In Situ Tungsten Carbide Formation in Nanostructured Copper Matrix Composite Using Mechanical Alloying and Sintering
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
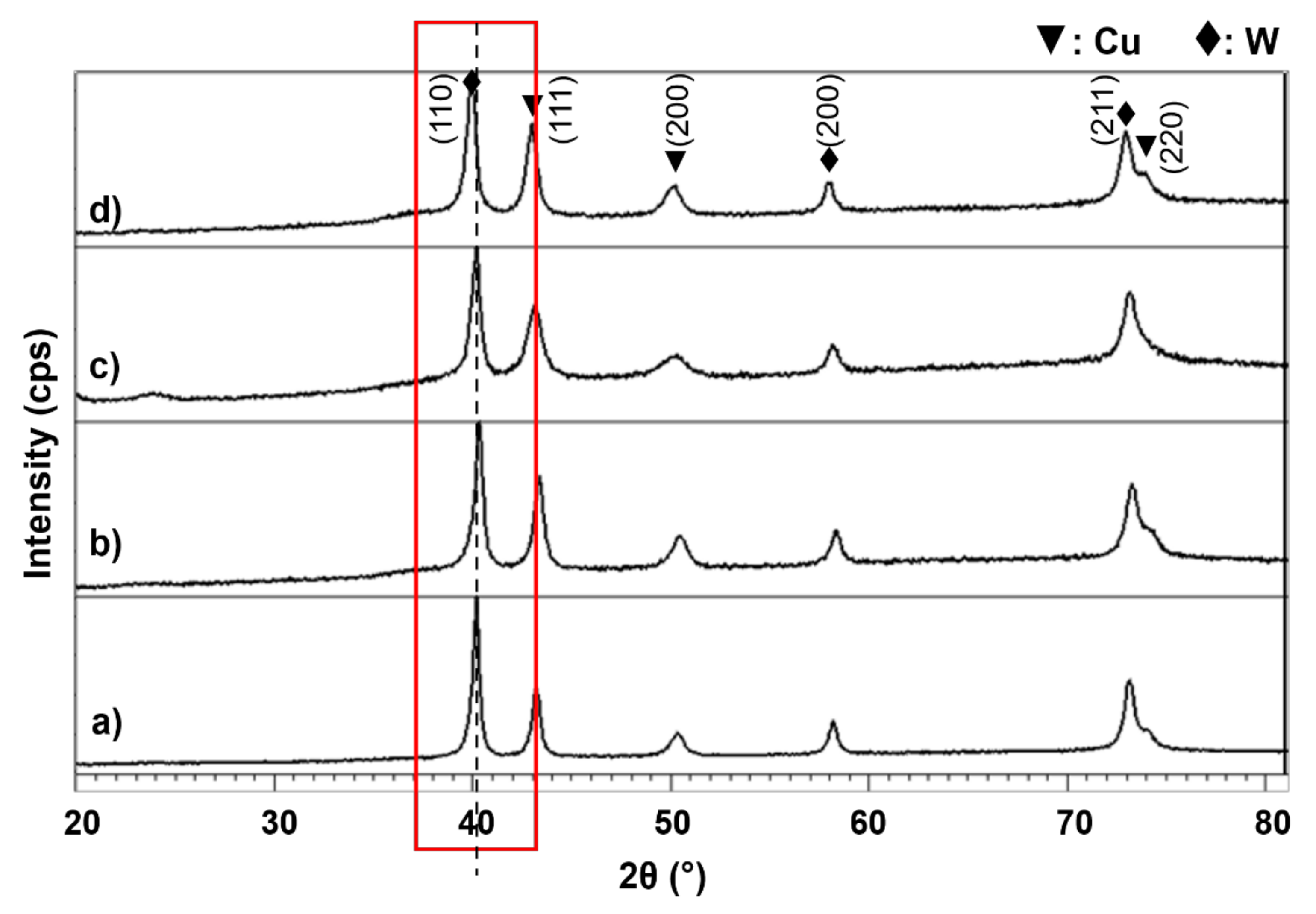
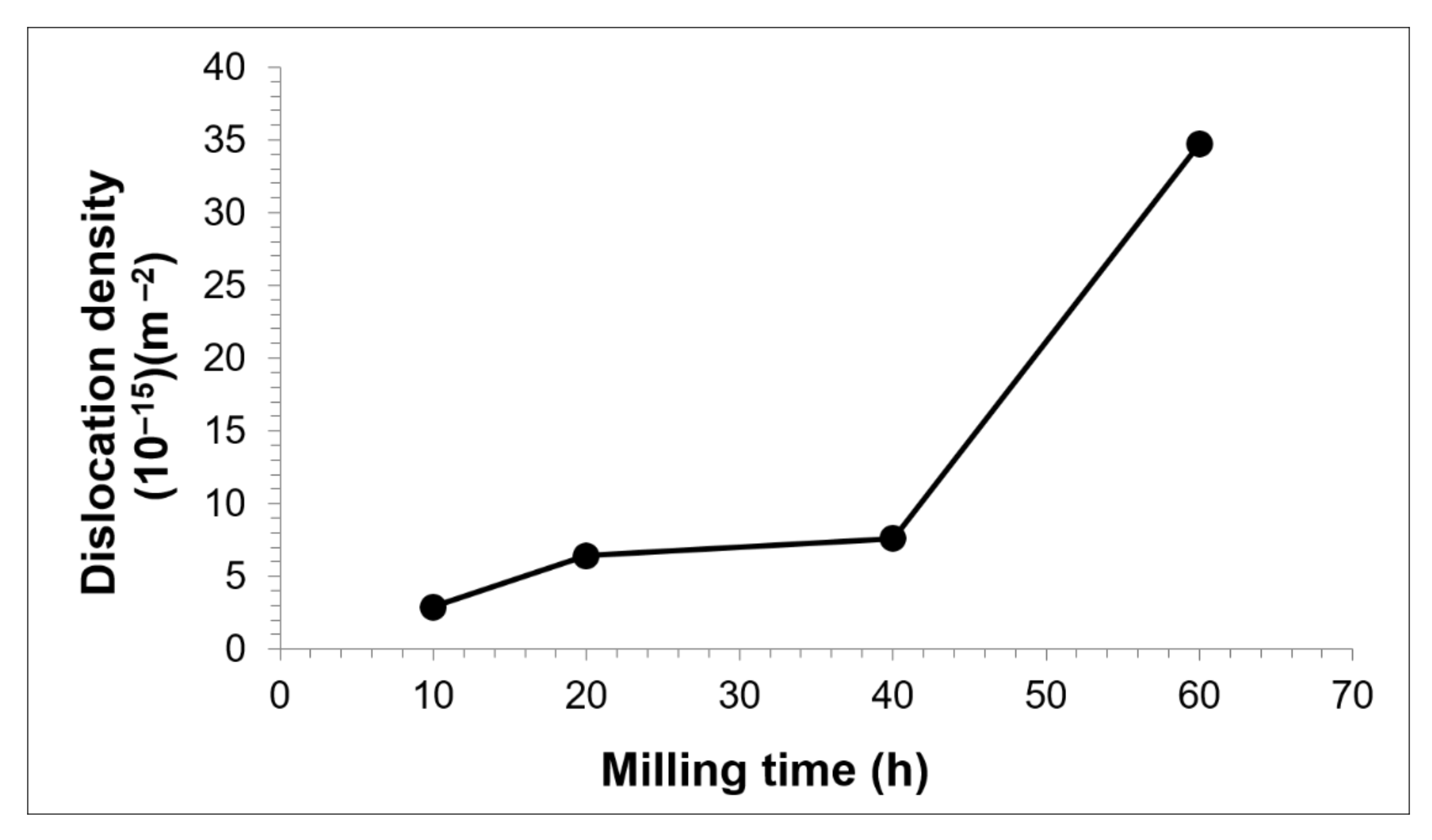
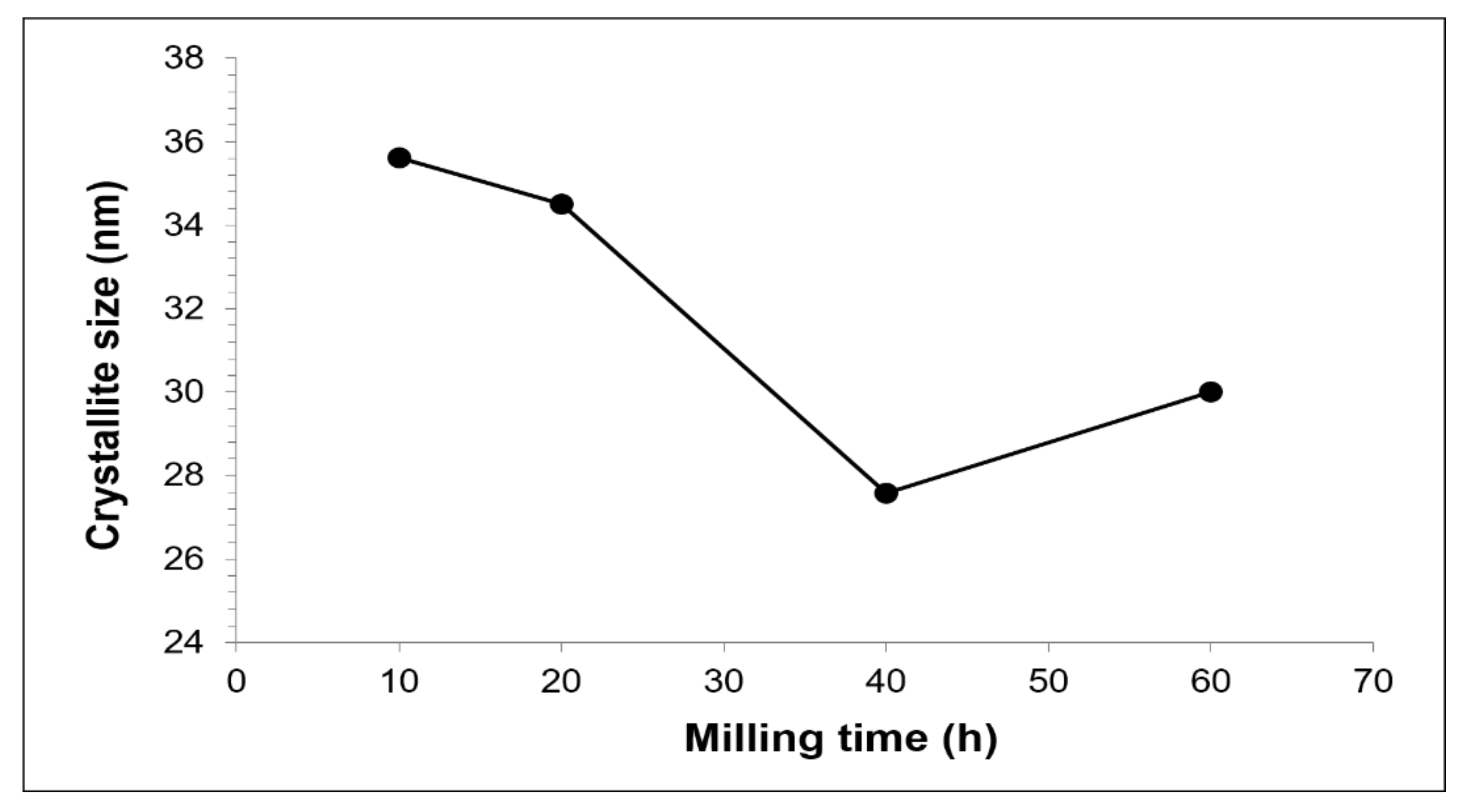
3.1. Cu-W-C as-Milled Powder
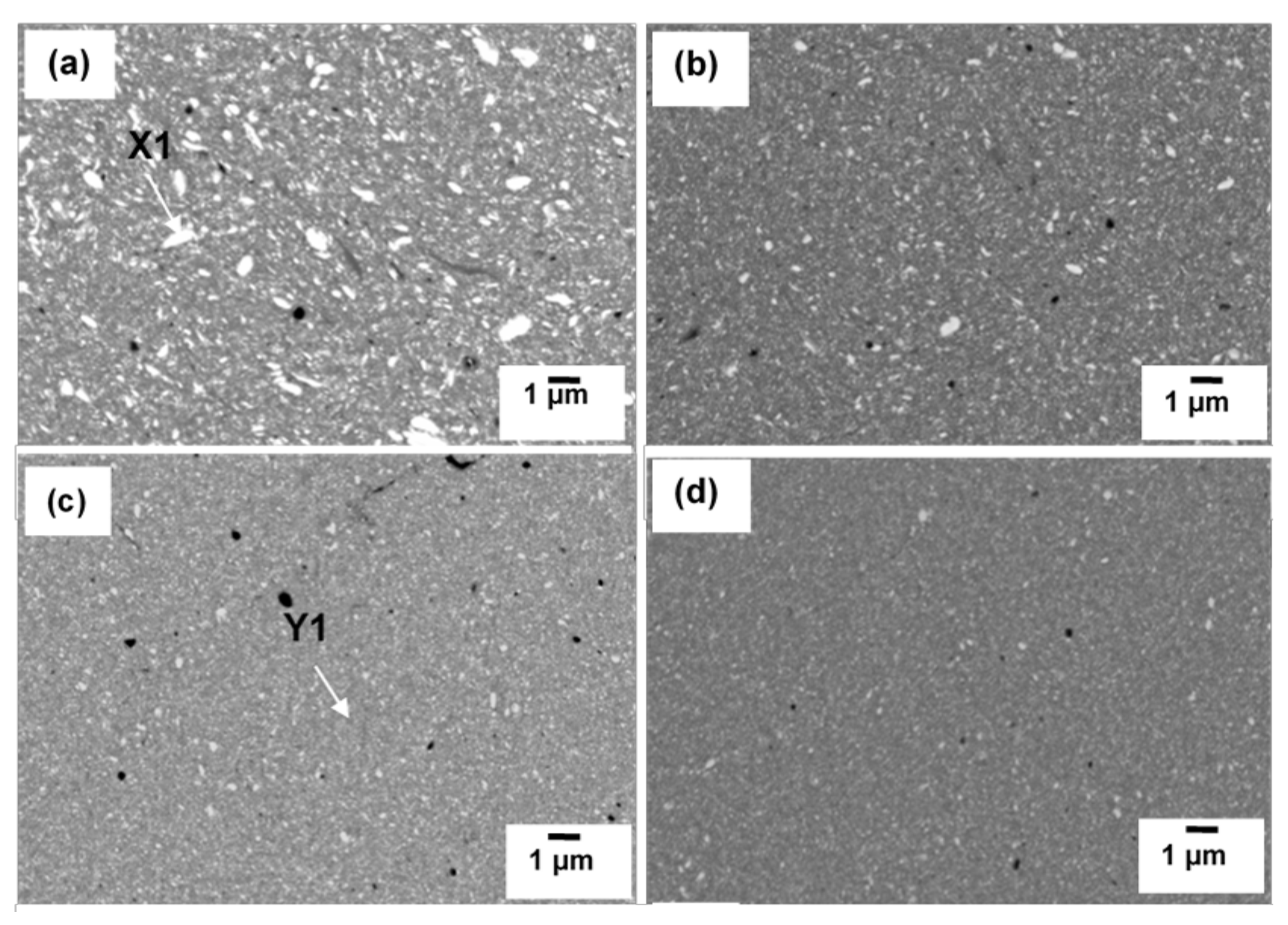
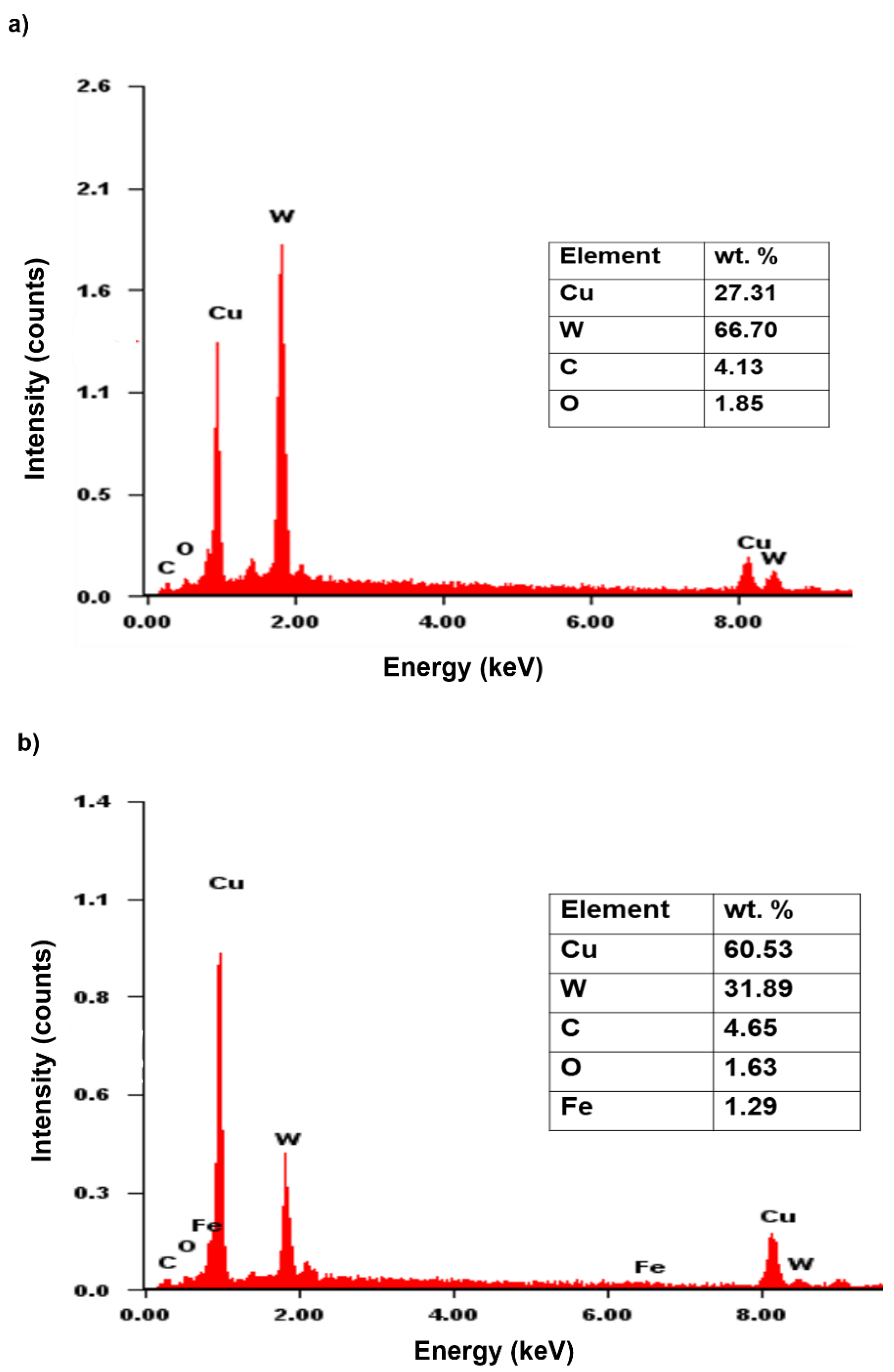
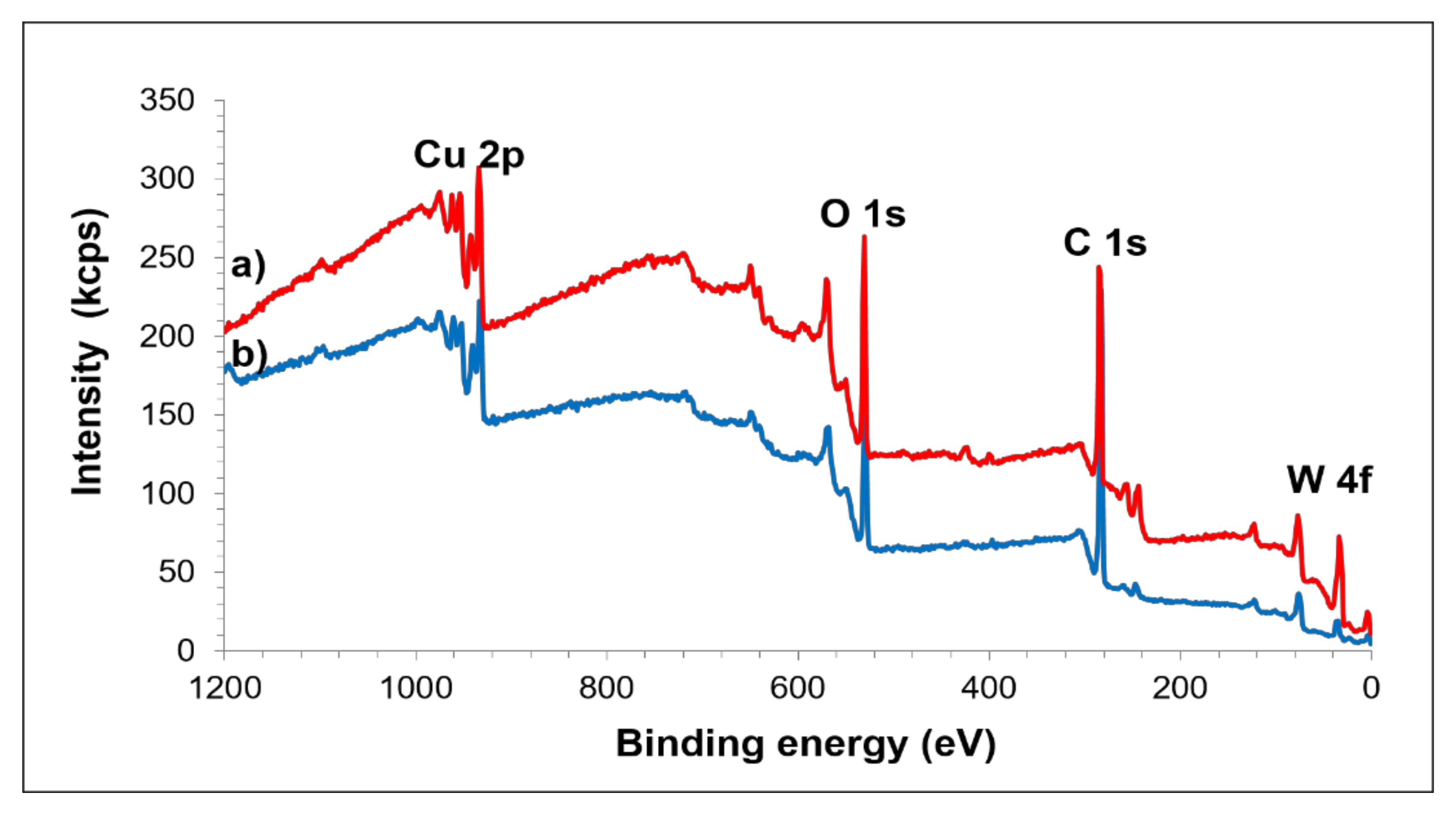
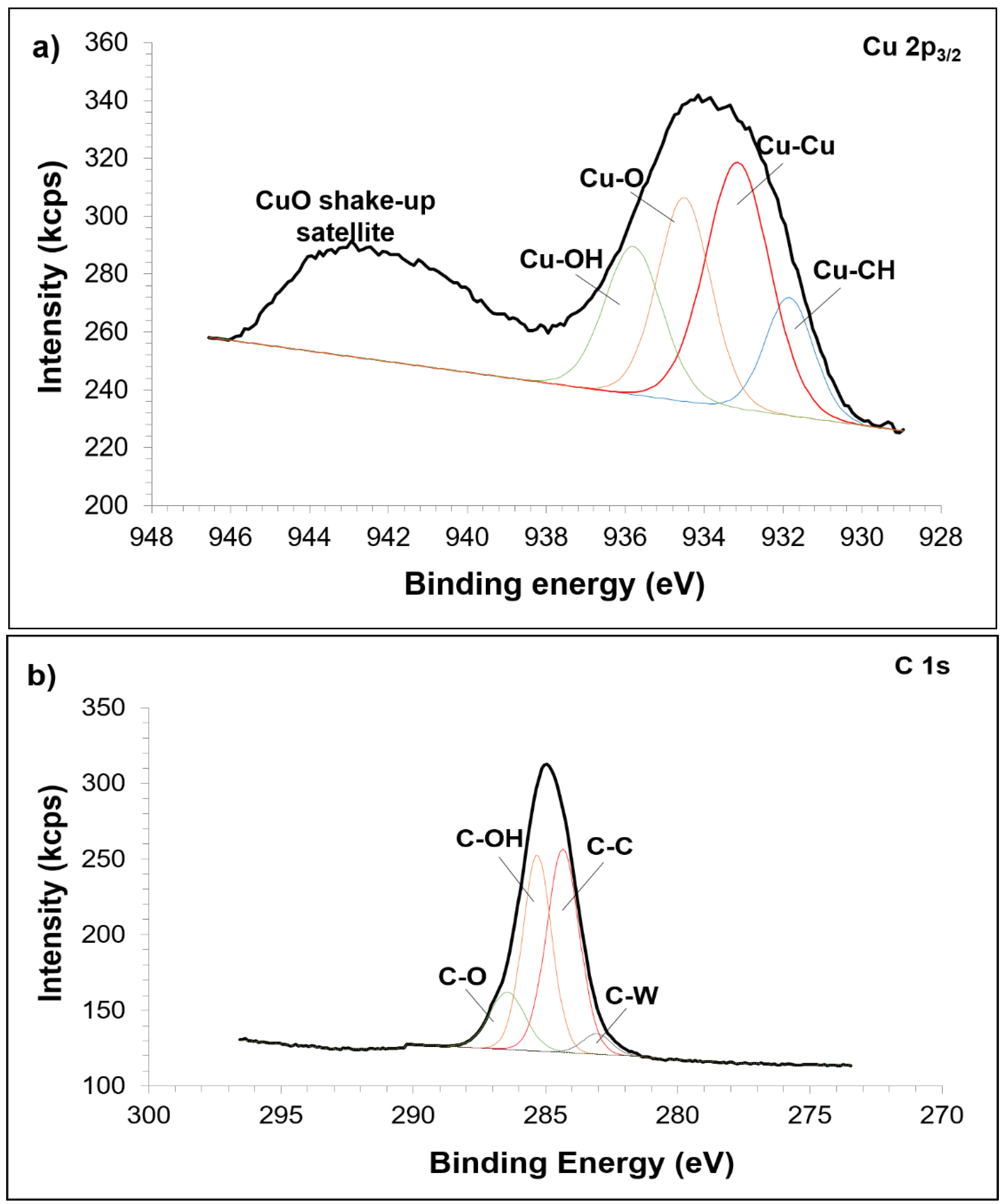
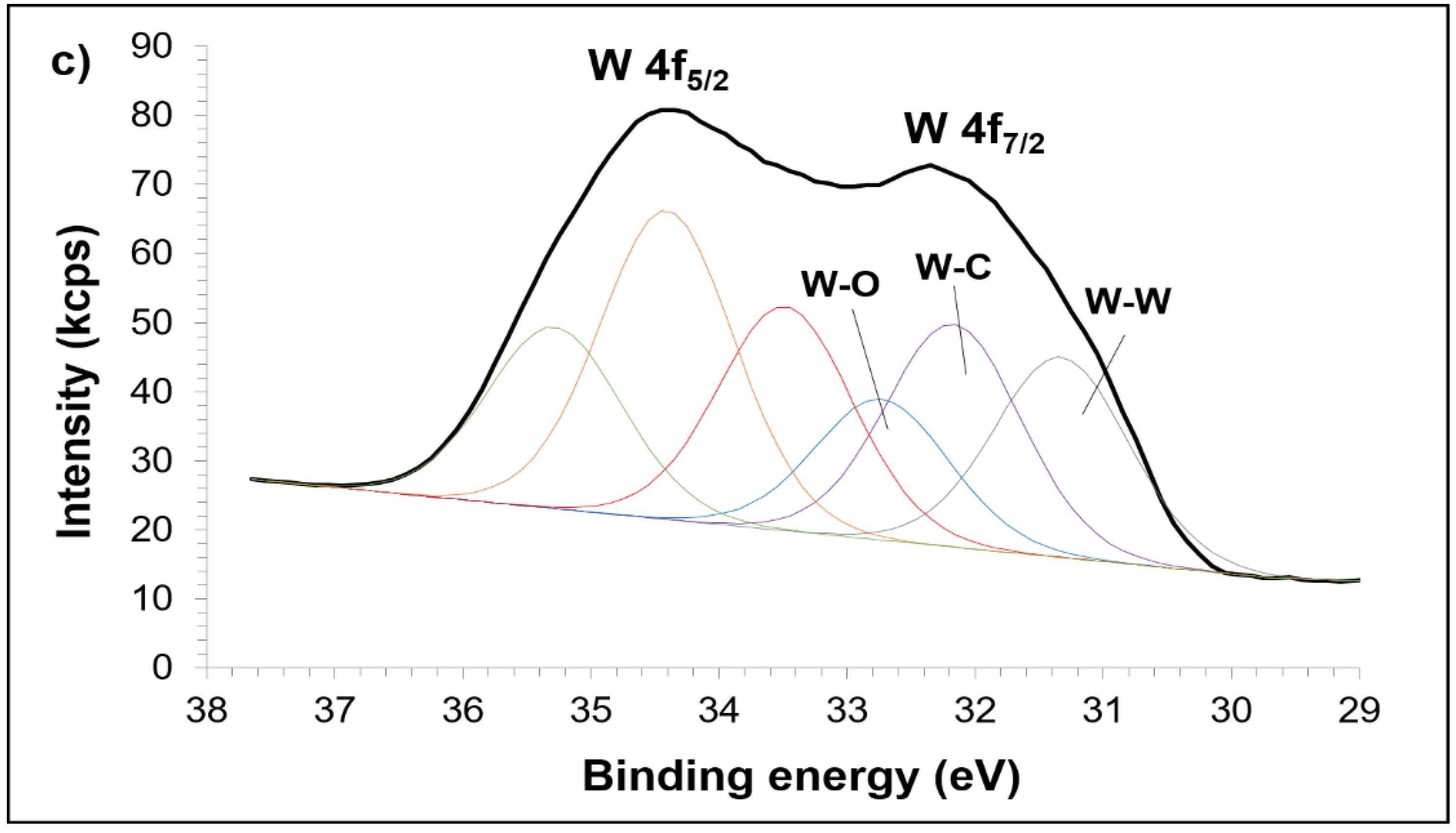
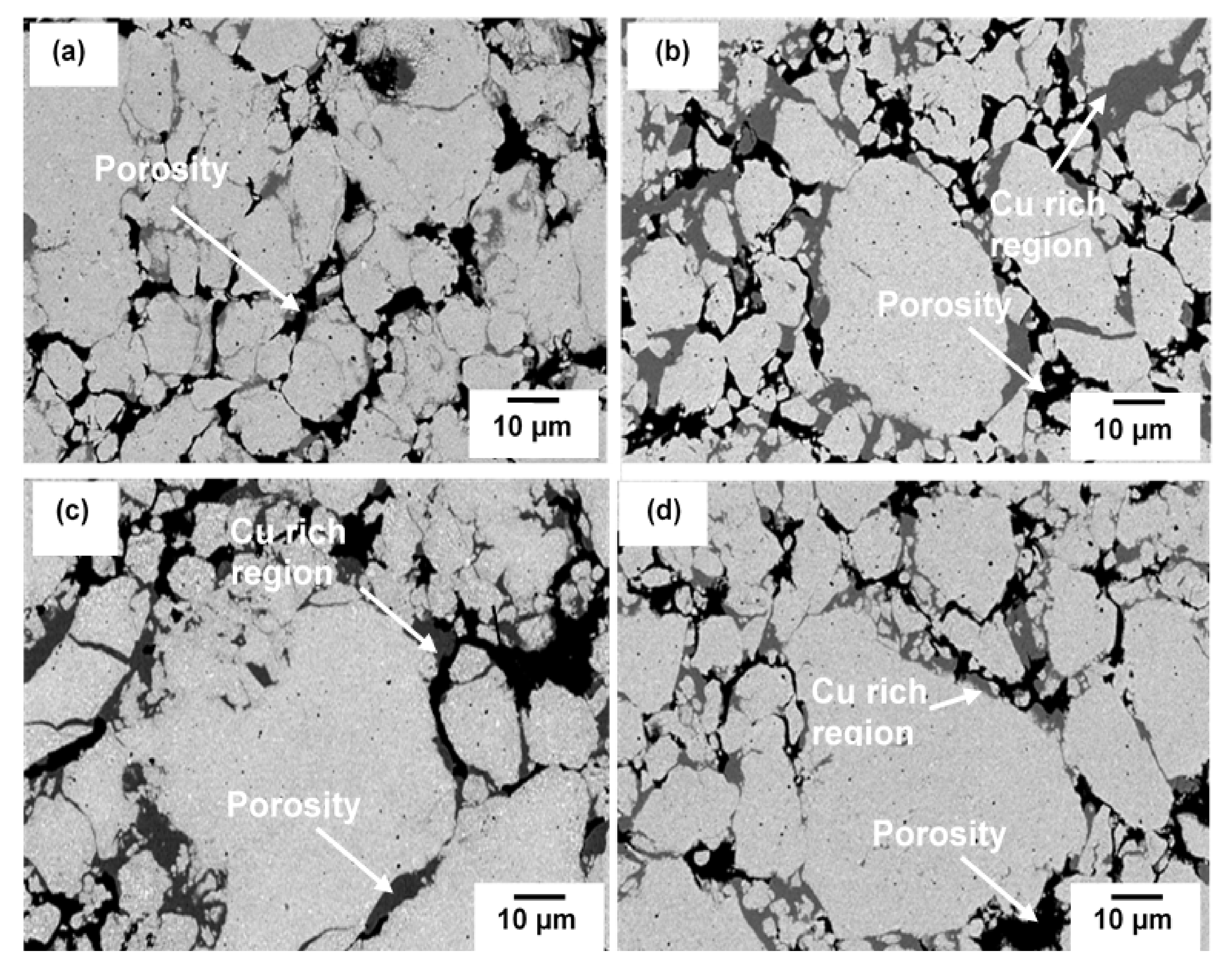
3.2. Sintered Cu-W-C Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khosravi, J.; Givi, M.K.B.; Barmouz, M.; Rahi, A. Microstructural, mechanical, and thermophysical characterization of Cu/WC composite layers fabricated via friction stir processing. Int. J. Adv. Manuf. Technol. 2014, 74, 1087–1096. [Google Scholar] [CrossRef]
- Islak, S.; Kır, D.; Buytoz, S. Effect of sintering temperature on electrical and microstructure properties of hot pressed Cu-TiC composites. Sci. Sinter. 2014, 46, 15–21. [Google Scholar] [CrossRef]
- Mallikarjuna, H.M.; Ramesh, C.S.; Koppad, P.G.; Keshavamurthy, R.; Sethuram, D. Nanoindentation and wear behaviour of copper based hybrid composites reinforced with SiC and MWCNTs synthesized by spark plasma sintering. Vacuum 2017, 145, 320–333. [Google Scholar] [CrossRef]
- Chen, W.; Dong, L.; Wang, J.; Zuo, Y.; Ren, S.; Fu, Y. Synergistic enhancing effect for mechanical and electrical properties of tungsten copper composites using spark plasma infiltrating sintering of copper-coated graphene. Sci. Rep. 2017, 7, 9. [Google Scholar] [CrossRef]
- Shiri, S.G.; Abachi, P.; Pourazarang, K.; Rahvard, M.M. Preparation of in-situ Cu/NbC nanocomposite and its functionally graded behavior for electrical contact applications. Trans. Nonferrous Met. Soc. China 2015, 25, 863–872. [Google Scholar] [CrossRef]
- Tian, Y.; Dou, Z.; Niu, L.; Zhang, T. Effect of nanoboron carbide particles on properties of copper-matrix/graphite composite materials. Mater. Res. Express 2019, 6, 17938–17950. [Google Scholar] [CrossRef]
- Akbarpour, M.R.; Mousa Mirabad, H.; Alipour, S. Microstructural and mechanical characteristics of hybrid SiC/Cu composites with nano- and micro-sized SiC particles. Ceram. Int. 2019, 45, 3276–3283. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Chaira, D. Fabrication and characterization of Cu–B4C metal matrix composite by powder metallurgy: Effect of B4C on microstructure, mechanical properties and electrical conductivity. Trans. Indian Inst. Met. 2019, 72, 673–684. [Google Scholar] [CrossRef]
- Xiang, S.; Hu, H.; Liang, Y.; Zhang, X. Fabrication of sub-stoichiometric titanium carbide-reinforced copper matrix composites using electropulsing-assisted flash sintering. Compos. Part A Appl. Sci. Manuf. 2021, 151, 106664. [Google Scholar] [CrossRef]
- Lin, H.; Guo, X.; Song, K.; Li, S.; Feng, J.; Zhang, X.; Feng, M. Synergistic strengthening effect of tungsten carbide (WC) particles and silicon carbide whiskers (SiCw) on mechanical properties of Cu–Al2O3 composite. J. Mater. Res. Technol. 2021, 15, 2837–2847. [Google Scholar] [CrossRef]
- Rajkumar, K.; Aravindan, S. Tribological performance of microwave sintered copper–TiC–graphite hybrid composites. Tribol. Int. 2011, 44, 347–358. [Google Scholar] [CrossRef]
- Umale, T.; Singh, A.; Reddy, Y.; Khatitrkar, R.; Sapate, S. In Abrasive wear behaviour of COPPER-SiC and COPPER-SiO2 composites. Int. J. Mod. Phys. Conf. Ser. World Sci. 2013, 22, 416–423. [Google Scholar] [CrossRef]
- Mishra, A. Dry sliding wear behaviourof titanium carbide with copper composites. Int. J. Mech. Eng. Robot. Res. 2014, 3, 4. [Google Scholar]
- Jha, P.; Gautam, R.K.; Tyagi, R. Friction and wear behavior of Cu–4 wt.% Ni–TiC composites under dry sliding conditions. Friction 2017, 5, 437–446. [Google Scholar] [CrossRef]
- Akbarpour, M.; Najafi, M.; Alipour, S.; Kim, H. Hardness, wear and friction characteristics of nanostructured Cu-SiC nanocomposites fabricated by powder metallurgy route. Mater. Today Commun. 2019, 18, 25–31. [Google Scholar] [CrossRef]
- Banthia, S.; Amid, M.; Sengupta, S.; Das, S.; Das, K. Reciprocating sliding wear of Cu, Cu-SiC functionally graded coating on electrical contact. J. Mater. Eng. Perform. 2020, 29, 3930–3940. [Google Scholar] [CrossRef]
- Deshpande, P.; Lin, R. Wear resistance of WC particle reinforced copper matrix composites and the effect of porosity. Mater. Sci. Eng. A 2006, 418, 137–145. [Google Scholar] [CrossRef]
- Toth, L. Transition Metal Carbides and Nitrides; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E. Mechanochemical Synthesis of Nanocrystalline Metal Powders. In Advances in Powder Metallurgy; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhang, G.; Gu, D. Synthesis of nanocrystalline TiC reinforced W nanocomposites by high-energy mechanical alloying: Microstructural evolution and its mechanism. Appl. Surf. Sci. 2013, 273, 364–371. [Google Scholar] [CrossRef]
- Zeng, W.; Zhou, D.; Zhang, D.; Li, X. Microstructural Evolution of Cu-10at% C Nanocomposite Powder During High Energy Mechanical Milling. Mater. Res. 2015, 18, 152–157. [Google Scholar] [CrossRef][Green Version]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Abdul Aziz, S.; Mamat, M.S. Effect of milling time on the microstructure, physical and mechanical properties of Al-Al2O3 nanocomposite synthesized by ball milling and powder metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Baghani, M.; Aliofkhazraei, M.; Seyfoori, A.; Askari, M. Mechanical alloying of CuFe-alumina nanocomposite: Study of microstructure, corrosion, and wear properties. Sci. Eng. Compos. Mater. 2018, 25, 1085–1094. [Google Scholar] [CrossRef]
- Şelte, A.; Özkal, B. Crystallite size and strain calculations of hard particle reinforced composite powders (Cu/Ni/Fe-WC) synthesized via mechanical alloying. Proc. Est. Acad. Sci. 2019, 68, 66–78. [Google Scholar] [CrossRef]
- Aghamiri, S.M.S.; Oono, N.; Ukai, S.; Kasada, R.; Noto, H.; Hishinuma, Y.; Muroga, T. Microstructure and mechanical properties of mechanically alloyed ODS copper alloy for fusion material application. Nucl. Mater. Energy 2018, 15, 17–22. [Google Scholar] [CrossRef]
- Sorkhe, Y.; Aghajani, H.; Tabrizi, A.T. Mechanical alloying and sintering of nanostructured TiO2 reinforced copper composite and its characterization. Mater. Des. 2014, 58, 168–174. [Google Scholar] [CrossRef]
- Taha, M.A.; Zawrah, M. Effect of nano ZrO2 on strengthening and electrical properties of Cu-matrix nanocomposits prepared by mechanical alloying. Ceram. Int. 2017, 43, 12698–12704. [Google Scholar] [CrossRef]
- Zang, J.; Li, H.; Sun, J.; Shen, Y.; Su, N.; Feng, X. Microstructure and thermal conductivity of Cu-Cu2AlNiZnAg/diamond coatings on pure copper substrate via high-energy mechanical alloying method. Surf. Interfaces 2020, 21, 100742. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Taha, M.A. Preparation of high strength graphene reinforced Cu-based nanocomposites via mechanical alloying method: Microstructural, mechanical and electrical properties. Appl. Phys. A 2020, 126, 220. [Google Scholar] [CrossRef]
- Sun, J.; Zang, J.; Li, H.; Feng, X.; Shen, Y. Influence of diamond content and milling duration on microstructure and thermal conductivity of Ti-coated diamond/copper composite coating on copper substrate. Mater. Chem. Phys. 2021, 259, 124017. [Google Scholar] [CrossRef]
- Li, J.; Ni, J.; Huang, B.; Chen, J.; Xu, Z.; Liao, S.; Wang, C.; Luo, W. Long-term ball milling and hot pressing of in-situ nanoscale tungsten carbides reinforced copper composite and its characterization. Mater. Charact. 2019, 152, 134–140. [Google Scholar] [CrossRef]
- Cullity, B.; Stock, S. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001; 388p. [Google Scholar]
- Zuo, B.; Saraswati, N.; Sritharan, T.; Hng, H.H. Production and annealing of nanocrystalline Fe–Si and Fe–Si–Al alloy powders. Mater. Sci. Eng. A 2004, 1–2, 210–216. [Google Scholar] [CrossRef]
- Kumar, R.; Bakshi, S.R.; Joardar, J.; Parida, S.; Raja, V.S.; Singh Raman, R.K. Structural evolution during milling, annealing, and rapid consolidation of nanocrystalline Fe–10Cr–3Al powder. Meterials 2017, 3, 272. [Google Scholar] [CrossRef] [PubMed]
- Berner, A.; Mundim, K.; Ellis, D.; Dorfman, S.; Fuks, D.; Evenhaim, R. Microstructure of Cu–C interface in Cu-based metal matrix composite. Sens. Actuators A Phys. 1999, 74, 86–90. [Google Scholar] [CrossRef]
- Cottrell, A. Chemical Bonding in Transition Metal Carbides; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Sohn, H.Y.; Ryu, T.; Choi, J.W.; Hwang, K.S.; Han, G.; Choi, Y.J.; Fang, Z.Z. The chemical vapor synthesis of inorganic nanopowders. J. Miner. Met. Mater. Soc. 2007, 59, 44–49. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, M.; Lee, Y.H.; Hong, S.H.; Kim, T.H.; Choi, S. Synthesis of tungsten carbide nanomaterials in triple DC thermal plasma jet system. J. Nanosci. Nanotechnol. 2019, 19, 6277–6284. [Google Scholar] [CrossRef]
- Tan, G.L.; Wu, X.J.; Zhao, M.H.; Zhang, H.F. Synthesis of nanocrystalline cubic substoichiometric WC1− z powders by mechanochemical technology. J. Mater. Sci. 2000, 35, 3151–3154. [Google Scholar] [CrossRef]
- Bolokang, S.; Banganayi, C.; Phasha, M. Effect of C and milling parameters on the synthesis of WC powders by mechanical alloying. Int. J. Refract. Met. Hard Mater. 2010, 28, 211–216. [Google Scholar] [CrossRef]
- Mehrizi, M.Z.; Momeni, M.; Beygi, R.; Kim, B.-H.; Kim, S.-K. Reaction pathway of NiAl/WC nanocomposite synthesized from mechanical activated NiAlWC powder system. Ceram. Int. 2019, 45, 11833–11837. [Google Scholar] [CrossRef]
- Zhong, L.; Deng, C.; Zhu, J.; Bai, H.; Xu, Y. Microstructure evolution and in-situ-formed mechanism of dual-scale WC-Fe reinforced iron matrix composites. J. Phys. Conf. Ser. 2020, 1605, 012185. [Google Scholar] [CrossRef]
- Ruoshan, L.; Guangrun, C.; Mingpu, W. Effect of Nb solute concentration on crystallite size refinement and strength enhancement in mechanically alloyed Cu-Nb Alloys. Rare Met. Mater. Eng. 2018, 47, 2607–2614. [Google Scholar] [CrossRef]
- Radajewski, M.; Schimpf, C.; Krüger, L. Study of processing routes for WC-MgO composites with varying MgO contents consolidated by FAST/SPS. J. Eur. Ceram. Soc. 2017, 37, 2031–2037. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, H.; Wang, H.; Tang, F.; Nie, H.; Hou, C.; Liu, X.; Song, X.; Nie, Z. Solid-solution hardening of WC by rhenium. J. Eur. Ceram. Soc. 2020, 40, 333–340. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusoff, M.; Zuhailawati, H. In Situ Tungsten Carbide Formation in Nanostructured Copper Matrix Composite Using Mechanical Alloying and Sintering. Materials 2022, 15, 2340. https://doi.org/10.3390/ma15072340
Yusoff M, Zuhailawati H. In Situ Tungsten Carbide Formation in Nanostructured Copper Matrix Composite Using Mechanical Alloying and Sintering. Materials. 2022; 15(7):2340. https://doi.org/10.3390/ma15072340
Chicago/Turabian StyleYusoff, Mahani, and Hussain Zuhailawati. 2022. "In Situ Tungsten Carbide Formation in Nanostructured Copper Matrix Composite Using Mechanical Alloying and Sintering" Materials 15, no. 7: 2340. https://doi.org/10.3390/ma15072340
APA StyleYusoff, M., & Zuhailawati, H. (2022). In Situ Tungsten Carbide Formation in Nanostructured Copper Matrix Composite Using Mechanical Alloying and Sintering. Materials, 15(7), 2340. https://doi.org/10.3390/ma15072340





