Production of Belite Based Clinker from Ornamental Stone Processing Sludge and Calcium Carbonate Sludge with Lower CO2 Emissions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
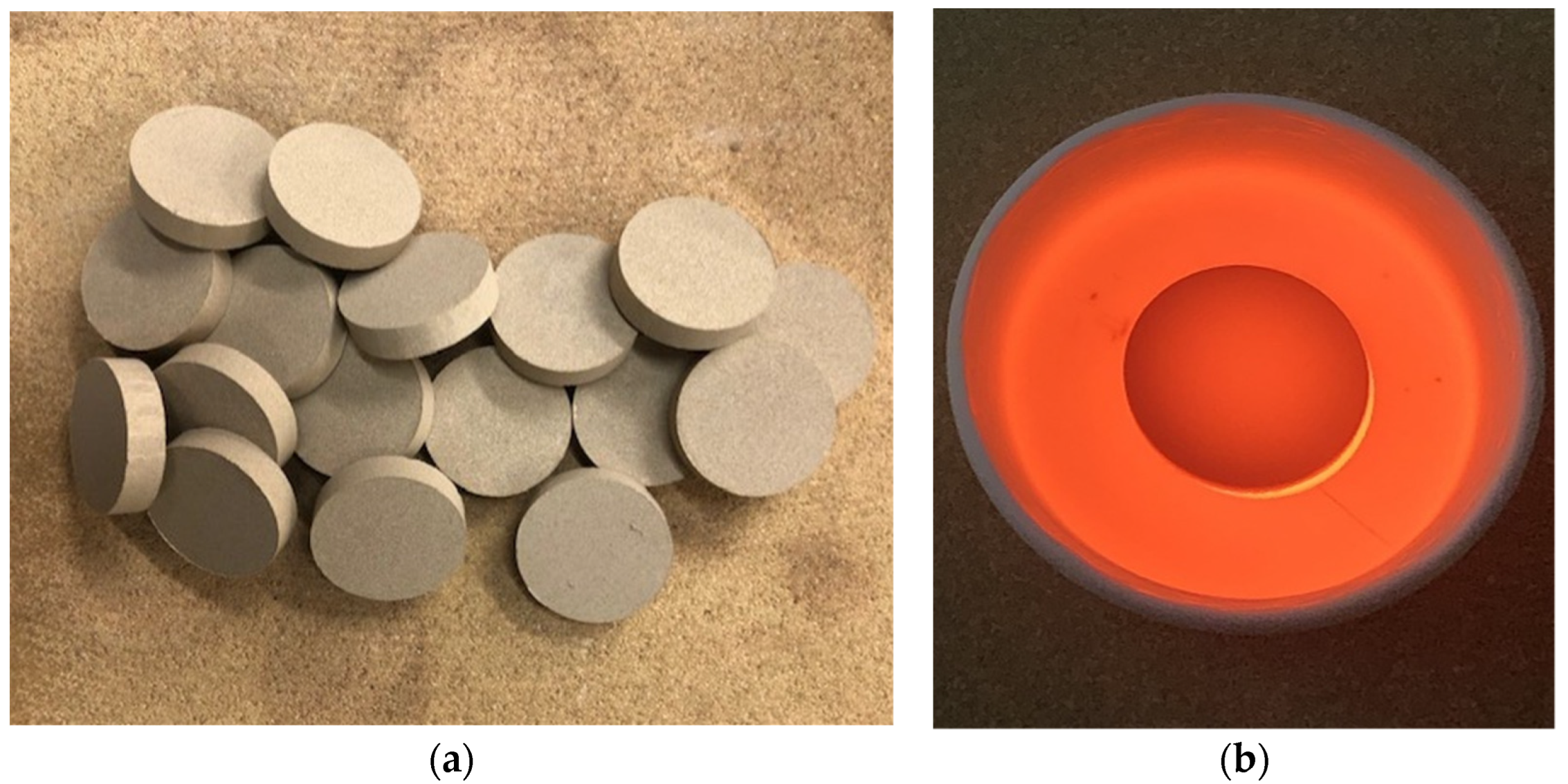
2.2.1. Dosage and Production of Belitic Clinker
2.2.2. Material Characterization
2.2.3. Evaluation of Carbon Dioxide Emissions during Clinker Production
3. Results and Discussion
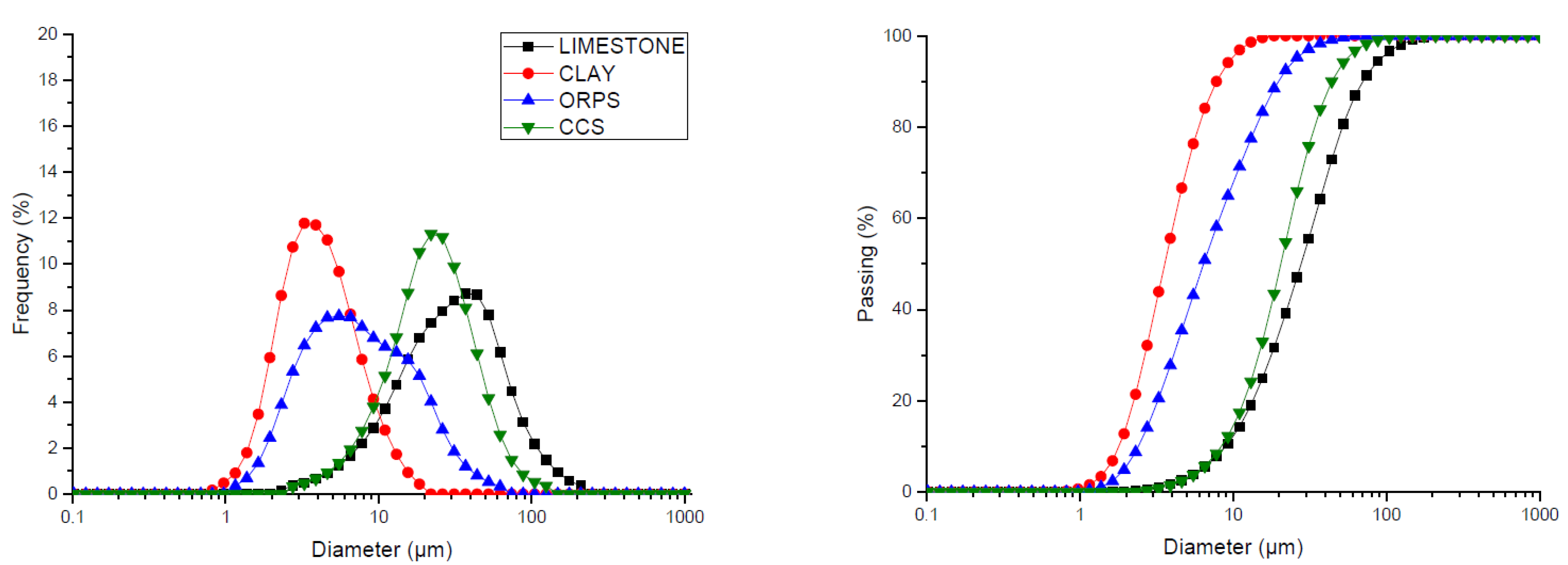
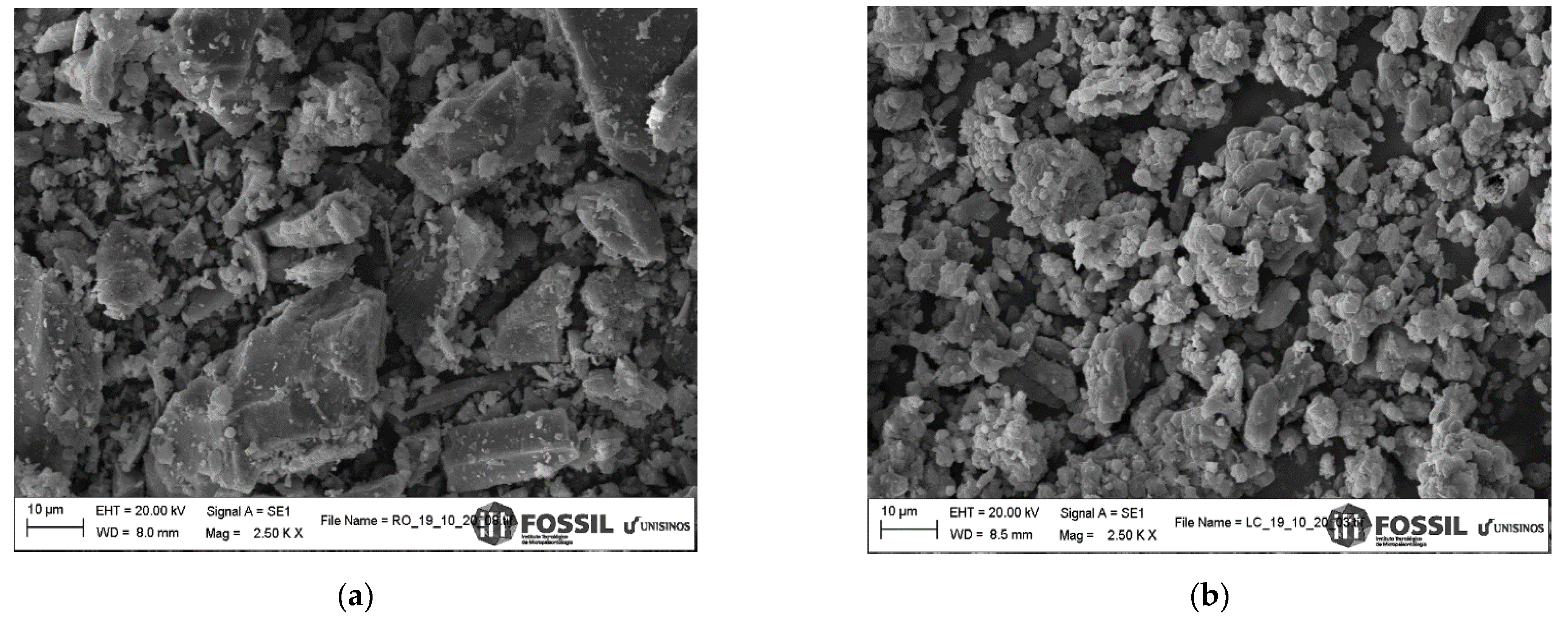
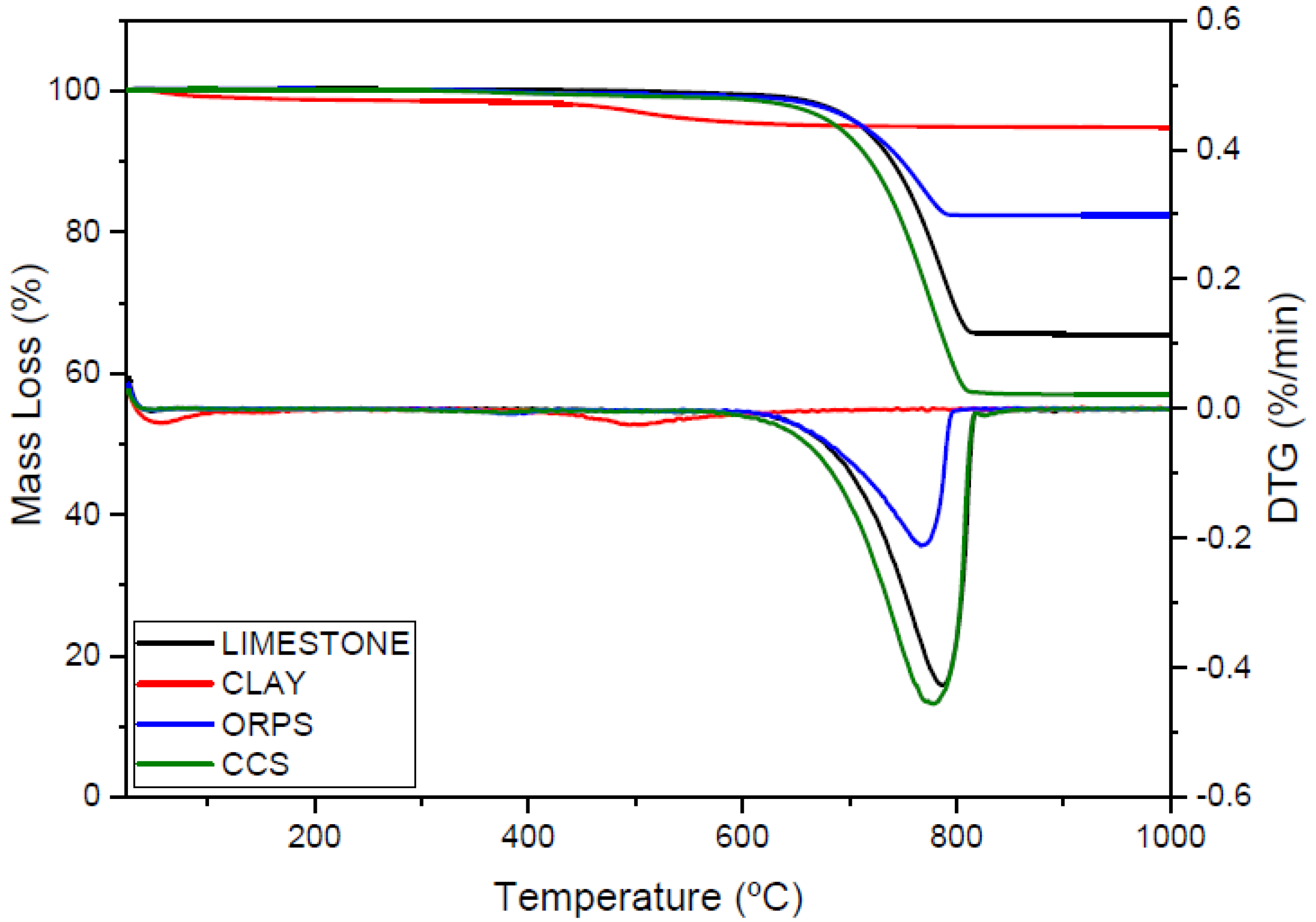
3.1. Characterization of Raw Materials
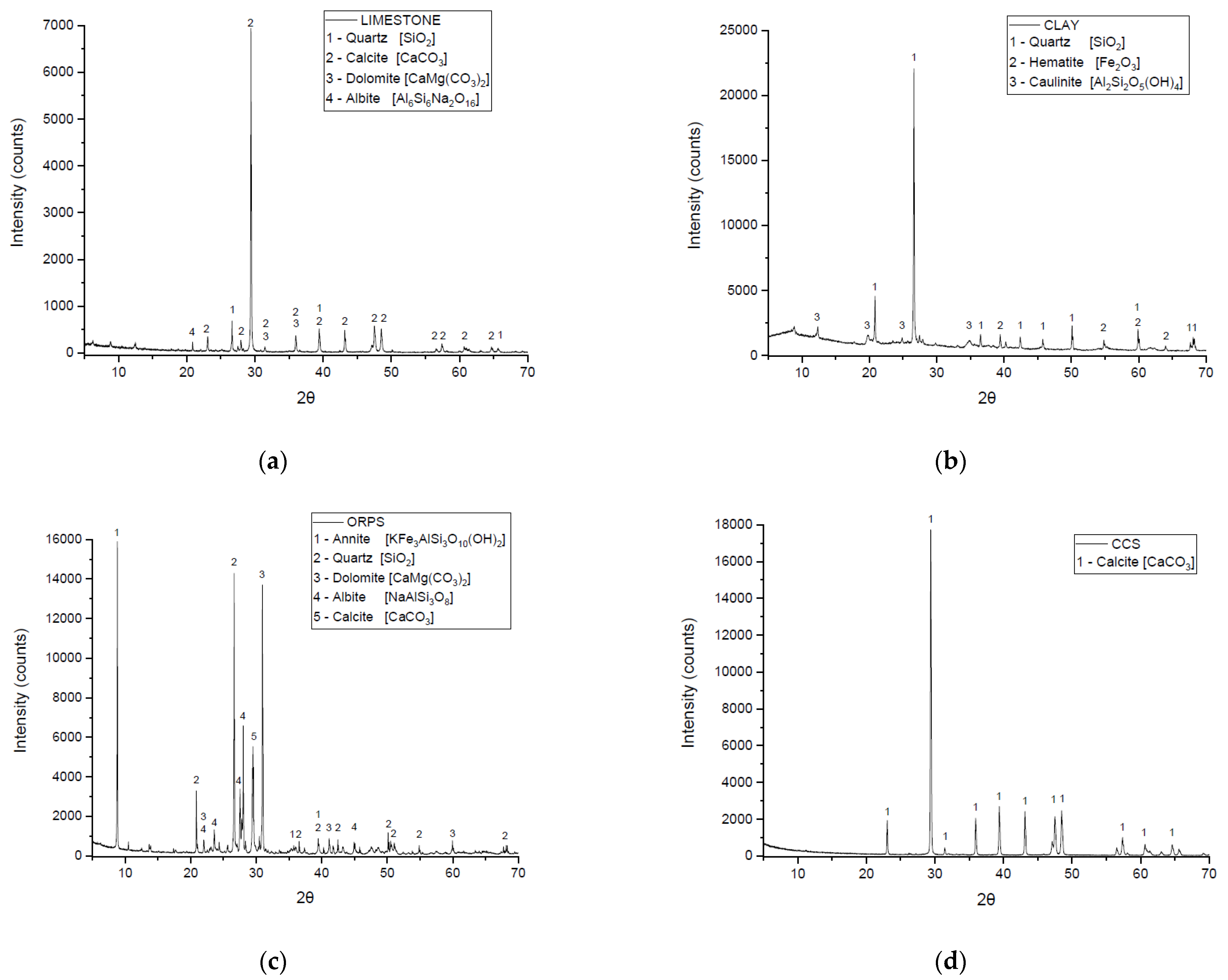
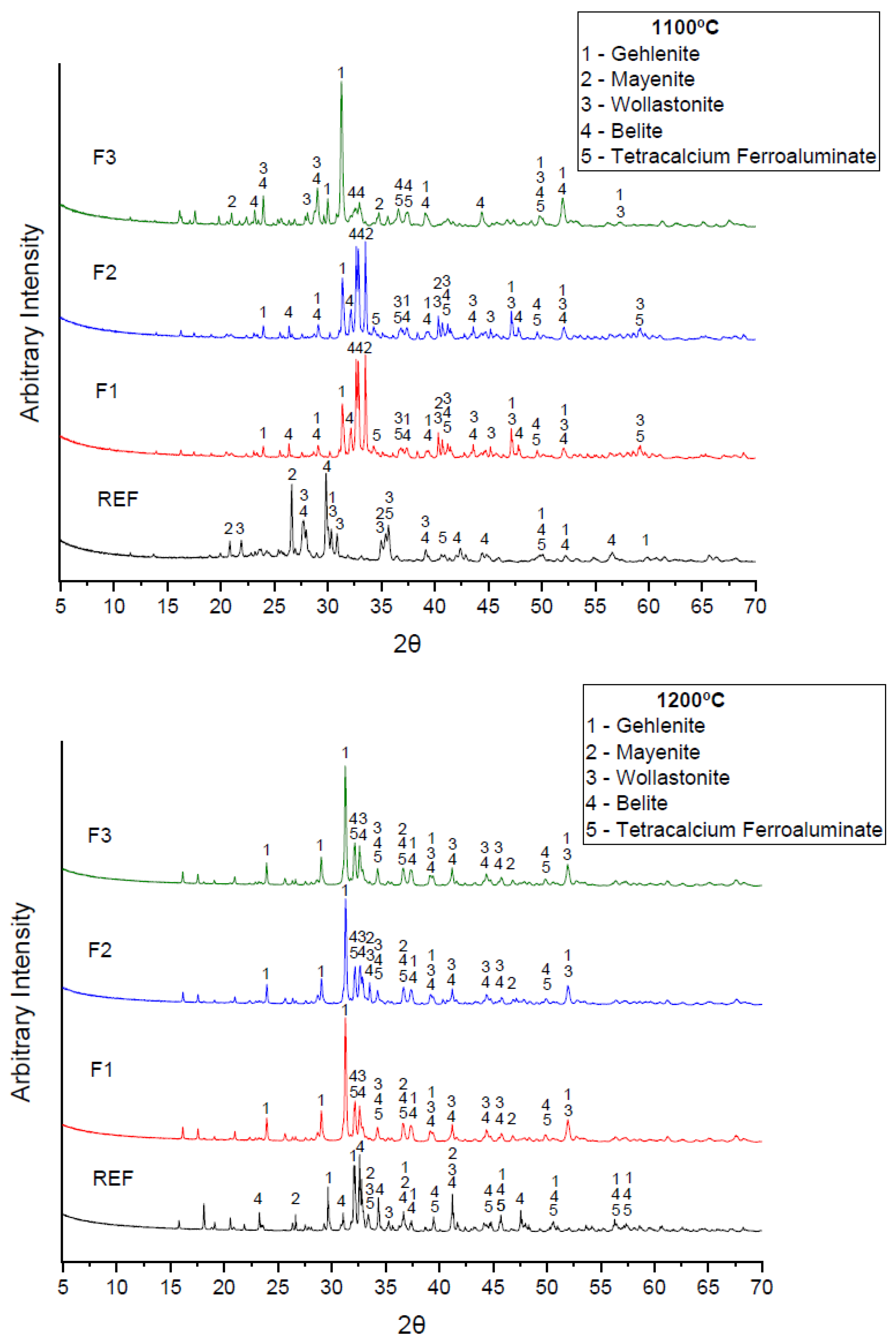
3.2. Mineral Characterization of Clinker Formulations
3.3. Clinker Particle Sizes
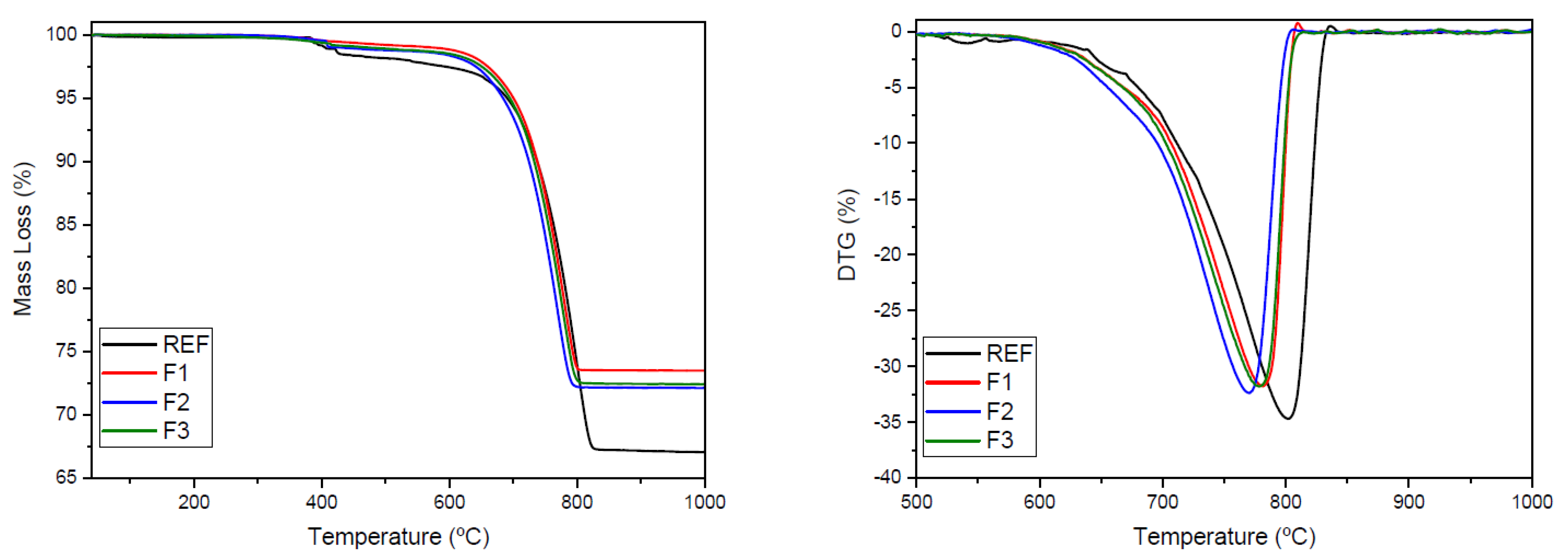
3.4. Evaluation of Carbon Dioxide Emissions during Clinker Production
4. Conclusions
- Both ORPS and CCS presented chemical and physical characteristics that favored clinkering;
- Mineral characterization identified predominantly belite in its meta-stable state (β-C2S), with prevalent crystalline peaks in formulations F1 and F2 at 1100 °C. The CaO/SiO2 ratio of the formulations tested in this study were within the pre-requisites for the production of belitic cements;
- The absence of a γ-C2S phase in the diffractograms attested to the efficiency of rapid clinker cooling, which prevented undesirable polymorph formation;
- Formulations REF and F3 formed gehlenite in both sintering temperatures, which was undesirable due to the fact of its lack of hydraulic properties;
- Clinker granulometry results were similar for all formulations and was related to the mineral characteristics;
- Formulations F1 and F2 presented lower CO2 emissions per ton of clinker produced. Formulations with 95–100% replacement by solid industrial waste could be used to produce belitic clinkers;
- Partial reduction or total substitution of limestone and clay could bring environmental and economic benefits to both cement and waste-generating industries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hache, E.; Simoën, M.; Seck, G.S.; Bonnet, C.; Jabberi, A.; Carcanague, S. The impact of future power generation on cement demand: An international and regional assessment based on climate scenarios. Int. Econ. 2020, 163, 114–133. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Liston, VA, USA, 2021.
- U.S. Geological Survey Production: Portland and masonry cement. Mineral Commodity Summaries; U.S. Geological Survey: Liston, VA, USA, 2017.
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Mikulčić, H.; Klemeš, J.J.; Vujanović, M.; Urbaniec, K.; Duić, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Liu, Y. Durability of High-strength Concrete Made with High Belite Cement. DEStech Trans. Eng. Technol. Res. 2017, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Harrison, T.; Jones, M.R.; Lawrence, D. The Production of Low Energy Cements, 5th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081007730. [Google Scholar]
- Sui, T.; Fan, L.; Wen, Z.; Wang, J.; Zhang, Z. Study on the properties of high strength concrete using high bellte cement. J. Adv. Concr. Technol. 2004, 2, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Preparation of clinker from paper pulp industry wastes. J. Hazard. Mater. 2015, 286, 252–260. [Google Scholar] [CrossRef]
- Ávalos-Rendón, T.L.; Chelala, E.A.P.; Mendoza Escobedo, C.J.; Figueroa, I.A.; Lara, V.H.; Palacios-Romero, L.M. Synthesis of belite cements at low temperature from silica fume and natural commercial zeolite. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 79–85. [Google Scholar] [CrossRef]
- Vashistha, P.; Singh, S.K.; Dutt, D.; Kumar, V. Sustainable utilization of paper mill solid wastes via synthesis of nano silica for production of belite based clinker. J. Clean. Prod. 2019, 224, 557–565. [Google Scholar] [CrossRef]
- Enríquez, M.K.; Tobón, J.I.; Ramírez, J.H. Use of industrial wastes for the synthesis of belite clinker. Mater. Constr. 2020, 70, e226. [Google Scholar] [CrossRef]
- Jang, H.; Lim, Y.; Kang, J.; So, S.; So, H. Influence of calcination and cooling conditions on pozzolanic reactivity of paper mill sludge. Constr. Build. Mater. 2018, 166, 257–270. [Google Scholar] [CrossRef]
- Sadek, D.M.; El-Attar, M.M.; Ali, H.A. Reusing of marble and granite powders in self-compacting concrete for sustainable development. J. Clean. Prod. 2016, 121, 19–32. [Google Scholar] [CrossRef]
- Shooshtarian, S.; Maqsood, T.; Caldera, S.; Ryley, T. Transformation towards a circular economy in the Australian construction and demolition waste management system. Sustain. Prod. Consum. 2022, 30, 89–106. [Google Scholar] [CrossRef]
- Ornamentais, R. Informe. January 2021, 55. Available online: https://abirochas.com.br/wp-content/uploads/2021/07/Informe-04_2021-Exportac%CC%A7o%CC%83es-1_semestre.pdf (accessed on 4 February 2022).
- Alyamaç, K.E.; Tuğrul, E. A durable, eco-friendly and aesthetic concrete work: Marble concrete. In Proceedings of the 11th International Congress on Advances in Civil Engineering (ACE 2014), Istanbul, Turkey, 21–25 October 2014; Volume 50, pp. 21–25. [Google Scholar]
- Mashaly, A.O.; El-Kaliouby, B.A.; Shalaby, B.N.; El-Gohary, A.M.; Rashwan, M.A. Effects of marble sludge incorporation on the properties of cement composites and concrete paving blocks. J. Clean. Prod. 2016, 112, 731–741. [Google Scholar] [CrossRef]
- Rana, A.; Kalla, P.; Verma, H.K.; Mohnot, J.K. Recycling of dimensional stone waste in concrete: A review. J. Clean. Prod. 2016, 135, 312–331. [Google Scholar] [CrossRef]
- Karaca, Z.; Pekin, A.; Deliormanlı, A.H. Classification of dimension stone wastes. Environ. Sci. Pollut. Res. 2012, 19, 2354–2362. [Google Scholar] [CrossRef]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Greenhouse gas emission by wastewater treatment plants of the pulp and paper industry—Modeling and simulation. Int. J. Greenh. Gas Control 2013, 17, 462–472. [Google Scholar] [CrossRef]
- Demirel, G.B.; Altın, A. Production of sorbent from paper industry solid waste for oil spill cleanup. Mar. Pollut. Bull. 2017, 125, 341–349. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Haq, I.; Raj, A. Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Bogue, R.H. Calculation of the compounds in Portland cement. Ind. Eng. Chem. Anal. Ed. 1929, 1, 192–197. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Ghouleh, Z.; Shao, Y. Production of eco-cement exclusively from municipal solid waste incineration residues. Resour. Conserv. Recycl. 2019, 149, 332–342. [Google Scholar] [CrossRef]
- Stark, J.; Mueller, A.; Schraeder, R.; Ruemmpler, K. Existence conditions of hydraulically active belite cement. Zem. Kalk Gips 1981, 34, 476–481. [Google Scholar]
- Bouzidi, M.A.; Tahakourt, A.; Bouzidi, N.; Merabet, D. Synthesis and Characterization of Belite Cement with High Hydraulic Reactivity and Low Environmental Impact. Arab. J. Sci. Eng. 2014, 39, 8659–8668. [Google Scholar] [CrossRef]
- Cynthia, C.; Agus, P.; Indra, P. Influence of calcium/silica ratio on the formation belite cement clinker from geothermal sludges. Mater. Sci. Forum 2019, 948, 249–253. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Academic Press: London, UK, 1997; Volume 20, p. 335. [Google Scholar]
- Costa, F.N.; Ribeiro, D.V. Reduction in CO2 emissions during production of cement, with partial replacement of traditional raw materials by civil construction waste (CCW). J. Clean. Prod. 2020, 276, 123302. [Google Scholar] [CrossRef]
- Bacarji, E.; Toledo Filho, R.D.; Koenders, E.A.; Figueiredo, E.P.; Lopes, J.L. Sustainability perspective of marble and granite residues as concrete fillers. Constr. Build. Mater. 2013, 45, 1–10. [Google Scholar] [CrossRef]
- Sato, V.Y.; Galina, A.P.L.; Teixeira, J.E.S.L. Contribution to the rheological study of cementitious pastes with addition of residues from the processing of ornamental rocks. Rev. Ibracon Estrut. E Mater. 2018, 11, 1284–1307. [Google Scholar] [CrossRef]
- Awad, A.H.; Aly Abd El-Wahab, A.; El-Gamsy, R.; Abdel-latif, M.H. A study of some thermal and mechanical properties of HDPE blend with marble and granite dust. Ain Shams Eng. J. 2019, 10, 353–358. [Google Scholar] [CrossRef]
- Abrahão, I.O. Rochas calcárias e sua ocorrência. Estud. Nac. Calcário Agrícola—Estud. FINEP 1983, 1. Available online: http://www.ia.ufrrj.br/cpacs/arquivos/teses_dissert/380_(ME-2013)_Edilene_Pereira_Ferreira.pdf (accessed on 4 February 2022).
- Mesquita, C.J.S.; Dall’Agnol, R.; De Almeida, J.D.A.C. Mineral chemistry and crystallization parameters of the A-type Paleoproterozoic Bannach Granite, Carajás Province, Pará, Brazil. Braz. J. Geol. 2018, 48, 575–601. [Google Scholar] [CrossRef]
- Lima, S.C.L.; Costa, L.C.B.; Defáveri, K.C.S.; Carvalho, J.M.F.; Peixoto, R.A.F.; Brigolini, G.J. Study on assessment of pozzolanic activity: Slate cutting waste. Mater. J. 2020, 117, 3–10. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Wang, S.; Tang, M.; Shen, X. Effect of SO3 and MgO on Portland cement clinker: Formation of clinker phases and alite polymorphism. Constr. Build. Mater. 2014, 58, 182–192. [Google Scholar] [CrossRef]
- Kacimi, L.; Simon-Masseron, A.; Salem, S.; Ghomari, A.; Derriche, Z. Synthesis of belite cement clinker of high hydraulic reactivity. Cem. Concr. Res. 2009, 39, 559–565. [Google Scholar] [CrossRef]
- MINDAT Larnite. Available online: https://www.mindat.org/min-2333.html (accessed on 8 December 2021).
- Elfami, N.; Ez-zaki, H.; Diouri, A.; Sassi, O.; Boukhari, A. Improvement of hydraulic and mechanical properties of dicalcium silicate by alkaline activation. Constr. Build. Mater. 2020, 247, 118589. [Google Scholar] [CrossRef]
- Saidani, S.; Smith, A.; El Hafiane, Y.; Ben Tahar, L. Role of dopants (B, P and S) on the stabilization of β-Ca2SiO4. J. Eur. Ceram. Soc. 2021, 41, 880–891. [Google Scholar] [CrossRef]
- Maheswaran, S.; Kalaiselvam, S.; Saravana Karthikeyan, S.K.S.; Kokila, C.; Palani, G.S. β-Belite cements (β-dicalcium silicate) obtained from calcined lime sludge and silica fume. Cem. Concr. Compos. 2016, 66, 57–65. [Google Scholar] [CrossRef]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Zhou, L.; Wang, W.; Zhou, K. Effect of Al2O3 on the Crystallization of Mold Flux for Casting High Al Steel. Metall. Mater. Trans. E 2015, 2, 99–108. [Google Scholar] [CrossRef]
- Odler, I. Special Inorganic Cements; CRC Press: Boca Raton, FL, USA, 2000; Volume 30, ISBN 0419227903. [Google Scholar]
- Ben Haha, M.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res. 2019, 123, 105778. [Google Scholar] [CrossRef]
- Han, B.; Wang, P.; Ke, C.; Yan, W.; Wei, Y.; Li, N. Hydration behavior of spinel containing high alumina cement from high titania blast furnace slag. Cem. Concr. Res. 2016, 79, 257–264. [Google Scholar] [CrossRef]
- Dovál, M.; Palou, M.; Mojumdar, S.C. Hydration behavior of C2S and C2AS nanomaterials, synthetized by sol-gel method. J. Therm. Anal. Calorim. 2006, 86, 595–599. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Qin, J.; Yang, C.; Cui, C.; Huang, J.; Hussain, A.; Ma, H. Ca2+ and OH− release of ceramsites containing anorthite and gehlenite prepared from waste lime mud. J. Environ. Sci. 2016, 47, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ideker, J.H.; Scrivener, K.L.; Fryda, H.; Touzo, B. Calcium Aluminate Cements. In Lea’s Chemistry of Cement and Concrete; Scientific Publications, 2019; ISBN 9780081007730. Available online: https://www.sciencedirect.com/topics/social-sciences/scientific-publications (accessed on 4 February 2022).
- Pöllmann, H. Composition of Cement Phases. Struct. Perform. Cem. 2002, 1, 25–56. [Google Scholar]
- Franco de Carvalho, J.M.; Campos, P.A.M.; Defáveri, K.; Brigolini, G.J.; Pedroti, L.G.; Peixoto, R.A.F. Low Environmental Impact Cement Produced Entirely from Industrial and Mining Waste. J. Mater. Civ. Eng. 2019, 31, 04018391. [Google Scholar] [CrossRef]
- El-Didamony, H.; Khalil, K.A.; Ahmed, I.A.; Heikal, M. Preparation of β-dicalcium silicate (β-C2S) and calcium sulfoaluminate (C3A3) phases using non-traditional nano-materials. Constr. Build. Mater. 2012, 35, 77–83. [Google Scholar] [CrossRef]
- Qu, B.; Martín, A.; Pastor, J.Y.; Palomo, A.; Fernández Jiménez, A. Microstructural characterisation of hybrid cement after exposure to high temperatures. Constr. Build. Mater. 2020, 262, 120843. [Google Scholar] [CrossRef]
- García-Maté, M.; Santacruz, I.; Cuesta, A.; León-Reina, L.; Aranda, M.A.G.; Baco, I.; Morin, V.; Walenta, G.; Gartner, E.; De La Torre, Á.G. Amorphous determination in calcium sulfoaluminate materials by external and internal methods. Adv. Cem. Res. 2015, 27, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Kotsay, G.N.; Jaskulski, R. Belite cement as an ecological alternative to Portland cement—A review. Mater. Struct. Technol. J. 2019, 2, 70–76. [Google Scholar] [CrossRef]
- Koumpouri, D.; Angelopoulos, G.N. Effect of boron waste and boric acid addition on the production of low energy belite cement. Cem. Concr. Compos. 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Zanelato, E.B.; Alexandre, J.; de Azevedo, A.R.G.; Marvila, M.T. Evaluation of roughcast on the adhesion mechanisms of mortars on ceramic substrates. Mater. and Struct 2019, 52, 89–109. [Google Scholar] [CrossRef]
| Source Materials, Chemical Moduli, and Theoretical Phase Content | Formulations and Respective Mix Ratios (w.t.%) | |||
|---|---|---|---|---|
| REF | F1 | F2 | F3 | |
| Limestone | 90.0 | 0.00 | 5.00 | 10.0 |
| Clay | 10.0 | 0.00 | 0.00 | 0.00 |
| ORPS | 0.00 | 52.5 | 50.0 | 47.5 |
| CCS | 0.00 | 47.5 | 45.0 | 42.5 |
| LSF | 77.44 | 77.64 | 77.92 | 79.50 |
| SM | 2.46 | 3.00 | 2.97 | 2.94 |
| AM | 2.49 | 2.66 | 2.63 | 2.60 |
| C3S | 11.44 | 15.68 | 18.55 | 21.42 |
| C2S | 64.80 | 64.01 | 61.15 | 58.30 |
| C3A | 14.67 | 12.91 | 12.82 | 12.74 |
| C4AF | 9.08 | 7.33 | 7.39 | 7.46 |
| Cycle A | Cycle B | Cycle C | Cycle D | Cycle E |
|---|---|---|---|---|
| 900 °C HR = 5 °C/min | 900 °C HT = 30 min | 1100 °C HR = 10 °C/min | 1100 °C HT = 60 min | Forced convection air cooling |
| 1200 °C HR = 10 °C/min | 1200 °C HT = 60 min |
| Chemical Characterization (%) | Limestone | Clay | ORPS | CCS | |
|---|---|---|---|---|---|
| SiO2 | 12.59 | 64.40 | 36.89 | ND | |
| Al2O3 | 3.57 | 19.86 | 8.51 | 0.36 | |
| Fe2O3 | 1.69 | 4.62 | 3.31 | 0.04 | |
| CaO | 43.84 | ND | 24.50 | 55.49 | |
| MgO | 1.08 | 1.24 | 5.13 | 0.71 | |
| SO3 | ND | ND | 0.01 | 0.05 | |
| Na2O | 0.28 | 0.20 | 1.62 | 0.56 | |
| K2O | 0.63 | 4.36 | 3.16 | 0.01 | |
| SrO | 0.12 | 0.03 | 0.06 | 0.25 | |
| MnO | 0.06 | 0.05 | 0.05 | 0.01 | |
| P2O5 | 0.12 | 0.46 | 0.09 | ND | |
| TiO2 | 0.24 | 0.65 | 0.81 | 0.01 | |
| Loss of Ignition (LOI) | 35.78 | 4.13 | 15.86 | 42.51 | |
| Specific Surface Area BET (cm2/g) | 33,491 | 26,687 | 26,819 | 12,566 | |
| Specific Mass (g/cm3) | 2.65 | 2.60 | 2.66 | 2.59 | |
| Granulometric Analysis | D10 (μm) | 8.96 | 1.81 | 2.42 | 8.41 |
| D50 (μm) | 27.82 | 3.58 | 6.41 | 20.47 | |
| D90 (μm) | 69.84 | 7.78 | 19.66 | 43.96 | |
| DM (μm) | 32.22 | 4.06 | 8.40 | 22.70 | |
| Formulations | Granulometric Analysis | Specific Surface Area BET (cm2/g) | ||||
|---|---|---|---|---|---|---|
| D10 (μm) | D50 (μm) | D90 (μm) | Dm (μm) | |||
| 1100 °C | REF | 1.83 | 4.59 | 10.51 | 5.27 | 4.418 |
| F1 | 1.34 | 3.83 | 9.47 | 4.51 | 4.052 | |
| F2 | 1.51 | 3.98 | 9.12 | 4.56 | 4.076 | |
| F3 | 1.25 | 2.89 | 6.44 | 3.30 | 4.787 | |
| 1200 °C | REF | 1.27 | 2.91 | 7.00 | 3.42 | 4.517 |
| F1 | 1.60 | 3.60 | 8.47 | 4.22 | 4.629 | |
| F2 | 1.59 | 3.17 | 6.59 | 3.57 | 4.821 | |
| F3 | 1.63 | 3.32 | 6.96 | 3.74 | 4.913 | |
| Formulation | Mass Loss from Decarbonation (%) | kg of CO2/ton of Clinker | Residual Mass at 1000 °C (%) | CO2 Emission (kg)/ton of Raw Mixture (t) | CO2 Emission (kg)/ton of Clinker (t) | Reduction in CO2/ton of Raw Mixture (%) | Reduction in CO2/ton of Raw Mixture (%) |
|---|---|---|---|---|---|---|---|
| REF | 30.68 | 1000 | 67.08 | 306.80 | 457.36 | - | - |
| F1 | 25.58 | 73.51 | 255.80 | 347.98 | 16.62 | 23.92 | |
| F2 | 26.33 | 72.15 | 263.30 | 364.93 | 14.18 | 20.21 | |
| F3 | 26.56 | 72.44 | 265.60 | 366.65 | 13.43 | 19.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, F.R.C.; Modolo, R.C.E.; Kulakowski, M.P.; Brehm, F.A.; Moraes, C.A.M.; Ferreira, V.M.; Mesquita, E.F.T.; de Azevedo, A.R.G.; Monteiro, S.N. Production of Belite Based Clinker from Ornamental Stone Processing Sludge and Calcium Carbonate Sludge with Lower CO2 Emissions. Materials 2022, 15, 2352. https://doi.org/10.3390/ma15072352
Ribeiro FRC, Modolo RCE, Kulakowski MP, Brehm FA, Moraes CAM, Ferreira VM, Mesquita EFT, de Azevedo ARG, Monteiro SN. Production of Belite Based Clinker from Ornamental Stone Processing Sludge and Calcium Carbonate Sludge with Lower CO2 Emissions. Materials. 2022; 15(7):2352. https://doi.org/10.3390/ma15072352
Chicago/Turabian StyleRibeiro, Francisco Roger Carneiro, Regina Célia Espinosa Modolo, Marlova Piva Kulakowski, Feliciane Andrade Brehm, Carlos Alberto Mendes Moraes, Victor Miguel Ferreira, Esequiel Fernandes Teixeira Mesquita, Afonso Rangel Garcez de Azevedo, and Sergio Neves Monteiro. 2022. "Production of Belite Based Clinker from Ornamental Stone Processing Sludge and Calcium Carbonate Sludge with Lower CO2 Emissions" Materials 15, no. 7: 2352. https://doi.org/10.3390/ma15072352
APA StyleRibeiro, F. R. C., Modolo, R. C. E., Kulakowski, M. P., Brehm, F. A., Moraes, C. A. M., Ferreira, V. M., Mesquita, E. F. T., de Azevedo, A. R. G., & Monteiro, S. N. (2022). Production of Belite Based Clinker from Ornamental Stone Processing Sludge and Calcium Carbonate Sludge with Lower CO2 Emissions. Materials, 15(7), 2352. https://doi.org/10.3390/ma15072352









