Recent Advances in Copper-Doped Titanium Implants
Abstract
1. Introduction
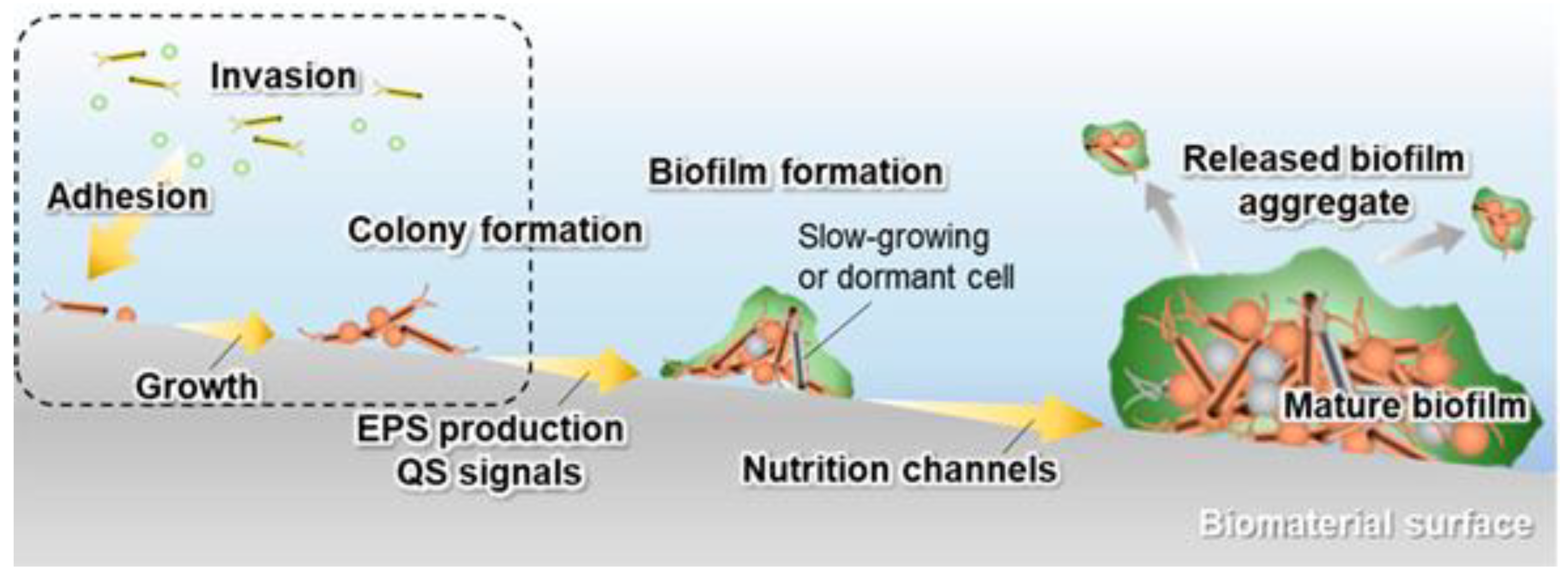
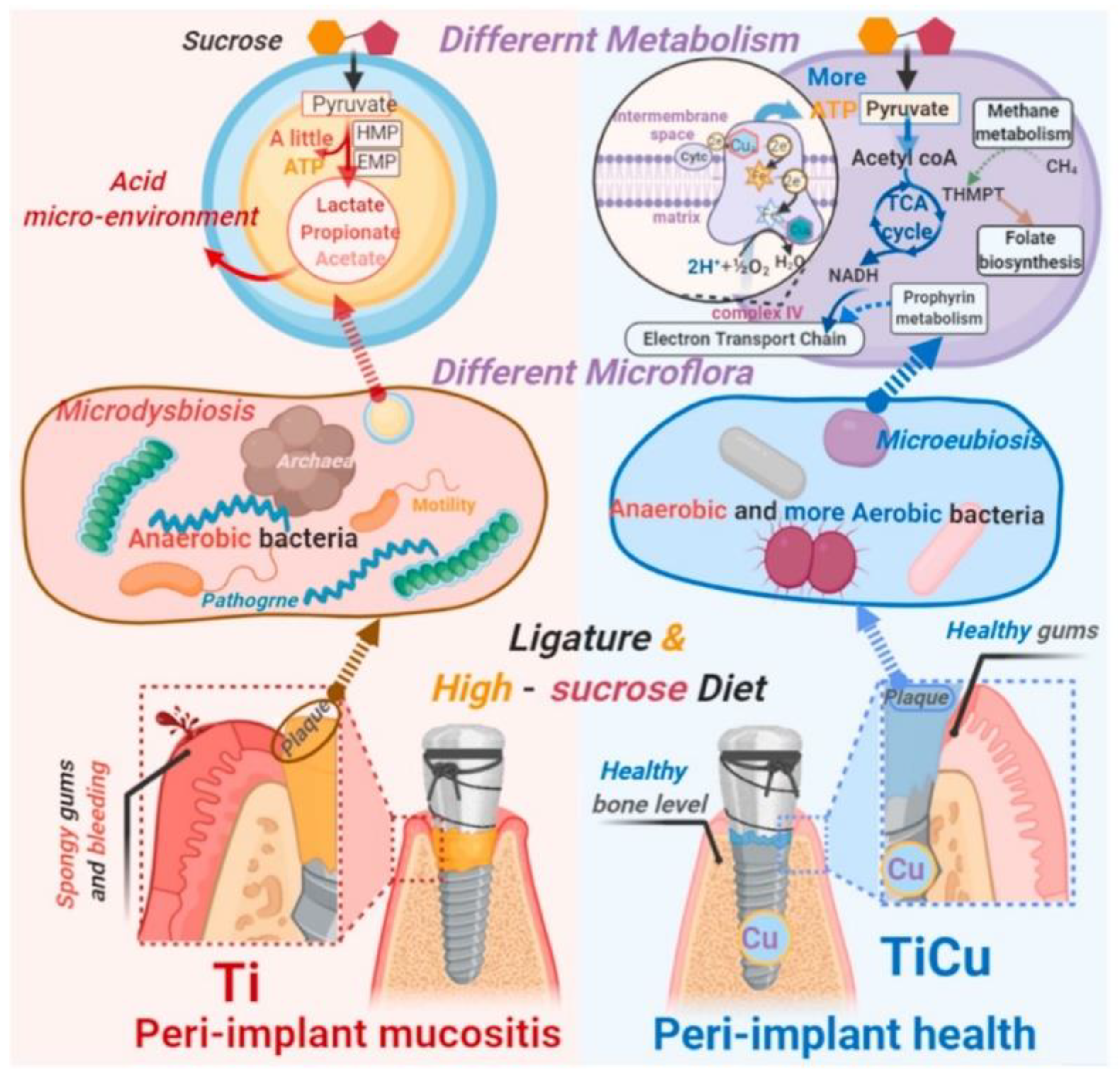
2. Implant–Bacteria Interactions
- The adhesion of bacteria to the surface of the material. This stage is heavily influenced by many variables, including the type of pathogen, the nature of the physiological fluid and the physicochemical properties of the material surface. Particularly, the roughness and surface topography have a strong influence at this stage [31]. The process of adhesion is reversible.
- The bacterial colonization of the implant surface, which is mediated by specific molecular and cellular interactions [18]. In addition, bacteria aggregate and undergo irreversible attachment, which completely changes the chemical properties of the implant surface through their metabolites.
- The biofilm maturation, microcolony formation and entrapment of planktonic bacteria in the extracellular polymeric substances (EPS). When bacteria form colonies on the surface, they produce exopolymer substances (mainly polysaccharides and other macromolecules), which contribute to biofilm formation. The biofilm can protect the bacteria from both fluid shear stress and the action of systemic pharmacological therapies [18].
- The proliferation of bacteria under biofilm protection until the entire surface of the material is covered.
3. Preparation Techniques and Related Properties of Cu-Doped Ti Implants
3.1. Ion Implantation
3.2. Alloy
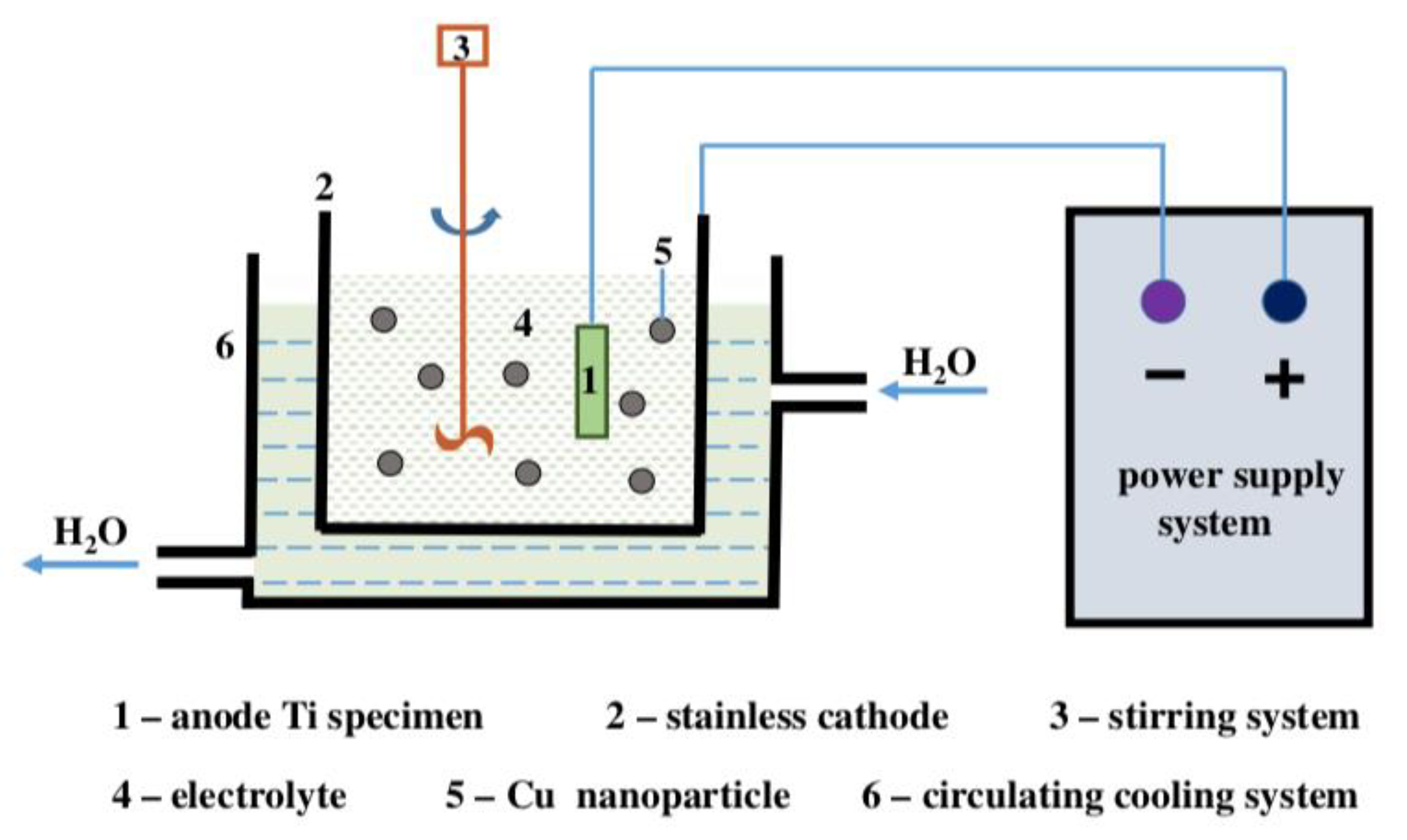
3.3. Electrochemical Techniques
3.4. Sputtering
3.5. Sol-Gel
4. Conclusions and Perspectives
- Combination of different surface modification methods. The above-summarized surface modification methods have pros and cons.
- (1)
- The process of ion implantation is more complex and thus difficult to operate but results in little damage to the material surface and does not change the original size and roughness of the implants. Therefore, it is very suitable for the processing of precision substrates. Due to its high strength and corrosion resistance, titanium alloy has been widely used in clinical applications. However, its wear resistance is poor, and the corrosion products of particles entering human tissue after wear may lead to implant failure. The ion implantation technology can effectively strengthen its surface wear resistance.
- (2)
- The MAO technology has the advantages of simple and fast processing process; however, its high energy consumption leads to high commercialization costs.
- (3)
- The magnetron sputtering coatings have a superior bond with the substrate, the coating thickness can be tuned by adjusting the process parameters, and the co-sputtering of different metals can be realized, which is suitable for industrialization. However, it faces problems, including low target utilization and difficulty in sputtering magnetic targets.
- (4)
- Although sol-gel methods easily achieve doping at the molecular level, they are expensive in principle and time consuming, which will increase the cost of commercialization. Overall, there is a trend to use different surface modification methods simultaneously to achieve better antibacterial effects and to promote osseointegration—for example, Ti-Cu alloys with combined sandblasting and acid etching technology and Ti-Cu alloys with combined anodic oxidation technology, magnetron sputtering and ion implantation technology.
In addition to the above-mentioned modification strategies, there are also hydrothermal methods, ion exchange methods and chemical vapor deposition that can effectively dope copper onto the titanium surface. Based on this, the development of a commercial titanium-based surface with excellent antibacterial effects that enhances tissue integration will be possible. Future research in the field of biomaterials should be directed toward combining multiple surface modification processes to provide long-term antimicrobial effects and to promote tissue integration. - Bactericidal ability and potential toxicity of Cu. Cu has been proven to be an effective antibacterial agent, and Cu-incorporated coatings show excellent antibacterial activity against S. aureus [15,36], E. coli [60,64], S. mutans [39] and P. gingivalis [68,69]. When the concentration of Cu2+ was 5 × 10−5 M, the bactericidal rate of Staphylococcus aureus was 92%, whereas when the concentration of Cu2+ was 5 × 10−6 M, the bactericidal rate of E. coli was 93% [132,133].Miyano et al. [134] evaluated the antibacterial activity of some pure metals using plate counting. The results of plate counting after 24 h incubation showed that the antibacterial effects from high to low were: Pb > Cu > Co > Zn > Ni > Zr > Mo. Compared with other metals, copper has better antibacterial effect and biocompatibility. Numerous in vitro tests have demonstrated the low ion release of Cu-doped Ti implants prepared by various methods and their excellent cytocompatibility without cytotoxicity to MG63 [15,37], MC3T3-E1 [56,71], L929 [33,88] and other cells.It was reported that the median toxic concentration of Cu ions on human gingival fibroblasts was 21.86 mg L−1 [135], and when the Cu ion concentration was higher than 9 mg/L, it was cytotoxic to MC3T3 cells [136]. Although the high concentration of Cu is thought to be toxic to mammalian cells, the concentration of Cu ions released from the surface of the Cu coating is low enough that the cytotoxicity is negligible.The effective concentration of antibacterial activity was much lower than that of cytotoxicity. However, the studied surface Cu ion release has certain problems, such as ion burst release (usually reaches a maximum within one day and then decreases rapidly in the next 10–30 days), and the antimicrobial effect increases with Cu ion concentration; however, there is potential cytotoxicity to cells when the Cu ion concentration is too high, which may cause long-term toxicity or side effects in humans.
- The antibacterial mechanism of Cu ions has yet to be studied. It is widely believed that metal ions can kill bacteria by inducing the production of reactive oxygen species (ROS) [137]. ROS are the oxygen reduction products, such as peroxides, superoxides, hydroxyl radicals and singlet state oxygen. However, many studies have shown that Cu-doped Ti-based implants still have antimicrobial effects when the concentration of Cu ions released is very low. For example, the release of Cu ions from the antimicrobial Ti6Al4V-5Cu alloy was 2.498 ± 0.755 μg/L after 20 days of immersion in 0.9% NaCl solution [138].This is due to the ability of Cu-containing particles to resist bacterial adhesion [138,139] and biofilm formation [35], which would kill bacteria on the surface. However, the exact antimicrobial mechanism in the contact sterilization mode is not known. Moreover, electron transfer in bacterial activity is another widely accepted antibacterial mechanism [140,141,142]. Although many antimicrobial mechanisms have been studied in detail, the antimicrobial mechanism of Cu-doped Ti-based implants is still not fully understood.
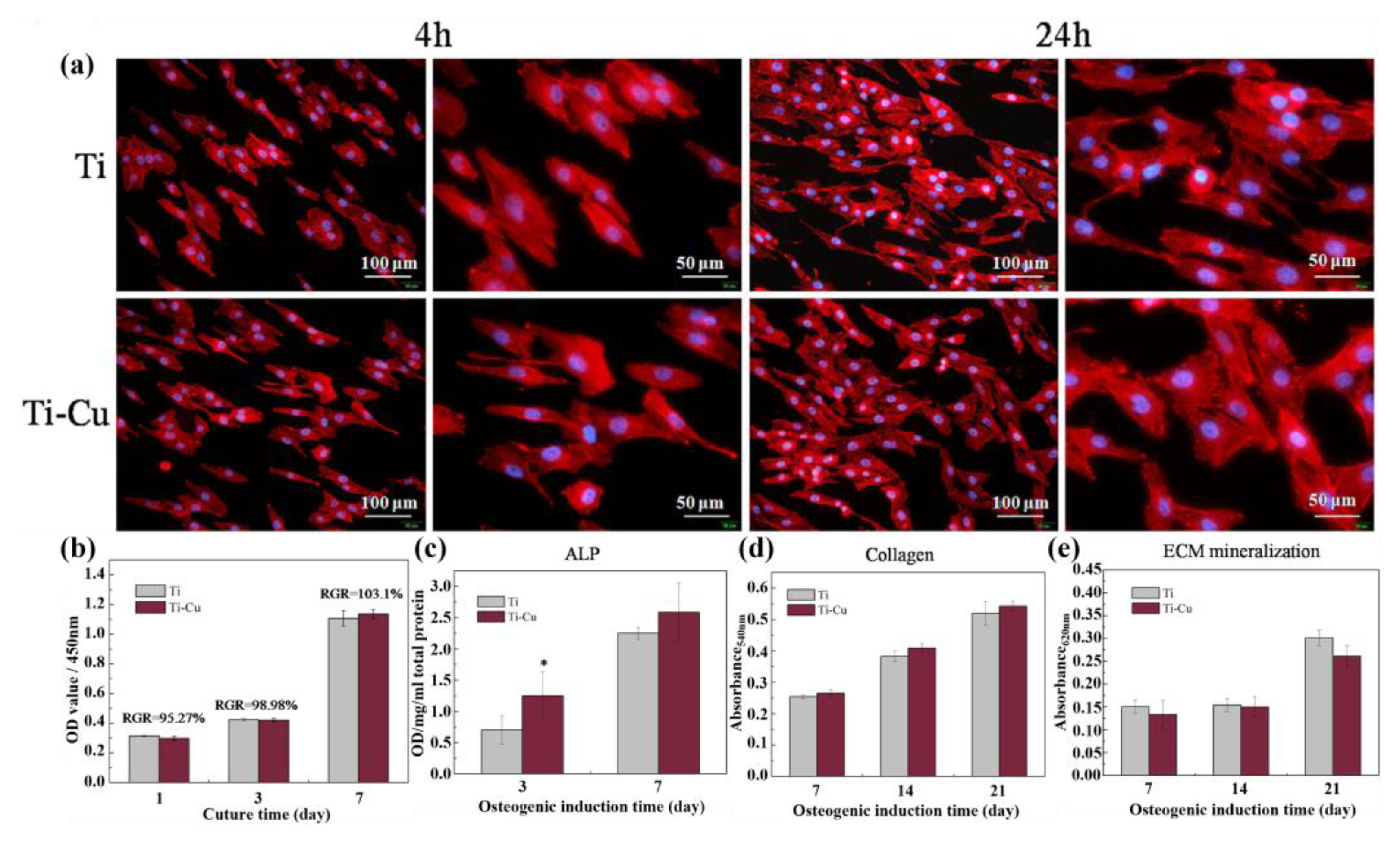
- Surface biological activity. The current research on Cu has focused on its antimicrobial effects, and there is a lack of research on whether the elemental Cu can enhance cellular and tissue activity. Although it has been shown that Ti-Cu alloys can promote the osteogenic differentiation of MG-63 cells by increasing the expression of osteogenic-related genes, such as ALP, Collagen I, OPN and OCN [67], few reports exist on the effects of cellular and tissue inertness of Cu-doped Ti implants prepared by other preparation methods on cells and tissues. It is also unclear whether a decrease in antimicrobial effects accompanies the increase in surface bioactivity. Thus, the effect of loading Cu on the implant surface in different ways on the cellular/tissue response and antimicrobial activity remains to be investigated.
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Wu, L.; Chen, X.; Sun, D.; Chen, Y.; Zhang, G.; Ding, X.; Pan, F. Enhanced protective coatings on Ti-10V-2Fe-3Al alloy through anodizing and post-sealing with layered double hydroxides. J. Mater. Sci. Technol. 2020, 37, 104–113. [Google Scholar]
- Cordeiro, J.; Nagay, B.; Dini, C.; Souza, J.; Rangel, E.; da Cruz, N. Copper source determines chemistry and topography of implant coatings to optimally couple cellular responses and antibacterial activity. Mater. Sci. Eng. C 2021, 112550. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar]
- Zheng, Y.; Li, J.; Liu, X.; Sun, J. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int. J. Nanomed. 2012, 7, 875. [Google Scholar]
- Dorkhan, M.; de Paz, L.E.C.; Skepö, M.; Svensäter, G.; Davies, J.R. Effects of saliva or serum coating on adherence of Streptococcus oralis strains to titanium. Microbiology 2012, 158, 390–397. [Google Scholar]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Norowski, P.A., Jr.; Bumgardner, J.D. Biomaterial and antibiotic strategies for peri-implantitis: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 530–543. [Google Scholar]
- Li, M.; Ma, Z.; Zhu, Y.; Xia, H.; Yao, M.; Chu, X.; Wang, X.; Yang, K.; Yang, M.; Zhang, Y. Toward a molecular understanding of the antibacterial mechanism of copper-bearing titanium alloys against Staphylococcus aureus. Adv. Healthc. Mater. 2016, 5, 557–566. [Google Scholar] [CrossRef]
- Helfet, D.L.; Haas, N.P.; Schatzker, J.; Matter, P.; Moser, R.; Hanson, B. AO philosophy and principles of fracture management-its evolution and evaluation. JBJS 2003, 85, 1156–1160. [Google Scholar]
- Laffer, R.R.; Graber, P.; Ochsner, P.E.; Zimmerli, W. Outcome of prosthetic knee-associated infection: Evaluation of 40 consecutive episodes at a single centre. Clin. Microbiol. Infect. 2006, 12, 433–439. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Chen, W.; Liu, S.; Zhang, Q.; Zhang, Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. J. Hosp. Infect. 2015, 89, 82–89. [Google Scholar] [CrossRef]
- Kessler, B.; Sendi, P.; Graber, P.; Knupp, M.; Zwicky, L.; Hintermann, B.; Zimmerli, W. Risk Factors for Periprosthetic Ankle Joint Infection: A Case-Control Study. J. Bone Jt. Surg.-Am. Vol. 2012, 94A, 1871–1876. [Google Scholar] [CrossRef]
- Goudouri, O.-M.; Kontonasaki, E.; Lohbauer, U.; Boccaccini, A.R. Antibacterial properties of metal and metalloid ions in chronic periodontitis and peri-implantitis therapy. Acta Biomater. 2014, 10, 3795–3810. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium–copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar]
- Hong, I.; Koo, C.H. Antibacterial properties, corrosion resistance and mechanical properties of Cu-modified SUS 304 stainless steel. Mater. Sci. Eng. A 2005, 393, 213–222. [Google Scholar] [CrossRef]
- Burghardt, I.; Luthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.G.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 61, 965–978. [Google Scholar]
- Xu, N.; Fu, J.J.; Zhao, L.Z.; Chu, P.K.; Huo, K.F. Biofunctional Elements Incorporated Nano/Microstructured Coatings on Titanium Implants with Enhanced Osteogenic and Antibacterial Performance. Adv. Healthc. Mater. 2020, 9, 2000681. [Google Scholar]
- Zhang, E.L.; Zhao, X.T.; Hu, J.L.; Wang, R.X.; Fu, S.; Qin, G.W. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar]
- Tesmer, M.; Wallet, S.; Koutouzis, T.; Lundgren, T. Bacterial colonization of the dental implant fixture–abutment interface: An in vitro study. J. Periodontol. 2009, 80, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Glauser, R.; Schüpbach, P.; Gottlow, J.; Hämmerle, C.H.; Research, R. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: A light-microscopic overview and histometric analysis. Clin. Implant Dent. Relat. Res. 2005, 7, s44–s51. [Google Scholar] [CrossRef] [PubMed]
- Dibart, S.; Warbington, M.; Su, M.F.; Skobe, Z.; Implants, M. In vitro evaluation of the implant-abutment bacterial seal: The locking taper system. Int. J. Oral Maxillofac. Implant. 2005, 20, 732–737. [Google Scholar]
- Costerton, J.W.; Cheng, K.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [PubMed]
- Lindsay, D.; Von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Liu, Y.J.C.; Biointerfaces, S.B. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B-Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.Q.; Huang, X.Y.; Zhang, Y.N.; Han, Y. The dual function of Cu-doped TiO2 coatings on titanium for application in percutaneous implants. J. Mater. Chem. B 2016, 4, 3788–3800. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar]
- Wang, X.Y.; Dong, H.; Liu, J.; Qin, G.W.; Chen, D.F.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 38–47. [Google Scholar]
- Zhang, E.L.; Zheng, L.L.; Liu, J.; Bai, B.; Liu, C. Influence of Cu content on the cell biocompatibility of Ti-Cu sintered alloys. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 46, 148–157. [Google Scholar]
- Liu, H.; Tang, Y.; Zhang, S.; Liu, H.; Wang, Z.; Li, Y.; Wang, X.; Ren, L.; Yang, K.; Qin, L. Anti-infection mechanism of a novel dental implant made of titanium-copper (TiCu) alloy and its mechanism associated with oral microbiology. Bioact. Mater. 2021, 8, 381–395. [Google Scholar]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar]
- Zhuang, Y.; Ren, L.; Zhang, S.; Wei, X.; Yang, K.; Dai, K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021, 119, 472–484. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Bilinski, A.; Lewandowska-Szumiel, M.; Rajchel, B. Effect of calcium-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 2001, 22, 2139–2151. [Google Scholar] [CrossRef]
- Shiau, D.K.; Yang, C.H.; Sun, Y.S.; Wu, M.F.; Pan, H.B.; Huang, H.H. Enhancing the blood response and antibacterial adhesion of titanium surface through oxygen plasma immersion ion implantation treatment. Surf. Coat. Technol. 2019, 365, 173–178. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Raman, S.; He, F.; Huang, Y. Surface modification of medical metals by ion implantation of silver and copper. Vacuum 2007, 81, 1114–1118. [Google Scholar] [CrossRef]
- Ji, X.M.; Zhao, M.L.; Dong, L.; Han, X.; Li, D.J. Influence of Ag/Ca ratio on the osteoblast growth and antibacterial activity of TiN coatings on Ti-6Al-4V by Ag and Ca ion implantation. Surf. Coat. Technol. 2020, 403, 9. [Google Scholar] [CrossRef]
- Han, X.; Ji, X.M.; Zhao, M.L.; Li, D.J. Mg/Ag ratios induced in vitro cell adhesion and preliminary antibacterial properties of TiN on medical Ti-6Al-4V alloy by Mg and Ag implantation. Surf. Coat. Technol. 2020, 397, 11. [Google Scholar] [CrossRef]
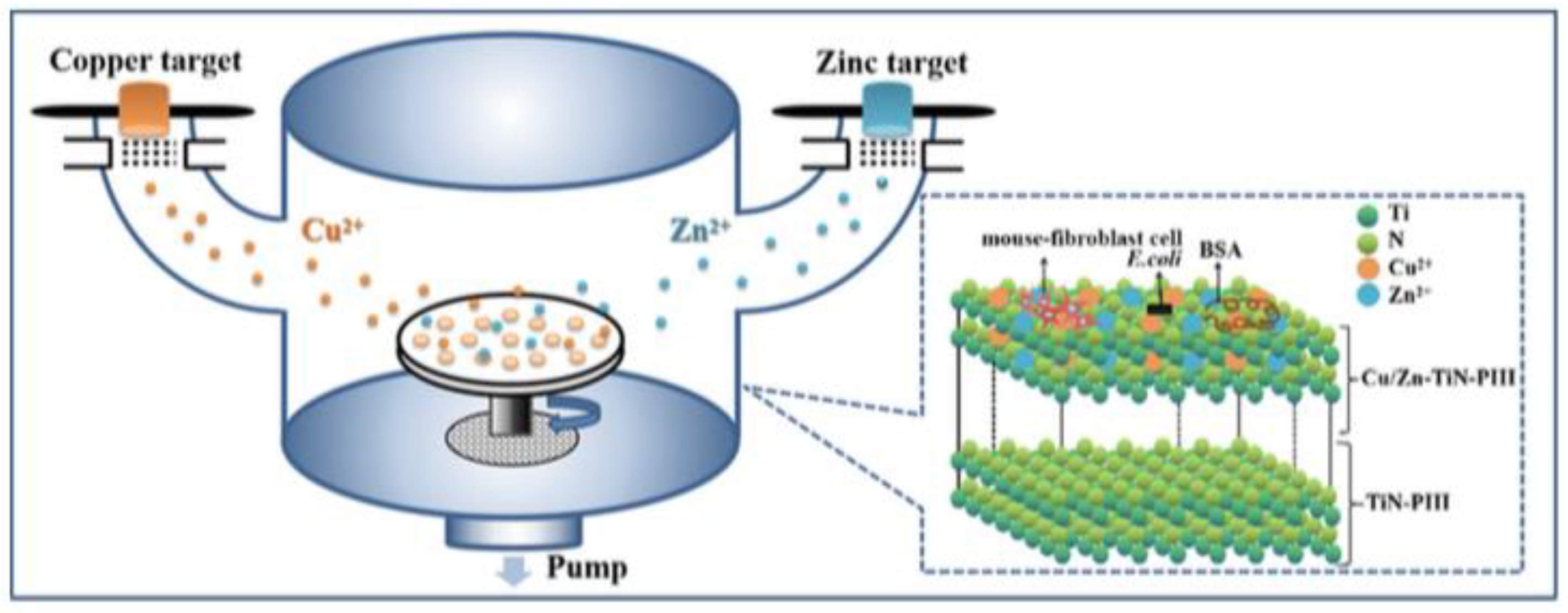
- Li, Q.L.; Li, L.; Zhao, M.L.; Dong, L.; Wu, J.; Li, D.J. Biological actions of Cu/Zn coimplanted TiN on Ti-6Al-4V alloy. Biointerphases 2019, 14, 051008. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qiao, Y.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S. aureus. Biomaterials 2015, 65, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Q.; Qiao, Y.Q.; Liu, X.Y. Titanium Modified by Copper Ion Implantation: Anti-bacterial and Cellular Behaviors. J. Inorg. Mater. 2020, 35, 158–164. [Google Scholar]
- Guo, C.R.; Li, L.; Li, S.S.; Wang, Y.P.; Yu, X.X. Preparation, characterization, bioactivity and degradation behavior in vitro of copper-doped calcium polyphosphate as a candidate material for bone tissue engineering. RSC Adv. 2017, 7, 42614–42626. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Xiong, G.Y.; Liang, H.; Raman, S.; He, F.; Huang, Y. Modification of medical metals by ion implantation of copper. Appl. Surf. Sci. 2007, 253, 9426–9429. [Google Scholar] [CrossRef]
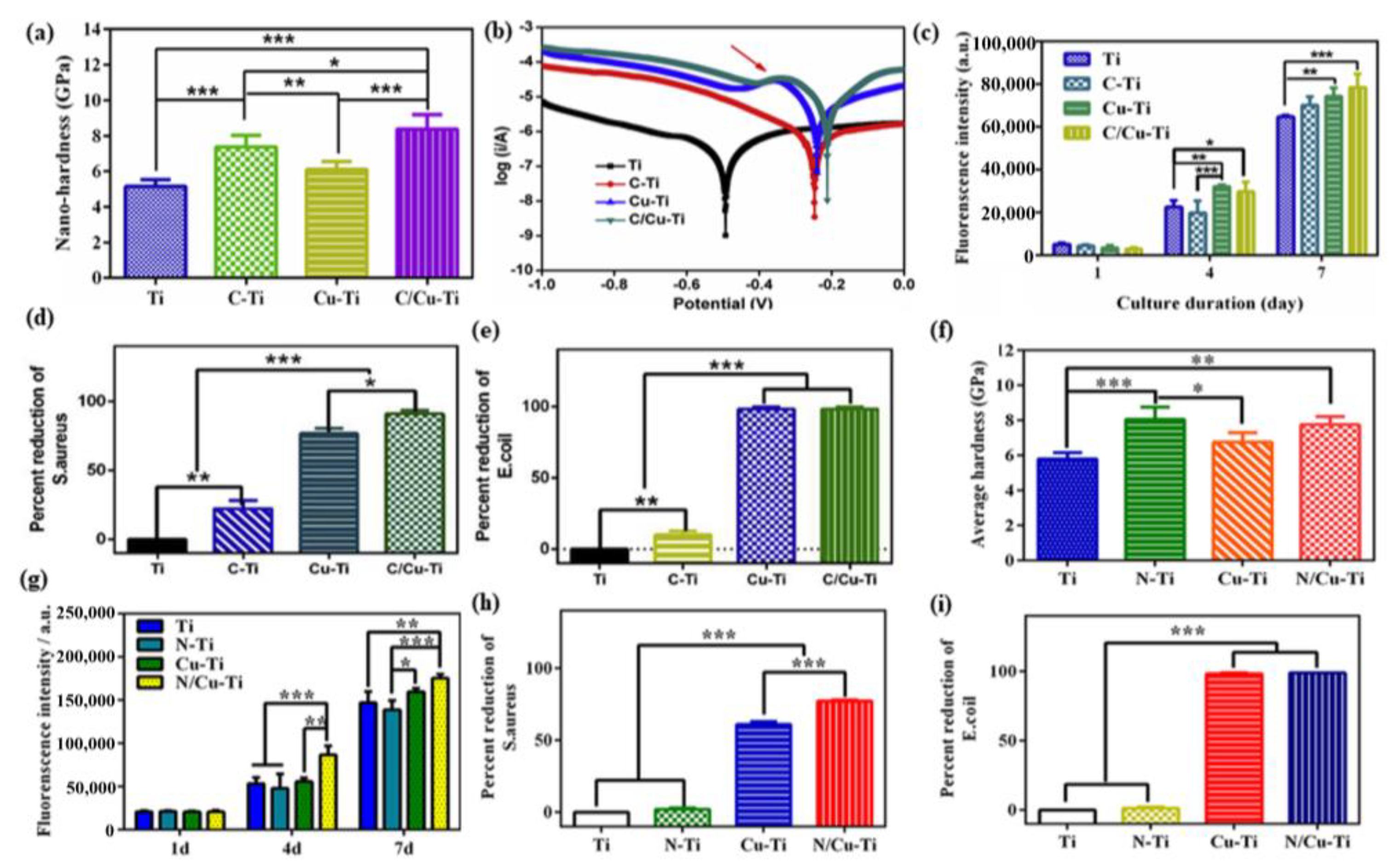
- Xia, C.; Ma, X.; Zhang, X.; Li, K.; Tan, J.; Qiao, Y.; Liu, X. Enhanced physicochemical and biological properties of C/Cu dual ions implanted medical titanium. Bioact. Mater. 2020, 5, 377–386. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Z.; Liao, Z.; Song, J.; Yang, K.; Liu, W. Study on improved tribological properties by alloying copper to CP-Ti and Ti–6Al–4V alloy. Mater. Sci. Eng. C 2015, 57, 123–132. [Google Scholar] [CrossRef]
- Xia, C.; Cai, D.S.; Tan, J.; Li, K.Q.; Qiao, Y.Q.; Liu, X.Y. Synergistic Effects of N/Cu Dual Ions Implantation on Stimulating Antibacterial Ability and Angiogenic Activity of Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3185–3193. [Google Scholar] [CrossRef]
- Jin, S.J.; Qi, X.; Zhang, B.; Sun, Z.Q.; Zhang, B.C.; Yang, H.; Wang, T.M.; Zheng, B.; Wang, X.G.; Shi, Q.P.; et al. Evaluation of promoting effect of a novel Cu-bearing metal stent on endothelialization process from in vitro and in vivo studies. Sci. Rep. 2017, 7, 17394. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhao, M.L.; Li, L.; Dong, L.; Wu, J.; Li, D.J. Co-regulation of Cu/Zn contents enhanced the biological and mechanical properties of TiN coated Ti-6Al-4V alloy. Surf. Coat. Technol. 2020, 395, 10. [Google Scholar] [CrossRef]
- Ma, N.; Liu, S.F.; Liu, W.; Xie, L.C.; Wei, D.X.; Wang, L.Q.; Li, L.J.; Zhao, B.B.; Wang, Y. Research Progress of Titanium-Based High Entropy Alloy: Methods, Properties, and Applications. Front. Bioeng. Biotechnol. 2020, 8, 603522. [Google Scholar] [CrossRef]
- Baldenebro-Lopez, F.; Herrera-Ramírez, J.; Arredondo-Rea, S.; Gómez-Esparza, C.; Martínez-Sánchez, R. Compounds. Simultaneous effect of mechanical alloying and arc-melting processes in the microstructure and hardness of an AlCoFeMoNiTi high-entropy alloy. J. Alloys Compd. 2015, 643, S250–S255. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hu, H.; Meng, J.; Zhao, X. Effect of Y2O3 content in the pack mixtures on the cyclic-oxidation of Y2O3-modified low temperature aluminide coatings on 309 stainless steel. Vacuum 2018, 158, 101–112. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Wei, Q.; Xiao, Y.; Chen, P.; Luo, G.; Shen, Q. Microstructure and mechanical properties of RexNbMoTaW high-entropy alloys prepared by arc melting using metal powders. J. Alloys Compd. 2020, 827, 154301. [Google Scholar] [CrossRef]
- Zhang, E.L.; Wang, X.Y.; Chen, M.; Hou, B. Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 69, 1210–1221. [Google Scholar] [CrossRef]
- Davies, R.A.; Ardalan, S.; Mu, W.-H.; Tian, K.; Farsaikiya, F.; Darvell, B.W.; Chass, G.A. Geometric, electronic and elastic properties of dental silver amalgam γ-(Ag3Sn), γ1-(Ag2Hg3), γ2-(Sn8Hg) phases, comparison of experiment and theory. Intermetallics 2010, 18, 756–760. [Google Scholar] [CrossRef]
- Liu, X.T.; Chen, S.Y.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, M.; Liu, R.; Ren, L.; Zhang, Y.; Pan, H.B.; Zhao, Y.; Yang, K. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016, 27, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.L.; Ren, J.; Li, S.Y.; Yang, L.; Qin, G.W. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of as-cast Ti-Cu alloys. Biomed. Mater. 2016, 11, 065001. [Google Scholar] [CrossRef] [PubMed]
- Krakhmalev, P.; Yadroitsev, I.; Yadroitsava, I.; De Smidt, O. Functionalization of Biomedical Ti6Al4V via In Situ Alloying by Cu during Laser Powder Bed Fusion Manufacturing. Materials 2017, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; Lu, Y.J.; Li, S.M.; Guo, S.; He, M.J.; Luo, K.; Lin, J.X. Copper-modified Ti6Al4V alloy fabricated by selective laser melting with pro-angiogenic and anti-inflammatory properties for potential guided bone regeneration applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 90, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, Z.; Kolawole, S.K.; Zeng, L.; Zhao, Y.; Ren, L.; Yang, K. In vitro study on cytocompatibility and osteogenesis ability of Ti-Cu alloy. J. Mater. Sci.-Mater. Med. 2019, 30, 75. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.; Ullah, I.; Zhang, S.Y.; Sun, Z.Q.; Ren, L.; Yang, K. Rough surface of copper-bearing titanium alloy with multifunctions of osteogenic ability and antibacterial activity. J. Mater. Sci. Technol. 2020, 48, 130–139. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.L.; Liu, H.; Zeng, L.L.; Ma, Z.; Li, J.; Zhao, Y.; Ren, L.; Yang, K. Effects of combined chemical design (Cu addition) and topographical modification (SLA) of Ti-Cu/SLA for promoting osteogenic, angiogenic and antibacterial activities. J. Mater. Sci. Technol. 2020, 47, 202–215. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, M.-C.; Xie, B.; Zhao, Y.-C.; Yin, D.; Gao, C.; Shuai, C.; Atrens, A. Corrosion and antibacterial performance of novel selective-laser-melted (SLMed) Ti-xCu biomedical alloys. J. Alloys Compd. 2021, 864, 158415. [Google Scholar] [CrossRef]
- Xu, D.; Lu, Z.; Wang, T.; Wang, S.; Jiang, Y.; Xu, Z.; Bi, Z.; Geng, S. Novel Ti-based alloys prepared with different heat treatment strategies as antibacterial biomedical implants. Mater. Des. 2021, 205, 109756. [Google Scholar] [CrossRef]
- Yi, C.B.; Yuan, Y.X.; Zhang, L.; Jiang, Y.H.; He, Z.Y. Antibacterial Ti-35Nb-7Zr-xCu alloy with excellent mechanical properties generated with a spark plasma sintering method for biological applications. J. Alloys Compd. 2021, 879, 10. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Liu, H.; Ren, L.; Wang, Q.; Zhang, Y. Effects of surface roughening on antibacterial and osteogenic properties of Ti-Cu alloys with different Cu contents. J. Mater. Sci. Technol. 2021, 88, 158–167. [Google Scholar] [CrossRef]
- Moore, B.; Asadi, E.; Lewis, G. Deposition Methods for Microstructured and Nanostructured Coatings on Metallic Bone Implants: A Review. Adv. Mater. Sci. Eng. 2017, 2017, 5812907. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Balcerzak, J. Tubular electrodeposition of chitosan–carbon nanotube implants enriched with calcium ions. J. Mech. Behav. Biomed. Mater. 2016, 60, 256–266. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, W.; Yu, H.; Zeng, Y.; Ming, F.; Liang, H.; Wang, Z. NiCo/NiCo–OH and NiFe/NiFe–OH core shell nanostructures for water splitting electrocatalysis at large currents. Appl. Catal. B Environ. 2020, 278, 119326. [Google Scholar] [CrossRef]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater. 2008, 4, 1518–1529. [Google Scholar] [CrossRef]
- Lin, D.-J.; Tsai, M.-T.; Shieh, T.-M.; Huang, H.-L.; Hsu, J.-T.; Ko, Y.-C.; Fuh, L.-J. In vitro antibacterial activity and cytocompatibility of bismuth doped micro-arc oxidized titanium. J. Biomater. Appl. 2013, 27, 553–563. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Martin, A.; Jorgensen, L.N.; Sampson, B.; Ågren, M.S. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biol. Trace Elem. Res. 2013, 153, 76–83. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Q.; Han, Y. Zn and Ag co-doped anti-microbial TiO2 coatings on Ti by micro-arc oxidation. J. Mater. Sci. Technol. 2016, 32, 919–924. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chung, C.-J.; Yang, T.-C.; Chiang, I.-P.; Tang, C.-H.; Chen, K.-C.; He, J.-L. Osteoblast growth behavior on micro-arc oxidized β-titanium alloy. Surf. Coat. Technol. 2010, 205, 1624–1629. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, M.; Wahafu, T.; Zhao, Y.; Qin, H.; Wang, J.; Zhang, X.; Wang, L. The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. Med. 2015, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Q.; Ivanisenko, Y.; Diemant, T.; Caron, A.; Chuvilin, A.; Jiang, J.Z.; Valiev, R.Z.; Qi, M.; Fecht, H.J. Synthesis and properties of hydroxyapatite-containing porous titania coating on ultrafine-grained titanium by micro-arc oxidation. Acta Biomater. 2010, 6, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, D.; Han, J.; Sun, L.; Zhu, H.; Yu, Z.; Dargusch, M.; Wang, G. Enhancing Antibacterial Performance and Biocompatibility of Pure Titanium by a Two-Step Electrochemical Surface Coating. ACS Appl. Mater. Interfaces 2020, 12, 44433–44446. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic cell responses and antibacterial efficacy of Cu/Zn co-substituted hydroxyapatite coatings on pure titanium using electrodeposition method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł.; Matýsek, D.; Valíček, J.; Harničárová, M. SEM and EDS analysis of surface layer formed on titanium after plasma electrolytic oxidation in H3PO4 with the addition of Cu(NO3)2. J. Nanosci. Nanotechnol. 2016, 16, 7814–7817. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P.; Dudek, Ł.J.S.; Analysis, I. GDOES, XPS, and SEM with EDS analysis of porous coatings obtained on titanium after plasma electrolytic oxidation. Surf. Interface Anal. 2017, 49, 303–315. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.Q.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, D.; Jiao, Y.; Wu, Y.; Peng, Z.; Zhou, J.; Wu, J.; Dong, Z. Synthesis and characterization of a bi-functional hydroxyapatite/Cu-doped TiO2 composite coating. Ceram. Int. 2019, 45, 6693–6701. [Google Scholar] [CrossRef]
- Zhao, Q.; Yi, L.; Hu, A.; Jiang, L.; Hong, L.; Dong, J. Antibacterial and osteogenic activity of a multifunctional microporous coating codoped with Mg, Cu and F on titanium. J. Mater. Chem. B 2019, 7, 2284–2299. [Google Scholar] [CrossRef]
- Shen, X.; Hu, W.; Ping, L.; Liu, C.; Yao, L.; Deng, Z.; Wu, G. Antibacterial and Osteogenic Functionalization of Titanium with Silicon/Copper-Doped High-Energy Shot Peening-Assisted Micro-Arc Oxidation Technique. Front. Bioeng. Biotechnol. 2020, 8, 573464. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Hiji, A.; Manaka, T.; Nozaki, K.; Chen, P.; Ashida, M.; Tsutsumi, Y.; Nagai, A.; Hanawa, T. Time-transient effects of silver and copper in the porous titanium dioxide layer on antibacterial properties. J. Funct. Biomater. 2020, 11, 44. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Investigation of antibacterial effect of copper introduced titanium surface by electrochemical treatment against facultative anaerobic bacteria. Dent. Mater. J. 2020, 39, 639–647. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Li, G.; Zhang, S.; Zhao, R.; Dong, A.; Zhang, R. Preparation and in vitro antibacterial properties of anodic coatings co-doped with Cu, Zn, and P on a Ti–6Al–4V alloy. Mater. Chem. Phys. 2020, 241, 122360. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Z.; Lu, X.; Lv, Y.; Cai, G.; Yang, L.; Dong, Z. Microstructural evolution and biological performance of Cu-incorporated TiO2 coating fabricated through one-step micro-arc oxidation. Appl. Surf. Sci. 2020, 508, 144766. [Google Scholar] [CrossRef]
- Hang, R.; Gao, A.; Huang, X.; Wang, X.; Zhang, X.; Qin, L.; Tang, B. Antibacterial activity and cytocompatibility of Cu–Ti–O nanotubes. J. Biomed. Mater. Res. A 2014, 102, 1850–1858. [Google Scholar] [CrossRef]
- Zong, M.; Bai, L.; Liu, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B. Antibacterial ability and angiogenic activity of Cu-Ti-O nanotube arrays. Mater. Sci. Eng. C 2017, 71, 93–99. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Rodríguez-Hernández, A.G.; Delgado, L.M.; Manero, J.M.; Javier Gil, F.; Rodríguez, D. Silver deposition on titanium surface by electrochemical anodizing process reduces bacterial adhesion of Streptococcus sanguinis and Lactobacillus salivarius. Clin. Oral Implant Res. 2015, 26, 1170–1179. [Google Scholar] [CrossRef]
- Komarova, E.G.; Sharkeev, Y.P.; Sedelnikova, M.B.; Prosolov, K.A.; Khlusov, I.A.; Prymak, O.; Epple, M. Zn- or Cu-Containing CaP-Based Coatings Formed by Micro-arc Oxidation on Titanium and Ti-40Nb Alloy: Part I—Microstructure, Composition and Properties. Materials 2020, 13, 4116. [Google Scholar] [CrossRef]
- Yang, L.; Perez-Amodio, S.; Barrère-de Groot, F.Y.; Everts, V.; van Blitterswijk, C.A.; Habibovic, P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials 2010, 31, 2976–2989. [Google Scholar] [CrossRef]
- Ewald, A.; Käppel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu (II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. Part A 2012, 100, 2392–2400. [Google Scholar] [CrossRef]
- Prinz, C.; Elhensheri, M.; Rychly, J.; Neumann, H.-G. Antimicrobial and bone-forming activity of a copper coated implant in a rabbit model. J. Biomater. Appl. 2017, 32, 139–149. [Google Scholar] [CrossRef]
- Ren, L.; Wong, H.M.; Yan, C.H.; Yeung, K.W.; Yang, K. Osteogenic ability of Cu-bearing stainless steel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Feurer, T.; Jager, T.; Avancini, E.; Bissig, B.; Yoon, S.; Buecheler, S.; Tiwari, A.N. Low-temperature-processed efficient semi-transparent planar perovskite solar cells for bifacial and tandem applications. Nat. Commun. 2015, 6, 8932. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.; Hultman, L.; Greene, J.J.S. Films. J. Vac. Sci. Technol. A Vac. 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Straňák, V.; Čada, M.; Quaas, M.; Block, S.; Bogdanowicz, R.; Kment, Š.; Hippler, R. Physical properties of homogeneous TiO2 films prepared by high power impulse magnetron sputtering as a function of crystallographic phase and nanostructure. J. Phys. D 2009, 42, 105204. [Google Scholar] [CrossRef]
- Christou, C.; Barber, Z.H. Ionization of sputtered material in a planar magnetron discharge. J. Vac. Sci. Technol. A 2000, 18, 2897–2907. [Google Scholar] [CrossRef]
- Savvides, N.; Window, B. Unbalanced magnetron ion-assisted deposition and property modification of thin films. J. Vac. Sci. Technol. A 1986, 4, 504–508. [Google Scholar] [CrossRef]
- Helmersson, U.; Lattemann, M.; Bohlmark, J.; Ehiasarian, A.P.; Gudmundsson, J.T. Ionized physical vapor deposition (IPVD): A review of technology and applications. Thin Solid Films 2006, 513, 1–24. [Google Scholar] [CrossRef]
- Straňák, V.; Quaas, M.; Wulff, H.; Hubička, Z.; Wrehde, S.; Tichý, M.; Hippler, R. Formation of TiOx films produced by high-power pulsed magnetron sputtering. J. Phys. D 2008, 41, 055202. [Google Scholar] [CrossRef]
- Stranak, V.; Cada, M.; Hubicka, Z.; Tichy, M.; Hippler, R. Time-resolved investigation of dual high power impulse magnetron sputtering with closed magnetic field during deposition of Ti–Cu thin films. J. Appl. Phys. 2010, 108, 043305. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Rebl, H.; Zietz, C.; Arndt, K.; Bogdanowicz, R.; Nebe, B.; Bader, R.; Podbielski, A.; Hubicka, Z.; et al. Deposition of thin titanium-copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater. Sci. Eng. C-Mater. Biol. Appl. 2011, 31, 1512–1519. [Google Scholar] [CrossRef]
- Finke, B.; Polak, M.; Hempel, F.; Rebl, H.; Zietz, C.; Stranak, V.; Lukowski, G.; Hippler, R.; Bader, R.; Nebe, J.B.; et al. Antimicrobial Potential of Copper-Containing Titanium Surfaces Generated by Ion Implantation and Dual High Power Impulse Magnetron Sputtering. Adv. Eng. Mater. 2012, 14, B224–B230. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Ksirova, P.; Zietz, C.; Drache, S.; Cada, M.; Hubicka, Z.; Bader, R.; Tichy, M.; Helm, C.A.; et al. Ionized vapor deposition of antimicrobial Ti-Cu films with controlled copper release. Thin Solid Films 2014, 550, 389–394. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Osekowska, M.; Kaczmarek, D.; Szponar, B.; Mazur, M.; Mazur, P.; Obstarczyk, A. Influence of Material Composition on Structure, Surface Properties and Biological Activity of Nanocrystalline Coatings Based on Cu and Ti. Coatings 2020, 10, 343. [Google Scholar] [CrossRef]
- Osękowska, M.; Wojcieszak, D.; Kaczmarek, D.; Mazur, M.; Obstarczyk, A.; Szponar, B. Multifunctional Nanocrystalline Cu–Ti Thin Films Enhance Survival and Induce Proliferation of Mouse Fibroblasts In Vitro. Coatings 2021, 11, 300. [Google Scholar] [CrossRef]
- Milan, P.B.; Khamseh, S.; Zarrintaj, P.; Ramezanzadeh, B.; Badawi, M.; Morisset, S.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Copper-enriched diamond-like carbon coatings promote regeneration at the bone-implant interface. Heliyon 2020, 6, e03798. [Google Scholar] [CrossRef]
- Wu, H.B.; Zhang, X.Y.; He, X.J.; Li, M.; Huang, X.B.; Hang, R.Q.; Tang, B. Wear and corrosion resistance of anti-bacterial Ti-Cu-N coatings on titanium implants. Appl. Surf. Sci. 2014, 317, 614–621. [Google Scholar] [CrossRef]
- He, X.J.; Zhang, G.N.; Wang, X.; Hang, R.Q.; Huang, X.B.; Qin, L.; Tang, B.; Zhang, X.Y. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Nikitenkov, N. Modern Technologies for Creating the Thin-film Systems and Coatings; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Prosolov, K.A.; Khimich, M.A.; Rau, J.V.; Lychagin, D.V.; Sharkeev, Y.P. Influence of oblique angle deposition on Cu-substituted hydroxyapatite nano-roughness and morphology. Surf. Coat. Technol. 2020, 394, 125883. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Belyavskaya, O.A.; Bolat-ool, A.A.; Khlusov, I.A.; Nikolaeva, O.A.; Prosolov, A.B.; Mitrichenko, D.V.; Komkov, A.R.; Sharkeev, Y.P. Antibacterial potential of Zn- and Cu-substituted hydroxyapatite-based coatings deposited by RF-magnetron sputtering. J. Phys. Conf. Ser. 2019, 1393, 012118. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Amna, T.; Al-Deyab, S.S.; Hassan, M.S.; Abdel-Hay, A.; Kim, H.Y. Inactivation of pathogenic Klebsiella pneumoniae by CuO/TiO2 nanofibers: A multifunctional nanomaterial via one-step electrospinning. Ceram. Int. 2012, 38, 4525–4532. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Kim, H.Y.; Khil, M.-S. Enhanced bactericidal effect of novel CuO/TiO2 composite nanorods and a mechanism thereof. Compos. Part B Eng. 2013, 45, 904–910. [Google Scholar] [CrossRef]
- Ikram, M.; Umar, E.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Hassan, J.; Ali, S. Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles. RSC Adv. 2020, 10, 24215–24233. [Google Scholar] [CrossRef]
- Sasani, N.; Khaki, J.V.; Zebarjad, S.M. Characterization and nanomechanical properties of novel dental implant coatings containing copper decorated-carbon nanotubes. J. Mech. Behav. Biomed. Mater. 2014, 37, 125–132. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Chen, S.; Ge, Z.; Yang, H.; Tang, J. Fabrication of Cu/TiO2 nanocomposite: Toward an enhanced antibacterial performance in the absence of light. Mater. Lett. 2012, 83, 154–157. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Espinosa-Cristobal, L.; Martinez-Castanon, G.; Martinez-Martinez, R.; Loyola-Rodriguez, J.; Patino-Marin, N.; Reyes-Macias, J.; Ruiz, F.J.L. Antibacterial effect of silver nanoparticles against Streptococcus mutans Mater. Mater. Lett. 2009, 63, 2603–2606. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Li, M.; Yu, P.; Chu, X.; Li, L.; Tan, G.; Wang, Y.; Chen, X.; Zhang, Y.; et al. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J. Inorg. Biochem. 2016, 163, 214–220. [Google Scholar] [CrossRef]
- Miyano, Y.; Koyama, K.; Sreekumari, K.; Sato, Y.; Kikuchi, Y. Evaluation of antibacterial ability of some pure metals. Tetsu-to-Hagane 2007, 93, 57–65. [Google Scholar] [CrossRef][Green Version]
- Issa, Y.; Brunton, P.; Waters, C.M.; Watts, D.C. Cytotoxicity of metal ions to human oligodendroglial cells and human gingival fibroblasts assessed by mitochondrial dehydrogenase activity. Dent. Mater. 2008, 24, 281–287. [Google Scholar] [CrossRef]
- Fowler, L.; Engqvist, H.; Öhman-Mägi, C. Effect of Copper Ion Concentration on Bacteria and Cells. Materials 2019, 12, 3798. [Google Scholar] [CrossRef]
- Johnson, L.; Hug, L. Distribution of reactive oxygen species defense mechanisms across domain bacteria. Free Radic. Biol. Med. 2019, 140, 93–102. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Sun, Z.; Ren, L.; Yang, K. Effect of annealing temperature on mechanical and antibacterial properties of Cu-bearing titanium alloy and its preliminary study of antibacterial mechanism. Materials 2018, 93, 495–504. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, G.; Li, H.; Yang, L.; Wang, X.; Qin, G.; Zhang, E. Anti-bacterium influenced corrosion effect of antibacterial Ti-3Cu alloy in Staphylococcus aureus suspension for biomedical application. Mater. Sci. Eng. C 2019, 94, 376–384. [Google Scholar] [CrossRef]
- De Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Souza Filho, A.G.; Alves, O.L.; Biointerfaces, S.B. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M.; Sannino, A.; Ambrosio, L. Metal-based antibacterial substrates for biomedical applications. Biomacromolecules 2015, 16, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cheng, H.; Xu, J.; Li, F.; Gao, B.; Li, Z.; Gao, C.; Huo, K.; Fu, J.; Xiong, W. Silver-loaded nanotubular structures enhanced bactericidal efficiency of antibiotics with synergistic effect in vitro and in vivo. Int. J. Nanomed. 2017, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Belyavskaya, O.A.; Bolat-ool, A.A.; Khlusov, I.A.; Nikolaeva, O.A.; Prosolov, A.B.; Mitrichenko, D.V.; Komkov, A.R.; Sharkeev, Y.P. Antibacterial potential of Zn- and Cu- substituted hydroxyapatite-based coatings deposited by RF-magnetron sputtering. In Proceedings of the 14th International Conference Gas Discharge Plasmas and Their Applications, Tomsk, Russia, 15–21 September 2019; Iop Publishing Ltd.: Bristol, UK, 2019; Volume 1393. [Google Scholar]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sun, M.; Wang, S.; He, J.; Wang, Y.; Qian, Y.; Liu, Y.; Dong, L.; Ma, L.; Cheng, K.; et al. Surface Modification by Divalent Main-Group-Elemental Ions for Improved Bone Remodeling to Instruct Implant Biofabrication. ACS Biomater. Sci. Eng. 2019, 5, 3311–3324. [Google Scholar] [CrossRef] [PubMed]


| Technique | Release | Tested Bacteria | Biocompatibility | Refs. |
|---|---|---|---|---|
| Powder Metallurgy | In 0.9% NaCl up to 72 h (0.05 mg/L) | S. aureus and E. coli | MG63 | [15,37] |
| Arc Melting | In 0.9% NaCl up to 24 h (7–27.5 μg/L) | S. aureus | No report | [60,64] |
| Laser Powder Bed Fusion | No report | S. aureus and E. coli | No report | [65] |
| Selective laser melting | In a study solution (pH = 2.3) consisting of (10.0 ± 0.1) g/L 90% C3H6O3 and (5.85 ± 0.005) g/L NaCl for 7 days ± 1 h (0.90 μg/cm2) | No report | RAW264.7 and HUVEC | [66] |
| Selective laser melting | In the Hanks’s solution for 24 h (40–90 μg/L) | E. coli | MG63 | [70] |
| Arc Melting | In 5 mL of PBS in a humidified atmosphere containing 5% CO2 for 7–28 days (2–15 ng/mL) | S. aureus and E. coli | MC3T3-E1 | [71] |
| Spark plasma sintering | In artificial body fluid for 1–28 days (0.4–1.6 μg/mL) | S. aureus and E. coli | No report | [72] |
| Arc Melting | In 0.9% NaCl solution for 1, 4, 7, 14, 21, and 35 days (substantially below the recommended daily intake of Cu, 3–7.5 μg/L) | S. mutans and P. gingivalis | rBMSCs | [39] |
| Arc Melting | In NS for 1–30 days (the rate of 8.3 μg/L per day in the first 10 days and 2.36 μg/L per day in the subsequent 20 days) | S. aureus and E. coli | MC3T3-E1 | [63] |
| Arc Melting | In 0.9% NaCl up to 24 h (3 μg/L) | S. aureus and E. coli | MG63 | [35,67] |
| Arc Melting then treated by sandblasting and large-grits etching | In 0.9% NaCl solution for 1, 3, 7, 14, and 21 days (after 21 days reached 83.5 μg/L) | S. mutans and P. gingivalis | MC3T3-E1 | [68,69] |
| Arc Melting | In PBS for 7, 14, 21, 28, 35, and 42 days (the rate of Cu2+ release was calculated as 0.106 mg/cm2/d) | MRSA | No report | [40] |
| Year | Elements | Tested Bacteria | Cell Culture | Ref. |
|---|---|---|---|---|
| 2016 | Cu Cu | No report S. aureus | No report L-929 | [86] [33] |
| 2017 | Cu | No report | No report | [87] |
| 2018 | Cu Cu and Zn Cu | S. aureus S. aureus S. aureus | RAW 264.7 and SaOS-2 L-929 MC3T3-E1 and Endothelial cell | [32] [88] [34] |
| 2019 | Cu Mg, Cu and F | No report S. aureus | No report MC3T3-E1 | [89] [90] |
| 2020 | Cu and Si Cu or Ag Cu Cu, Zn, and P Cu | S. aureus and S. mutans E. coli S. aureus and E. coli MRSA, S. aureus and E. coli S. aureus | MC3T3-E1 No report MC3T3-E1 MG63 MC3T3-E1 | [91] [92] [93] [94] [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhou, H.; Zeng, Y.; Xie, H.; Ma, D.; Wang, Z.; Liang, H. Recent Advances in Copper-Doped Titanium Implants. Materials 2022, 15, 2342. https://doi.org/10.3390/ma15072342
Wu Y, Zhou H, Zeng Y, Xie H, Ma D, Wang Z, Liang H. Recent Advances in Copper-Doped Titanium Implants. Materials. 2022; 15(7):2342. https://doi.org/10.3390/ma15072342
Chicago/Turabian StyleWu, Yuncheng, Hao Zhou, Ye Zeng, Hongxing Xie, Dongxu Ma, Zhoucheng Wang, and Hanfeng Liang. 2022. "Recent Advances in Copper-Doped Titanium Implants" Materials 15, no. 7: 2342. https://doi.org/10.3390/ma15072342
APA StyleWu, Y., Zhou, H., Zeng, Y., Xie, H., Ma, D., Wang, Z., & Liang, H. (2022). Recent Advances in Copper-Doped Titanium Implants. Materials, 15(7), 2342. https://doi.org/10.3390/ma15072342







