.2–1.25V | 165 at 0.1 A g−1 | 88% after 800 cycles at 2.0 A g−1 | [41] |
| MoS2−x | 3M Zn (CF3SO3)2 | 0.25–1.25V | 138.6 at 0.1 A g−1 | 87.8% after 1000 cycles at 1.0 A g−1 | [38] |
| VS2·NH3 | 2M Zn (CF3SO3)2 | 0.2–1.7V | 390 at 0.1 A g−1 | 110% after 2000 cycles at 3.0 A g−1 | [49] |
| VS2/VOx | 25M ZnCl2 | 0.1–1.8V | 260 at 0.1 A g−1 | 75% after 3000 cycles at 1.0 A g−1 | [50] |
| rGO-VS2 | 3M Zn (CF3SO3)2 | 0.4–1.7V | 238 at 0.1 A g−1 | 93% after 1000 cycles at 5.0 A g−1 | [47] |
| VS2@SS | 1M ZnSO4 | 0.4–1.0V | 187 at 0.1 A g−1 | 80% after 2000 cycles at 2.0 A g−1 | [46] |
| VS2@VOOH | 3M Zn (CF3SO3)2 | 0.4–1.0V | 165 at 0.1 A g−1 | 86% after 200 cycles at 0.5 A g−1 | [48] |
| VS2 | 1M ZnSO4 | 0.4–1.0V | 159.1 at 0.1 A g−1 | 98% after 200 cycles at 0.5 A g−1 | [45] |
| WS-200 | 1M ZnSO4 | 0.1–1.5V | 206.25 at 0.1 A g−1 | 0% after 100 cycles at 0.2 A g−1 | [51] |
| VSe2 | 2M ZnSO4 | 0.1–1.6V | 131.8 at 0.1 A g−1 | 80.8% after 500 cycles at 0.1 A g−1 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, H.; Yuan, J.; Xia, Y.; Liu, Y.; Sun, A. Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries. Materials 2022, 15, 2654. https://doi.org/10.3390/ma15072654
Li T, Li H, Yuan J, Xia Y, Liu Y, Sun A. Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries. Materials. 2022; 15(7):2654. https://doi.org/10.3390/ma15072654
Chicago/Turabian StyleLi, Tao, Haixin Li, Jingchen Yuan, Yong Xia, Yuejun Liu, and Aokui Sun. 2022. "Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries" Materials 15, no. 7: 2654. https://doi.org/10.3390/ma15072654
APA StyleLi, T., Li, H., Yuan, J., Xia, Y., Liu, Y., & Sun, A. (2022). Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries. Materials, 15(7), 2654. https://doi.org/10.3390/ma15072654







