Effects of Blast Furnace Slag Powder and Limestone Powder on the Mechanical Properties and Durability of Shotcrete Using Monocalcium Aluminate Setting Accelerator
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Plan and Mix Proportion
3. Results and Discussion
3.1. Properties of the Mortar
3.1.1. Test Methods of Mortar Experiment
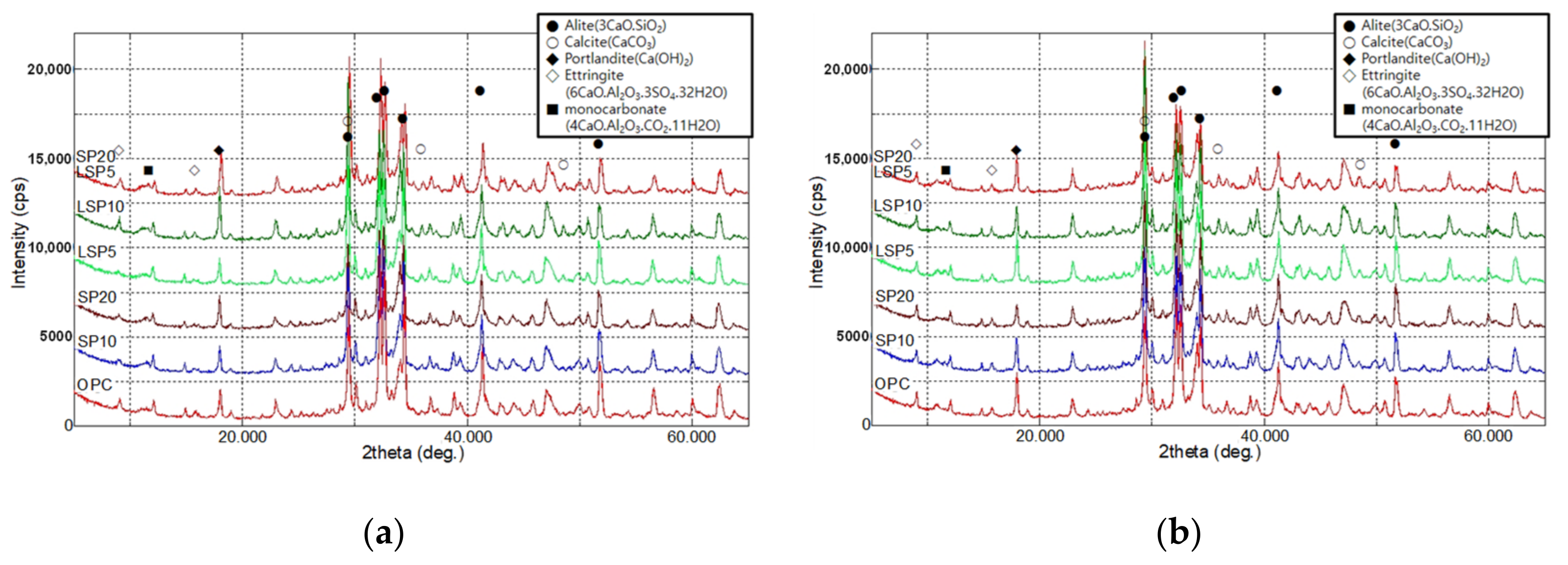
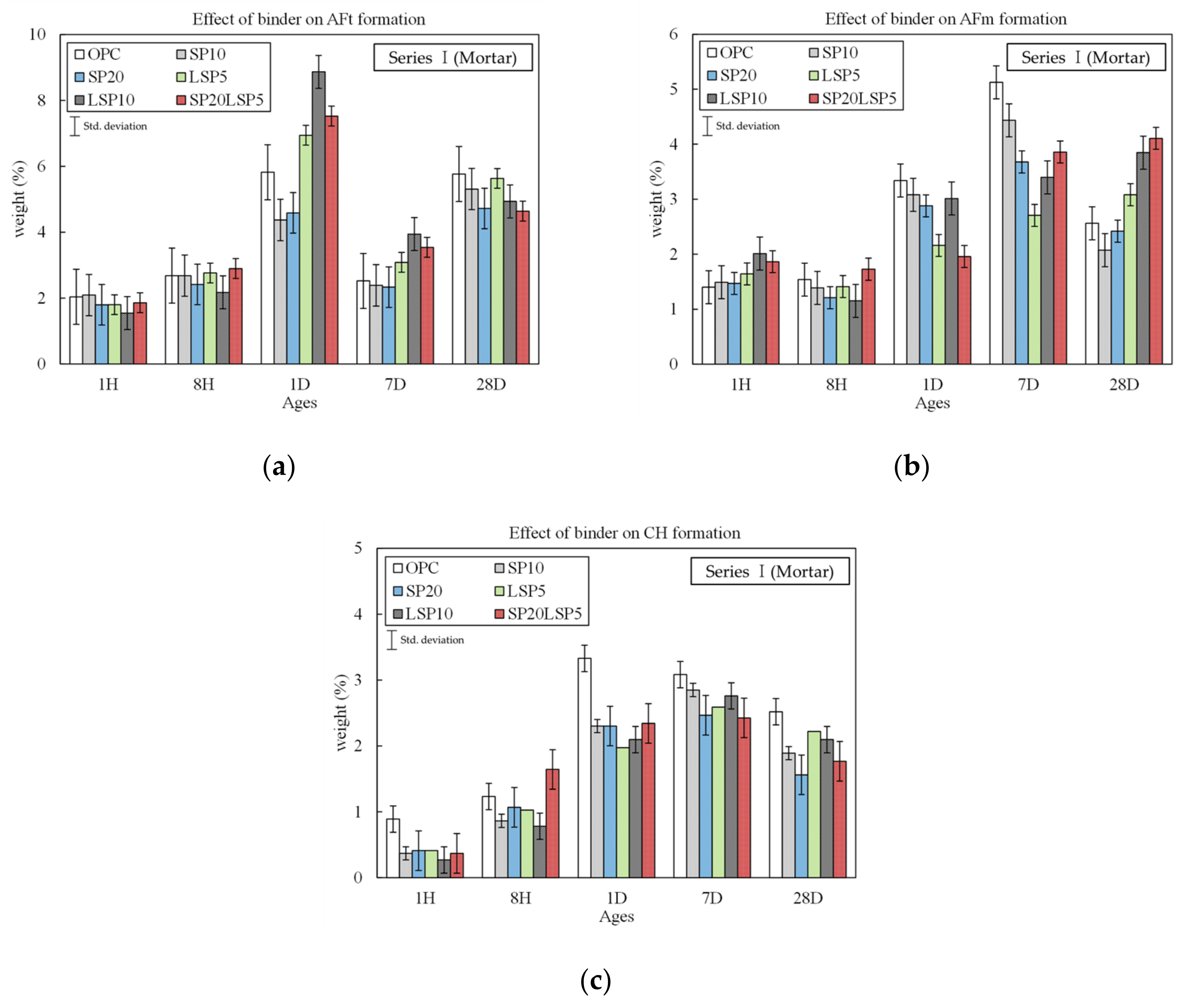
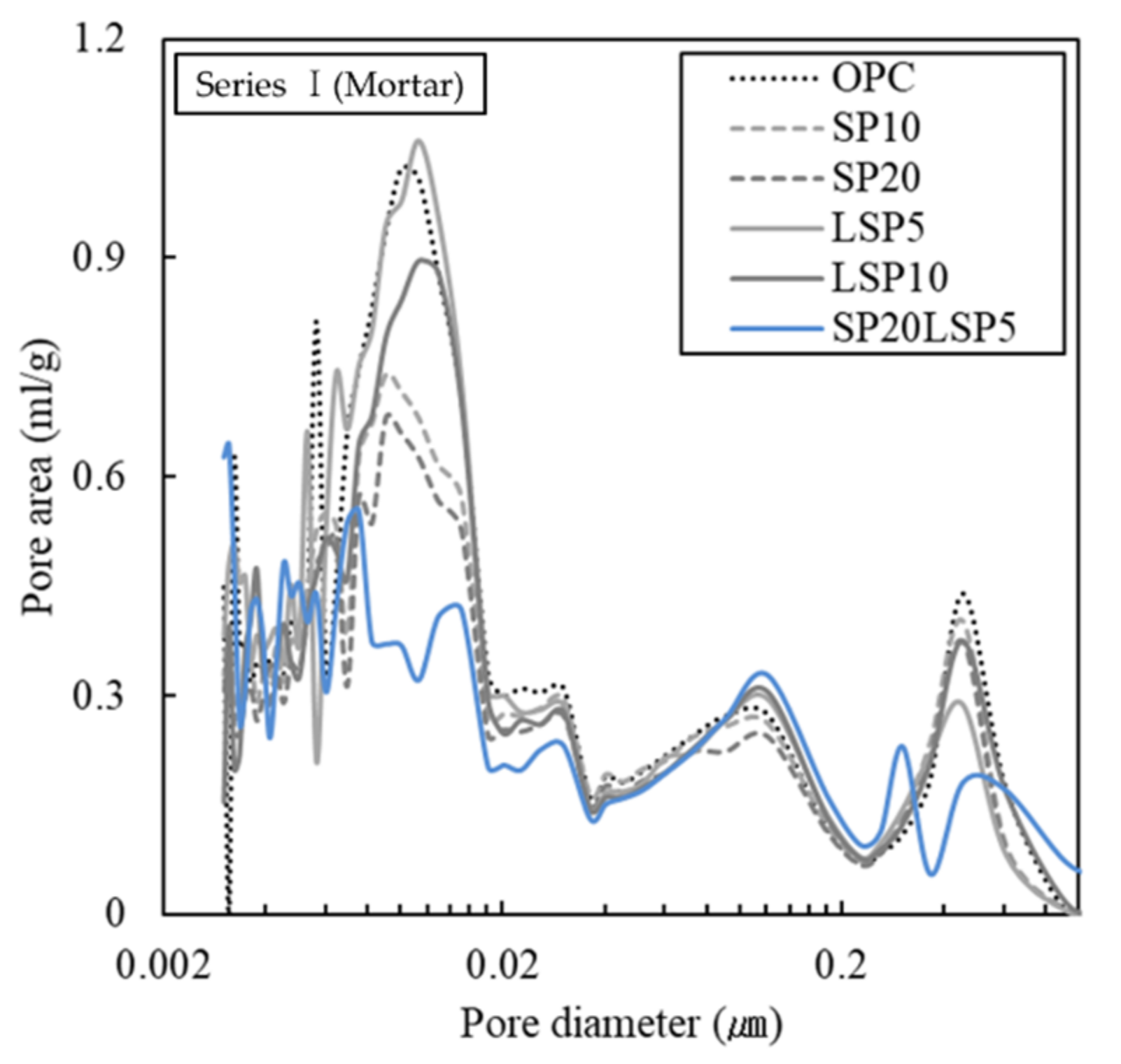
3.1.2. Analysis Result of Hydrate and Pore Distribution
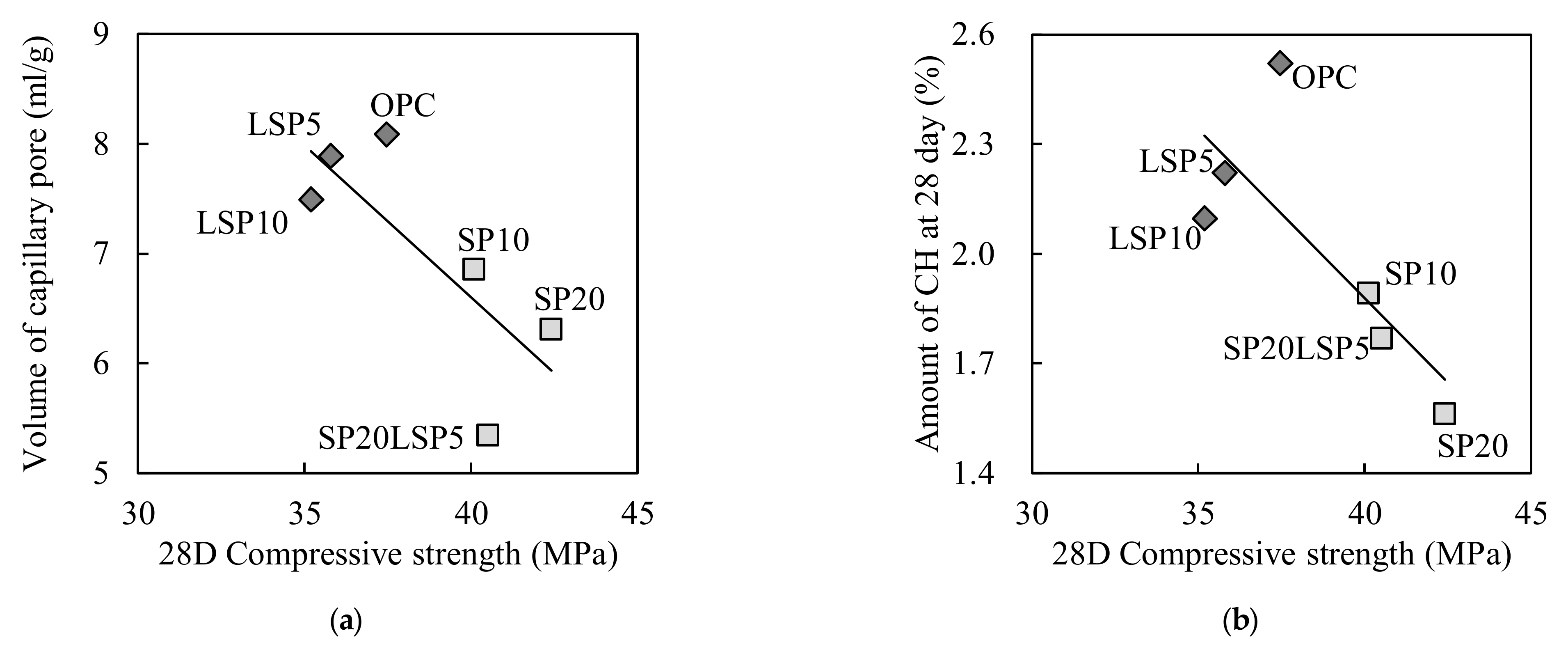
3.1.3. Analysis Result of Penetration Resistance and Compressive
3.2. Hardened Properties and Durability of the Shotcrete
3.2.1. Methods of Shotcrete Experiment
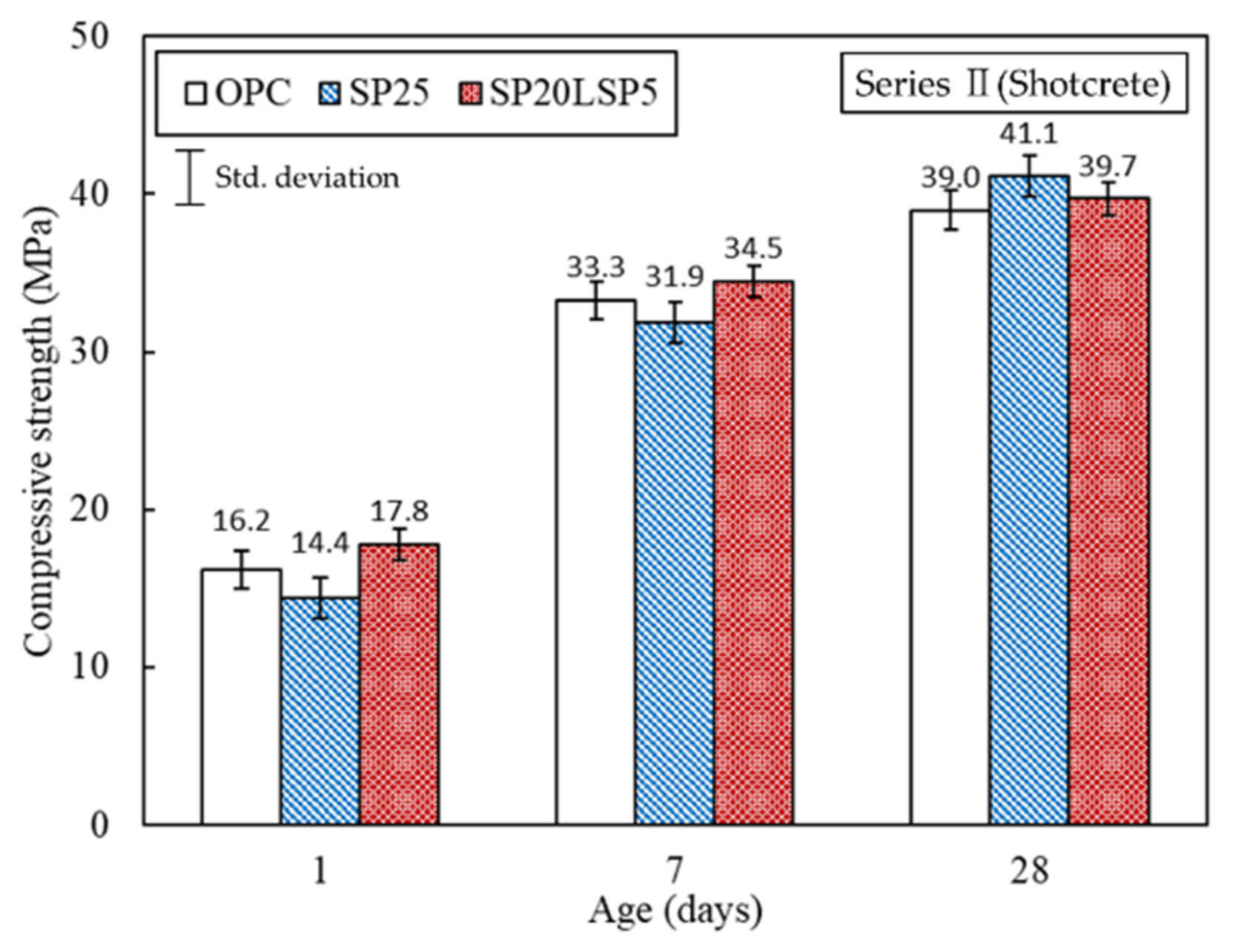
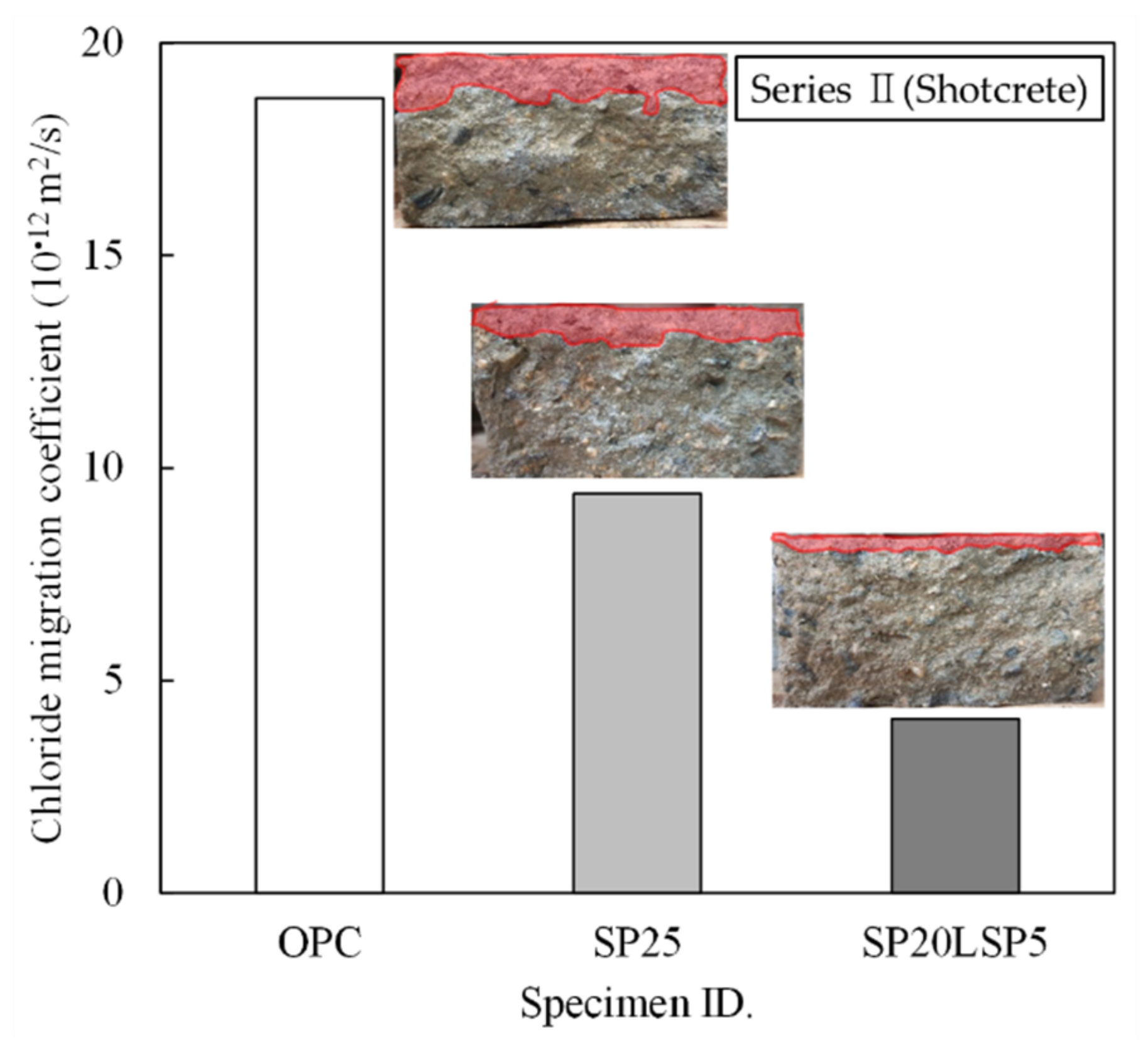
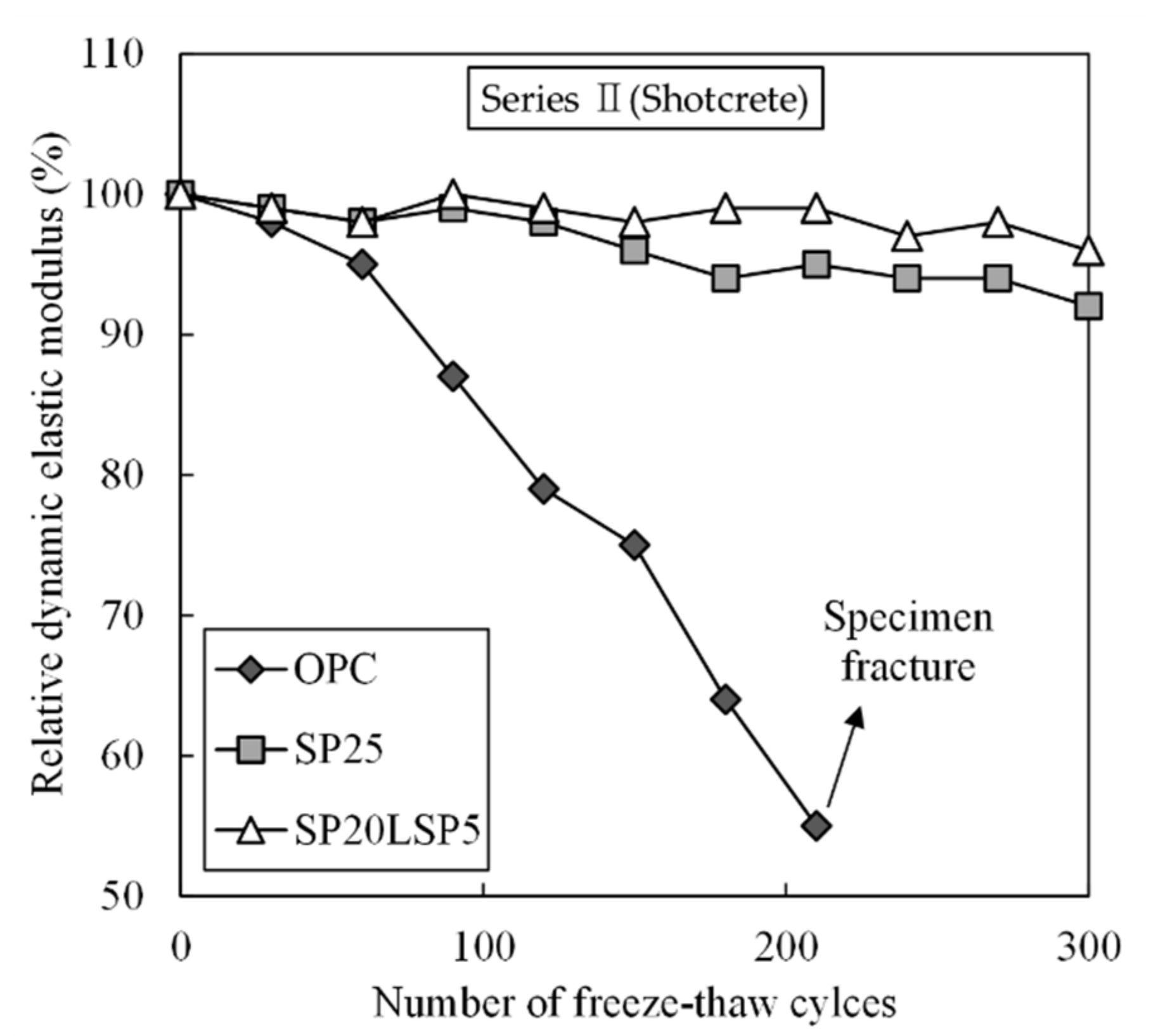
3.2.2. Results of Shotcrete Experiment
4. Conclusions
- Hydration analysis showed that the ettringite was preferentially produced by hydration reaction with CA (accelerator used for rapid setting) and the amount of CH produced was similar in all samples. In addition, LSP showed excellent performance at early ages, and when used with SP, the reactivity at the early stages of hydration was improved.
- Incorporation of SP improves 28 days compressive strength caused by capillary pores and gel pores reduction. Partial substitution of LSP stabilized the ettringite and reduced the pores as reacted with aluminate minerals.
- No delay in the setting of mortar was observed with the addition of SP addition, and the setting was increased as the LSP was added, increasing the penetration resistance. When the combination of SP and LSP were mixed, the setting time increased, increasing the penetration resistance and strength of shotcrete mortar.
- Specimen containing SP and LSP together exhibits high chloride resistance and freeze–thaw resistance due to the decrease in capillary pores. The combined use of SP and LSP was found to be effective in improving the durability of shotcrete.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thomas, A. Sprayed Concrete Lined Tunnel; Taylor & Francis: New York, NY, USA, 2012; p. 9. [Google Scholar] [CrossRef]
- American Concrete Institute. Specification for Materials, Proportioning, and Application of Shotcrete, ACI Committee 506, ACI 506.2-77; American Concrete Institute: Detroit, MI, USA, 1990. [Google Scholar]
- Hemphill, G.B. Practical Tunnel Construction; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 309–322. [Google Scholar]
- Maltese, C.; Pistolesi, C.; Bravo, A.; Cella, F.; Cerulli, T.; Salvioni, D. A case history: Effect of moisture on the setting behaviour of a Portland cement reacting with an alkali-free accelerator. Cem. Concr. Res. 2007, 37, 856–865. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Li, X.; Du, G.P.; Song, Z.P. Experimental research on single-layer tunnel of steel fiber shotcrete. Rock Soil Mech. 2009, 30, 2319–2323. (In Chinese) [Google Scholar]
- Ghosh, S.N. Cement and Concrete Science & Technology; Thomas Telford: New Delhi, India, 1991. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer Science & Business: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Lea, F.M. The Chemistry of Cement and Concrete, 3rd ed.; Chemical Publishing Co.: London, UK, 1971. [Google Scholar]
- Leung, C.K.Y.; Lai, R.; Lee, A.Y.F. Properties of wet-mixed fiber reinforced shotcrete and fiber reinforced concrete with similar composition. Cem. Concr. Res. 2005, 35, 788–795. [Google Scholar] [CrossRef]
- Kim, H.; Moon, H.; Kim, J.-H.; Chung, C.-W. Influences of Slag Replacement on the Properties of Shotcrete Using a Slurry-Type Set Accelerator. J. Korea Inst. Build. Constr. 2014, 14, 389–396. [Google Scholar] [CrossRef][Green Version]
- Edmonds, R.N.; Majumdar, A.J. The hydration of mixtures of monocalcium aluminate and blastfurnace slag. Cem. Concr. Res. 1989, 19, 779–782. [Google Scholar] [CrossRef]
- Gosselin, C. Microstructural Development of Calcium Aluminate Cement Based Systems with and without Supplementary Cementitious Materials (No. THESIS); EPFL: Lausanne, Switzerland, 2009. [Google Scholar]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H. A review on effects of limestone powder on the properties of concrete. Constr. Build. Mater. 2018, 192, 153–166. [Google Scholar] [CrossRef]
- Schmidt, M.; Harr, K.; Boeing, R. Blended cement according to ENV 197 and experiences in Germany. Cem. Concr. Aggreg. 1993, 15, 156–164. [Google Scholar] [CrossRef]
- Moir, G.K.; Kelham, S. Developments in the manufacture and use of Portland limestone cement. Spec. Publ. 1999, 172, 797–820. [Google Scholar] [CrossRef]
- Cussino, L.; Negro, A. Hydration of aluminous cement in the presence of silicic and calcareous aggregates. In International Congress on the Chemistry of Cement; Editions Septima: Paris, France, 1980; Volume 62, pp. 62–67. [Google Scholar]
- Fentiman, C.H. Hydration of carbo-aluminous cement at different temperatures. Cem. Concr. Res. 1985, 15, 622–630. [Google Scholar] [CrossRef]
- Juradin, S.; Vlajić, D. Influence of cement type and mineral additions, silica fume and metakaolin, on the properties of fresh and hardened self-compacting concrete. Adv. Struct. Mater. 2015, 70, 251–267. [Google Scholar] [CrossRef]
- Uysal, M.; Sumer, M. Performance of self-compacting concrete containing different mineral admixtures. Constr. Build. Mater. 2011, 25, 4112–4120. [Google Scholar] [CrossRef]
- ASTM C778; Standard Specification for Standard Sand. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM C1436-13; Standard Specification for Materials for Shotcrete. ASTM: West Conshohocken, PA, USA, 2016.
- ASTM C1365; Standard Test. Method for Determination of the Proportion of Phases in Portland Cement and Portland-Cement Clinker Using X-ray Powder Diffraction Analysis. ASTM: West Coshohocken, PA, USA, 2018; pp. 1–11.
- ASTM D4404; Standard Test. Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry. ASTM Standards: Conshohocken, PA, USA, 2018.
- ASTM C403/C403M; Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. ASTM: West Conshohocken, PA, USA, 2016; pp. 1–7.
- ASTM C109/C109M REV A; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM: West Conshohocken, PA, USA, 2017; pp. 1–10.
- Balonis, M.; Glasser, F.P. The density of cement phases. Cem. Concr. Res. 2009, 39, 733–739. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Rashad, A.M.; Sadek, D.M. An investigation on Portland cement replaced by high-volume GGBS pastes modified with micro-sized metakaolin subjected to elevated temperatures. Int. J. Sustain. Built Environ. 2016, 6, 91–101. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.; Justnes, H. Synergy between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Soroka, I.; Setter, N. The effect of fillers on strength of cement mortars. Cem. Concr. Res. 1977, 7, 449–456. [Google Scholar] [CrossRef]
- ASTM C873/C873M; Standard Test Method for Compressive Strength of Concrete Cylinders Cast in Place in Cylindrical Molds. ASTM: West Conshohocken, PA, USA, 2015; pp. 1–4.
- ASTM C39/C39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM: West Conshohocken, PA, USA, 2018; pp. 1–8.
- NT BUILD 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments. Nordtest Method: Espoo, Finland, 1999.
- ASTM C666/C666M; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM: West Conshohocken, PA, USA, 2015; pp. 1–5.
- Lee, S.T.; Kim, S.S.; Kim, D.G.; Park, K.P. Effect of Types of Accelerators and Replacement Levels of GGBFS on the Performance of Shotcrete Mortars. J. Korea Inst. Struct. Maint. Insp. 2013, 17, 76–84. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Z. Influences of limestone powder on the resistance of concretes to the chloride ion penetration and sulfate attack. Powder Technol. 2018, 338, 725–733. [Google Scholar] [CrossRef]
- Panesar, D.K.; Zhang, R. Performance comparison of cement replacing materials in concrete: Limestone fillers and supplementary cementing materials—A review. Constr. Build. Mater. 2020, 251, 118866. [Google Scholar] [CrossRef]
- Deja, J. Freezing and de-icing salt resistance of blast furnace slag concretes. Cem. Concr. Compos. 2003, 25, 357–361. [Google Scholar] [CrossRef]
- Stark, J.; Ludwig, H.M. Freeze-thaw and freeze-deicing salt resistance of concretes containing cement rich in granulated blast furnace slag. ACI Mater. J. 1997, 94, 47–55. [Google Scholar]


| Materials | Chemical Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | L.O.I e | |
| OPC a | 63.9 | 21.9 | 5.2 | 3.6 | 2.2 | 2.0 | 1.0 | 0.2 |
| SP b | 37.4 | 34.1 | 14.9 | 1.4 | 3.2 | 6.8 | 0.6 | 1.6 |
| LSP c | 43.7 | 10.9 | 3.7 | 1.8 | 0.7 | 1.3 | 1.0 | 36.9 |
| SC d | 47.8 | 3.3 | 35.1 | 0.9 | 0.1 | 0.9 | 0.2 | 11.7 |
| Series | Specimen ID. | Type | Binder (wt.%) | Experimental Plan | ||
|---|---|---|---|---|---|---|
| OPC | SP | LSP | ||||
| I | OPC | Mortar | 100 |
| ||
| SP10 | 90 | 10 | ||||
| SP20 | 80 | 20 | ||||
| LSP5 | 95 | 5 | ||||
| LSP10 | 90 | 10 | ||||
| SP20LSP5 | 75 | 20 | 5 | |||
| II | OPC | shotcrete | 100 |
| ||
| SP25 | 75 | 25 | ||||
| SP20LSP5 | 75 | 20 | 5 | |||
| Series | W/B a (%) | B:S b | Binder (g) | Water (g) | SC c (B × wt.%) |
|---|---|---|---|---|---|
| I (Mortar) | 50 | 1:3 | 450 | 225 | 5.0 |
| W/B | S/a a (%) | Unit Weight (N/m3) | Admixture (B × wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Binder | Sand | Gravel | AD b | SC c | ||||
| OPC | SP | LSP | |||||||
| 0.43 | 60 | 190 | 441 | 1015 | 695 | 1.0 | 5.0 | ||
| 331 | 110 | ||||||||
| 331 | 88 | 22 | |||||||
| Classification of Pores | OPC | SP10 | SP20 | LSP5 | LSP10 | SP20 LSP5 | |
|---|---|---|---|---|---|---|---|
| Capillary pores | Large (0.05–10 μm) | 2.39 | 2.34 | 2.15 | 2.23 | 2.36 | 2.32 |
| Medium (0.01–0.05 μm) | 5.70 | 4.52 | 4.15 | 5.66 | 5.13 | 3.02 | |
| Gel pores (≤0.01 μm) | 9.79 | 8.79 | 7.93 | 10.6 | 8.76 | 8.91 | |
| Total pores | 17.9 | 15.6 | 14.2 | 18.5 | 16.3 | 14.3 | |
| OPC | SP25 | SP20LSP5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 cycle | 120 cycles | 210 cycles | 300 cycles | 0 cycle | 120 cycles | 210 cycles | 300 cycles | 0 cycle | 120 cycles | 210 cycles | 300 cycles |
 |  |  | - |  |  |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Kim, G.; Lee, T.; Koo, K.; Lee, S.; Son, M.; Sasui, S.; Eu, H. Effects of Blast Furnace Slag Powder and Limestone Powder on the Mechanical Properties and Durability of Shotcrete Using Monocalcium Aluminate Setting Accelerator. Materials 2022, 15, 2495. https://doi.org/10.3390/ma15072495
Kang B, Kim G, Lee T, Koo K, Lee S, Son M, Sasui S, Eu H. Effects of Blast Furnace Slag Powder and Limestone Powder on the Mechanical Properties and Durability of Shotcrete Using Monocalcium Aluminate Setting Accelerator. Materials. 2022; 15(7):2495. https://doi.org/10.3390/ma15072495
Chicago/Turabian StyleKang, Bonghee, Gyuyong Kim, Taegyu Lee, Kyungmo Koo, Sangkyu Lee, Minjae Son, Sasui Sasui, and Hamin Eu. 2022. "Effects of Blast Furnace Slag Powder and Limestone Powder on the Mechanical Properties and Durability of Shotcrete Using Monocalcium Aluminate Setting Accelerator" Materials 15, no. 7: 2495. https://doi.org/10.3390/ma15072495
APA StyleKang, B., Kim, G., Lee, T., Koo, K., Lee, S., Son, M., Sasui, S., & Eu, H. (2022). Effects of Blast Furnace Slag Powder and Limestone Powder on the Mechanical Properties and Durability of Shotcrete Using Monocalcium Aluminate Setting Accelerator. Materials, 15(7), 2495. https://doi.org/10.3390/ma15072495










