Cubic Iron Core–Shell Nanoparticles Functionalized to Obtain High-Performance MRI Contrast Agents
Abstract
:1. Introduction
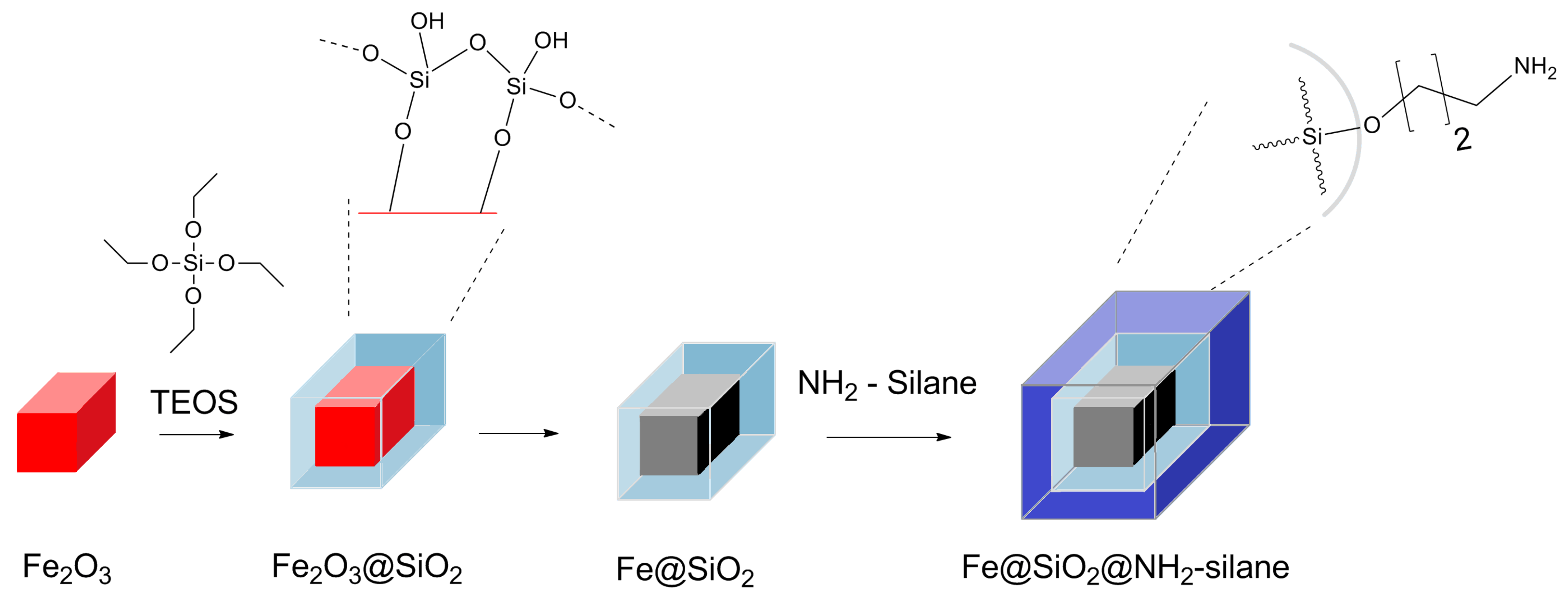
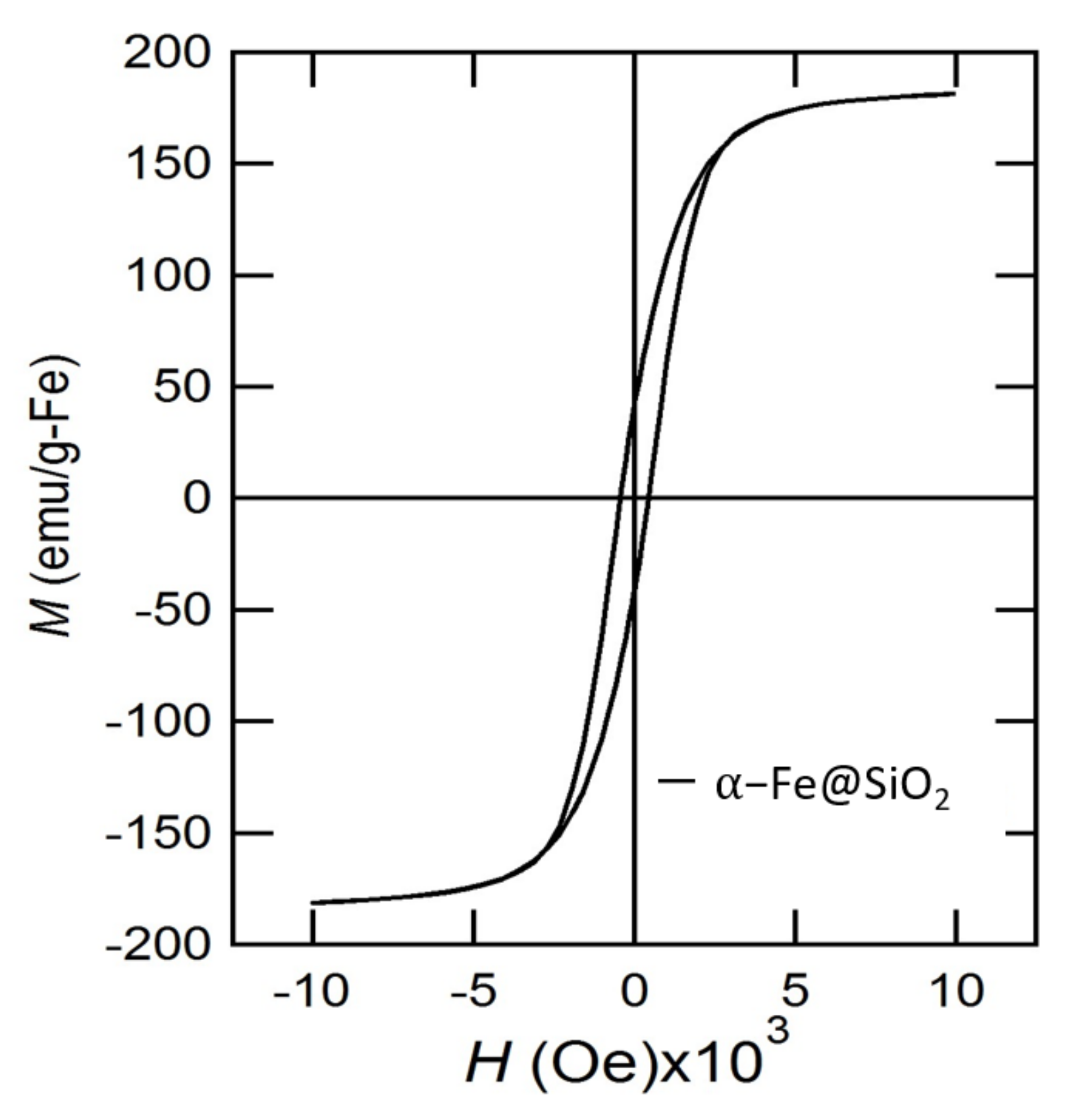
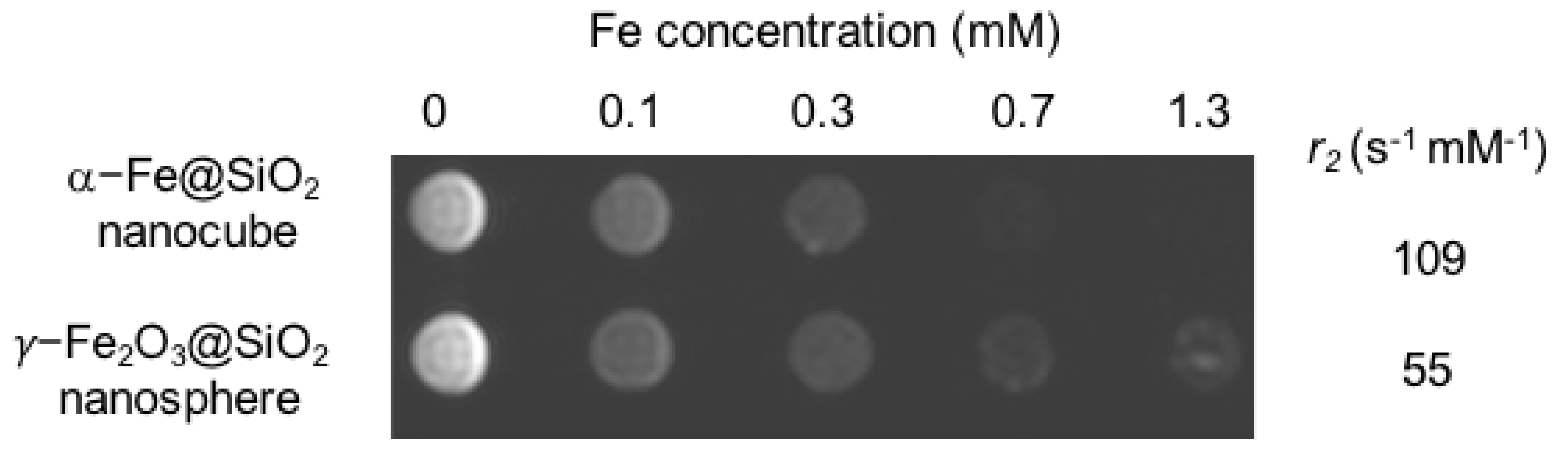
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652–1660. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Bonder, M.J.; Balakrishnan, S.; Wang, X.; Mao, H.; Hadjipanayis, G.C. Metallic Iron Nanoparticles for MRI Contrast Enhancement and Local Hyperthermia. Small 2008, 4, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Z.Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; Gao, J. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266–2273. [Google Scholar]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef]
- Kohara, K.; Yamamoto, S.; Seinberg, L.; Murakami, T.; Tsujimoto, M.; Ogawa, T.; Kurata, H.; Kageyama, H.; Takano, M. Carboxylated SiO2-coated α-Fe nanoparticles: Towards a versatile platform for biomedical applications. Chem. Commun. 2013, 49, 2566–2573. [Google Scholar] [CrossRef]
- Merbach, A.S.; Helm, L.; Tóth, E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Huber, D.L. Synthesis, Properties, and Applications of Iron Nanoparticles. Small 2005, 1, 482–501. [Google Scholar]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, C.S.; Mudakavi, R.J.; Kishore, S.; Kashyap, S.; Elumalai, R.; Chakravortty, D.; Raichurac, A.M.; Chattopadhyay, K. Magnetic iron nanoparticles for in vivo targeted delivery and as biocompatible contrast agents. RSC Adv. 2016, 6, 114344–114352. [Google Scholar] [CrossRef]
- Tartaj, P.; Serna, C.J. Synthesis of Monodisperse Superparamagnetic Fe/Silica Nanospherical Composites. J. Am. Chem. Soc. 2003, 125, 15754–15755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, S.; Lee, I.Y.; Park, S.; Willis, J.; Haldolaarachchige, N.; Young, D.P.; Luo, Z.; Guo, Z. Silica stabilized iron particles toward anti-corrosion magnetic polyurethane nanocomposites. Rsc Adv. 2012, 2, 1136–1143. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ruwan, G.; Tamada, Y.; Kohara, K.; Kusano, Y.; Sasano, T.; Ohno, K.; Tsujii, Y.; Kageyama, H.; Ono, T. Transformation of Nano- to Mesosized Iron Oxide Cores to α-Fe within Organic Shells Preserved Intact. Chem. Mater. 2011, 23, 1564–1569. [Google Scholar] [CrossRef]
- Matsumoto, A.; Sugiura, T.; Kobashi, M.; Yamamoto, S. Preparation and Magnetic Properties of Nano-Sized Iron Powder Particles Coated with Silica Film by Calcium Hydride Reduction Method. Mater. Trans. 2020, 61, 1404–1408. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Palma, R.D.; Peeters, S.; Bael, M.J.V.; den Rul, H.V.; Bonroy, K.; Laureyn, W.; Mullens, J.; Borghs, G.; Maes, G. Silane ligand exchange to make hydrophobic superparamagnetic nanoparticles water-dispersible. Chem. Mater. 2007, 19, 1821–1831. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.; Zhang, H.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar] [CrossRef]
- Zhu, X.M.; Wang, Y.X.; Leung, K.C.; Lee, S.F.; Zhao, F.; Wang, D.W.; Lai, J.M.; Wan, C.; Cheng, C.H.; Ahuja, A.T. Preparation and characterization of magnetite nanoparticles coated by amino silane. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar]
- Mahmoudi, M.; Laurent, S.; Shokrgozar, M.A.; Hosseinkhani, M. Toxicity Evaluations of Superparamagnetic Iron Oxide Nanoparticles: Cell “Vision” versus Physicochemical Properties of Nanoparticles. ACS Nano 2011, 5, 7263–7276. [Google Scholar] [CrossRef] [PubMed]
- Voinov, M.A.; Pagán, J.O.S.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-Mediated Production of Hydroxyl Radicals as a Mechanism of Iron Oxide Nanoparticle Biotoxicity. J. Am. Chem. Soc. 2010, 133, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Decome, L.; Rose, J.; Orsiere, T.; Meo, M.D.; Briois, V.; Chaneac, C.; Olivi, L.; Berge-Lefranc, J.L.; Botta, A. In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: A physicochemical and cyto-genotoxical study. Environ. Sci. Technol. 2006, 40, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar]
- Kunzmann, A.; Andersson, B.; Vogt, C.; Feliu, N.; Ye, F.; Gabrielsson, S.; Toprak, M.S.; Buerki-Thurnherr, T.; Laurent, S.; Vahter, M. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicol. Appl. Pharmacol. 2011, 253, 81–93. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Ivask, A.; Kahru, A.; Vija, H.; Titma, T.; Visnapuu, M.; Joost, U.; Pudova, K.; Adamberg, S.; Visnapuu, T. Bacterial polysaccharide levan as stabilizing, non-toxic and functional coating material for microelement-nanoparticles. Carbohydr. Polym. 2016, 136, 710–720. [Google Scholar] [CrossRef]
- Mesarosova, M.; Ciampor, F.; Zavisova, V.; Koneracka, M.; Ursinyova, M.; Kozics, K.; Tomasovicova, N.; Hashim, A.; Vavra, I.; Krizanova, Z. The intensity of internalization and cytotoxicity of superparamagnetic iron oxide nanoparticles with different surface modifications in human tumor and diploid lung cells. Neoplasma 2012, 59, 584–597. [Google Scholar] [CrossRef]
- Seinberg, L.; Volokhova, M. Metal-Based Core Nanoparticles, Synthesis and Use. WO2021/144006A1, 22 July 2020. [Google Scholar]
- Volokhova, M.; Boldin, A.; Link, J.; Tsujimoto, M.; Stern, R.; Seinberg, L. Synthesis of Ni@SiO2 and Co@SiO2 nanomagnets by using calcium hydride as a reducing agent. J. Mater. Res. Tech. 2021, 12, 988–992. [Google Scholar] [CrossRef]
- Lai, S.M.; Hsiao, J.K.; Yu, H.P.; Lu, C.W.; Huang, C.C.; Shieh, M.J.; Lai, P.S. Polyethylene glycol-based biocompatible and highly stable superparamagnetic iron oxide nanoclusters for magnetic resonance imaging†. J. Mater. Chem. 2012, 22, 15160–15167. [Google Scholar] [CrossRef]
- Carroll1, M.R.J.; Woodward1, R.C.; House1, M.J.; Teoh, W.Y.; Amal, R.; Hanley, T.L.; Pierre1, T.G.S. Experimental validation of proton transverse relaxivity models for superparamagnetic nanoparticle MRI contrast agents. Nanotech 2010, 21, 35103. [Google Scholar] [CrossRef]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.M. Magnetic Nanoparticles and Their Applications in Medicine. Nanomed 2006, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Madler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci-Nano. 2015, 2, 630–644. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volokhova, M.; Shugai, A.; Tsujimoto, M.; Kubo, A.-L.; Telliskivi, S.; Nigul, M.; Uudeküll, P.; Vija, H.; Bondarenko, O.M.; Adamson, J.; et al. Cubic Iron Core–Shell Nanoparticles Functionalized to Obtain High-Performance MRI Contrast Agents. Materials 2022, 15, 2228. https://doi.org/10.3390/ma15062228
Volokhova M, Shugai A, Tsujimoto M, Kubo A-L, Telliskivi S, Nigul M, Uudeküll P, Vija H, Bondarenko OM, Adamson J, et al. Cubic Iron Core–Shell Nanoparticles Functionalized to Obtain High-Performance MRI Contrast Agents. Materials. 2022; 15(6):2228. https://doi.org/10.3390/ma15062228
Chicago/Turabian StyleVolokhova, Maria, Anna Shugai, Masahiko Tsujimoto, Anna-Liisa Kubo, Sven Telliskivi, Mait Nigul, Peep Uudeküll, Heiki Vija, Olesja M. Bondarenko, Jasper Adamson, and et al. 2022. "Cubic Iron Core–Shell Nanoparticles Functionalized to Obtain High-Performance MRI Contrast Agents" Materials 15, no. 6: 2228. https://doi.org/10.3390/ma15062228
APA StyleVolokhova, M., Shugai, A., Tsujimoto, M., Kubo, A.-L., Telliskivi, S., Nigul, M., Uudeküll, P., Vija, H., Bondarenko, O. M., Adamson, J., Kahru, A., Stern, R., & Seinberg, L. (2022). Cubic Iron Core–Shell Nanoparticles Functionalized to Obtain High-Performance MRI Contrast Agents. Materials, 15(6), 2228. https://doi.org/10.3390/ma15062228







