Bioinspired Topographic Surface Modification of Biomaterials
Abstract
:1. Introduction
2. Bioinspiration from Animal and Insect Surfaces
2.1. Topographic Features from Animal and Insect Models
2.2. Microorganisms Adhesion and Colonization
2.3. Cellular Adhesion and Biocompatibility
3. Bioinspiration from Vegetal Surfaces
3.1. Topographic Features from Vegetal Models
3.2. Microorganisms Adhesion and Colonization
3.3. Cellular Adhesion and Biocompatibility
4. Mechanisms Involved in Reduction in Bacterial Adhesion and Improvement of Cell Attachment
4.1. Reduction in Bacterial Adhesion and Bactericidal Mechanisms
4.2. Mechanisms Related to Enhancemente in Cell Attachment
5. Conclusions, Challenges and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar] [CrossRef] [Green Version]
- Drack, M.; Limpinsel, M.; De Bruyn, G.; Nebelsick, J.H.; Betz, O. Towards a theoretical clarification of biomimetics using conceptual tools from engineering design. Bioinspir. Biomim. 2018, 13, 016007. [Google Scholar] [CrossRef] [PubMed]
- French, J.R.J.; Ahmed, B.M. The challenge of biomimetic design for carbon-neutral buildings using termite engineering. Insect Sci. 2010, 17, 154–162. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Asrar, W.; Sulaeman, E. Literature review: Biomimetic and conventional aircraft wing tips. Int. J. Aviat. Aeronaut. Aerosp. 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Fish, F.E.; Kocak, D.M. Biomimetics and marine technology: An introduction. Mar. Technol. Soc. J. 2011, 45, 8–13. [Google Scholar] [CrossRef]
- Yan, H.; Wu, Q.; Yu, C.; Zhao, T.; Liu, M. Recent Progress of Biomimetic Antifouling Surfaces in Marine. Adv. Mater. Interfaces 2020, 7, 20. [Google Scholar] [CrossRef]
- Wijegunawardana, I.D.; de Mel, W.R. Biomimetic Designs for Automobile Engineering: A Review. Int. J. Automot. Mech. Eng. 2021, 18, 9029–9041. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Adv. Sci. 2017, 4, 1700401. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef]
- Mahtabian, S.; Mirhadi, S.M.; Tavangarian, F. From rose petal to bone scaffolds: Using nature to fabricate osteon-like scaffolds for bone tissue engineering applications. Ceram. Int. 2021, 47, 21633–21641. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z.; Yang, P.; Cheng, G. In vitro bioactivity and biocompatibility of bio-inspired Ti-6Al-4V alloy surfaces modified by combined laser micro/nano structuring. Molecules 2020, 25, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Trans. 2019, 17, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [Green Version]
- Asha, A.B.; Chen, Y.; Zhang, H.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef]
- Choi, S.H.; Jang, Y.S.; Jang, J.H.; Bae, T.S.; Lee, S.J.; Lee, M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Freitas, S.C.; Correa-Uribe, A.; Martins, M.C.L.; Pelaez-Vargas, A. Self-Assembled Monolayers for Dental Implants. Int. J. Dent. 2018, 2018, 4395460. [Google Scholar] [CrossRef]
- Zabara, M.; Ren, Q.; Amenitsch, H.; Salentinig, S. Bioinspired Antimicrobial Coatings from Peptide-Functionalized Liquid Crystalline Nanostructures. ACS Appl. Bio Mater. 2021, 4, 5295–5303. [Google Scholar] [CrossRef]
- Zhang, Z.; Kou, N.; Ye, W.; Wang, S.; Lu, J.; Lu, Y.; Liu, H.; Wang, X. Construction and Characterizations of Antibacterial Surfaces Based on Self-Assembled Monolayer of Antimicrobial Peptides (Pac-525) Derivatives on Gold. Coatings 2021, 11, 9. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Khan, M.; Adil, S.F. Green synthesis of ZnO hierarchical microstructures by Cordia myxa and their antibacterial activity. Saudi J. Biol. Sci. 2019, 26, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, M.T.; Xu, S.; Yang, H.; Sun, H.B. Bioinspired Superhydrophobic Surfaces via Laser-Structuring. Front. Chem. 2020, 16, 835. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef]
- Patil, D.; Overland, M.; Stoller, M.; Chatterjee, K. Bioinspired nanostructured bactericidal surfaces. Curr. Opin. Chem. Eng. 2021, 34, 100741. [Google Scholar] [CrossRef]
- Shimomura, M. The New Trends in Next Generation Biomimetics Material Technology: Learning from Biodiversity. Sci. Technol. Trends 2010, 37, 53–75. [Google Scholar]
- O’Neill, P.; Barrett, A.; Sullivan, T.; Regan, F.; Brabazon, D. Rapid Prototyped Biomimetic Antifouling Surfaces for Marine Applications. Biomimetics 2016, 5, 58. [Google Scholar] [CrossRef]
- Bai, X.Q.; Xie, G.T.; Fan, H.; Peng, Z.X.; Yuan, C.Q.; Yan, X.P. Study on biomimetic preparation of shell surface microstructure for ship antifouling. Wear 2012, 306, 285–295. [Google Scholar] [CrossRef]
- Munther, M.; Palma, T.; Angeron, I.A.; Salari, S.; Ghassemi, H.; Vasefi, M.; Beheshti, A.; Davami, K. Microfabricated Biomimetic placoid Scale-Inspired surfaces for antifouling applications. Appl. Surf. Sci. 2018, 453, 166–172. [Google Scholar] [CrossRef]
- Han, X.; Wu, J.; Zhang, X.; Shi, J.; Wei, J.; Yang, Y.; Wu, B.; Feng, Y. The progress on antifouling organic coating: From biocide to biomimetic surface. J. Mater. Sci. Technol. 2021, 61, 46–62. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Sun, D.W. Biomimetic modification of freezing facility surfaces to prevent icing and frosting during freezing for the food industry. Trends Food Sci. Technol. 2021, 111, 581–594. [Google Scholar] [CrossRef]
- Zouaghi, S.; Bellayer, S.; Thomy, V.; Dargent, T.; Coffinier, Y.; Andre, C.; Delaplace, G.; Jimenez, M. Biomimetic surface modifications of stainless steel targeting dairy fouling mitigation and bacterial adhesion. Food Bioprod. Process. 2019, 113, 32–38. [Google Scholar] [CrossRef]
- Ibrahim, U.H.; Devnarain, N.; Govender, T. Biomimetic strategies for enhancing synthesis and delivery of antibacterial nanosystems. Int. J. Pharm. 2021, 596, 120276. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arango-Santander, S.; Serna, L.; Sanchez-Garzon, J.; Franco, J. Evaluation of Streptococcus mutans Adhesion to Stainless Steel Surfaces Modified Using Different Topographies Following a Biomimetic Approach. Coatings 2021, 11, 829. [Google Scholar] [CrossRef]
- Arango-Santander, S.; Gonzalez, C.; Aguilar, A.; Cano, A.; Castro, S.; Sanchez-Garzon, J.; Franco, J. Assessment of streptococcus mutans adhesion to the surface of biomimetically-modified orthodontic archwires. Coatings 2020, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef] [PubMed]
- Hayles, A.; Hasan, J.; Bright, R.; Palms, D.; Brown, T.; Barker, D.; Vasilev, K. Hydrothermally etched titanium: A review on a promising mechano-bactericidal surface for implant applications. Mater. Today Chem. 2021, 22, 100622. [Google Scholar] [CrossRef]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Bettinger, C.; Langer, R.; Borenstein, J. Engineering substrate micro- and nanotopography to control cell function. Angew. Chem. Int. Ed. Engl. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaez-Vargas, A.; Gallego-Perez, D.; Magallanes-Perdomo, M.; Fernandes, M.H.; Hansford, D.J.; De Aza, A.H.; Pena, P.; Monteiro, F.J. Isotropic micropatterned silica coatings on zirconia induce guided cell growth for dental implants. Dent. Mater. 2011, 27, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chi, Z.; Cao, L.; Weng, Z.; Wang, L.; Li, L.; Saeed, S.; Lian, Z.; Wang, Z. Fabrication of biomimetic superhydrophobic and anti-icing Ti6Al4V alloy surfaces by direct laser interference lithography and hydrothermal treatment. App. Surf. Sci. 2020, 534, 147576. [Google Scholar] [CrossRef]
- Kuczynska-Zemla, D.; Sotniczuk, A.; Pisarek, M.; Chlanda, A.; Garbacz, H. Corrosion behavior of titanium modified by direct laser interference lithography. Surf. Coat. Technol. 2021, 418, 127219. [Google Scholar] [CrossRef]
- Lohse, M.; Heinrich, M.; Grützner, S.; Haase, A.; Ramos, I.; Salado, C.; Thesen, M.W.; Grützner, G. Versatile fabrication method for multiscale hierarchical structured polymer masters using a combination of photo- and nanoimprint lithography. Micro Nano Eng. 2021, 10, 100079. [Google Scholar] [CrossRef]
- Ponomarev, V.A.; Shvindina, N.V.; Permyakova, E.S.; Slukin, P.V.; Ignatov, S.G.; Sirota, B.; Voevodin, A.A.; Shtansky, D.V. Structure and antibacterial properties of Ag-doped micropattern surfaces produced by photolithography method. Coll. Surf. B Biointerfaces. 2019, 173, 719–724. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Nagay, B.E.; Barao, V.A.R.; Sukotjo, C.; Jursich, G.; Takoudis, C.G. Atomic layer deposition of TiO2, ZrO2 and TiO2/ZrO2 mixed oxide nanofilms on PMMA for enhanced biomaterial functionalization. Appl. Surf. Sci. 2022, 578, 151891. [Google Scholar] [CrossRef]
- Xia, D.-H.; Pan, C.; Qin, Z.; Fan, B.; Song, S.; Jin, W.; Hu, W. Covalent surface modification of LY12 aluminum alloy surface by self-assembly dodecyl phosphate film towards corrosion protection. Prog. Org. Coat. 2020, 143, 105638. [Google Scholar] [CrossRef]
- Ortiz-Cárdenas, J.E.; Zatorski, J.M.; Arneja, A.; Montalbine, A.N.; Munson, J.M.; Luckey, C.J.; Pompano, R.R. Towards spatially-organized organs-on-chip: Photopatterning cell-laden thiolene and methacryloyl hydrogels in a microfluidic device. Organs-on-a-Chip 2022, 4, 100018. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B. Nanomanufacturing of bioinspired surfaces. Tribol. Int. 2019, 129, 67–74. [Google Scholar] [CrossRef]
- Chien, H.W.; Chen, X.Y.; Tsai, W.P. Poly(methyl methacrylate)/titanium dioxide (PMMA/TiO2) nanocomposite with shark-skin structure for preventing biofilm formation. Mater. Lett. 2021, 285, 129098. [Google Scholar] [CrossRef]
- Chien, H.W.; Chen, X.Y.; Tsai, W.P.; Lee, M. Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerfaces 2020, 186, 110738. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, S.N. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Faustino, C.M.C.; Lemos, S.M.C.; Monge, N.; Ribeiro, I.A.C. A scope at antifouling strategies to prevent catheter-associated infections. Adv. Colloid Interface Sci. 2020, 284, 102230. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, G.; Cong, Q.; Chen, G.; Ren, L. Effects of Methanol on Wettability of the Non-Smooth Surface on Butterfly Wing. J. Bionic. Eng. 2008, 5, 127–133. [Google Scholar] [CrossRef]
- Bhushan, B.; Sayer, R.A. Surface characterization and friction of a bio-inspired reversible adhesive tape. Microsyst. Technol. 2007, 13, 71–78. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Liao, G.; Yang, Y.; Ren, L.; Yang, H.; Chen, X. The Sound Suppression Characteristics of Wing Feather of Owl (Bubo bubo). J. Bionic Eng. 2012, 9, 192–199. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, H.S.; Cho, I.J.; Yoon, E.S. The role of bio-inspired hierarchical structures in wetting. Bioinspiration Biomim. 2015, 10, 026009. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Jin, J.; Liu, J.; Yan, Y.; Han, Z.; Ren, L. Anti-icing property of bio-inspired micro-structure superhydrophobic surfaces and heat transfer model. Appl. Surf. Sci. 2017, 400, 498–505. [Google Scholar] [CrossRef]
- Liu, Q.; Brookbank, L.; Ho, A.; Coffey, J.; Brennan, A.B.; Jones, C.J. Surface texture limits transfer of S. aureus, T4 bacteriophage, influenza B virus and human coronavirus. PLoS ONE 2020, 15, e0244518. [Google Scholar] [CrossRef]
- Román-Kustas, J.; Hoffman, J.B.; Reed, J.H.; Gonsalves, A.E.; Oh, J.; Li, L.; Hong, S.; Jo, K.D.; Dana, C.E.; Miljkovic, N.; et al. Molecular and Topographical Organization: Influence on Cicada Wing Wettability and Bactericidal Properties. Adv. Mater. Interfaces 2020, 7, 2000112. [Google Scholar] [CrossRef] [Green Version]
- Román-Kustas, J.; Hoffman, J.B.; Alonso, D.; Reed, J.H.; Gonsalves, A.E.; Oh, J.; Hong, S.; Jo, K.D.; Dana, C.E.; Alleyne, M.; et al. Analysis of cicada wing surface constituents by comprehensive multidimensional gas chromatography for species differentiation. Microchem. J. 2020, 158, 105089. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, F.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.E.; Yang, Y.; Yuen, M.-F.; Zhang, W.; Nobbs, A.H.; Su, B. Bactericidal activity of biomimetic diamond nanocone surfaces. Biointerphases 2016, 11, 011014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhang, J.; Xie, G.; Liu, Z.; Shao, H. Cicada Wings: A Stamp from Nature for Nanoimprint Lithography. Small 2006, 2, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, F.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commum. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.H.T.; Webb, H.K.; Hasan, J.; Tobin, M.J.; Crawford, R.J.; Ivanova, E.P. Dual role of outer epicuticular lipids in determining the wettability of dragonfly wings. Colloids Surf. B Biointerfaces 2013, 106, 126–134. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure –A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Badge, I.; Stark, A.Y.; Paoloni, E.L.; Niewiarowski, P.H.; Dhinojwala, A. The Role of Surface Chemistry in Adhesion and Wetting of Gecko Toe Pads. Sci. Rep. 2014, 4, 6643. [Google Scholar] [CrossRef] [Green Version]
- Watson, G.S.; Green, D.W.; Cribb, B.W.; Brown, C.L.; Meritt, C.R.; Tobin, M.J.; Vongsvivut, J.; Sun, M.; Liang, A.P.; Watson, J.A. Insect Analogue to the Lotus Leaf: A Planthopper Wing Membrane Incorporating a Low-Adhesion, Nonwetting, Superhydrophobic, Bactericidal, and Biocompatible Surface. ACS Appl. Mater. Interfaces 2017, 9, 24381–24392. [Google Scholar] [CrossRef]
- Pu, X.; Li, G.; Huang, H. Preparation, anti-biofouling and drag-reduction properties of a biomimetic shark skin surface. Biol. Open 2016, 5, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Magyar, A.; Arthanareeswaran, V.K.A.; Soós, L.; Nagy, K.; Dobák, A.; Szilágyi, I.M.; Justh, N.; Chandra, A.R.; Köves, B.; Tenke, P. Does micropattern (sharklet) on urinary catheter surface reduce urinary tract infections? Results from phase I randomized open label interventional trial. Eur. Urol. Suppl. 2017, 16, e146–e148. [Google Scholar] [CrossRef]
- Shahali, H.; Hasan, J.; Mathews, A.; Wang, H.; Yan, C.; Tesfamichael, T.; Yarlagadda, P.K.D.V. Multi-biofunctional properties of three species of cicada wings and biomimetic fabrication of nanopatterned titanium pillars. J. Mater. Chem. B 2019, 7, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Hazell, G.; Fisher, L.; Murray, A.; Nobbs, A.; Su, B. Bioinspired bactericidal surfaces with polymer nanocone arrays. J. Colloids Interf. 2018, 528, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magin, C.M.; May, R.M.; Drinker, M.C.; Cuevas, K.H.; Brennan, A.B.; Reddy, S.T. Micropatterned Protective Membranes Inhibit Lens Epithelial Cell Migration in Posterior Capsule Opacification Model. Transl. Vis. Sci. Technol. 2015, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, E.E.; Manna, D.; Mettetal, M.R.; May, R.M.; Dannemiller, E.M.; Chung, K.K.; Brennan, A.B.; Reddy, S.T. Surface micropattern limits bacterial contamination. Antimicrob. Resist. Infect. Control 2014, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, E.E.; Magin, C.M.; Mettetal, M.R.; May, R.M.; Henry, M.K.M.; DeLoid, H.; Prater, J.; Sullivan, L.; Thomas, J.G.; Twite, M.D.; et al. Micropatterned Endotracheal Tubes Reduce Secretion-Related Lumen Occlusion. Ann. Biomed. Eng. 2016, 44, 3645–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.T.; Chung, K.K.; McDaniel, C.J.; Darouiche, R.O.; Landman, J.; Brennan, A.B. Micropatterned surfaces for reducing the risk of catheter-associated urinary tract infection: An in vitro study on the effect of sharklet micropatterned surfaces to inhibit bacterial colonization and migration of uropathogenic Escherichia coli. J. Endourol. 2011, 25, 1547–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, R.M.; Magin, C.M.; Mann, E.E.; Drinker, M.C.; Fraser, J.C.; Siedlecki, C.A.; Brennan, A.B.; Reddy, S.T. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin. Transl. Med. 2015, 26, 9. [Google Scholar] [CrossRef] [Green Version]
- Arisoy, F.D.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, I.D.; Watkins, J.J. Bioinspired Photocatalytic Shark-Skin Surfaces with Antibacterial and Antifouling Activity via Nanoimprint Lithography. ACS Appl. Mater. Interfaces 2018, 10, 20055–20063. [Google Scholar] [CrossRef]
- Rostami, S.; Puza, F.; Ucak, M.; Ozgur, E.; Gul, O.; Ercan, U.K.; Garipcan, B. Bifunctional sharkskin mimicked chitosan/graphene oxide membranes: Reduced biofilm formation and improved cytocompatibility. Appl. Surf. Sci. 2021, 544, 148828. [Google Scholar] [CrossRef]
- Dehghani, S.; Mashreghi, M.; Nezhad, A.H.N.; Karimi, J.; Hosseinpour, S.; Davoodi, A. Exploring mechano-bactericidal nature of Psalmocharias cicadas wings: An analytical nanotopology investigation based on atomic force microscopy characterization. Surf. Interfaces 2021, 26, 101407. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.H.; Al Kobaisi, M.; Senituinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nanopatterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yang, L.; Liu, N.; Yang, Y.; Zhao, J.; Yang, P.; Cheng, G. Bioinspired surface hierarchical microstructures of Ti6Al4V alloy with a positive effect on osteoconduction. Surf. Coat. Technol. 2020, 388, 125594. [Google Scholar] [CrossRef]
- Mobini, S.; Kuliasha, C.A.; Siders, Z.A.; Bohmann, N.A.; Jamal, S.M.; Judy, J.W.; Schmidt, C.E.; Brennan, A.B. Microtopographical Patterns Promote Different Responses in Fibroblasts and Schwann Cells: A Possible Feature for Neural Implants. J. Biomed. Mater. Res. A 2021, 109, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Liu, W.; Su, B.L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface. Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shao, R.; Wang, Q.; Sun, S. Hierarchical hydrophobic surfaces with controlled dual transition between rose petal effect and lotus effect via structure tailoring or chemical modification. Coll. Surf. A Physichem. Eng. Asp. 2021, 622, 126661. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. The rose petal effect and the modes of superhydrophobicity. Philos. Trans. R Soc. A 2010, 368, 4713–4728. [Google Scholar] [CrossRef]
- Cao, Y.; Jana, S.; Bowen, L.; Tan, X.; Liu, H.; Rostami, N.; Brown, J.; Jakubovics, N.S.; Chen, J. Hierarchical Rose Petal Surfaces Delay the Early-Stage Bacterial Biofilm Growth. Langmuir 2019, 35, 14670–14680. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food. Agric. 2013, 94, 3. [Google Scholar] [CrossRef]
- Mitharwal, S.; Kumar, A.; Chauhan, K.; Taneja, N.K. Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta L.) leaves: A review. Food Chem. 2022, 383, 132406. [Google Scholar] [CrossRef]
- Tricinci, O.; Terencio, T.; Mazzolai, B.; Pugno, N.M.; Greco, F.; Mattoli, V. 3D Micropatterned Surface Inspired by Salvinia molesta via Direct Laser Lithography. ACS Appl. Mater. Interfaces 2015, 7, 25560–25567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, R.; Hao, L.; Song, L.; Tian, L.; Fan, Y.; Zhao, J.; Liu, C.; Ming, W.; Ren, L. Lotus-leaf-inspired hierarchical structured surface with non-fouling and mechanical bactericidal performances. Chem. Eng. J. 2020, 398, 125609. [Google Scholar] [CrossRef]
- Öztürk-Öncel, M.Ö.; Ercok-Biradli, F.; Rasier, R.; Marcali, M.; Elbuken, C.; Garipcan, B. Rose petal topography mimicked poly(dimethylsiloxane) substrates for enhanced corneal endothelial cell behavior. Mater. Sci. Eng. C 2021, 126, 112147. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Roohani, I.; No, Y.J.; Madafiglio, G.; Chang, F.; Zhao, F.; Lu, Z.; Zreiqat, H. Nature-inspired topographies on hydroxyapatite surfaces regulate stem cells behavior. Bioactive Mater. 2021, 6, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Ivvala, J.; Arora, H.S.; Ghosh, S.K.; Grewal, H.S. Bioinspired micro/nano structured aluminum with multifaceted applications. Colloids Surf. B Interfaces 2022, 211, 112311. [Google Scholar] [CrossRef]
- Hwang, G.B.; Page, K.; Patir, A.; Nair, S.P.; Allan, E.; Parkin, I.P. The Anti-Biofouling Properties of Superhydrophobic Surfaces are Short-Lived. ACS Nano 2018, 12, 6050–6058. [Google Scholar] [CrossRef]
- Xue, F.; Liu, J.; Guo, L.; Zhang, L.; Li, Q. Theoretical study on the bactericidal nature of nanopatterned surfaces. J. Theor. Biol. 2015, 385, 1–7. [Google Scholar] [CrossRef]
- Velic, A.; Hasan, J.; Li, Z.; Yarlagadda, P.K.D.V. Mechanics of Bacterial Interaction and Death on Nanopatterned Surfaces. Biophys. J. 2020, 120, 217–231. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commum. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Turner, A.M.; Dowell, N.; Kam, L.; Isaacson, M.; Turner, J.N.; Craighead, H.G.; Shain, W. Attachment of astroglial cells to microfabricated pillar arrays of different geometries. J. Biomed. Mater. Res. 2000, 51, 430–441. [Google Scholar] [CrossRef]
- Hasturk, O.; Ermis, M.; Demirci, U.; Hasirci, N.; Hasirci, V. Square prism micropillars improve osteogenicity of poly(methyl methacrylate) surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 53. [Google Scholar] [CrossRef] [PubMed]
- Radotic, V.; Bedalov, A.; Drvis, P.; Braeken, D.; Kovacic, D. Guided growth with aligned neurites in adult spiral ganglion neurons cultured in vitro on silicon micro-pillar substrates. J. Neural. Eng. 2019, 16, 066037. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, K.S.; Ullmann, S.; Vinje, J.; Sikorski, P. Influence of Nanopillar Arrays on Fibroblast Motility, Adhesion, and Migration Mechanisms. Small 2019, 15, e1902514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryszak, B.; Szustakiewicz, K.; Dzienny, P.; Junka, A.; Paleczny, J.; Szymczyk-Ziolkowska, P.; Hoppe, V.; Grzymajlo, M.; Antonczak, A. ‘Cookies on a tray’: Superselective hierarchical microstructured poly(L-lactide) surface as a decoy for cells. Mater. Sci. Eng. C 2022, 132, 112648. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Lekka, M.; Marzec, M.; Harhay, K.; Ohar, H.; Ostapiv, D.; Sharan, M.; Yaremchuk, I.; et al. Temperature-responsive grafted polymer brushes obtained from renewable sources with potential application as substrates for tissue engineering. Appl. Surf. Sci. 2017, 407, 546–554. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Gu, Y.; Ding, J. Nonmonotonic self-deformation of cell nuclei on topological surfaces with micropillar array. ACS Appl. Mater. Interfaces 2017, 9, 18521–18530. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Zhang, A.; Jin, S.; Liang, X.; Tang, Z.; Liu, X.; Chen, H. Vascular cell behavior on heparin-like polymers modified silicone surfaces: The prominent role of the lotus leaf-like topography. J. Colloid Interface Sci. 2021, 603, 501–510. [Google Scholar] [CrossRef]
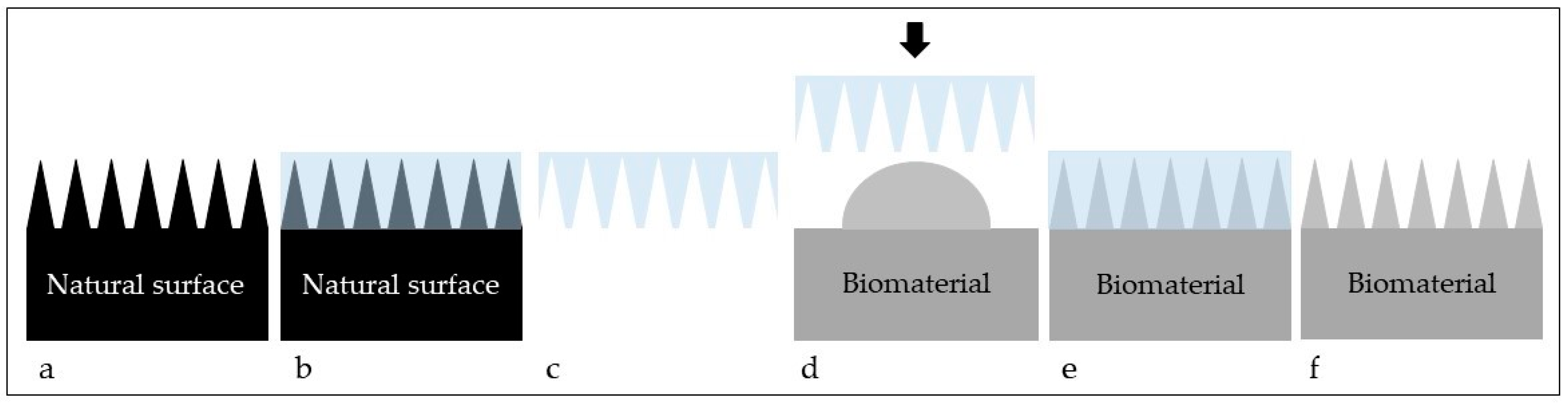



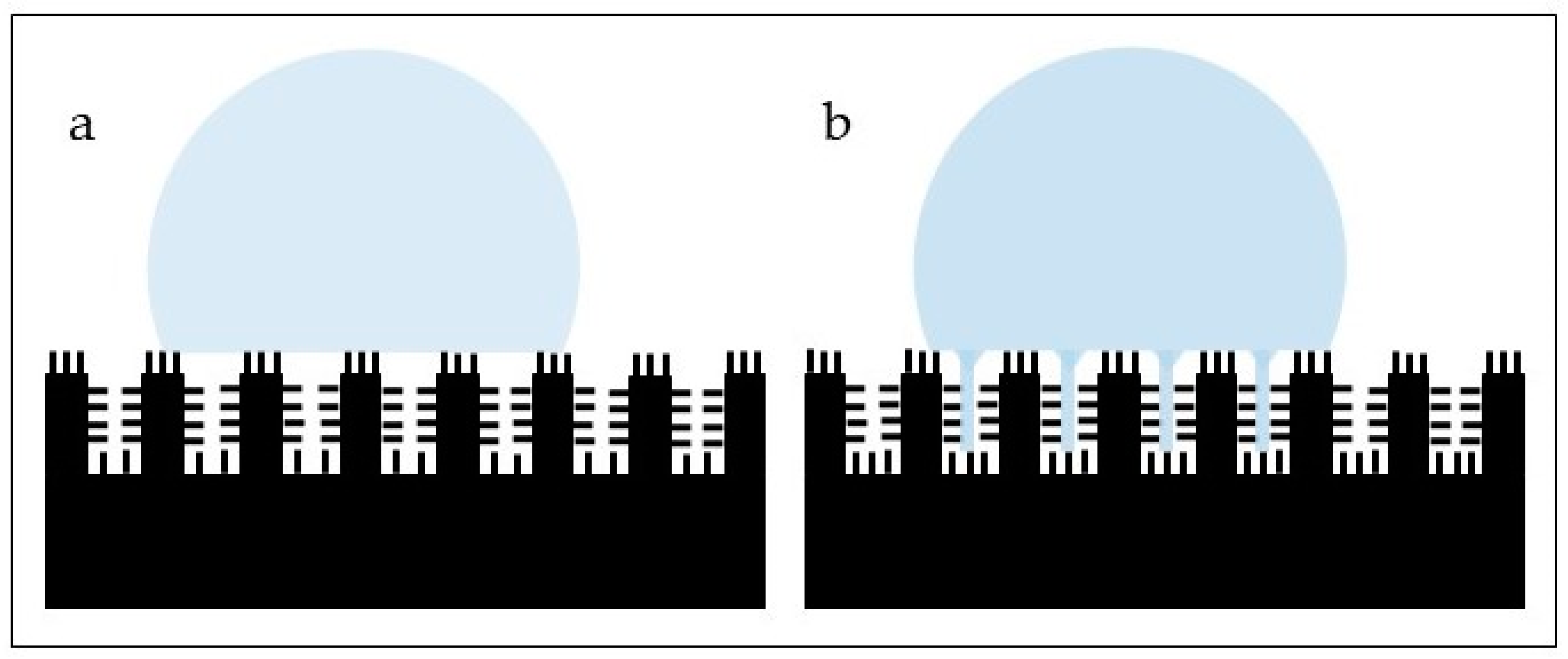
| Animal/Insect | Topography | Applications in Biomaterials | References |
|---|---|---|---|
| Sharkskin | Denticles: scales of diamond-shape with a raised ridge and concave groove that show some nanostructures. The Sharklet model is made of rectangular features of 4–16 µm in length, around 2 µm of width and a height of 3 µm at a spacing of around 2 μm between adjacent features. | Reduction in bacterial adhesion alone or coupled with other chemical and photocatalytic compounds | [79,80] |
| Cicada wings | Highly ordered array of nanopillars or nanocones of different sizes, heights and spatial distribution depending on the species. | Antibacterial | [62,81,82] |
| Dragonfly skin (Diplacodes bipunctata) | Nanopillar clusters of random size, height and spacing | Antibacterial | [74] |
| Gecko skin (Lucasium steindachneri) | Dome-shaped pigmented scales arranged in a hexagonal patterning. Scales from 100–190 µm in diameter and around 50 µm in height at the back, larger scales with more spacing in the abdominal area. Spinules (hairs) up to 4 µm in length, with sub-micron spacing and a small radius of curvature typically from 10 to 20 nm. | Antibacterial | [76] |
| Planthopper wing (Desudaba danae) | Hindwing: micro asperities of around 6 µm in height, 500 nm in length, 45–50 nm in diameter at a spacing of around 14 µm. Forewing: grouped structures of various roughness dimensions. | Antibacterial Cell compatibility | [78] |
| Butterfly wing (Morpho aega) | The wing is covered with micro scales, parallel ridges and tile-like microstructures, nanoscale ribs and lamella-stacking nano-stripe structures | Easy cleaning coatings | [79] |
| Tree frog toe pad (Litoria caerulea) | Peg-studded hexagonal cells separated by channels and by finer pegs on the flattened surface of each hexagonal cell | Enhanced attachment | [83] |
| Vegetal | Topography | Applications in Biomaterials | References |
|---|---|---|---|
| Lotus leaf (Nelumbo nucifera) | Hierarchical surface with protrusions and valleys ranging from 3–10 µm. Nanometric particles (70–100 nm in size) of a hydrophobic wax-like material in the protrusions. Subsurface layer has nano sticks with diameters around 50 nm randomly distributed | Reduction in bacterial adhesion Antibacterial | [95,96,97] |
| Rice leaves | Papillae around 5–8 µm in height on the surface arranged in one-dimensional parallel order. Sublayer shows nanometric pins proportionally distributed | Reduction in bacterial adhesion | [95] |
| Rose petals | Hierarchical structures with micro-papillae of around 20 µm in diameter. Nanometric cuticular folds of around 730 nm in width | Reduction in bacterial adhesion Cell attachment | [95] |
| Taro leaves (Colocasia esculenta) | Hierarchical structure with elliptic protrusions with diameters of around 10 µm uniformly distributed in nest-like caves. Nanometric pins disseminated on the surface | Reduction in bacterial adhesion | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arango-Santander, S. Bioinspired Topographic Surface Modification of Biomaterials. Materials 2022, 15, 2383. https://doi.org/10.3390/ma15072383
Arango-Santander S. Bioinspired Topographic Surface Modification of Biomaterials. Materials. 2022; 15(7):2383. https://doi.org/10.3390/ma15072383
Chicago/Turabian StyleArango-Santander, Santiago. 2022. "Bioinspired Topographic Surface Modification of Biomaterials" Materials 15, no. 7: 2383. https://doi.org/10.3390/ma15072383
APA StyleArango-Santander, S. (2022). Bioinspired Topographic Surface Modification of Biomaterials. Materials, 15(7), 2383. https://doi.org/10.3390/ma15072383






