Effect of Ultraviolet Light C (UV-C) Radiation Generated by Semiconductor Light Sources on Human Beta-Coronaviruses’ Inactivation
Abstract
:1. Introduction
2. Materials and Methods
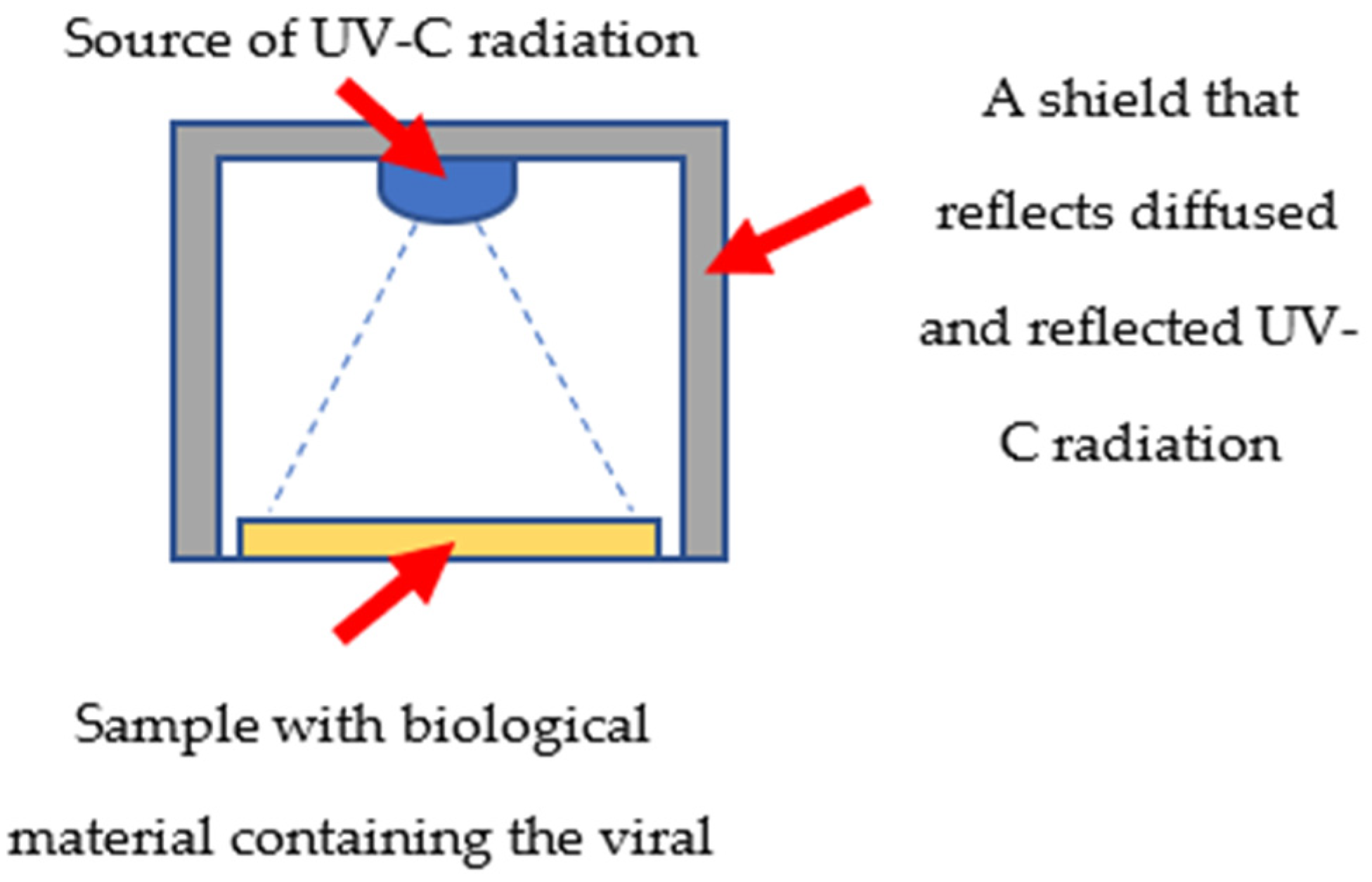
2.1. Virus Irradiation and Cytopathic Effect Evaluation
2.2. Determination of the HoV-OC43 Virus Particles after Irradiation Using UV Absorbance
2.3. RT-qPCR Viral Quantification after Irradiation
3. Results
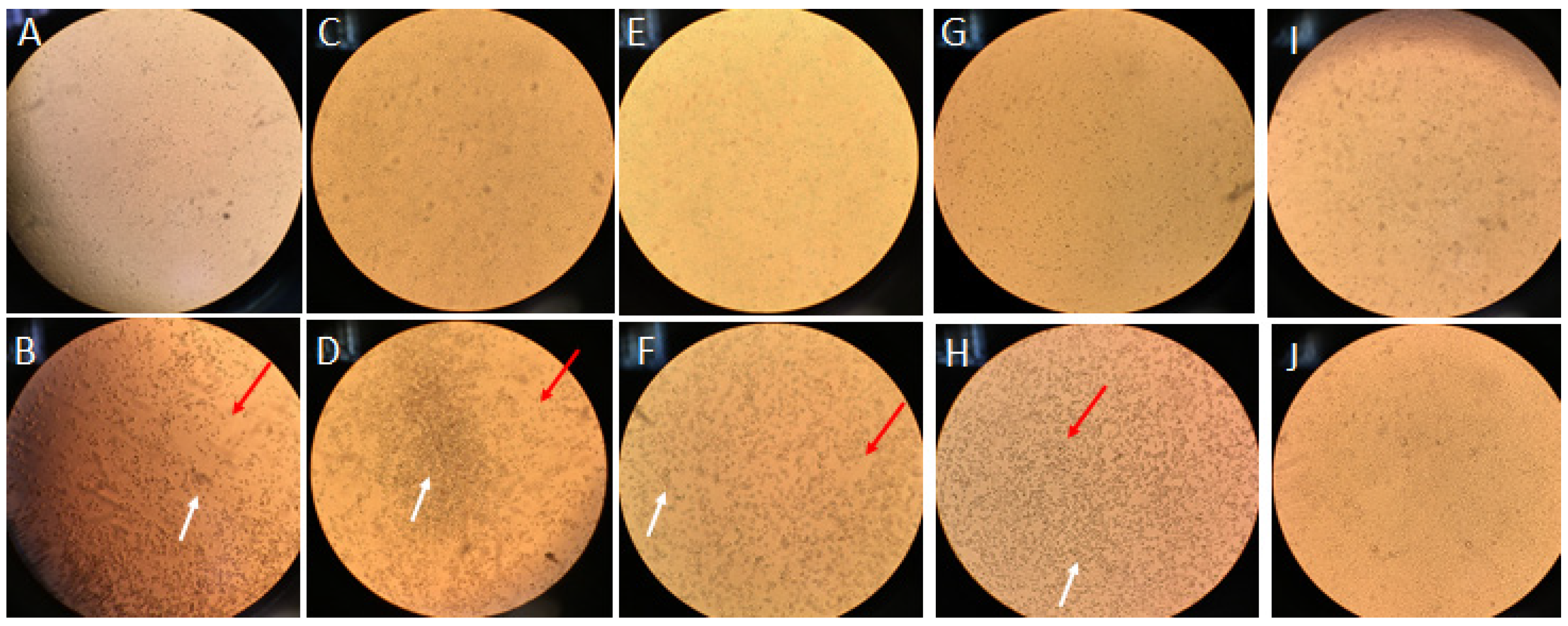
3.1. Inactivation of Human Coronavirus HCoV-OC43 by Ultraviolet C (UVC) Exposure in Infectivity Assay
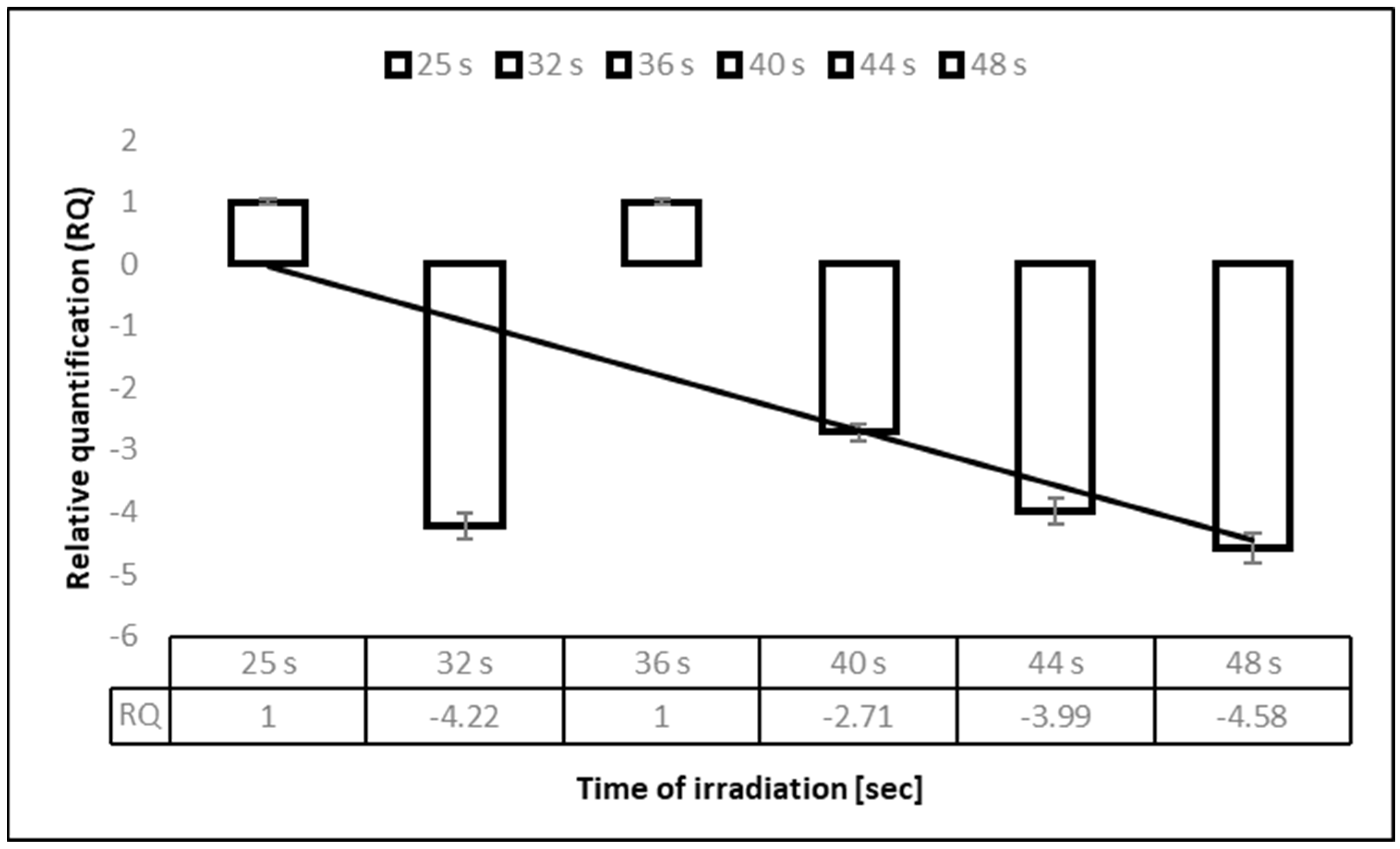
3.2. Deactivation of Human Coronavirus HCoV-OC43 by Ultraviolet C (UV-C) 275 nm in the Time Exposure Experiment, Assessed by an Infectivity Reduction Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Sayah, M.H. Chemical disinfectants of COVID-19: An overview. J. Water Health 2020, 18, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Saito, A.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg. Microbes Infect. 2020, 9, 1744–1747. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. UVC LED irradiation effectively inactivates aerosolized viruses, bacteria, and fungi in a chamber-type air disinfection system. Appl. Environ. Microbiol. 2018, 84, e00944-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattanakul, S.; Oguma, K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms. Water Res. 2018, 130, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, D.K.; Kang, D.H. Using UVC light-emitting diodes at wavelengths of 266 to 279 nanometers to inactivate foodborne pathogens and pasteurize sliced cheese. Appl. Environ. Microbiol. 2016, 82, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Nishisaka-Nonaka, R.; Mawatari, K.; Yamamoto, T.; Kojima, M.; Shimohata, T.; Uebanso, T.; Nakahashi, M.; Emoto, T.; Akutagawa, M.; Kinouchi, Y.; et al. Irradiation by ultraviolet light-emitting diodes inactivates influenza a viruses by inhibiting replication and transcription of viral RNA in host cells. J. Photochem. Photobiol. B 2018, 189, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W. UV Surface Disinfection. In Ultraviolet Germicidal Irradiation Handbook; Springer: Berlin, Germany, 2009. [Google Scholar]
- Bergman, R.S. Germicidal UV sources and systems. Photochem. Photobiol. 2021, 97, 466–470. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Shatalov, M.; Jain, R.; Saxena, T.; Dobrinsky, A.; Shur, M. Development of deep UV leds and current problems in material and device technology in III-Nitride Semiconductor Optoelectronics. In Semiconductors and Semimetals; Elsevier: Amsterdam, The Netherlands, 2017; Volume 96, pp. 45–83. [Google Scholar]
- Trivellin, N.; Piva, F.; Fiorimonte, D.; Buffolo, M.; De Santi, C.; Orlandi, V.T.; Dughiero, F.; Meneghesso, G.; Zanoni, E.; Meneghini, M. UV-based technologies for sars-cov2 inactivation: Status and perspectives. Electronics 2021, 10, 1703. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-COV-2 to uv irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gerchman, Y.; Mamane, H.; Friedman, N.; Mandelboim, M. Uv-led disinfection of coronavirus: Wavelength effect. J. Photochem. Photobiol. B Biol. 2020, 212, 112044. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W.; et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cavanagh, D. Coronavirus IBV-induced membrane fusion occurs at near-neutral pH. Arch. Virol. 1992, 122, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Polio Laboratory Manual; WHO/IVB/04.10; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Porterfield, J.Z.; Zlotnick, A. A simple and general method for determining the protein and nucleic acid content of viruses by UV absorbance. Virology 2010, 407, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maizel, J.V.; White, D.O.; Scharff, M.D. The polypeptides of adenovirus: I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 1968, 36, 115–125. [Google Scholar] [CrossRef]
- Sender, R.; Bar-On, Y.M.; Gleizer, S.; Bernsthein, B.; Flamholz, A.; Phillips, R.; Milo, R. The total number and mass of SARS-CoV-2 virions. medRxiv, 2021; preprint. [Google Scholar] [CrossRef]
- UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus. Available online: www.fda.gov (accessed on 10 March 2022).
- Ultraviolet Radiation Safety, Environmental Health & Safety. Available online: www.unr.edu (accessed on 10 March 2022).
- Criscuolo, E.; Diotti, R.A.; Ferrarese, R.; Alippi, C.; Viscardi, G.; Signorelli, C.; Mancini, N.; Clementi, M.; Clementi, N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021, 10, 206–209. [Google Scholar] [CrossRef]
- Calgua, B.; Carratalà, A.; Guerrero-Latorre, L.; de Abreu Corrêa, A.; Kohn, T.; Sommer, R.; Girones, R. UVC Inactivation of dsDNA and ssRNA Viruses in Water: UV Fluences and a qPCR-Based Approach to Evaluate Decay on Viral Infectivity. Food Environ. Virol. 2014, 6, 260–268. [Google Scholar] [CrossRef]
- Pecson, B.M.; Valério-Martin, L.; Kohn, T. Quantitative PCR for determining the infectivity of bacteriophage MS2 upon inactivation by heat, UV-B radiation and singlet oxygen: Advantages and limitations of an enzymatic treatment to reduce false-positive results. Appl. Environ. Microbiol. 2009, 68, 5544–5554. [Google Scholar] [CrossRef] [Green Version]
- Mamane-Gravetz, H.; Linden, K.; Cabaj, A.; Sommer, R. Spectral sensitivity of Bacillus subtilis spores and MS2 coliphage for validation testing of ultraviolet reactors for water disinfection. Environ. Sci. Technol. 2005, 39, 7845–7852. [Google Scholar] [CrossRef] [PubMed]
| Number | Manufacturer | Central Wavelength of the Light (nm) | Optical Power (μW) | Light Intensity Value in the Peak (lx) | Lens | |
|---|---|---|---|---|---|---|
| 1 | REFOND | 275 | 7.75 | 0.247 | 1955 | NO |
| 2 | THORLABS | 275 | 0.90 | 0.023 | 49 | YES |
| 3 | THORLABS | 260 | 0.41 | 0.014 | 76 | YES |
| 4 | THORLABS | 255 | 1.40 | 0.044 | 79 | YES |
| 5 | THORLABS | 250 | 0.49 | 0.014 | 45 | YES |
| Diode/Irradiation Time | 1 Control (NonIrradiated) Virus | 2 CCDI50 | 3 ITR |
|---|---|---|---|
| 275 J/40″ | 104.0 | 100.7 | 99.9 |
| 260/11′40″ | 100.5 | 99.9 | |
| 255 J/3′45″ | 101.25 | 99.9 | |
| 250 J/10′17″ | 101.5 | 99.9 |
| Diode/ Irradiation Time | Control (NIVPC) | Irradiated Virus Particle Concentration (IVPC) | ITR |
|---|---|---|---|
| 275 J/40″ | 2.5 × 1013 | 1.5 × 1011 | 99.9 |
| 260/11′40″ | 9.5 × 1010 | 99.9 | |
| 255 J/3′45″ | 1.0 × 1011 | 99.9 | |
| 250 J/10′17″ | 4.0 × 1011 | 99.9 |
| Irradiation Time | 1 Kärber’s Titer | 3 ITR | 2 UV Spectrophotometry | 3 ITR | ||
|---|---|---|---|---|---|---|
| Control NIV | CCDI50 | Control NIVPC | IVPC | |||
| 25″ | 103.5 | 103.3 | 99.9 | 1 × 1012 | 1.1 × 1011 | 99.9999 |
| 32″ | 101.9 | 99.9 | 4.6 × 1011 | 99.9999 | ||
| 36″ | 101.5 | 99.9 | 4.2 × 1011 | 99.9999 | ||
| 40″ | 101.1 | 99.9 | 1.9 × 1011 | 99.9999 | ||
| 44″ | 100.7 | 99.9 | 5.3 × 1010 | 99.9999 | ||
| 48″ | 100.5 | 99.9 | 3.7 × 107 | 99.9999 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobotka, P.; Przychodzki, M.; Uściło, K.; Woliński, T.R.; Staniszewska, M. Effect of Ultraviolet Light C (UV-C) Radiation Generated by Semiconductor Light Sources on Human Beta-Coronaviruses’ Inactivation. Materials 2022, 15, 2302. https://doi.org/10.3390/ma15062302
Sobotka P, Przychodzki M, Uściło K, Woliński TR, Staniszewska M. Effect of Ultraviolet Light C (UV-C) Radiation Generated by Semiconductor Light Sources on Human Beta-Coronaviruses’ Inactivation. Materials. 2022; 15(6):2302. https://doi.org/10.3390/ma15062302
Chicago/Turabian StyleSobotka, Piotr, Maciej Przychodzki, Konrad Uściło, Tomasz R. Woliński, and Monika Staniszewska. 2022. "Effect of Ultraviolet Light C (UV-C) Radiation Generated by Semiconductor Light Sources on Human Beta-Coronaviruses’ Inactivation" Materials 15, no. 6: 2302. https://doi.org/10.3390/ma15062302
APA StyleSobotka, P., Przychodzki, M., Uściło, K., Woliński, T. R., & Staniszewska, M. (2022). Effect of Ultraviolet Light C (UV-C) Radiation Generated by Semiconductor Light Sources on Human Beta-Coronaviruses’ Inactivation. Materials, 15(6), 2302. https://doi.org/10.3390/ma15062302








