Self-Healing Systems in Silicon Anodes for Li-Ion Batteries
Abstract
:1. Introduction
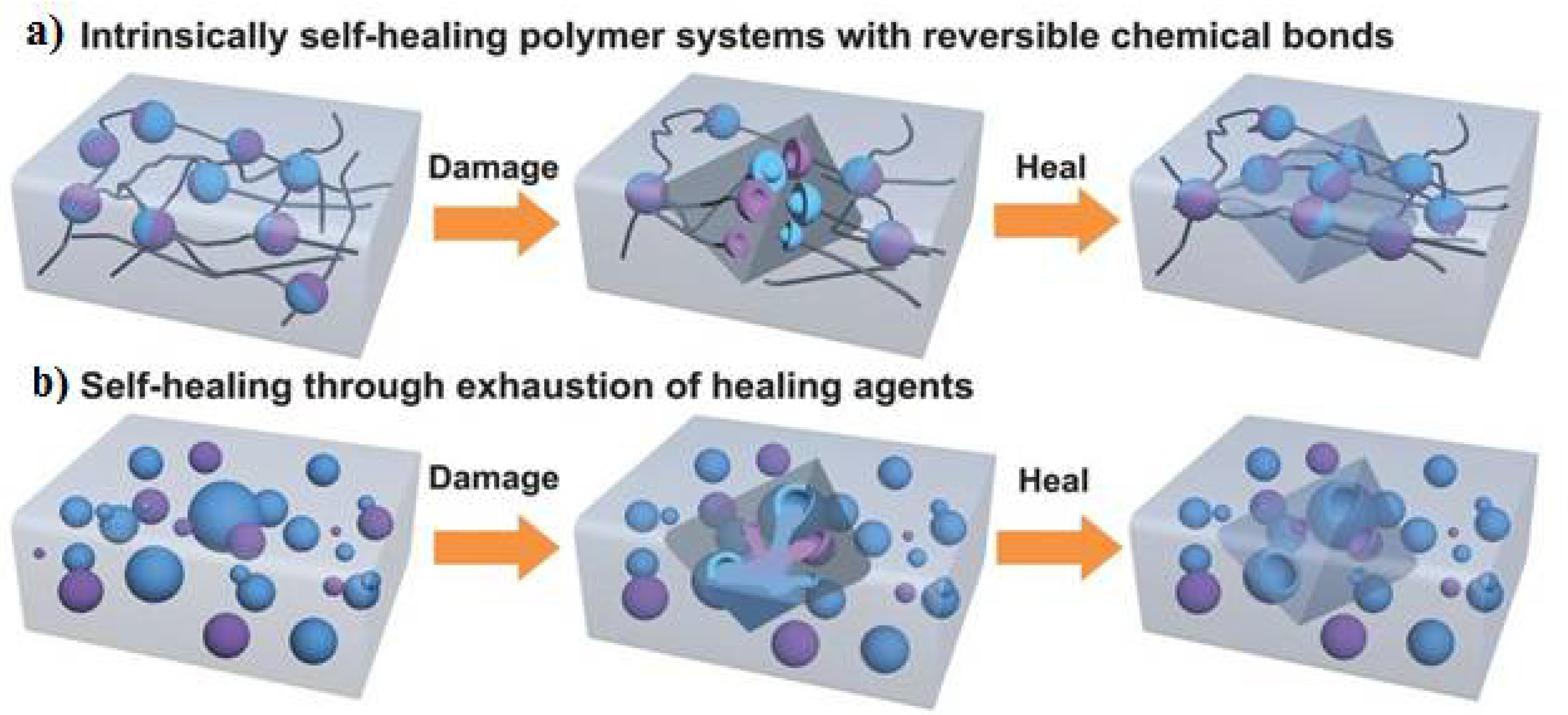
1.1. Various Approaches of Self-Healing Polymers
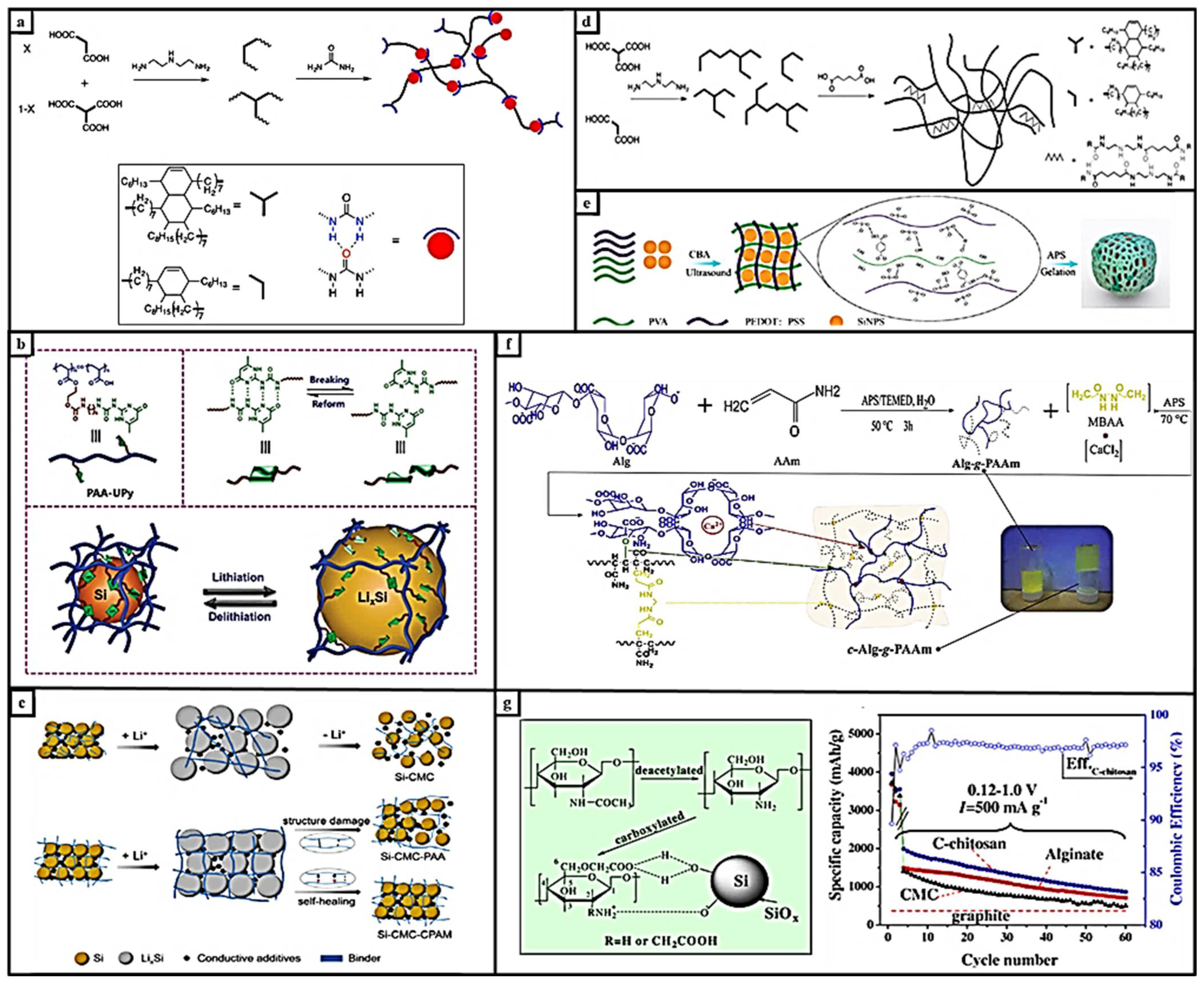
1.1.1. π-π-Stacking-Interaction-Based Self-Healing Polymers
1.1.2. Metal–Ligand-Based Self-Healing Polymers
1.1.3. Ionic-Interaction-Based Self-Healing Polymers
1.1.4. Hydrogen-Bond-Based Self-Healing Polymers
1.2. Effect of Nanoparticle Additive on Self-Healing Properties
2. Recently Reported Self-Healing Anode Systems
2.1. Physical-Interaction-Based Self-Healing Materials
2.1.1. Hydrogen-Bonded Supramolecular Self-Healing
| Self-Healing Material | Anode Active Materials | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| Na-alginate | Si-C composite anodes, 100 nm | 1 M LiPF6 in EC/DEC/EMC | 2850 mAh g−1 (first cycle capacity), 1250 mAh g−1 after 50 cycles at 0.1 A g−1 | [60] |
| PAAH0.2Na0.8 | Si/graphite < 100 nm | 1 M LiPF6 in EC/DEC/EMC | 1100 mAh g−1 after 30 cycles at 50 mA g−1, | [68] |
| Self-healing polymers (SHPs) | Silicon microparticle (SiMP) | 1 M LiPF6 in EC/DEC/EMC | 2617 mAh g−1 after 90 cycles at 0.4 A g−1 | [62] |
| Carboxymethyl chitosan | Silicon, 100 nm | 1 M LiPF6 in EC/DEC/EMC | 950 mAh g−1 over 50 cycles at 500 mA g−1 | [64] |
| Self-healing polymers (SHPs) | Silicon microparticle (SiMP), 1–3 µm | 1 M LiPF6 in EC/DEC/VC/FEC | 2736 mAh g−1 at C/20 after 500 cycles | [62] |
| Self-healing-type binder PAABS content binder 9 PAABS+6 CMC | Silicon 20–30 nm/graphite electrode | 1 M LiPF6 in EC/DEC/EMC | 1150 mAh g−1 (First cycle capacity), About 500 mAh g−1 after 50 cycles at 0.5 C | [69] |
| Native-XG, Na-CMC, alginate | Si/graphite, 100 nm Si | 1 M LiPF6 in EC/DEC/EMC | 14.2%, 22.8%, and 34.6% of capacities after 200 cycles at 1 C, respectively | [70] |
| Urea via hydrogen bonds | Silicon particles (~0.9 µm) | 1 M LiPF6 in EC/DEC/VC/FEC | 1700 mAh g−1; 80% retained after 175 cycles at C/20 | [24] |
| Self-healing polymers (SHPs) | Silicon, 100 nm | 1 M LiPF6 in EC/DEC | 719 mAh g−1; 81% retained after 100 cycles at 1 C | [29] |
| Crosslinked chitosan (CS-GA) | Silicon, 100 nm | 1 M LiPF6 in EC/DEC/EMC | 1969 mAh g−1 after 100 cycles at 500 mA g−1 | [71] |
| Pyrene–poly(acrylic acid)– polyrotaxane | Silicon | 1 M LiPF6 in EC/DEC/EMC | 82.5% retained after 150 cycles at 0.5 C | [40] |
| Dual-crosslinked network binder of alginate | Silicon/graphite anodes | 1 M LiPF6 in EC/DEC/VC/FEC | 1743 mAh g−1; 74% retained after 200 cycles at 2000 mA g−1 | [66] |
| PAA-UPy | Silicon | 1 M LiPF6 in EC/EMC/DMC | 2000 mAh g−1; 74% retained after 110 cycles at 1 C | [40] |
| UPy-functionalized PEG | Silicon | 1 M LiPF6 in EC/DMC | 1847 mAh g−1; 81% ICE at 1 C | [72] |
| CMC-CPAM | Silicon-based | 1 M LiPF6 in EC/DEC/EMC | 2103 mAh g−1; 92% ICE at 2 C | [67] |
| PEDOT-PSS-PVA | Silicon | 1 M LiPF6 in EC/DEC/EMC | 1743 mAh g−1; 74% retained after 200 cycles at 2000 mA g−1 | [35] |
| PAU-g-PEG, | Silicon | 1 M LiPF6 in EC/DEC/EMC | 2831 mAh g−1/3.2 mAh cm−2; 85% retained after 120 cycles at 0.1 C | [41] |
| Self-healing polymers (SHPs) | Silicon microparticle (SiMP) | 1 M LiPF6 in EC/DEC/EMC | 2100 mAh g−1; 91.8% capacity retention after 100 cycles at C/10 | [63] |
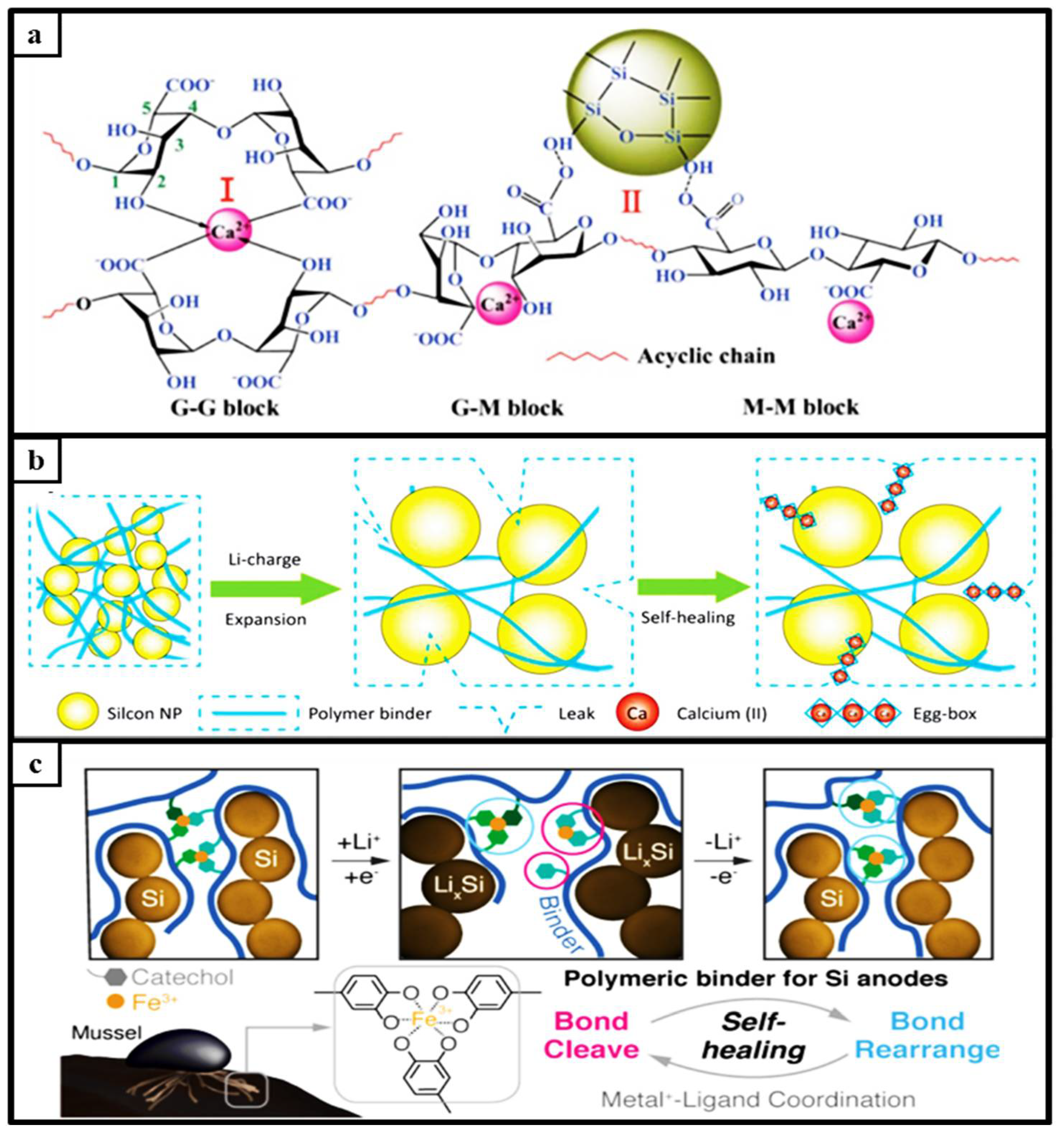
2.1.2. Metal Interaction of Self-Healing Materials
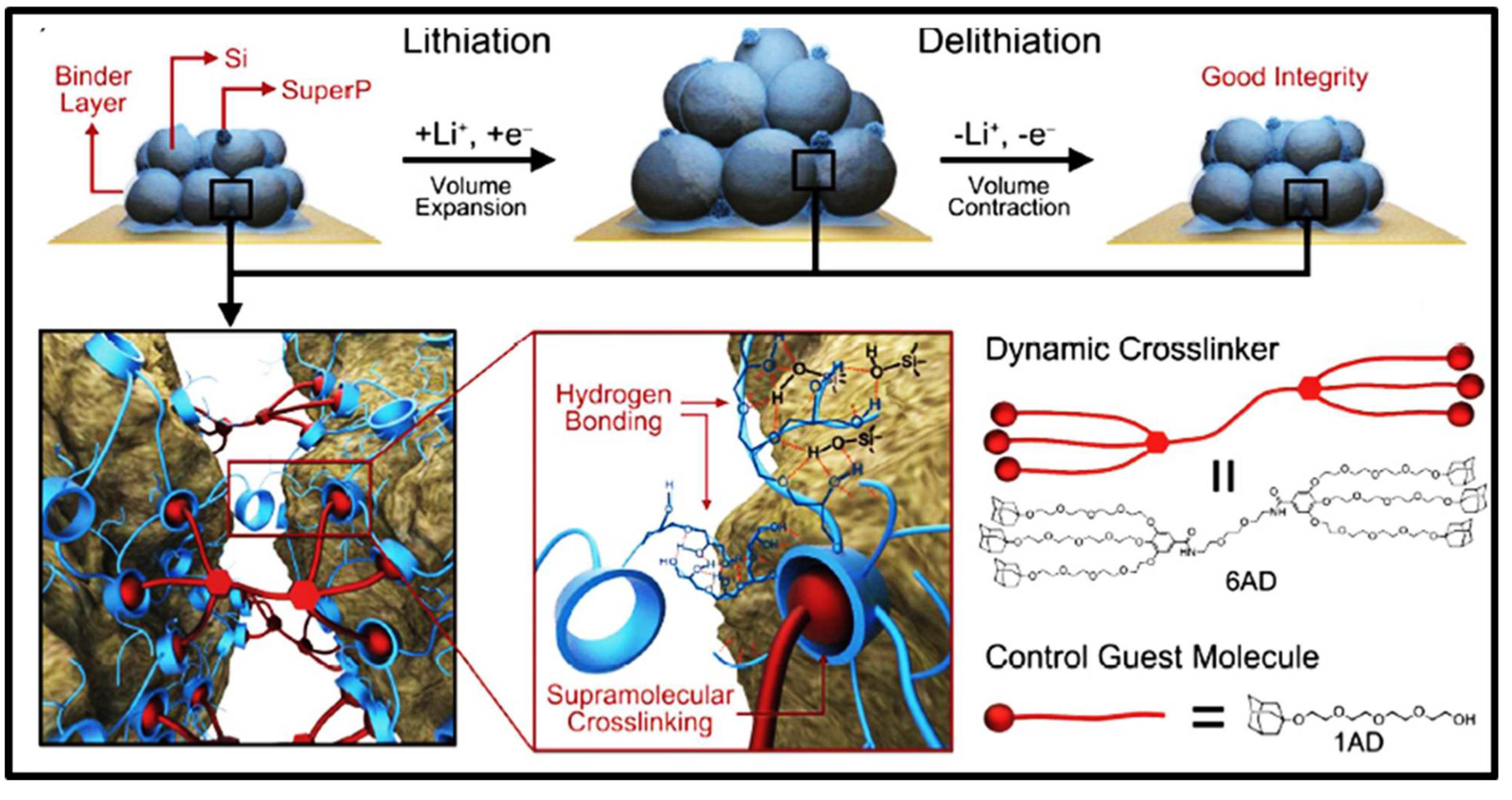
2.1.3. Host–Guest Interactions
2.1.4. Ionically Bonded Interaction
2.1.5. Multiple Functional Interactions of Self-healing Mechanism
2.2. Chemical-Interaction-Based Self-Healing Materials
2.2.1. Imine-Bond-Based Self-Healing Systems
2.2.2. Ester-Bond-Based Self-Healing Systems
2.2.3. Disulfide-Bond-Based Self-Healing Systems
2.2.4. Diels–Alder-Reaction-Based Self-Healing Systems
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AMPS | 2-Acrylamido-2-methylpropanesulfonic acid |
| AN | acrylonitrile |
| BA | acrylic acid n-butyl ester |
| CBA | 4-carboxybenzaldehyde |
| CE | Coulombic efficiency |
| CMC | carboxymethyl cellulose |
| CPAM | cationic polyacrylamides |
| DC | discharge capacity |
| DEC | diethyl carbonate |
| DMC | dimethyl carbonate |
| EC | ethylene carbonate |
| EMC | ethyl methyl carbonate |
| FEC | fluoroethylene carbonate |
| Ga-In-Sn | gallium–indium–tin alloy |
| IC | ionic conductivity |
| ICE | initial coulombic efficiency |
| LiPF6 | lithium hexafluorophosphate |
| LM | liquid metal |
| PEDOT | poly (3, 4-ethylene- dioxythiophene) |
| PSS | poly (styrenesulfonate) |
| PEGMA | poly (ethylene glycol) methyl ether methacrylate |
| PAA | poly(acrylic acid) |
| PVA | polyvinyl alcohol |
| PEG | polyethylene glycol |
| PEGA | poly (ethylene glycol) methyl ether acrylate |
| RT | room temperature |
| UPy | ureido-pyrimidinone |
| UPyMA | (2-(3-(6-methyl-4-oxo-1,4-dihydropyrimidin-2-yl)ureido)ethyl methacrylate) |
| VC | vinyl carbonate |
References
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-healing of chemical gels cross-linked by diarylbibenzofuranone-based trigger-free dynamic covalent bonds at room temperature. Angew. Chem. Int. Ed. 2012, 51, 1138–1142. [Google Scholar]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [PubMed]
- Taskin, O.S.; Hubble, D.; Zhu, T.; Liu, G. Biomass-derived polymeric binders in silicon anodes for battery energy storage applications. Green Chem. 2021, 23, 7890–7901. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Sun, H.; Dong, X.; Cao, J.; Wang, L.; Xu, Y.; Ren, J.; Hwang, Y.; Son, I.H.; et al. A self-healing aqueous lithium-ıon battery. Angew. Chem. Int. Ed. 2016, 55, 14384–14388. [Google Scholar]
- Urban, M.W.; Davydovich, D.; Yang, Y.; Demir, T.; Zhang, Y.; Casabianca, L. Key-and-lock commodity self-healing copolymers. Science 2018, 362, 220–225. [Google Scholar]
- Mai, W.; Yu, Q.; Han, C.; Kang, F.; Li, B. Self-healing materials for energy-storage devices. Adv. Funct. Mater. 2020, 30, 1909912. [Google Scholar]
- Taskin, O.S.; Yuca, N.; Papavasiliou, J.; Avgouropoulos, G. Interconnected conductive gel binder for high capacity silicon anode for Li-ion batteries. Mater. Lett. 2020, 273, 127918. [Google Scholar]
- Chen, H.; Wu, Z.; Su, Z.; Chen, S.; Yan, C.; Al-Mamun, M.; Tang, Y.; Zhang, S. A mechanically robust self-healing binder for silicon anode in lithiumion batteries. Nano Energy 2021, 81, 105654. [Google Scholar]
- Taskin, O.S.; Kiskan, B.; Weber, J.; Yagci, Y. One-pot, one-step strategy for the preparation of clickable melamine based microporous organic polymer network. Macromol. Mater. Eng. 2015, 300, 1116–1122. [Google Scholar]
- Huang, S.; Ren, J.; Liu, R.; Bai, Y.; Li, X.; Huang, Y.; Yue, M.; He, X.; Yuan, G. Low addition amount of self-healing ionomer binder for Si/graphite electrodes with enhanced cycling. New J. Chem. 2018, 42, 6742–6749. [Google Scholar]
- Güzel, E.; Koçyiğit, Ü.M.; Taslimi, P.; Erkan, S.; Taskin, O.S. Biologically active phthalocyanine metal complexes: Preparation, evaluation of α-glycosidase and anticholinesterase enzyme inhibition activities, and molecular docking studies. J. Biochem. Mol. Toxicol. 2021, 35, e22765. [Google Scholar]
- Taskin, O.S.; Kiskan, B.; Aksu, A.; Balkis, N.; Yagci, Y. Copper(II) removal from the aqueous solution using microporous benzidine-based adsorbent material. J. Environ. Chem. Eng. 2016, 4, 899–907. [Google Scholar]
- Qin, J.; Lin, F.; Hubble, D.; Wang, Y.; Li, Y.; Murphy, I.A.; Jang, S.-H.; Yang, J.; Jen, A.K.Y. Tuning self-healing properties of stiff, ion-conductive polymers. J. Mater. Chem. A 2019, 7, 6773–6783. [Google Scholar]
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S.-I. Silicon microparticle anodes with self-healing multiple network binder. Joule 2018, 2, 950–961. [Google Scholar]
- Li, F.; Xu, J.; Hou, Z.; Li, M.; Yang, R. Silicon anodes for high-performance storage devices: Structural design, material compounding, advances in electrolytes and binders. ChemNanoMat 2020, 6, 720–738. [Google Scholar]
- Chen, D.; Wang, D.; Yang, Y.; Huang, Q.; Zhu, S.; Zheng, Z. Self-healing materials for next-generation energy harvesting and storage devices. Adv. Energy Mater. 2017, 7, 1700890. [Google Scholar]
- Luo, C.; Fan, X.; Ma, Z.; Gao, T.; Wang, C. Self-healing chemistry between organic material and binder for stable sodium-ıon batteries. Chem 2017, 3, 1050–1062. [Google Scholar]
- Yuca, N.; Cetintasoglu, M.E.; Dogdu, M.F.; Akbulut, H.; Tabanli, S.; Colak, U.; Taskin, O.S. Highly efficient poly(fluorene phenylene) copolymer as a new class of binder for high-capacity silicon anode in lithium-ion batteries. Int. J. Energy Res. 2018, 42, 1148–1157. [Google Scholar]
- Wu, Z.-H.; Yang, J.-Y.; Yu, B.; Shi, B.-M.; Zhao, C.-R.; Yu, Z.-L. Self-healing alginate–carboxymethyl chitosan porous scaffold as an effective binder for silicon anodes in lithium-ion batteries. Rare Met. 2019, 38, 832–839. [Google Scholar]
- Wang, Z.; Tao, F.; Pan, Q. A self-healable polyvinyl alcohol-based hydrogel electrolyte for smart electrochemical capacitors. J. Mater. Chem. A 2016, 4, 17732–17739. [Google Scholar]
- Rajeev, K.K.; Nam, J.; Jang, W.; Kim, Y.; Kim, T.-H. Polysaccharide-based self-healing polymer binder via Schiff base chemistry for high-performance silicon anodes in lithium-ion batteries. Electrochim. Acta 2021, 384, 138364. [Google Scholar]
- Bulut, E.; Güzel, E.; Yuca, N.; Taskin, O.S. Novel approach with polyfluorene/polydisulfide copolymer binder for high-capacity silicon anode in lithium-ion batteries. J. Appl. Polym. Sci. 2020, 137, 48303. [Google Scholar]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar]
- Xu, J.H.; Ye, S.; Ding, C.D.; Tan, L.H.; Fu, J.J. Autonomous self-healing supramolecular elastomer reinforced and toughened by graphitic carbon nitride nanosheets tailored for smart anticorrosion coating applications. J. Mater. Chem. A 2018, 6, 5887–5898. [Google Scholar]
- Xu, J.; Ye, S.; Fu, J. Novel sea cucumber-inspired material based on stiff, strong yet tough elastomer with unique self-healing and recyclable functionalities. J. Mater. Chem. A 2018, 6, 24291–24297. [Google Scholar]
- Li, X.; Zhang, H.; Zhang, P.; Yu, Y. A sunlight-degradable autonomous self-healing supramolecular elastomer for flexible electronic devices. Chem. Mater. 2018, 30, 3752–3758. [Google Scholar]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar]
- Lopez, J.; Chen, Z.; Wang, C.; Andrews, S.C.; Cui, Y.; Bao, Z. The effects of cross-linking in a supramolecular binder on cycle life in silicon microparticle anodes. ACS Appl. Mater. Interfaces 2016, 8, 2318–2324. [Google Scholar]
- Kwon, T.W.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145. [Google Scholar]
- Frischmann, P.D.; Gerber, L.C.; Doris, S.E.; Tsai, E.Y.; Fan, F.Y.; Qu, X.; Jain, A.; Persson, K.A.; Chiang, Y.M.; Helms, B.A. Supramolecular perylene bisimide-polysulfide gel networks as nanostructured redox mediators in dissolved polysulfide lithium–sulfur batteries. Chem. Mater. 2015, 27, 6765. [Google Scholar]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977. [Google Scholar] [PubMed]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042. [Google Scholar] [PubMed]
- Rajeev, K.K.; Nam, J.; Kim, E.; Kim, Y.; Kim, T.H. A self-healable polymer binder for Si anodes based on reversible Diels–Alder chemistry. Electrochim. Acta 2020, 364, 137311. [Google Scholar]
- Hu, S.; Wang, L.; Huang, T.; Yu, A. A conductive self-healing hydrogel binder for high-performance silicon anodes in lithium-ion batteries. J. Power Source 2020, 449, 227472. [Google Scholar]
- Fox, J.; Wie, J.J.; Greenland, B.W.; Burattini, S.; Hayes, W.; Colquhoun, H.M.; Mackay, M.E.; Rowan, S.J. High-strength, healable, supramolecular polymer nanocomposites. J. Am. Chem. Soc. 2012, 134, 5362–5368. [Google Scholar]
- Burattini, S.; Colquhoun, H.M.; Greenland, B.W.; Hayes, W. A novel self-healing supramolecular polymer system. Faraday Discuss. 2009, 143, 251–264. [Google Scholar]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A conductive self-healing hybrid gel enabled by metal–ligand supramolecule and nanostructured conductive polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar]
- Ryu, J.; Kim, S.; Kim, J.; Park, S.; Lee, S.; Yoo, S.; Kim, J.; Choi, N.-S.; Ryu, J.-H.; Park, S. Room-temperature crosslinkable natural polymer binder for high-rate and stable silicon anodes. Adv. Funct. Mater. 2020, 30, 1908433. [Google Scholar]
- Zhang, G.; Yang, Y.; Chen, Y.; Huang, J.; Zhang, T.; Zeng, H.; Wang, C.; Liu, G.; Deng, Y. A Quadruple-hydrogen-bonded supramolecular binder for high-performance silicon anodes in lithium-ıon batteries. Small 2018, 14, 1801189. [Google Scholar]
- Nam, J.; Kim, E.; Kk, R.; Kim, Y.; Kim, T.H. A conductive self healing polymeric binder using hydrogen bonding for Si anodes in lithiumion batteries. Sci. Rep. 2020, 10, 14966. [Google Scholar] [PubMed]
- Taskin, O.S.; Kiskan, B.; Yagci, Y. Polybenzoxazine precursors as self-healing agents for polysulfones. Macromolecules 2013, 46, 8773–8778. [Google Scholar]
- Yuca, N.; Taskin, O.S.; Arici, E. An overview on efforts to enhance the Si electrode stability for lithiumion batteries. Energy Storage 2020, 2, e94. [Google Scholar]
- Alfaro, P.; Palavicini, A.; Wang, C. Hydrogen, oxygen and hydroxyl on porous silicon surface: A joint density-functional perturbation theory and ınfrared spectroscopy approach. Thin Solid Film. 2014, 571, 206. [Google Scholar]
- Tasdelen, M.A.; Taskin, O.S.; Celik, C. Orthogonal synthesis of block copolymer via photoinduced cuaac and ketene chemistries. Macromol. Rapid Commun. 2016, 37, 521–526. [Google Scholar]
- Lee, J.Y.; Buxton, G.A.; Balazs, A.C. Using nanoparticles to create self-healing composites. J. Chem. Phys. 2004, 121, 5531–5540. [Google Scholar]
- Gupta, S.; Zhang, Q.; Emrick, T.; Balazs, A.; Russell, T. Entropy-driven segregation of nanoparticles to cracks in multilayered composite polymer structures. Nat. Mater. 2006, 5, 229–233. [Google Scholar]
- Han, B.; Yang, Y.; Shi, X.; Zhang, G.; Gong, L.; Xu, D.; Zeng, H.; Wang, C.; Gu, M.; Deng, Y. Spontaneous repairing liquid metal/Si nanocomposite as a smart conductive-additive-free anode for lithium-ion battery. Nano Energy 2018, 50, 359–366. [Google Scholar]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar]
- Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2011, 3, 34–37. [Google Scholar]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [PubMed] [Green Version]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized hydrogel: Self-healing properties of supramolecular hydrogels formed by polymerization of host–guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [PubMed]
- Deng, G.; Tang, C.; Li, F.; Jiang, H.; Chen, Y. Covalent cross-linked polymer gels with reversible sol−gel transition and self-healing properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar]
- Nicolaÿ, R.; Kamada, J.; Van Wassen, A.; Matyjaszewski, K. Responsive gels based on a dynamic covalent trithiocarbonate cross-linker. Macromolecules 2010, 43, 4355–4361. [Google Scholar]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar]
- Reutenauer, P.; Buhler, E.; Boul, P.J.; Candau, S.J.; Lehn, J.M. Room temperature dynamic polymers based on Diels-Alder chemistry. Chemistry 2009, 15, 1893–1900. [Google Scholar]
- Korth, M. Density functional theory: Not quite the right answer for the right reason yet. Angew. Chem. Int. Ed. 2017, 56, 5396–5398. [Google Scholar]
- Yan, B.; Huang, J.; Han, L.; Gong, L.; Li, L.; Israelachvili, J.N.; Zeng, H. Duplicating dynamic strain-stiffening behavior and nanomechanics of biological tissues in a synthetic self-healing flexible network hydrogel. ACS Nano 2017, 11, 11074–11081. [Google Scholar]
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z. Silyl ether as a robust and thermally stable dynamic covalent motif for malleable polymer design. J. Am. Chem. Soc. 2017, 139, 14881–14884. [Google Scholar]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar]
- Aslan, E.; Aksu, A.; Korkmaz, N.E.; Taskin, O.S.; Caglar, N.B. Monitoring the antioxidant activities by extracting the polyphenolic contents of algae collected from the Bosphorus. Mar. Pollut. Bull. 2019, 141, 313–317. [Google Scholar] [PubMed]
- Chen, Z.; Wang, C.; Lopez, J.; Lu, Z.; Cui, Y.; Bao, Z. High-areal-capacity silicon electrodes with low-cost silicon particles based on spatial control of self-healing binder. Adv. Energy Mater. 2015, 5, 1401826. [Google Scholar]
- Kim, D.; Hyun, S.; Han, S.M. Freestanding silicon microparticle and self-healing polymer composite design for effective lithiation stress relaxation. J. Mater. Chem. A 2018, 6, 11353–11361. [Google Scholar]
- Yue, L.; Zhang, L.; Zhong, H. Carboxymethyl chitosan: A new water soluble binder for si anode of li-ıon batteries. J. Power Source 2014, 247, 327. [Google Scholar]
- Sun, Y.; Lopez, J.; Lee, H.W.; Liu, N.; Zheng, G.; Wu, C.L.; Sun, J.; Liu, W.; Chung, J.W.; Bao, Z.; et al. A stretchable graphitic carbon/si anode enabled by conformal coating of a self-healing elastic polymer. Adv. Mater. 2016, 28, 2455–2461. [Google Scholar]
- Gendensuren, B.; Oh, E.-S. Dual-crosslinked network binder of alginate with polyacrylamide for silicon/graphite anodes of lithiumion battery. J. Power Source 2018, 384, 379–386. [Google Scholar]
- Zhang, J.; Wang, N.; Zhang, W.; Fang, S.; Yu, Z.; Shi, B.; Yang, J. A cycling robust network binder for high performance Si–based negative electrodes for lithium-ion batteries. J. Colloid Interface Sci. 2020, 578, 452–460. [Google Scholar]
- Han, Z.J.; Yabuuchi, N.; Shimomura, K.; Murase, M.; Yui, H.; Komaba, S. High-capacity si–graphite composite electrodes with a self-formed porous structure by a partially neutralized polyacrylate for Li-ion batteries. Energy Environ. Sci. 2012, 5, 9014. [Google Scholar]
- Nguyen, M.H.T.; Oh, E.S. Application of a new acrylonitrile/butylacrylate water-based binder for negative electrodes of lithium-ion batteries. Electrochem. Commun. 2013, 35, 45. [Google Scholar]
- Jeong, Y.K.; Kwon, T.W.; Lee, I.; Kim, T.S.; Coskun, A.; Choi, J.W. Hyperbranched β-cyclodextrin polymer as an effective multidimensional binder for silicon anodes in lithium rechargeable batteries. Nano Lett. 2014, 14, 864. [Google Scholar]
- Chen, C.; Lee, S.H.; Cho, M.; Kim, J.; Lee, Y. Cross-linked chitosan as an efficient binder for si anode of Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 2658. [Google Scholar] [PubMed]
- Yang, J.; Zhang, L.; Zhang, T.; Wang, X.; Gao, Y.; Fang, Q. Self-healing strategy for si nanoparticles towards practical application as anode materials for Li-ıon batteries. Electrochem. Commun. 2018, 87, 22. [Google Scholar]
- Deshpande, R.; Li, J.; Cheng, Y.-T.; Verbrugge, M. Liquid metal alloys as self-healing negative electrodes for lithiumion batteries. J. Electrochem. Soc. 2011, 158, A845–A849. [Google Scholar]
- Zhang, L.; Zhang, L.; Chai, L.; Xue, P.; Hao, W.; Zheng, H. A coordinatively cross-linked polymeric network as a functional binder for high-performance silicon submicro-particle anodes in lithium-ion batteries. J. Mater. Chem. A 2014, 2, 19036. [Google Scholar]
- Yoon, J.; Oh, D.X.; Jo, C.; Lee, J.; Hwang, D.S. Improvement of desolvation and resilience of alginate binders for si-based anodes in a lithiumion battery by calcium-mediated cross-linking. Phys. Chem. Chem. Phys. 2014, 16, 25628. [Google Scholar]
- Jeong, Y.K.; Choi, J.W. Mussel-inspired self-healing metallopolymers for silicon nanoparticle anodes. ACS Nano 2019, 13, 8364–8373. [Google Scholar]
- Shi, Y.; Song, M.; Zhang, Y.; Zhang, C.; Gao, H.; Niu, J.; Ma, W.; Qin, J.; Zhang, Z. A self-healing CuGa2 anode for high-performance Li ion batteries. J. Power Source 2019, 437, 226889. [Google Scholar]
- Kwon, T.W.; Jeong, Y.K.; Deniz, E.; AlQaradawi, S.Y.; Choi, J.W.; Coskun, A. Dynamic cross-linking of polymeric binders based on host–guest ınteractions for silicon anodes in lithiumion batteries. ACS Nano 2015, 9, 11317. [Google Scholar]
- Kwon, T.W.; Jeong, Y.K.; Lee, I.; Kim, T.S.; Choi, J.W.; Coskun, A. Systematic molecular-level design of binders ıncorporating meldrum’s acid for silicon anodes in lithium rechargeable batteries. Adv. Mater. 2014, 26, 7979. [Google Scholar]
- Zeng, W.; Wang, L.; Peng, X.; Liu, T.; Jiang, Y.; Qin, F.; Hu, L.; Chu, P.K.; Huo, K.; Zhou, Y. Enhanced Ion Conductivity in Conducting Polymer Binder for High-Performance Silicon Anodes in Advanced Lithium-ion Batteries. Adv. Energy Mater. 2018, 8, 1702314. [Google Scholar]
- Lim, S.; Chu, H.; Lee, K.; Yim, T.; Kim, Y.J.; Mun, J.; Kim, T.H. Physically cross-linked polymer binder ınduced by reversible acid–base ınteraction for high-performance silicon composite anodes. ACS Appl. Mater. Interfaces 2015, 7, 23545. [Google Scholar] [PubMed]
- Kim, S.; Jeong, Y.K.; Wang, Y.; Lee, H.; Choi, J.W. A “Sticky” mucin-ınspired dna-polysaccharide binder for silicon and silicon–graphite blended anodes in lithium-ıon batteries. Adv. Mater. 2018, 30, 1707594. [Google Scholar]
- Kim, S.-M.; Jeon, H.; Shin, S.-H.; Park, S.-A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior toughness and fast self-healing at room temperature engineered by transparent elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar]
- Engle, L.P.; Wagener, K.B. A review of thermally controlled covalent bond formation in polymer chemistry. J. Macromol. Sci. Part C 1993, 33, 239–257. [Google Scholar]
- Kennedy, J.P.; Castner, K.F. Thermally reversible polymer systems by cyclopentadienylation. II. The synthesis of cyclopentadiene-containing polymers. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 2055–2070. [Google Scholar]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar]
- Lee, D.H.; Heo, G.; Pyo, K.-h.; Kim, Y.; Kim, J.-W. Mechanically robust and healable transparent electrode fabricated via vapor-assisted solution process. ACS Appl. Mater. Interfaces 2016, 8, 8129–8136. [Google Scholar]
- Pyo, K.-h.; Lee, D.H.; Kim, Y.; Kim, J.-W. Extremely rapid and simple healing of a transparent conductor based on Ag nanowires and polyurethane with a Diels–Alder network. J. Mater. Chem. C 2016, 4, 972–977. [Google Scholar]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-healing materials based on disulfide links. Macromolecules 2011, 44, 2536–2541. [Google Scholar]
- Tesoro, G.C.; Sastri, V. Reversible crosslinking in epoxy resins. I. Feasibility studies. J. Appl. Polym. Sci. 1990, 39, 1425–1437. [Google Scholar]
- Sastri, V.R.; Tesoro, G.C. Reversible crosslinking in epoxy resins. II. New approaches. J. Appl. Polym. Sci. 1990, 39, 1439–1457. [Google Scholar]
- Tsarevsky, N.V.; Matyjaszewski, K. Reversible redox cleavage/coupling of polystyrene with disulfide or thiol groups prepared by atom transfer radical polymerization. Macromolecules 2002, 35, 9009–9014. [Google Scholar]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as reversible covalent crosslinkers for self-healing polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965. [Google Scholar]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Influence of stoichiometry on the glass transition and bond exchange reactions in epoxy thermoset polymers. RSC Adv. 2014, 4, 48682–48690. [Google Scholar]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic covalent polymer networks based on degenerative imine bond exchange: Tuning the malleability and self-healing properties by solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar]
- Cao, P.-F.; Yang, G.; Li, B.; Zhang, Y.; Zhao, S.; Zhang, S.; Erwin, A.; Zhang, Z.; Sokolov, A.P.; Nanda, J.; et al. Rational design of a multifunctional binder for high-capacity silicon-based anodes. ACS Energy Lett. 2019, 4, 1171–1180. [Google Scholar]
- Jung, C.H.; Kim, K.H.; Hong, S.H. Stable silicon anode for lithium-ion batteries through covalent bond formation with a binder via esterification. ACS Appl Mater Interfaces 2019, 11, 26753–26763. [Google Scholar]
- Jiao, Y.; Chen, W.; Lei, T.; Dai, L.; Chen, B.; Wu, C.; Xiong, J. A novel polar copolymer design as a multi-functional binder for strong affinity of polysulfides in lithium-sulfur batteries. Nanoscale Res. Lett. 2017, 12, 195. [Google Scholar]
- Jiao, X.; Yin, J.; Xu, X.; Wang, J.; Liu, Y.; Xiong, S.; Zhang, Q.; Song, J. Highly energy-dissipative, fast self-healing binder for stable Si anode in lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2005699. [Google Scholar]





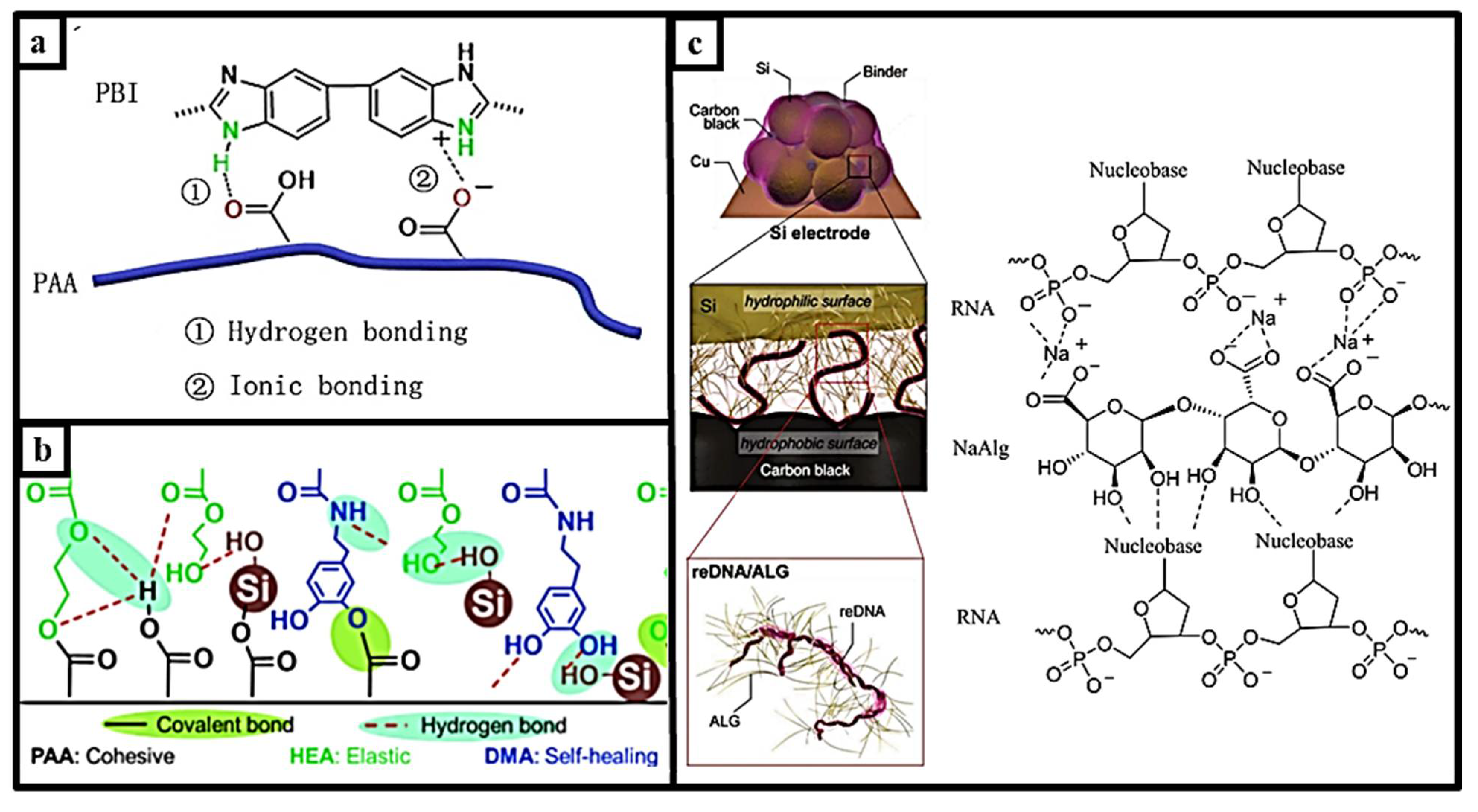
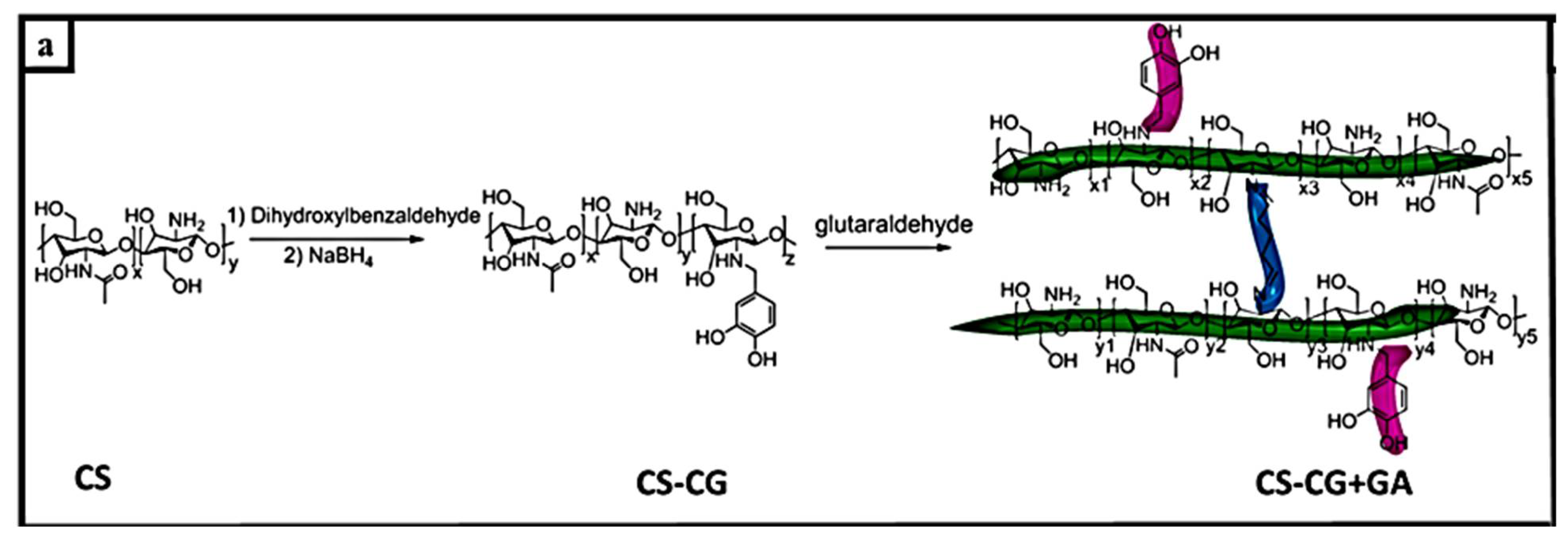

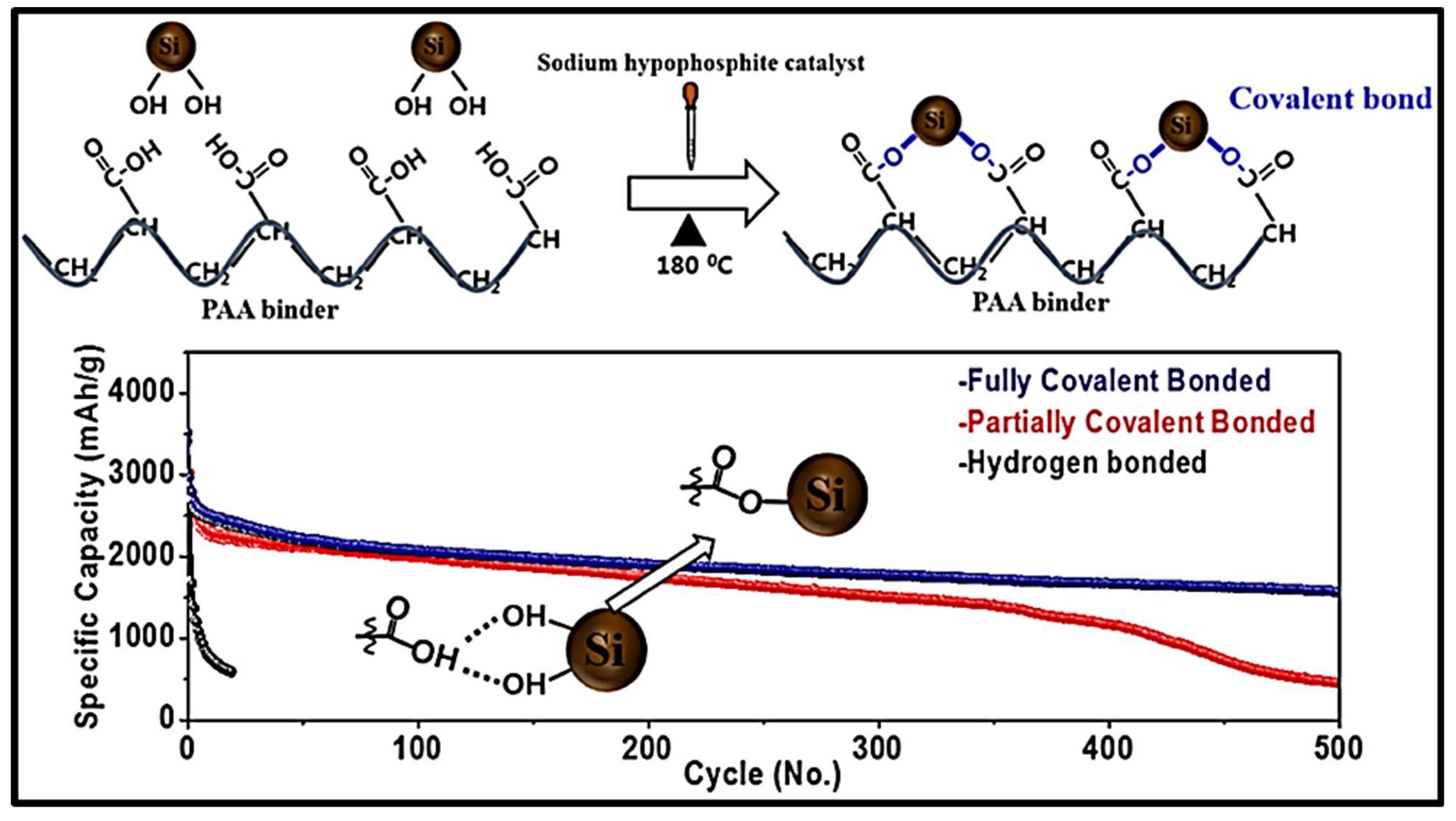
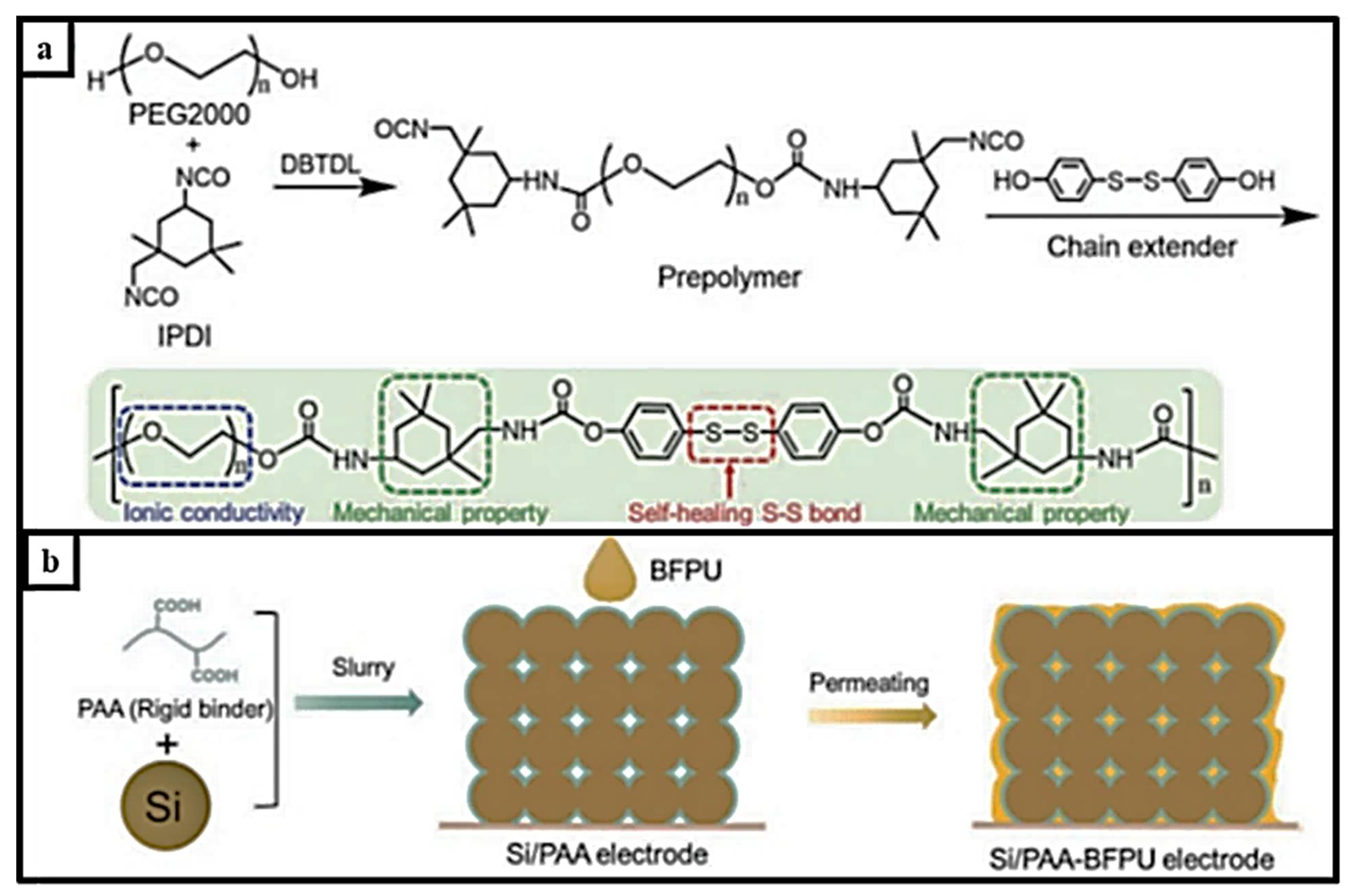

| Self-Healing Properties | Anode Active Material | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| Solid–liquid transition of gallium | Liquid gallium | 1M LiPF6 EC/DEC/DMC | 626 mAh g−1 at C/5 | [73] |
| Alginate binder with Ca2+ ions | Silicon sub-microparticule 200 nm | 1M LiPF6 EC/DEC/DMC (1:1:1 volume ratio) | 2522 mAh g−1 after 500 cycles at 20 C | [74] |
| Ca-alginate binder | Silicon, 100 nm | 1.3 M LiPF6 EC/EMC, 3:7 10wt% FEC | 1711 mAh g−1 at 0.2 A g−1 1 after 300 cycles | [75] |
| Low melting temperature | Ga-In-Sn alloy | 1M LiPF6 in EC/EMC/DMC | 2300 mAh g−1 at 0.25 C | [48] |
| Fe-ß-catechol coordination bonds | Silicon microparticule. 300 nm | 1M LiPF6 in DMC/EC | 81.9% capacity retention after 350 cycles at 1 C | [76] |
| The lithiation–delithiation mechanism | Liquid CuGa2/Si nanocomposite | 1M LiPF6 in EC/DEC | 630 mAh g−1 at 200 mA g−1 | [77] |
| Self-Healing Material | Anode Active Materials | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| Meldrum’s acid | Silicon | 1M LiPF6 in EC/DEC | 1743 mAh g−1; 74% retained after 200 cycles at 2000 mA g−1 | [79] |
| Alg–C-chitosan | Silicon | 1M LiPF6 in EC/DEC | 750 mAh g−1 after the 100th cycle | [20] |
| Ionic polymer with PEDOT:PSS, PEO, and PEI | Silicon | 1M LiPF6 EC/DEC (1:1 volume ratio with 5% FEC | over 2000 mAh g−1 after 500 cycles at 1.0 A g−1 | [80] |
| Content of binder/ conductive additive (%) 1.8/8 | Si/graphite | 1M LiPF6 EC/DEC/EMC (2:3:1 volume ratio) | 71.7% capacity retention after 100 cycles at 0.5 C | [11] |
| Self-Healing Material | Anode Active Materials | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| PAA-PBI with H-bond–ionic bond | Silicon, 100 nm | 1M LiPF6 in EC/DEC | 1376.7 mAh g−1 and improved capacity retention of 54.6% after 100 cycles at 1 C | [81] |
| reDNA/NaAlg Hydrophobic interaction | Silicon, 50 nm | 1M LiPF6 in EC/DEC | 80.1% capacity retention after 160 cycles at 1.75 A g−1 | [82] |
| PAA-P (HEAco-DMA) H bond–covalent bond | Silicon, 50–100 nm | 1M LiPF6 EC/DEC | 1855 mAh g−1 under Si loading of 1 mg cm−2 at 5 A g−1 | [15] |
| Self-Healing Material | Anode Active Materials | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| Catechol-functionalized chitosan crosslinked by glutaraldehyde | Silicon nanoparticle, 100 nm | 1M LiPF6 in EC/EMC | 2144 ± 14 mAh g−1; 91.5% capacity retention after 100 cyclesat 1 C | [98] |
| GCS-I-OSA | Silicon powder 50 nm | 1M LiPF6 in EC/DEC | 1364 mAh g−1 after 100 cycles at 0.2 C | [34] |
| Self-Healing Material | Anode Active Materials | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| BC-g | Silicon powder 50 nm | 1.3 M LiPF6 in DEC/EC | 2750 mAh g−1; 87.3% capacity retention after 100 cycles at 0.2 C | [34] |
| Esterificated PAA | Silicon powder 50 nm | 1M LiPF6 in DEC/EC/DMC 1:1:1 10% FEC | 1500 mAh g−1 after 500 cycles at 1000 mA g−1 | [99] |
| Self-Healing Material | Anode Active Materials | Electrolyte | Electrochemical Performance | Ref. |
|---|---|---|---|---|
| 1,6-bismaleimide (BMI) functionalized poly(acrylic acid) (FPAA) DA-PAA | Silicon powder 50 nm | 1 M LiPF6 in EC/EMC 1:2 (v/v) with 10% FEC | 1076 mAh g−1; 99.7% capacity retention after 200 cycles at 1 C | [22] |
| Poly(ether-thioureas) | Silicon powder, 100 nm | 1 M LiPF6 in DEC/EC/DMC | 1325 mAh g−1 at 1 C | [100] |
| PAA–BFPU binder | Silicon powder, 100 nm | 1 M LiPF6 in DEC/EC/DMC | over 88% capacity retention after 200 cyclesat 3.5 mAh cm−2 | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuca, N.; Kalafat, I.; Guney, E.; Cetin, B.; Taskin, O.S. Self-Healing Systems in Silicon Anodes for Li-Ion Batteries. Materials 2022, 15, 2392. https://doi.org/10.3390/ma15072392
Yuca N, Kalafat I, Guney E, Cetin B, Taskin OS. Self-Healing Systems in Silicon Anodes for Li-Ion Batteries. Materials. 2022; 15(7):2392. https://doi.org/10.3390/ma15072392
Chicago/Turabian StyleYuca, Neslihan, Ilknur Kalafat, Emre Guney, Busra Cetin, and Omer S. Taskin. 2022. "Self-Healing Systems in Silicon Anodes for Li-Ion Batteries" Materials 15, no. 7: 2392. https://doi.org/10.3390/ma15072392
APA StyleYuca, N., Kalafat, I., Guney, E., Cetin, B., & Taskin, O. S. (2022). Self-Healing Systems in Silicon Anodes for Li-Ion Batteries. Materials, 15(7), 2392. https://doi.org/10.3390/ma15072392






