Novel Composite Nitride Nanoceramics from Reaction-Mixed Nanocrystalline Powders in the System Aluminum Nitride AlN/Gallium Nitride GaN/Titanium Nitride TiN (Al:Ga:Ti = 1:1:1)
Abstract
:1. Introduction
2. Experimental Section
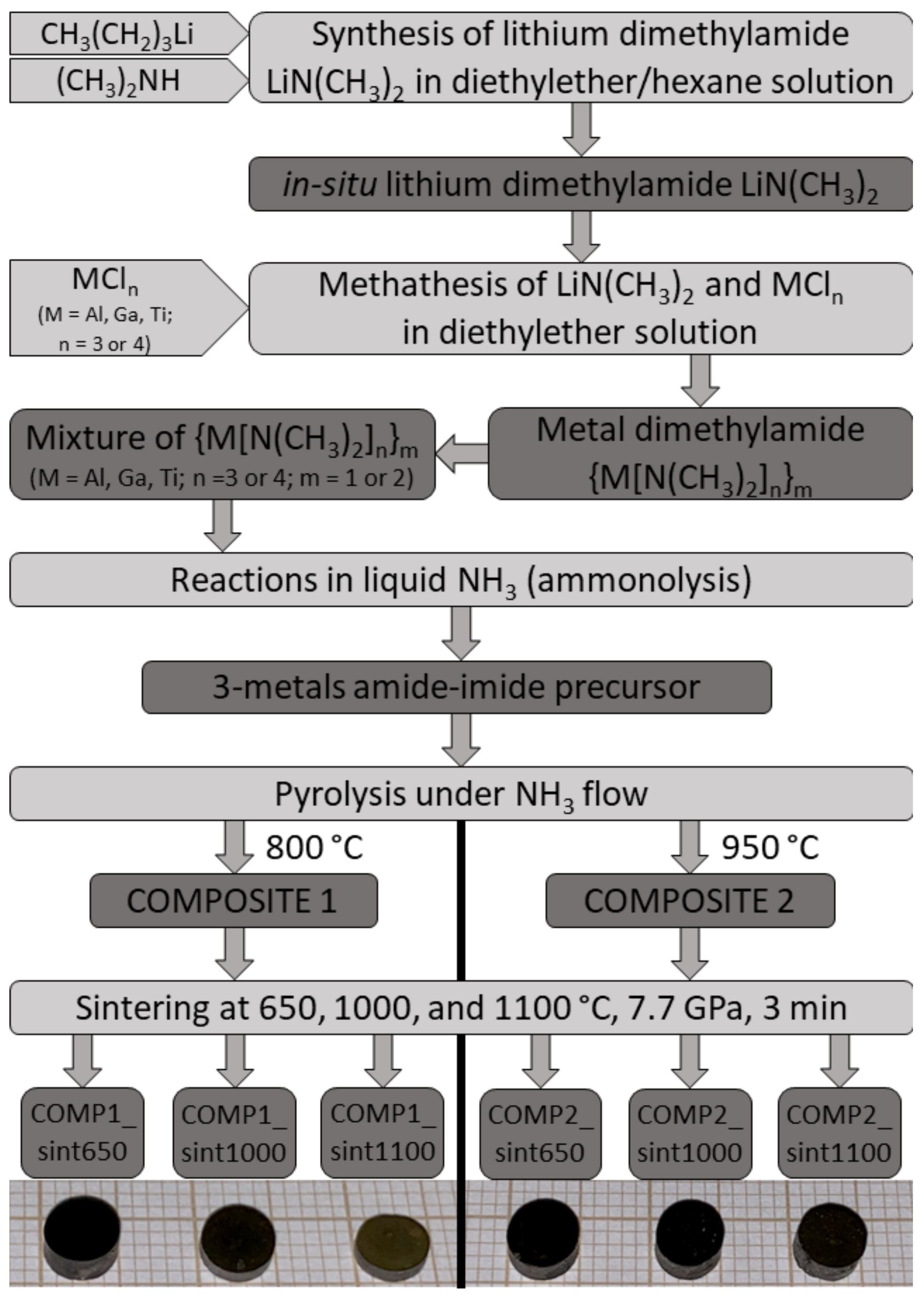
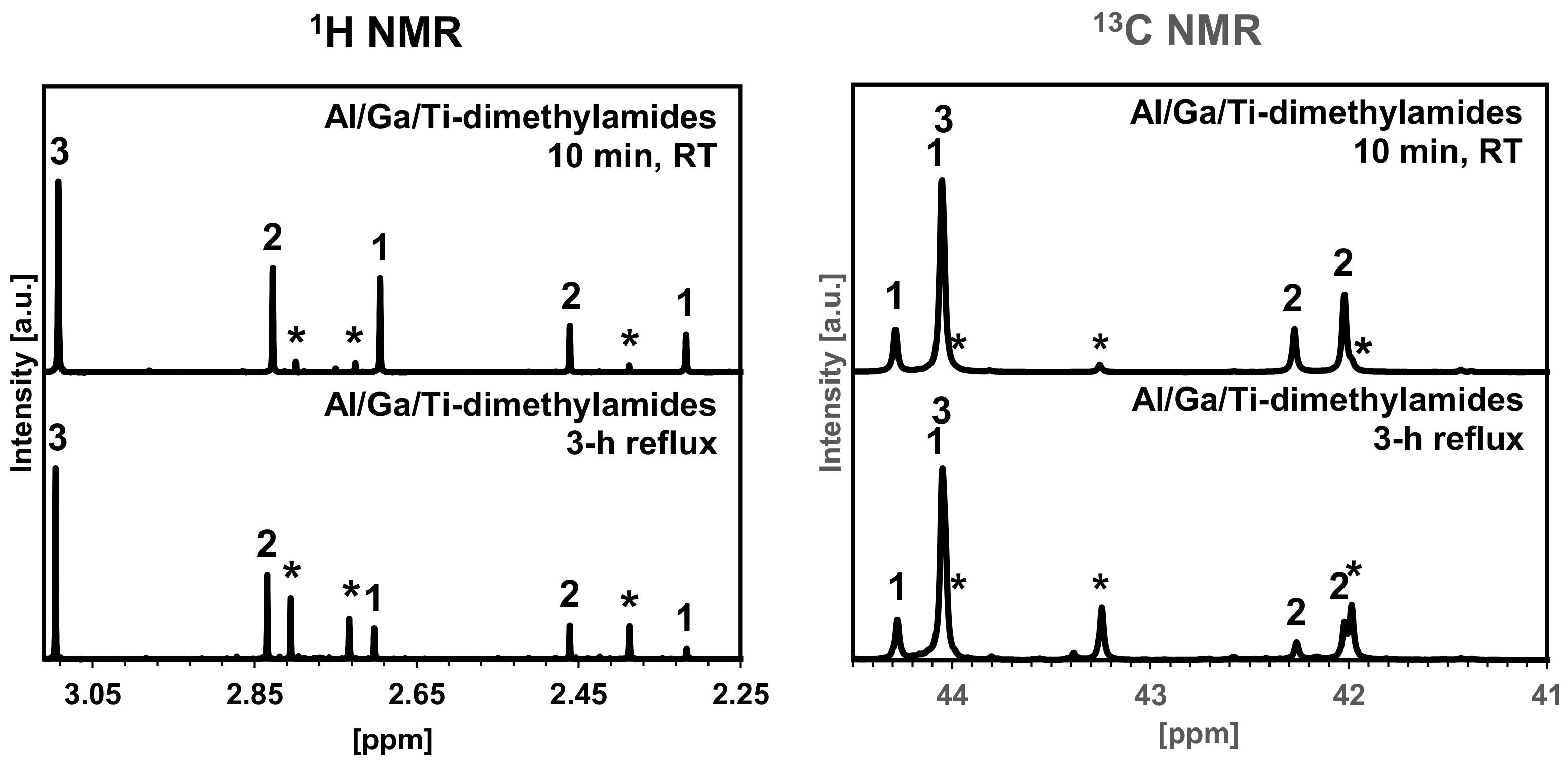
2.1. Preparation of The Mixed Metal–Amide/Imide Precursor via Reflux in Hexane/Effective Equilibration of Ternary Solution in The Metal–Dimethylamide System {Al[N(CH3)2]3}2/{Ga[N(CH3)2]3}2/Ti[N(CH3)2]4, Atomic Ratio Al:Ga:Ti = 1:1:1
2.2. Nitridation Pyrolysis
2.3. No-Additive High-Pressure and High-Temperature Sintering
2.4. Nitride Sample Labeling
2.5. Characterization
3. Results and Discussion
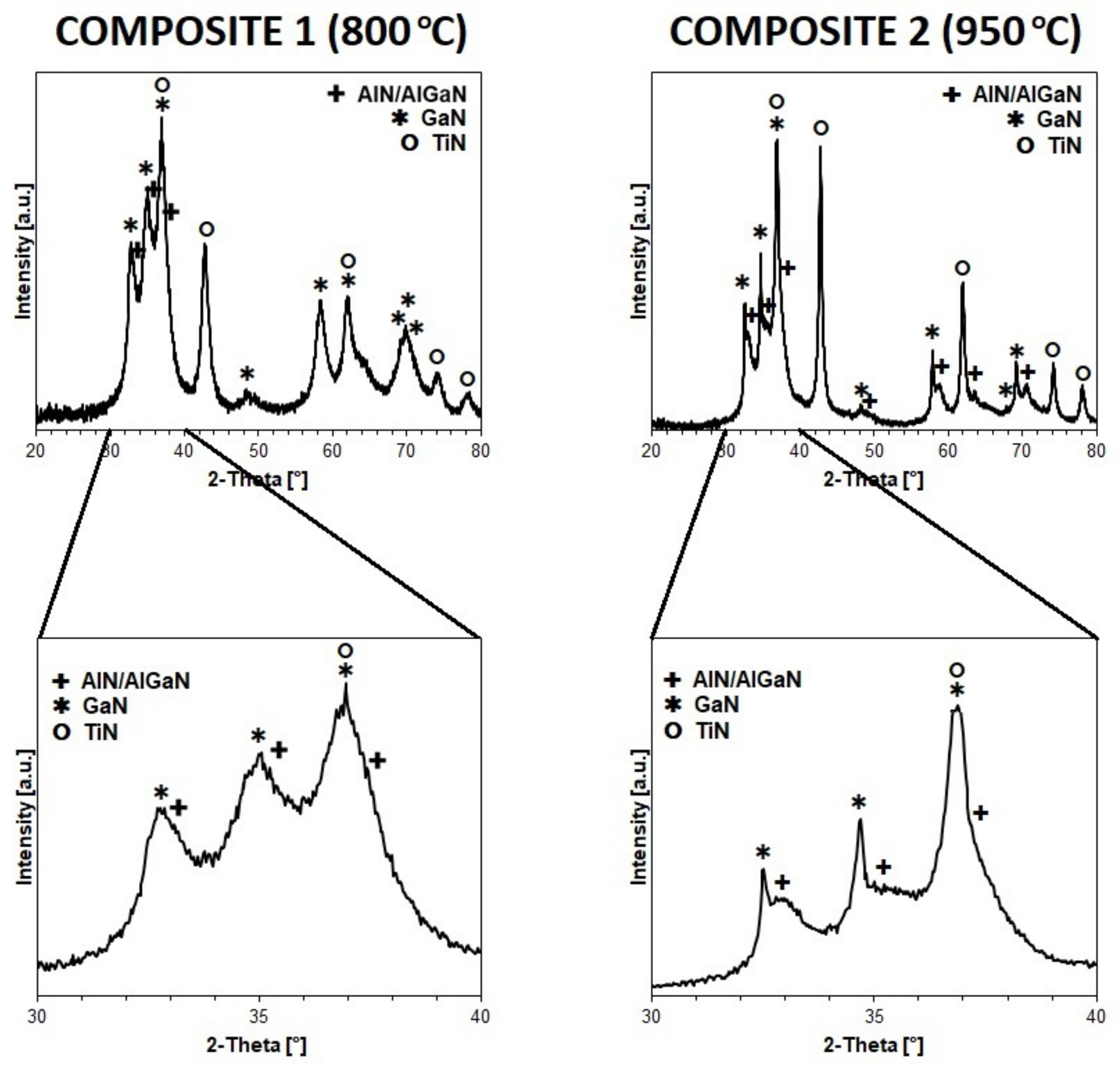
3.1. From Molecular Precursors to Preparation of Nanopowders
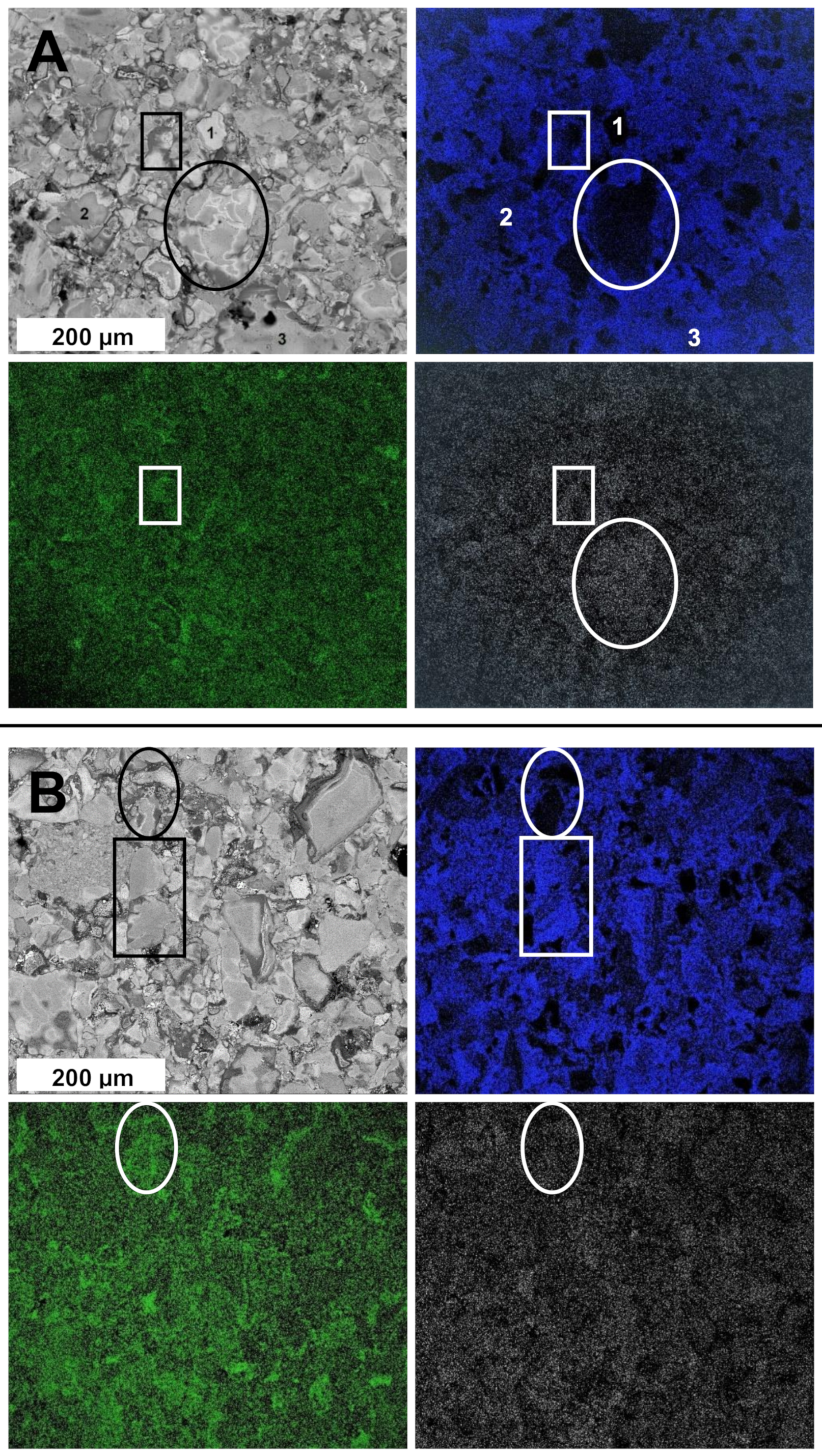
3.2. From Reaction-Mixed Nitride Nanopowders to Sintered Composite Nanoceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parwizian, M.; De Roo, J. Precursor chemistry of metal nitride nanocrystals. Nanoscale 2021, 13, 18865. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S. Background Story of the Invention of Efficient InGaN Blue-Light-Emitting Diodes (Nobel Lecture). Angew. Chem. Int. Ed. 2015, 54, 7770–7788. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Akedo, J. Hexagonal to cubic crystal structure transformation during aerosol deposition of aluminum nitride. J. Cryst. Growth 2005, 275, e1269–e1273. [Google Scholar] [CrossRef]
- Elagin, A.A.; Beketov, A.R.; Baranov, M.V.; Shishkin, R.A. Aluminum nitride. Preparation methods. Refract. Ind. Ceram. 2013, 54, 44. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.F.; Weng, Q.H.; Wang, X.B.; Li, X.; Zhang, J.; Golberg, D.; Bando, Y. Recent progress on fabrications and appli-cations of boron nitride nanomaterials: A review. J. Mater. Sci. Technol. 2015, 31, 589. [Google Scholar] [CrossRef]
- Yang, L.; Ditta, A.; Feng, B.; Zhang, Y.; Xie, Z. Study of the Comparative Effect of Sintering Methods and Sintering Additives on the Microstructure and Performance of Si3N4 Ceramic. Materials 2019, 12, 2142. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Xu, X.; Kimoto, K.; Hirosaki, N.; Tanaka, H. Fabrication of silicon nitride nanoceramics—Powder preparation and sintering: A review. Sci. Technol. Adv. Mater. 2007, 8, 635–643. [Google Scholar] [CrossRef]
- Alhussain, H.; Mise, T.; Matsuo, Y.; Kiyono, H.; Nishikiori, K.; Akashi, T. Influence of ammonia gas exposure on microstructure of nanocrystalline titanium nitride powder synthesized from titanium dioxide. J. Ceram. Soc. Jpn. 2019, 127, 824–829. [Google Scholar] [CrossRef]
- Piprek, J. Efficiency models for GaN-based light-emitting diodes: Status and challenges. Materials 2020, 13, 5174. [Google Scholar] [CrossRef]
- Vecchia, M.D.; Ravyts, S.; Broeck, G.V.D.; Driesen, J. Gallium-Nitride Semiconductor Technology and Its Practical Design Challenges in Power Electronics Applications: An Overview. Energies 2019, 12, 2663. [Google Scholar] [CrossRef] [Green Version]
- Drygas, M.; Kapusta, K.; Janik, J.F.; Bucko, M.M.; Gierlotka, S.; Stelmakh, S.; Palosz, B.; Olejniczak, Z. Novel nanoceramics from in situ made nanocrystalline powders of pure nitrides and their composites in the system aluminum nitride AlN/gallium nitride GaN/aluminum gallium nitride Al0.5Ga0.5N. J. Eur. Ceram. Soc. 2020, 40, 5339–5348. [Google Scholar] [CrossRef]
- Janik, J.F.; Wells, R.L.; Coffer, J.L.; St. John, J.V.; Pennington, W.T.; Schimek, G.L. Nanocrystalline Aluminum Nitride and Aluminum/Gallium Nitride Nanocomposites via Transamination of [M(NMe2)3]2, M = Al, Al/Ga (1/1). Chem. Mater. 1998, 10, 1613–1622. [Google Scholar] [CrossRef]
- Drygas, M.; Lejda, K.; Janik, J.F.; Musielak, B.; Gierlotka, S.; Stelmakh, S.; Palosz, B. Composite nitride nanoceramics in the system titanium nitride (TiN)–aluminum nitride (AlN) through high pressure and high temperature sintering of synthesis-mixed nanocrystalline powders. Materials 2021, 14, 588. [Google Scholar] [CrossRef]
- Drygaś, M.; Lejda, K.; Janik, J.; Łyszczarz, K.; Gierlotka, S.; Stelmakh, S.; Pałosz, B. New Nitride Nanoceramics from Synthesis-Mixed Nanopowders in the Composite System Gallium Nitride GaN–Titanium Nitride TiN. Materials 2021, 14, 3794. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, K.M.; Olmstead, M.M.; Power, P.P. Structural and spectroscopic characterization of the compounds [Al(NMe2)3]2, [Ga(NMe2)3]2, [(Me2N)2Al{μ-N(H)1-Ad}]2 (1-Ad = 1-adamantanyl) and [{Me(μ-NPh2)Al}2NPh(μ-C6H4)]. Polyhedron 1990, 9, 257. [Google Scholar] [CrossRef]
- Nöth, H.; Konrad, P. Darstellung, Struktur und einige Reaktionen von Tris(dimethylamino)gallan/Preparation, Structure and Some Reactions of Trisdimethylaminogallane. Z. Für Nat. B 1975, 30, 681–687. [Google Scholar] [CrossRef]
- Bradley, D.C.; Gitlitz, M.H. Metallo-organic compounds containing metal-nitrogen bonds. Part VI. Infrared and nuclear magnetic resonance of dialkylamido-derivatives of titanium. J. Chem. Soc. A 1969, 980–984. [Google Scholar] [CrossRef]
- Borysiuk, J.; Caban, P.; Strupiński, W.; Gierlotka, S.; Stelmakh, S.; Janik, J.F. TEM investigations of GaN layers grown on silicon and sintered GaN nano-ceramic substrates. Cryst. Res. Technol. 2007, 42, 1291–1296. [Google Scholar] [CrossRef]
- Drygas, M.; Janik, J.F.; Gosk, J.; Gierlotka, S.; Palosz, B.; Twardowski, A. Structural and magnetic properties of ceramics prepared by high-pressure high-temperature sintering of manganese-doped gallium nitride nanopowders. J. Eur. Ceram. Soc. 2016, 36, 1033–1044. [Google Scholar] [CrossRef]
- Maya, L. Synthetic Approaches to Aluminum Nitride via Pyrolysis of a Precursor. Adv. Ceram. Mater. 1986, 1, 150–153. [Google Scholar] [CrossRef]
- Janik, J.F.; Wells, R.L. Gallium Imide, {Ga(NH)3/2}n, a New Polymeric Precursor for Gallium Nitride Powders. Chem. Mater. 1996, 8, 2708–2711. [Google Scholar] [CrossRef]
- Brown, G.M.; Maya, L. Ammonolysis Products of the Dialkylamides of Titanium, Zirconium, and Niobium as Precursors to Metal Nitrides. J. Am. Ceram. Soc. 1988, 71, 78–82. [Google Scholar] [CrossRef]
- Belousov, A.; Katrych, S.; Hametner, K.; Gunther, D.; Karpinski, J.; Batlogg, B. AlxGa1-xN bulk crystal growth: Crystallographic properties and p–T phase diagram. J. Cryst. Growth 2010, 312, 2585. [Google Scholar] [CrossRef]
- Drygas, M.; Olejniczak, Z.; Grzanka, E.; Bucko, M.M.; Paine, R.T.; Janik, J.F. Probing the Structural/Electronic Diversity and Thermal Stability of Various Nanocrystalline Powders of Gallium Nitride GaN. Chem. Mater. 2008, 20, 6816–6828. [Google Scholar] [CrossRef]
- Stoehr, M.; Shin, C.S.; Petrov, I.; Greene, J.E. Raman scattering from TiNx (0.67 ≤ × ≤ 1.00) single crystals grown on MgO (001). J. Appl. Phys. 2011, 110, 83503. [Google Scholar] [CrossRef]
- Ponon, N.K.; Appleby, D.J.; Arac, E.; King, P.; Ganti, S.; Kwa, K.S.; O’Neill, A. Effect of deposition conditions and post deposition anneal on reactively sputtered titanium nitride thin films. Thin Solid Film. 2015, 578, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Xie, Y.; Wang, X.; Lv, S.; Hou, T.; Bai, C. Synthesis of Uniform Titanium Nitride Nanocrystalline Powders via a Reduction-Hydrogenation-Dehydrogenation-Nitridation Route. J. Am. Ceram. Soc. 2004, 88, 249–251. [Google Scholar] [CrossRef]
- Coffer, J.L.; Waldek Zerda, T.; Appel, R.; Wells, R.L.; Janik, J.F. Micro-Raman investigation of nanocrystalline GaN, AlN, and an AlGaN composite prepared from pyrolysis of metal amide-imide precursors. Chem. Mater. 1999, 11, 20. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.D.; Fung, S.; Beling, C.D.; Ling, C.C. Spatial characterization of a 2 in GaN wafer by Raman spectroscopy and capacitance–voltage measurements. J. Phys. D Appl. Phys. 2004, 37, 2814–2818. [Google Scholar] [CrossRef]
- Bernard, M.; Deneuville, A.; Thomas, O.; Gergaud, P.; Sandstrom, P.; Birch, J. Raman spectra of TiN/AlN superlattices. Thin Solid Film. 2000, 380, 252–255. [Google Scholar] [CrossRef]
- Spengler, W.; Kaiser, R.; Christensen, A.N.; Müller-Vogt, G. Raman scattering, superconductivity, and phonon density of states of stoichiometric and nonstoichiometric TiN. Phys. Rev. B 1978, 17, 1095–1101. [Google Scholar] [CrossRef]
- Chen, W.-H.; Qin, Z.-Y.; Tian, X.-Y.; Zhong, X.-H.; Sun, Z.-H.; Li, B.-K.; Zheng, R.-S.; Guo, Y.; Wu, H.-L. The Physical Vapor Transport Method for Bulk AlN Crystal Growth. Molecules 2019, 24, 1562. [Google Scholar] [CrossRef] [Green Version]
- Yonenaga, I. Mechanical stability of power device materials, high temperature hardness of SiC, AlN and GaN. Chem. Sustain. Dev. 2001, 9, 19. [Google Scholar]
- Kishore, N.; Nagarajan, V.; Chandiramouli, R. Mechanical and electronic properties under high pressure on ternary AlGaN and InGaN compound—A first-principles perspective. Mater. Res. Express 2019, 6, 15052. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Lin, Y.-T.; Chan, A.; Chang, J.-T. High Temperature Wear Behavior of Titanium Nitride Coating Deposited Using High Power Impulse Magnetron Sputtering. Coatings 2019, 9, 555. [Google Scholar] [CrossRef] [Green Version]
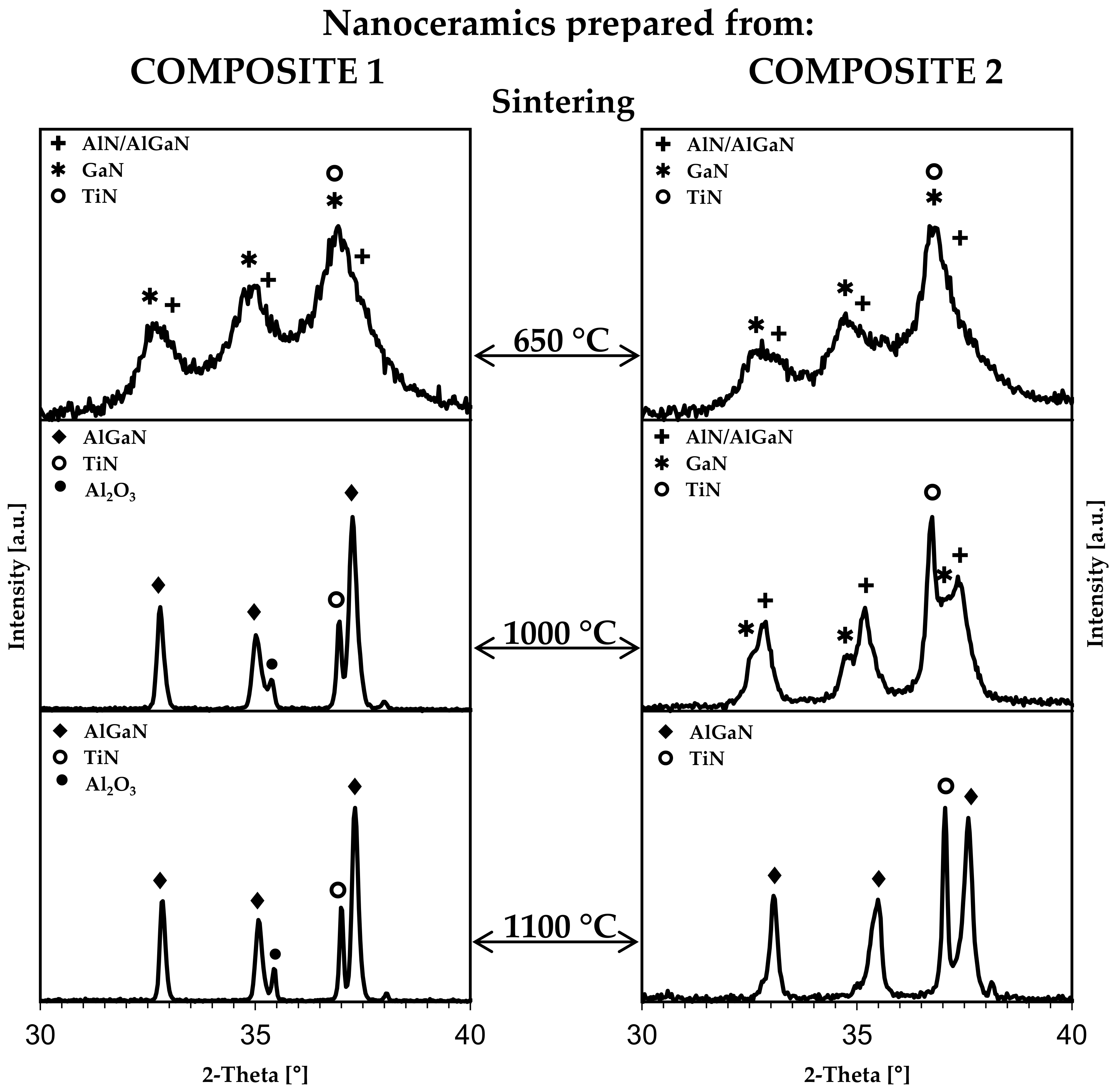

| Composite Nanoceramics from: | Sintering Temperature | ||||
|---|---|---|---|---|---|
| 650 °C | 1000 °C | 1100 °C | |||
| COMPOSITE 1: | Comp1_sint650 | Comp1_sint1000 | Comp1_sint1100 | ||
| (i) hexagonal phase: | GaN | “AlN/AlGaN” | Al0.5Ga0.5N | Al0.5Ga0.5N | |
| a (Å) | 3.20 | 3.17 | 3.18 | 3.18 | |
| c (Å) | 5.19 | 5.11 | 5.16 | 5.16 | |
| Dav (nm) | 6 | – | 58 | 77 | |
| (ii) cubic phase: | c-TiN | c-TiN | c-TiN | ||
| a (Å) | 4.24 | 4.24 | 4.24 | ||
| Dav (nm) | 8 | 93 | 182 | ||
| COMPOSITE 2: | Comp2_sint650 | Comp2_sint1000 | Comp2_sint1100 | ||
| (i) hexagonal phase: | GaN | “AlN/AlGaN” | GaN | ”AlN/AlGaN” | Al0.5Ga0.5N |
| a (Å) | 3.18 | 3.13 | 3.18 | 3.15 | 3.17 |
| c (Å) | 5.17 | 5.06 | 5.15 | 5.10 | 5.13 |
| Dav (nm) | 7 | – | 51 | 23 | 53 |
| (ii) cubic phase: | c-TiN | c-TiN | c-TiN | ||
| a (Å) | 4.24 | 4.24 | 4.25 | ||
| Dav (nm) | 15 | 43 | 86 | ||
| Composite Nanoceramics from: | Sintering Temperature | ||
|---|---|---|---|
| 650 °C | 1000 °C | 1100 °C | |
| COMPOSITE 1 (800 °C) | |||
| dHe (SD) (g/cm3): | 4.66 (0.003) | 5.02 (0.002) | 5.04 (0.003) |
| % theor.: | 95 | 102 | 103 |
| HV (SD) (GPa): | 13.5 (1.3) | 12.1 (0.7) | 12.2 (2.4) |
| COMPOSITE 2 (950 °C) | |||
| dHe (SD) (g/cm3): | 4.71 (0.003) | 4.68 (0.006) | 4.84 (0.004) |
| % theor.: | 96 | 95 | 98 |
| HV (SD) (GPa): | 10.8 (1.7) | 12.1 (1.9) | 10.6 (1.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drygas, M.; Lejda, K.; Janik, J.F.; Stelmakh, S.; Palosz, B. Novel Composite Nitride Nanoceramics from Reaction-Mixed Nanocrystalline Powders in the System Aluminum Nitride AlN/Gallium Nitride GaN/Titanium Nitride TiN (Al:Ga:Ti = 1:1:1). Materials 2022, 15, 2200. https://doi.org/10.3390/ma15062200
Drygas M, Lejda K, Janik JF, Stelmakh S, Palosz B. Novel Composite Nitride Nanoceramics from Reaction-Mixed Nanocrystalline Powders in the System Aluminum Nitride AlN/Gallium Nitride GaN/Titanium Nitride TiN (Al:Ga:Ti = 1:1:1). Materials. 2022; 15(6):2200. https://doi.org/10.3390/ma15062200
Chicago/Turabian StyleDrygas, Mariusz, Katarzyna Lejda, Jerzy F. Janik, Svitlana Stelmakh, and Bogdan Palosz. 2022. "Novel Composite Nitride Nanoceramics from Reaction-Mixed Nanocrystalline Powders in the System Aluminum Nitride AlN/Gallium Nitride GaN/Titanium Nitride TiN (Al:Ga:Ti = 1:1:1)" Materials 15, no. 6: 2200. https://doi.org/10.3390/ma15062200
APA StyleDrygas, M., Lejda, K., Janik, J. F., Stelmakh, S., & Palosz, B. (2022). Novel Composite Nitride Nanoceramics from Reaction-Mixed Nanocrystalline Powders in the System Aluminum Nitride AlN/Gallium Nitride GaN/Titanium Nitride TiN (Al:Ga:Ti = 1:1:1). Materials, 15(6), 2200. https://doi.org/10.3390/ma15062200







