Effect of Tempering Temperature on Microstructure and Sulfide Stress Cracking of 125 Ksi Grade Casing Steel
Abstract
:1. Introduction
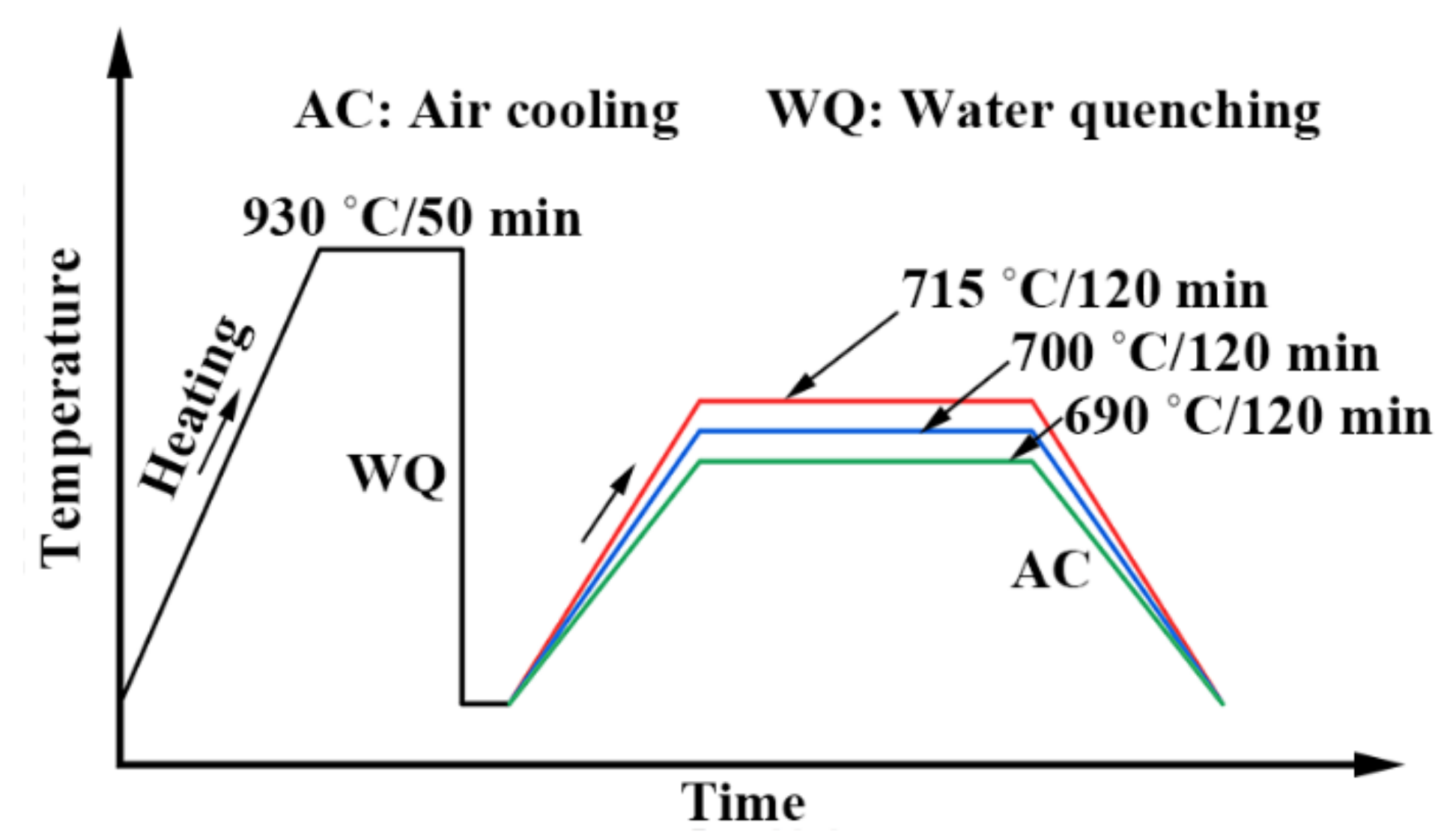
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
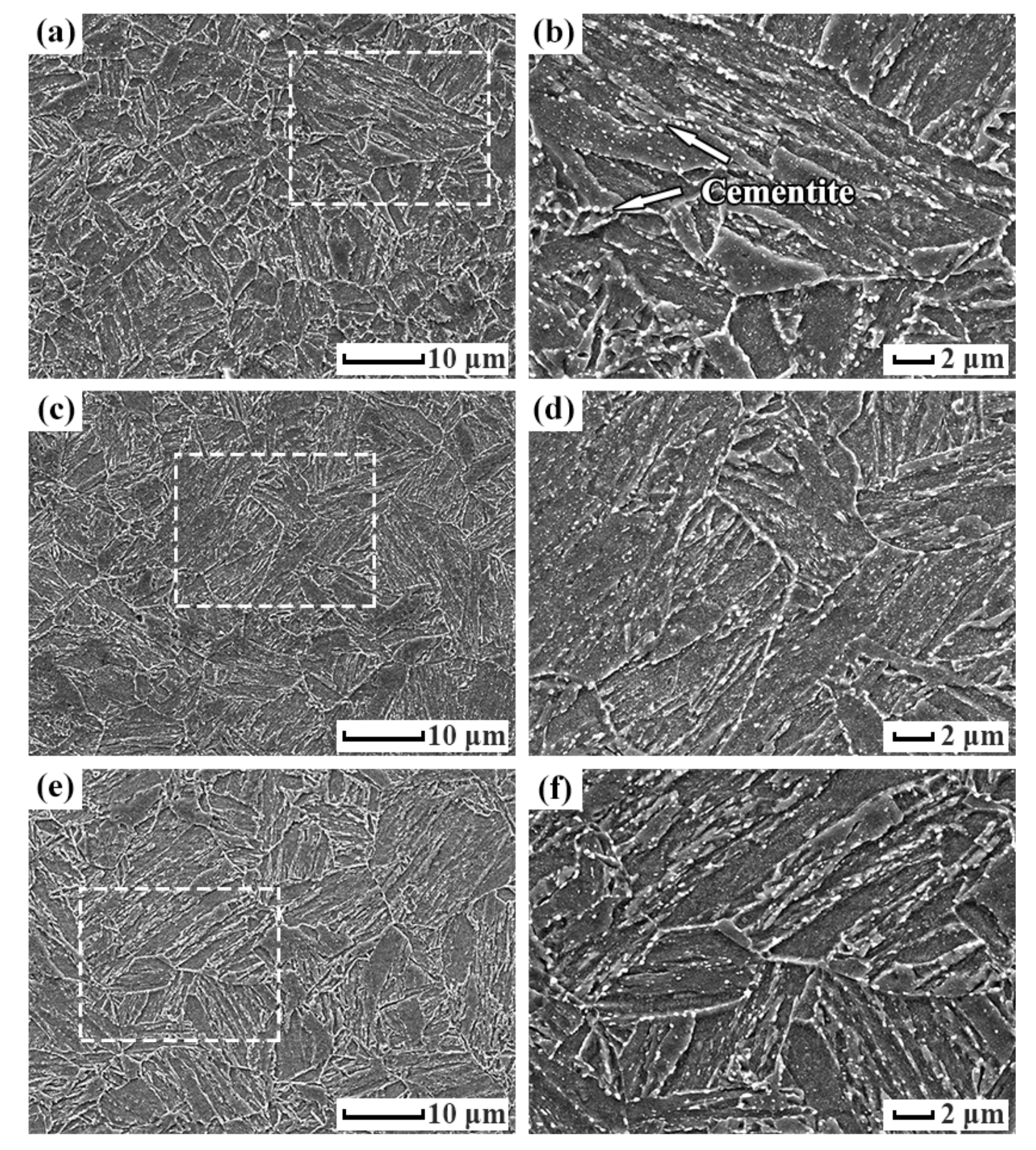
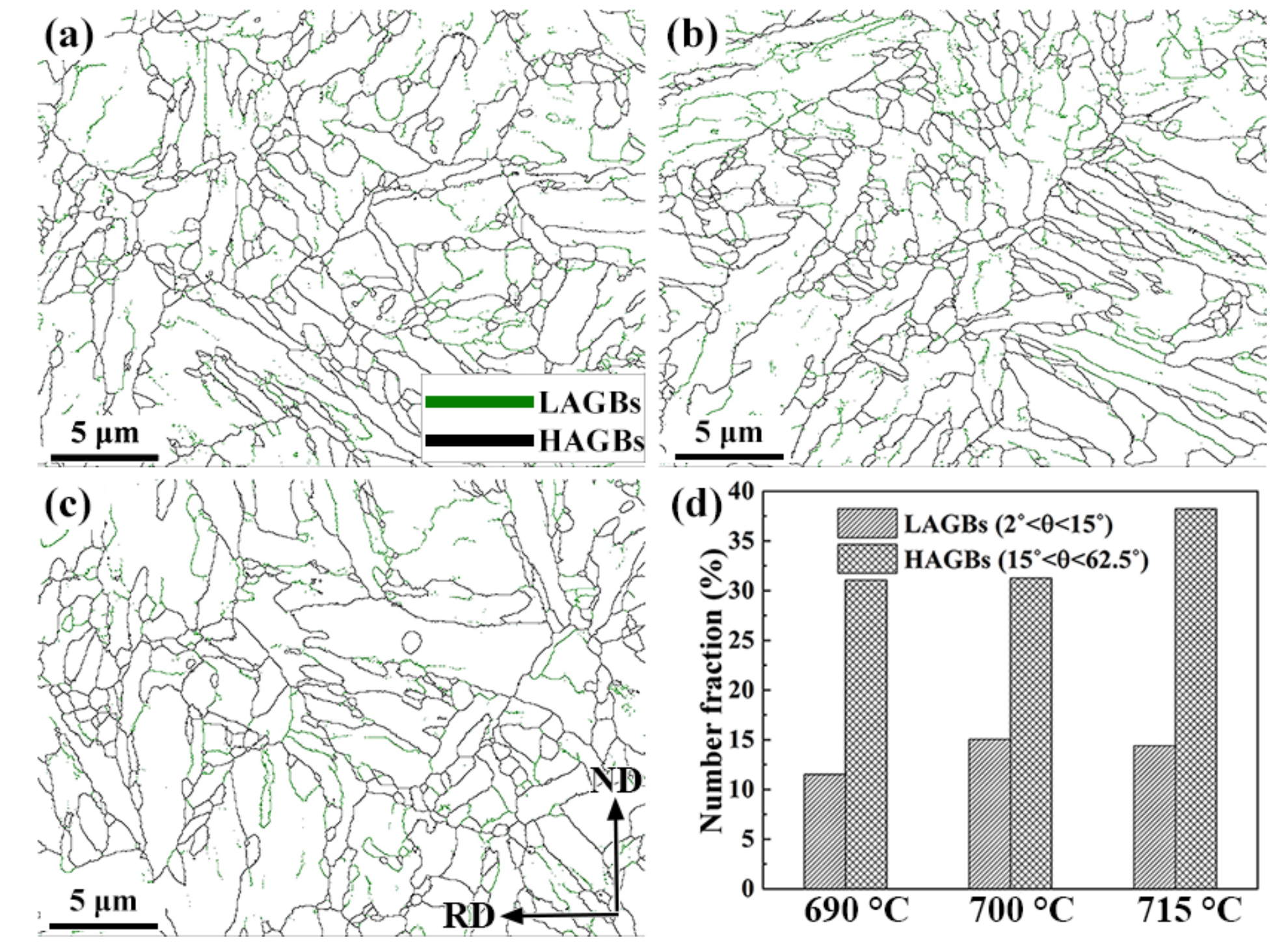
- The microstructures of the 0.5Cr0.4W casing steel tempered at 690 °C, 700 °C, and 715 °C were all tempered martensites, with good anti-SSC performance.
- (2)
- With the price of deterioration in mechanical properties and hardness, the KISSC of steel tempered at 715 °C rose to 34.58 MPa·m0.5. Restricted by the strength limitation of 125 ksi grade casing steel, 700 °C is a more suitable tempering temperature option.
- (3)
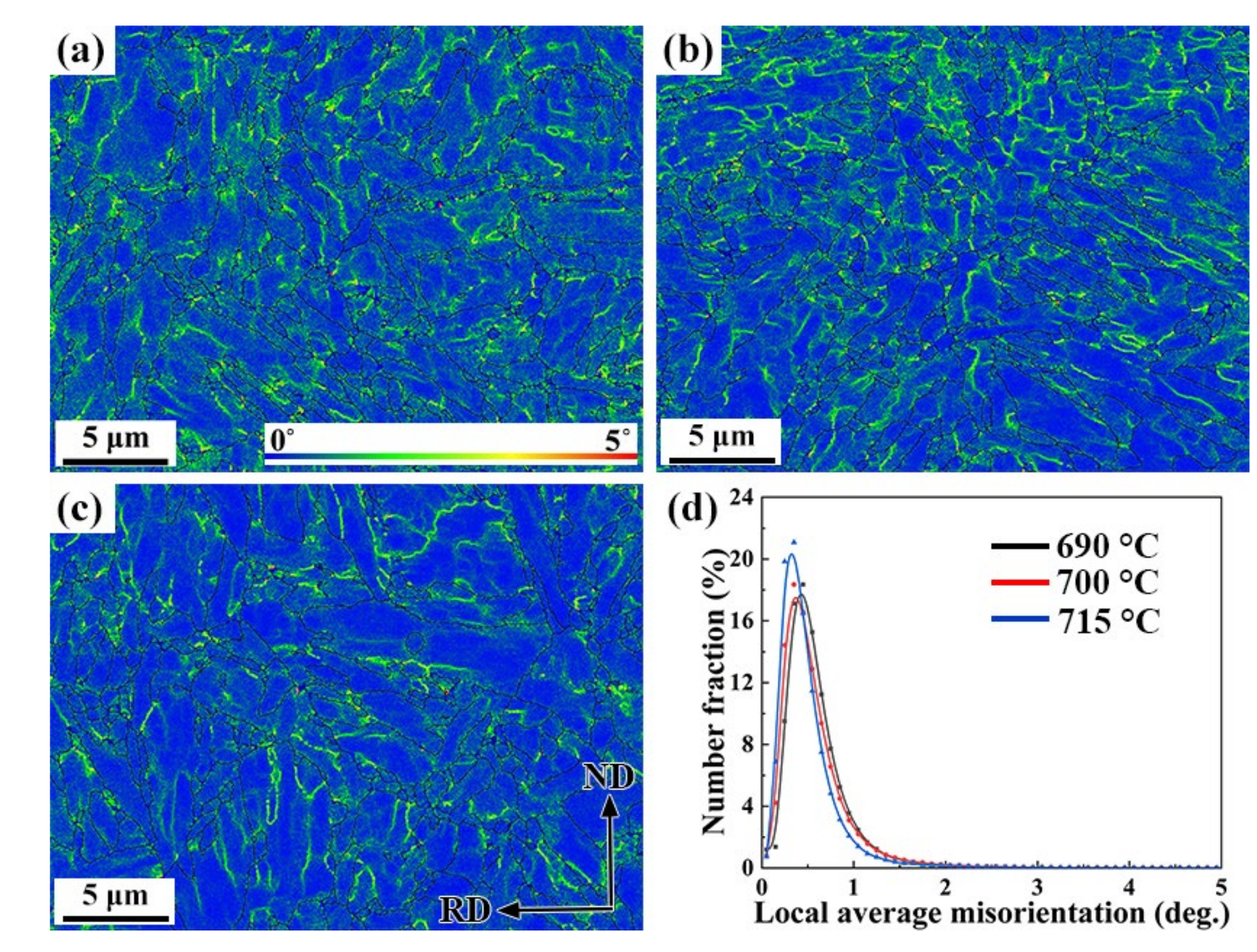
- The lower LAM exhibited a superior SSC performance; as the movable dislocation reduced under the external stress, less hydrogen was transported to the crack-sensitive zone weakening the detriments from local hydrogen concentration and SSC susceptibility.
- (4)
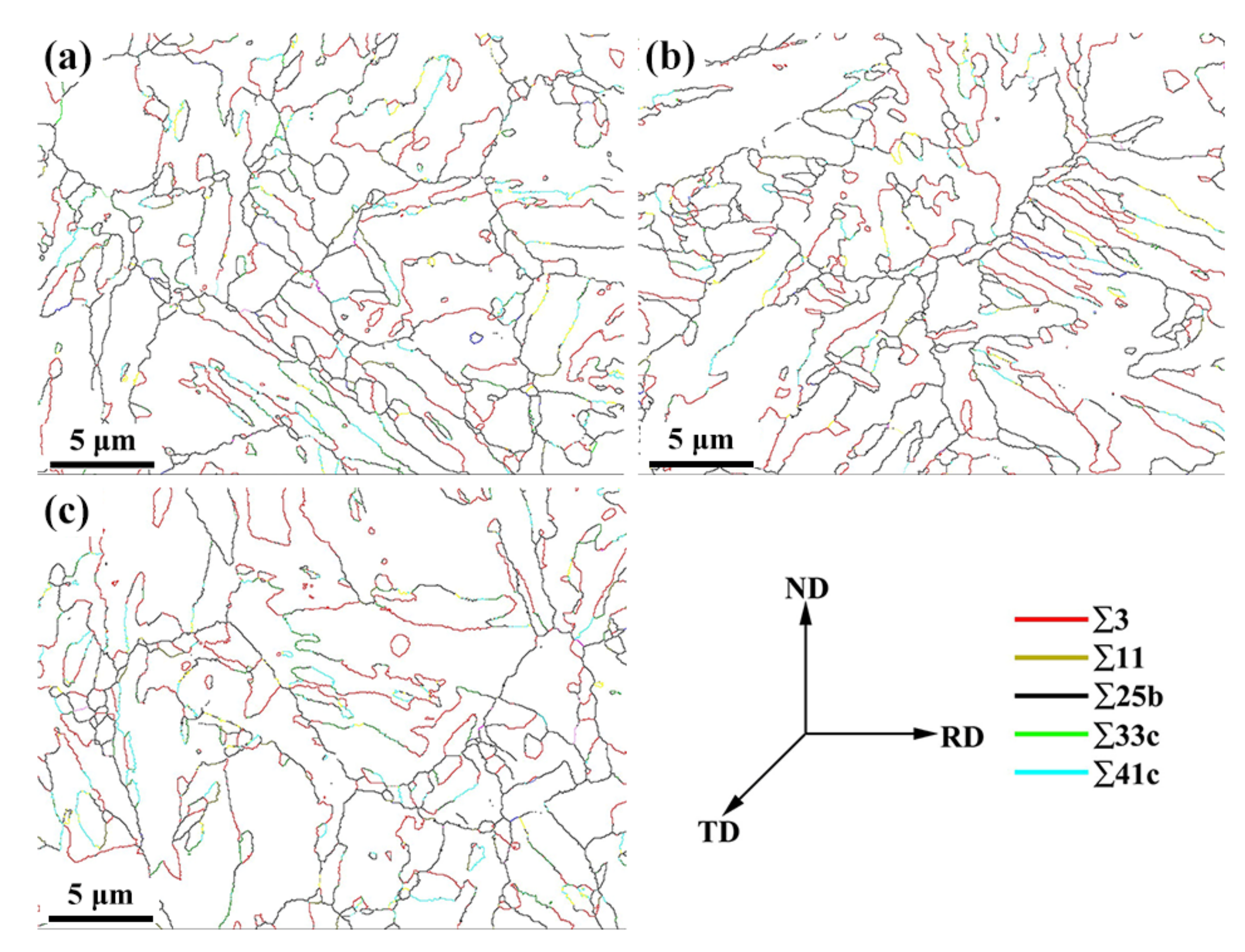
- The average value of the Taylor factor went down as the tempering temperature rose, while for grain with a low Taylor factor, the slip is more likely to occur; effectively avoiding the stress concentration caused by entangled dislocation and inhibiting crack propagation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Ma, L. Current situation and future development of oil and gas field in China. Chem. Eng. Des. Commun. 2017, 43, 53. [Google Scholar]
- Yang, Y.; Bo, T.T.; Ye, M.; Huang, Y.C.; Zhang, M. Present situation and development trend of oil and gas field development in China. China Petrol. Chem. Stand. Qual. 2014, 34, 183. [Google Scholar]
- Zhang, Y.C.; Zhang, B.M.; Li, B.L.; Zhu, G.Y.; Su, J.; Wang, X.M. History of hydrocarbon accumulations spanning important tectonic phases in marine sedimentary basins of China: Taking the Tarim Basin as an example. Petrol. Explor. Dev. 2011, 38, 1–13. [Google Scholar]
- Ma, Y.S.; Cai, X.Y.; Li, G.X. Basic characteristics and concentration of the Puguang gas field in the Sichuan basin. Acta. Geol. Sin. 2005, 79, 858–865. [Google Scholar]
- Li, Y.; Xue, Z.J. Challenges and development tendency of engineering technology in oil and gas development in Sinopec. Petrol. Drill. Tech. 2016, 44, 1–5. [Google Scholar]
- Li, X.Y.; Wen, J.B.; Li, Q.A. Anti-corrosion technologies for tubings in oil and gas wells. Corros. Sci. Prot. Tech. 2003, 15, 272–276. [Google Scholar]
- Kermani, M.B.; Harrop, D. The impact of corrosion on the oil and gas industry. SPE Prod. Facil. 1996, 11, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, A.; Hashimoto, K.; Shimodaira, S. Hydrogen electrode reaction and hydrogen embrittlement of mild steel in hydrogen sulfide solutions. Corrosion 1976, 32, 321–331. [Google Scholar] [CrossRef]
- Capelle, J.; Dmytrakh, I.; Pluvinage, G. Comparative assessment of electrochemical hydrogen absorption by pipeline steels with different strength. Corros. Sci. 2010, 52, 1554–1559. [Google Scholar] [CrossRef]
- González-Arévalo, N.E.; Velázquez, J.C.; Díaz-Cruz, M.; Cervantes-Tobón, A.; Terán, G.; Hernández-Sanchez, E.; Capula-Colindres, S. Influence of aging steel on pipeline burst pressure prediction and its impact on failure probability estimation. Eng. Fail. Anal. 2020, 120, 104950. [Google Scholar] [CrossRef]
- Watkins, M.; Ayer, R. Microstructure—The critical variable controlling the SSC resistance of low-alloy steels. In Proceedings of the Corrosion’s 95: National Association of Corrosion Engineers (NACE) International Annual Conference and Corrosion Show, Orlando, FL, USA, 26–31 March 1995. [Google Scholar]
- Ohnuma, M.; Suzuki, J.; Wei, F.G.; Tsuzaki, K. Direct observation of hydrogen trapped by NbC in steel using small-angle neutron scattering. Scr. Mater. 2008, 58, 142–145. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, P.; Xiang, S.D.; Li, J.X. Some views on development of domestic OCTG-making industry. Steel Pipe 2011, 40, 12–16. [Google Scholar]
- Gao, X.; Xiao, G.Z.; Guan, S.H.; Wang, J. Research on high collapse resistance casing and production status in China. Welded Pipe 2011, 40, 12–16. [Google Scholar]
- Zhang, Z.H.; Zhang, C.X.; Yin, G.H.; Tan, Z.L. Development of Baosteel’s corrosion-resistant series of oil country tubular goods. Baosteel Tech. 2009, S1, 62–66. [Google Scholar]
- Wang, X.T.; Liu, M.; Zhou, G.Y.; Jiang, H.; Li, X.; Luo, M.; Liu, Y.H.; Zhang, Z.H.; Cao, G.H. Effects of chromium and tungsten on sulfide stress cracking in high strength low alloy 125 ksi grade casing steel. Corros. Sci. 2019, 160, 108163. [Google Scholar] [CrossRef]
- NACE Standard TM 0177-2016; Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments. NACE International: Houston, TX, USA, 2016.
- Liu, M.; Wang, C.H.; Dai, Y.C.; Li, X.; Cao, G.H.; Russell, A.M.; Liu, Y.H.; Dong, X.M.; Zhang, Z.H. Effect of quenching and tempering process on sulfide stress cracking susceptibility in API-5CT-C110 casing steel. Mater. Sci. Eng. A 2017, 688, 378–387. [Google Scholar] [CrossRef]
- Carneiro, R.A.; Ratnapuli, R.C.; de Freitas Cunha Lins, V. The influence of chemical composition and microstructure of API linepipe steels on hydrogen induced cracking and sulfide stress corrosion cracking. Mater. Sci. Eng. A 2003, 357, 104–110. [Google Scholar] [CrossRef]
- Dvoracek, L.M. Sulfide stress corrosion cracking of steels. Corrosion 1970, 26, 177–188. [Google Scholar] [CrossRef]
- Mendibide, C.; Sourmail, T. Composition optimization of high-strength steels for sulfide stress cracking resistance improvement. Corros. Sci. 2009, 51, 2878–2884. [Google Scholar] [CrossRef]
- Badji, R.; Chauveau, T.; Bacroix, B. Texture, misorientation and mechanical anisotropy in a deformed dual phase stainless steel weld joint. Mater. Sci. Eng. A 2013, 575, 94–103. [Google Scholar] [CrossRef]
- Britton, T.B.; Birosca, S.; Preuss, M.; Wilkinson, A.J. Electron backscatter diffraction study of dislocation content of a macrozone in hot-rolled Ti-6Al-4V alloy. Scr. Mater. 2010, 62, 639–642. [Google Scholar] [CrossRef]
- Pressouyre, G.M. A classification of hydrogen traps in steel. Metall. Trans. A 1979, 10, 1571–1573. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Response of hydrogen trapping capability to microstructural change in tempered Fe-0.2C martensite. Scr. Mater. 2005, 52, 467–472. [Google Scholar] [CrossRef]
- Oriani, R.A.; Josephic, P.H. Equilibrium aspects of hydrogen-induced cracking of steels. Acta Metall. 1974, 22, 1065–1074. [Google Scholar] [CrossRef]
- Liang, Y.; Sofronis, P.; Aravas, N. On the effect of hydrogen on plastic instabilities in metals. Acta Mater. 2003, 51, 2717–2730. [Google Scholar] [CrossRef]
- Nibur, K.A.; Somerday, B.P.; Balch, D.K.; Marchi, C.S. The role of localized deformation in hydrogen-assisted crack propagation in 21Cr-6Ni-9Mn stainless steel. Acta Mater. 2009, 57, 3795–3809. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen content on the embrittlement in a Fe-Mn-C twinning-induced plasticity steel. Corros. Sci. 2012, 59, 277–281. [Google Scholar] [CrossRef]
- Novak, P.; Yuan, R.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. A statistical, physical-based, micro-mechanical model of hydrogen-induced intergranular fracture in steel. J. Mech. Phys. Solids 2010, 58, 206–226. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lu, H.Z.; Liang, J.T.; Rosenthal, A.; Liu, H.W.; Sneddon, G.; McCarroll, I.; Zhao, Z.Z.; Li, W.; Guo, A.M.; et al. Observation of hydrogen trapping at dislocations, grain boundaries, and precipitates. Science 2020, 367, 171–175. [Google Scholar] [CrossRef]
- Caron, R.N.; Krauss, G. The tempering of Fe-C lath martensite. Metall. Mater. Trans. A 1972, 3, 2381–2389. [Google Scholar] [CrossRef]
- Jothi, S.; Merzlikin, S.V.; Croft, T.N.; Andersson, J.; Brown, S.G.R. An investigation of micro-mechanisms in hydrogen induced cracking in nickle-based superalloy 718. J. Alloys. Compd. 2016, 664, 664–681. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Inomata, T.; Kobayashi, H.; Tsurekawa, S.; Watanabe, T. Effects of grain boundary- and triple junction-character on intergranular fatigue crack nucleation in polycrystalline. J. Mater. Sci. 2008, 43, 3792–3799. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, M.F.; Zhu, G.C.; Li, N.; Xie, J.F.; Wang, P.B.; Yu, Y.; Song, S.Y.; Yin, C.X. Effect of heat treatment on microstructure and properties of Ti-6Al-4V-0.5Ni-0.05Ru titantium alloy used in oil and gas exploration. Rare Met. Mater. Eng. 2021, 50, 2557–2567. [Google Scholar]
- Rollett, A.; Humphreys, F.; Rohrer, G.S. Recrystallization and Related Annealing Phenomena, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 0-08-042685-9. [Google Scholar]
- Grimmer, H.; Bollmann, W.; Warrington, D.H. Coincidence-site lattices and complete pattern-shift lattices in cubic crystals. Acta Crystallogr. A 1974, 30, 197–207. [Google Scholar] [CrossRef]
- Randle, V. Twinning-related grain boundary engineering. Acta Mater. 2004, 52, 4067–4081. [Google Scholar] [CrossRef]
- Seita, M.; Hanson, J.P.; Gradecak, S.; Demkowica, M.J. The dual role of coherent twin boundaries in hydrogen embrittlement. Nat. Commun. 2015, 6, 6164. [Google Scholar] [CrossRef]
- Shen, J.H.; Li, Y.L.; Wei, Q. Statistic derivation of Taylor factors for polycrystalline metals with application to pure magnesium. Mater. Sci. Eng. A 2013, 582, 270–275. [Google Scholar] [CrossRef]
- Wang, Z.H.; Sun, S.H.; Wang, B.; Shi, Z.P.; Fu, W.T. Importance and role of grain size in free surface cracking prediction of heavy forgings. Mater. Sci. Eng. A 2015, 625, 321–330. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Basu, R.; Eskandari, M. The mechanism of failure by hydrogen induced cracking in an acidic environment for API 5L X70 pipeline steel. Int. J. Hydrogen Energy 2015, 40, 1096–1107. [Google Scholar] [CrossRef]




| C | Si | Mn | Cr | Mo | W | Nb + V + Ti | Fe |
|---|---|---|---|---|---|---|---|
| 0.26 ± 0.02 | 0.29 ± 0.03 | 0.52 ± 0.05 | 0.50 ± 0.04 | 0.80 ± 0.06 | 0.40 ± 0.04 | (0.10–0.18) ± 0.03 | Bal. |
| Tempering Temperature (°C) | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Hardness (HRC) | KISSC (MPa·m0.5) |
|---|---|---|---|---|
| 690 | 960/6.94 | 994/3.27 | 33.2/0.37 | 21.36/0.32 |
| 700 | 887/4.55 | 939/3.10 | 30.1/0.09 | 31.16/2.45 |
| 715 | 800/8.99 | 861/4.19 | 25.9/0.33 | 34.58/0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Zhou, G.-Y.; Shen, H.; Wang, X.-T.; Li, M.-C.; Zhang, Z.-H.; Cao, G.-H. Effect of Tempering Temperature on Microstructure and Sulfide Stress Cracking of 125 Ksi Grade Casing Steel. Materials 2022, 15, 2589. https://doi.org/10.3390/ma15072589
Luo M, Zhou G-Y, Shen H, Wang X-T, Li M-C, Zhang Z-H, Cao G-H. Effect of Tempering Temperature on Microstructure and Sulfide Stress Cracking of 125 Ksi Grade Casing Steel. Materials. 2022; 15(7):2589. https://doi.org/10.3390/ma15072589
Chicago/Turabian StyleLuo, Ming, Gao-Yang Zhou, Han Shen, Xin-Tian Wang, Mou-Cheng Li, Zhong-Hua Zhang, and Guang-Hui Cao. 2022. "Effect of Tempering Temperature on Microstructure and Sulfide Stress Cracking of 125 Ksi Grade Casing Steel" Materials 15, no. 7: 2589. https://doi.org/10.3390/ma15072589
APA StyleLuo, M., Zhou, G.-Y., Shen, H., Wang, X.-T., Li, M.-C., Zhang, Z.-H., & Cao, G.-H. (2022). Effect of Tempering Temperature on Microstructure and Sulfide Stress Cracking of 125 Ksi Grade Casing Steel. Materials, 15(7), 2589. https://doi.org/10.3390/ma15072589







