Biodegradable Mg–Zn–Ca-Based Metallic Glasses
Abstract
:1. Introduction
2. Microstructure and Mechanical Properties of Mg–Zn–Ca-Based MGs
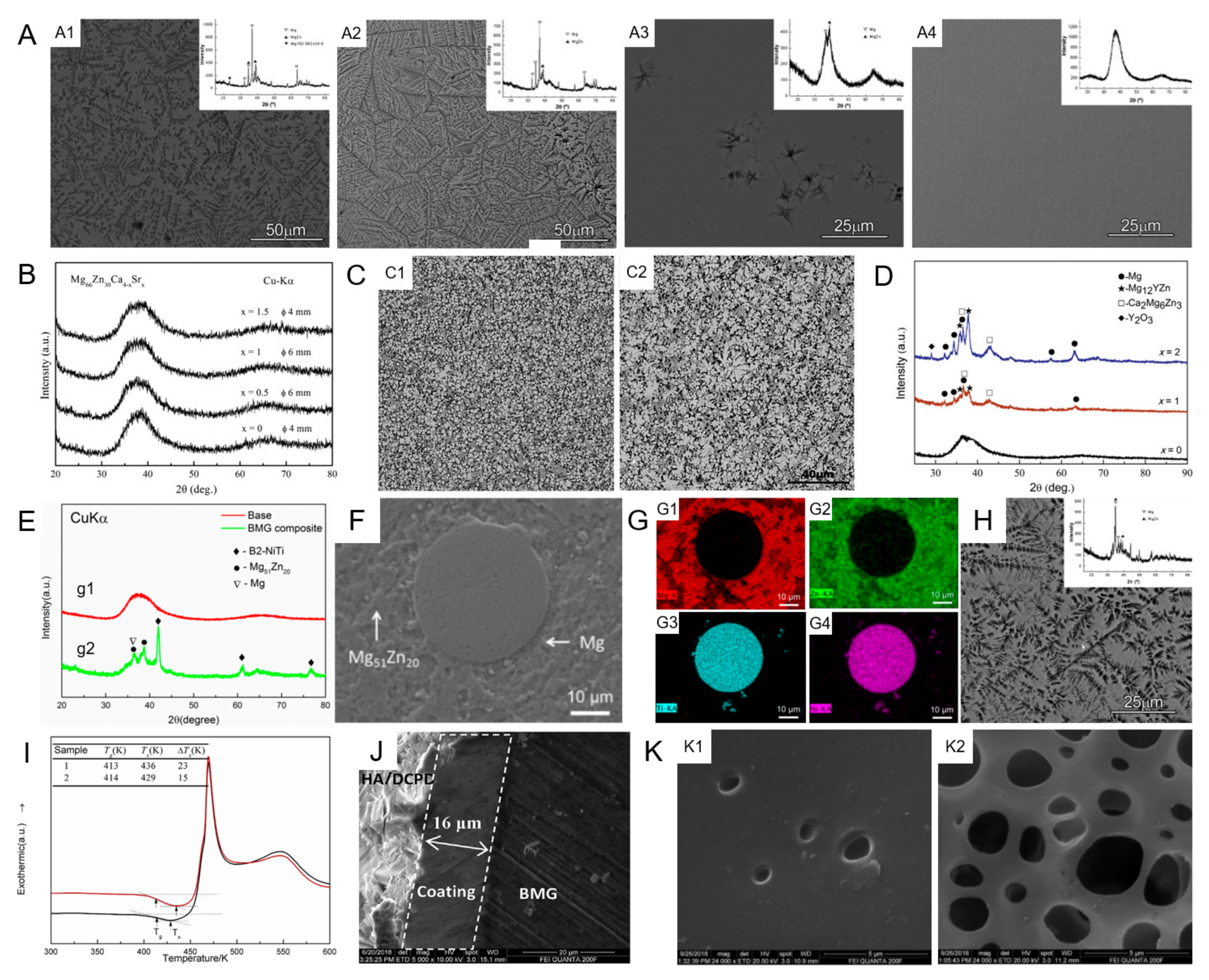
2.1. Microstructure
2.1.1. Mg–Zn–Ca MGs
2.1.2. Mg–Zn–Ca MGs with Minor Alloying Additions
2.1.3. Mg–Zn–Ca MGMCs
2.1.4. Surface Coating of Mg–Zn–Ca MGs
2.1.5. Crystallization Kinetics and Thermal Stability of Mg–Zn–Ca MGs
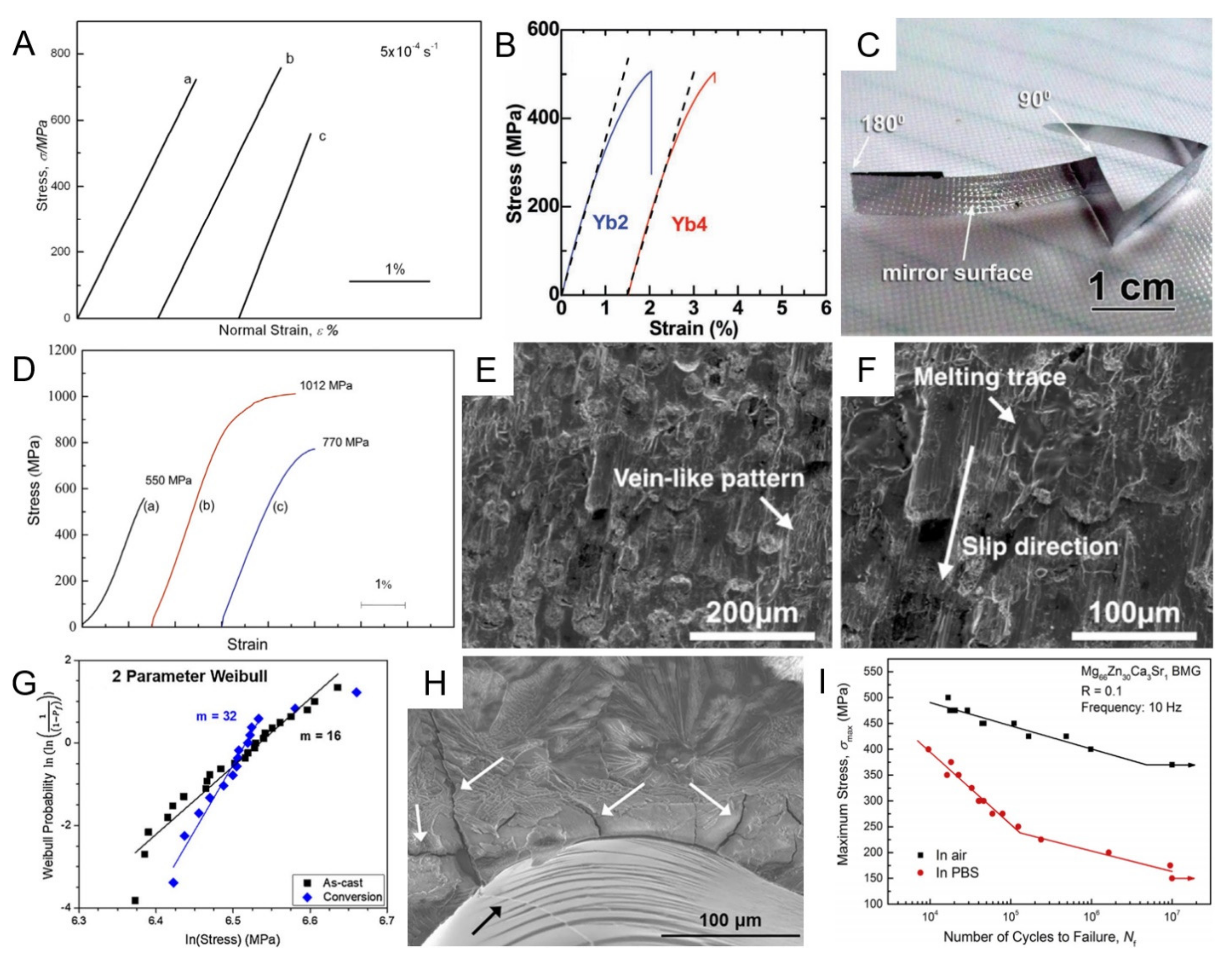
2.2. Mechanical Properties
3. Biocorrosion and Biocompatibility of Mg–Zn–Ca-Based MGs
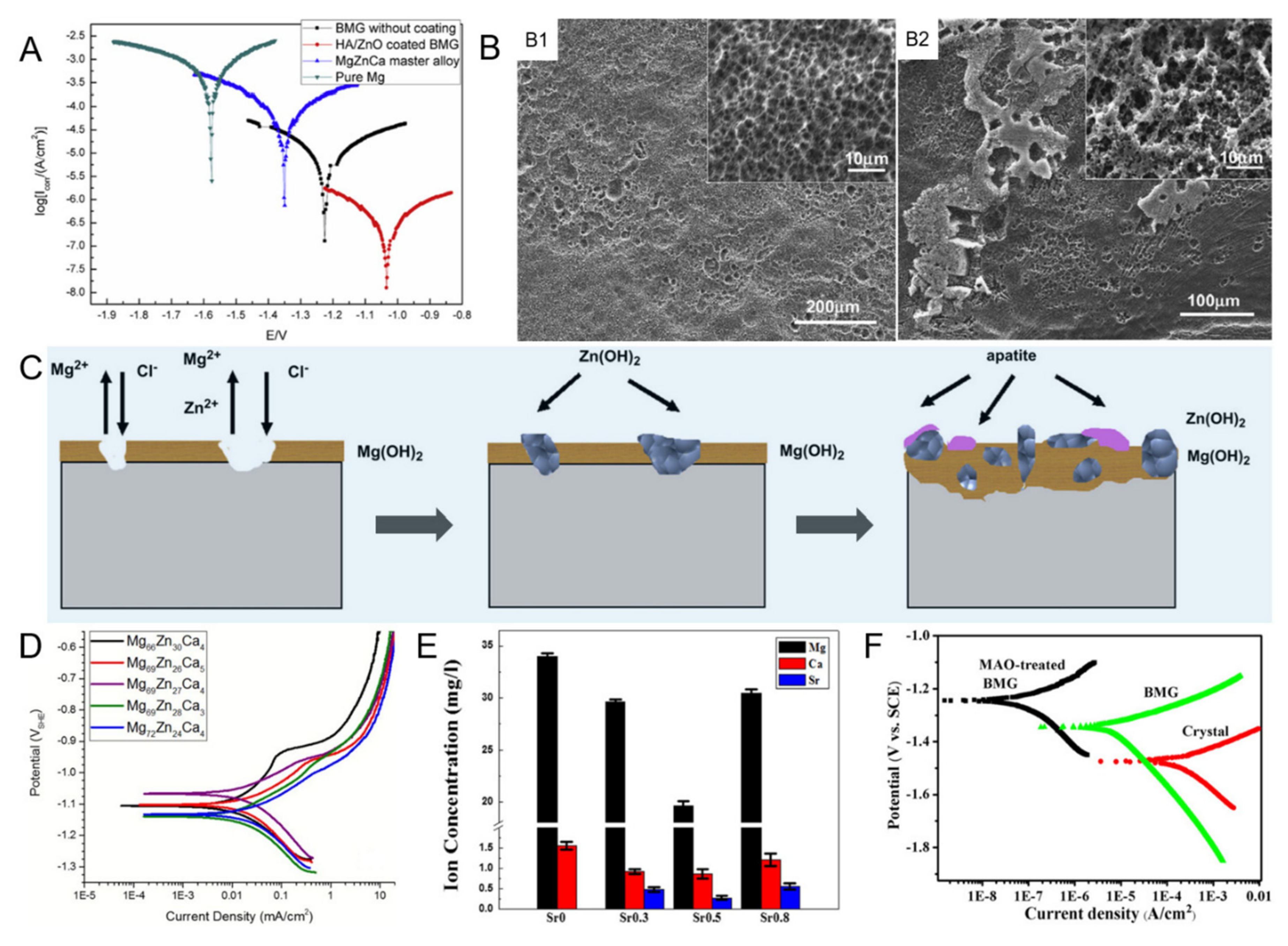
3.1. Biocorrosion
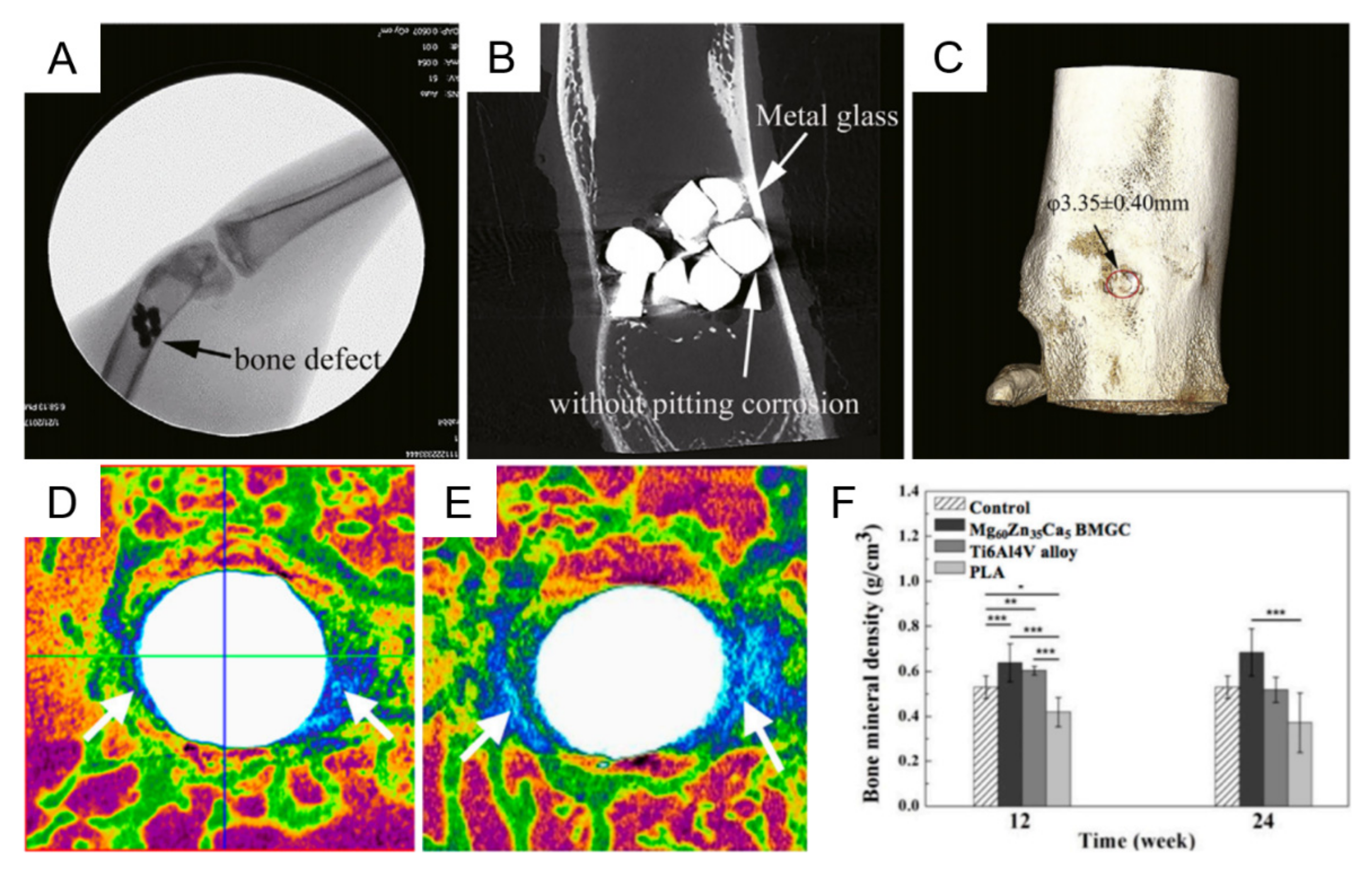
3.2. Biocompatibility
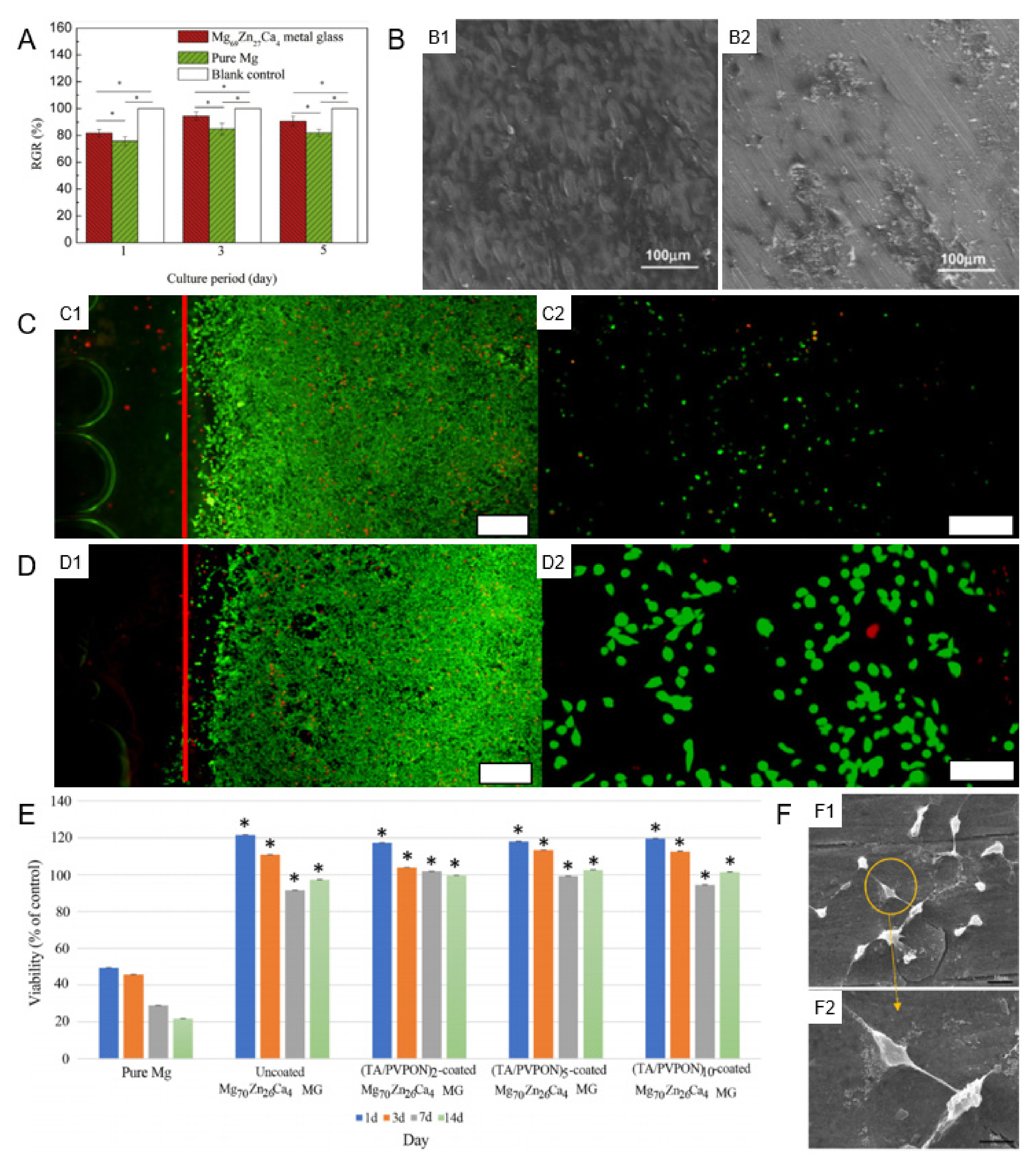
3.2.1. Cellular Biocompatibility
3.2.2. Tissue Biocompatibility
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable magnesium-based implants in orthopedics—A general review and perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Design. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Li, K.H.; Ge, J.C.; Liu, S.N.; Fu, S.; Yin, Z.X.; Zhang, W.T.; Chen, G.X.; Wei, S.C.; Ji, H.; Feng, T.; et al. In situ scattering study of multiscale structural evolution during liquid–liquid phase transition in Mg-based metallic glasses. Rare Met. 2021, 40, 3107–3116. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Role of magnesium in genomic stability. Mut. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Meagher, P.; O’Cearbhaill, E.D.; Byrne, J.H.; Browne, D.J. Bulk metallic glasses for implantable medical devices and surgical tools. Adv. Mater. 2016, 28, 5755–5762. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.; Xu, J. Biodegradable alloys: The glass window of opportunities. Nat. Mater. 2009, 8, 855–857. [Google Scholar] [CrossRef]
- Chen, M. A brief overview of bulk metallic glasses. NPG Asia Mater. 2011, 3, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.A.; Wang, W.H. The fracture of bulk metallic glasses. Prog. Mater. Sci. 2015, 74, 211–307. [Google Scholar] [CrossRef]
- Trexler, M.M.; Thadhani, N.N. Mechanical properties of bulk metallic glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Khan, M.M.; Nemati, A.; Rahman, Z.U.; Shah, U.H.; Asgar, H.; Haider, W. Recent advancements in bulk metallic glasses and their applications: A review. Crit. Rev. Solid State 2017, 43, 233–268. [Google Scholar] [CrossRef]
- Gu, X.; Shiflet, G.J.; Guo, F.Q.; Poon, S.J. Mg–Ca–Zn bulk metallic glasses with high strength and significant ductility. J. Mater. Res. 2005, 20, 1935–1938. [Google Scholar] [CrossRef]
- Zheng, Q.; Cheng, S.; Strader, J.H.; Ma, E.; Xu, J. Critical size and strength of the best bulk metallic glass former in the Mg–Cu–Gd ternary system. Scr. Mater. 2007, 56, 161–164. [Google Scholar] [CrossRef]
- Hsieh, P.J.; Yang, L.C.; Su, H.C.; Lu, C.C.; Jang, J.S.C. Improvement of mechanical properties in MgCuYNdAg bulk metallic glasses with adding Mo particles. J. Alloy. Compd. 2010, 504, S98–S101. [Google Scholar] [CrossRef]
- Zhang, X.L.; Chen, G.; Bauer, T. Mg-based bulk metallic glass composite with high bio-corrosion resistance and excellent mechanical properties. Intermetallics 2012, 29, 56–60. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef]
- Cao, J.D.; Martens, P.; Laws, K.J.; Boughton, P.; Ferry, M. Quantitative in vitro assessment of Mg65Zn30Ca5 degradation and its effect on cell viability. J. Biomed. Mater. Res. B 2013, 101, 43–49. [Google Scholar] [CrossRef]
- Li, Q.F.; Weng, H.R.; Suo, Z.Y.; Ren, Y.L.; Yuan, X.G.; Qiu, K.Q. Microstructure and mechanical properties of bulk Mg–Zn–Ca amorphous alloys and amorphous matrix composites. Mater. Sci. Eng. A 2008, 487, 301–308. [Google Scholar] [CrossRef]
- Nowosielski, R.; Cesarz-Andraczke, K. Impact of Zn and Ca on dissolution rate, mechanical properties and GFA of resorbable Mg–Zn–Ca metallic glasses. Arch. Civ. Mech. Eng. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, N. New bulk metallic glasses for applications as magnetic-sensing, chemical, and structural materials. MRS Bull. 2007, 32, 651–658. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Hofmann, D.; Jarvis, D.J.; Fecht, H.J. Low-density high-strength bulk metallic glasses and their composites: A review. Adv. Eng. Mater. 2015, 17, 761–780. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Qin, C.; Yu, H.; Xia, X.; Wang, C.; Zhang, Y.; Hu, Q.; Zhao, W. Dealloying of Cu-Based metallic glasses in acidic solutions: Products and energy storage applications. Nanomaterials 2015, 5, 697–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.C.; Liang, S.X. Fe-based metallic glasses in functional catalytic applications. Chem. Asian J. 2018, 13, 3575–3592. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Sun, C.; Sun, C. Functional applications of metallic glasses in electrocatalysis. ChemCatChem 2019, 11, 2401–2414. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Nozaki, A.; Navlani-García, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Recent applications of amorphous alloys to design skeletal catalysts. Bull. Chem. Soc. Jpn. 2020, 93, 438–454. [Google Scholar] [CrossRef]
- Qin, C.; Zhao, W.; Inoue, A. Glass formation, chemical properties and surface analysis of Cu-based bulk metallic glasses. Int. J. Mol. Sci. 2011, 12, 2275–2293. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Lu, Z.C.; Wang, S.L.; Wu, Y.; Lu, Z.P. Fe-based bulk metallic glasses: Glass formation, fabrication, properties and applications. Prog. Mater. Sci. 2019, 103, 235–318. [Google Scholar] [CrossRef]
- Qin, C.; Hu, Q.; Li, Y.; Wang, Z.; Zhao, W.; Louzguine-Luzgin, D.V.; Inoue, A. Novel bioactive Fe-based metallic glasses with excellent apatite-forming ability. Mater. Sci. Eng. C 2016, 69, 513–521. [Google Scholar] [CrossRef]
- Xie, G.; Qin, F.; Zhu, S. Recent progress in Ti-based metallic glasses for application as biomaterials. Mater. Trans. 2013, 54, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Zhou, X.; Liu, X. Application of Zr and Ti-based bulk metallic glasses for orthopaedic and dental device materials. Metals 2020, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Schroers, J.; Kumar, G.; Hodges, T.M.; Chan, S.; Kyriakides, T.R. Bulk metallic glasses for biomedical applications. JOM 2009, 61, 21–29. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Yahaya, B.; Lim, J.Y.; Kurniawan, D. Mg-based bulk metallic glasses for biodegradable implant materials: A review on glass forming ability, mechanical properties, and biocompatibility. J. Non Cryst. Solids 2015, 426, 110–115. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, X.; Xiong, H.; Zhou, J.; Yu, H.; Qin, C.; Wang, Z. Stearic acid coated MgO nanoplate arrays as effective hydrophobic films for improving corrosion resistance of Mg-based metallic glasses. Nanomaterials 2020, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Kiani, F.; Wen, C.; Li, Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites-A review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef]
- Song, M.S.; Zeng, R.C.; Ding, Y.F.; Li, R.W.; Easton, M.; Cole, I.; Birbilis, N.; Chen, X.B. Recent advances in biodegradation controls over Mg alloys for bone fracture management: A review. J. Mater. Sci. Technol. 2019, 35, 535–544. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, S.S.; Li, X.M.; Fan, Y.B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. B 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, J.; Yu, H.; Chen, C. Degradable magnesium-based alloys for biomedical applications: The role of critical alloying elements. J. Biomater. Appl. 2019, 33, 1348–1372. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. Biometals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Shabib, I.; Haider, W. The current trends of Mg alloys in biomedical applications—A review. J. Biomed. Mater. Res. B 2019, 107, 1970–1996. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, J.; Fu, H.; Zhang, H.; Tan, L.; Zhao, D.; Yang, K. In vitro and in vivo studies on the biodegradable behavior and bone response of Mg69Zn27Ca4 metal glass for treatment of bone defect. J. Mater. Sci. Technol. 2019, 35, 2254–2262. [Google Scholar] [CrossRef]
- Matias, T.B.; Roche, V.; Nogueira, R.P.; Asato, G.H.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Jorge, A.M. Mg-Zn-Ca amorphous alloys for application as temporary implant: Effect of Zn content on the mechanical and corrosion properties. Mater. Des. 2016, 110, 188–195. [Google Scholar] [CrossRef]
- Fu, W.; Sun, Y.; Zhang, W. The effect of cooling rate on microstructure and mechanical properties of Zr-Based bulk metallic glasses. Adv. Mater. Sci. Eng. 2013, 2013, 826758. [Google Scholar] [CrossRef] [Green Version]
- Song, S.M.; Wong, P.C.; Chiang, C.W.; Tsai, P.H.; Jang, J.S.C.; Chen, C.H. A bi-phase core–shell structure of Mg-based bulk metallic glass for application in orthopedic fixation implants. Mater. Sci. Eng. C 2020, 111, 110783. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg–Zn–Ca–Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, S.; Wei, Y.; Xi, X.; Cai, K.; Pan, F. Effects of Y on the microstructure, mechanical and bio-corrosion properties of Mg–Zn–Ca bulk metallic glass. J. Mater. Sci. Technol. 2014, 30, 1255–1261. [Google Scholar] [CrossRef]
- Guo, W.; Kato, H.; Lü, S.; Wu, S. Porous NiTi particle dispersed Mg-Zn-Ca bulk metallic glass matrix composites. Materials 2018, 11, 1959. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, K.; Wang, B.; Ai, F. Nano-hydroxyapatite/ZnO coating prepared on a biodegradable Mg–Zn–Ca bulk metallic glass by one-step hydrothermal method in acid situation. Ceram. Int. 2020, 46, 6958–6964. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Zhou, J.; Li, S.Y.; Huang, P.R. In vitro corrosion resistance and cytocompatibility of Mg66Zn28Ca6 amorphous alloy materials coated with a double-layered nHA and PCL/nHA coating. Colloid Surf. B Biointerfaces 2020, 196, 111251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Ma, E.; Xu, J. Reliability of compressive fracture strength of Mg–Zn–Ca bulk metallic glasses: Flaw sensitivity and Weibull statistics. Scr. Mater. 2008, 58, 496–499. [Google Scholar] [CrossRef]
- Wang, W.H. Roles of minor additions in formation and properties of bulk metallic glasses. Prog. Mater. Sci. 2007, 52, 540–596. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Guo, S.; Jiang, W.; Liu, Q. Effect of Sr on the microstructure and biodegradable behavior of Mg–Zn–Ca-Mn alloys for implant application. Mater. Des. 2018, 153, 308–316. [Google Scholar] [CrossRef]
- Zai, W.; Man, H.C.; Su, Y.; Li, G.; Lian, J. Impact of microalloying element Ga on the glass-forming ability (GFA), mechanical properties and corrosion behavior of Mg–Zn–Ca bulk metallic glass. Mater. Chem. Phys. 2020, 255, 123555. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Li, Y.; Wei, Y.; Xi, X.; Cai, K. Microstructure, mechanical and bio-corrosion properties of Mn-doped Mg–Zn–Ca bulk metallic glass composites. Mater. Sci. Eng. C 2013, 33, 3832–3838. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhu, J.; Chang, L.; Song, J.G.; Chen, X.H.; Hui, X.D. Influence of Cu content on the mechanical properties and corrosion resistance of Mg-Zn-Ca bulk metallic glasses. Int. J. Min. Met. Mater. 2014, 21, 487–493. [Google Scholar] [CrossRef]
- Gonzalez, S.; Pellicer, E.; Fornell, J.; Blanquer, A.; Barrios, L.; Ibanez, E.; Solsona, P.; Surinach, S.; Baro, M.D.; Nogues, C.; et al. Improved mechanical performance and delayed corrosion phenomena in biodegradable Mg–Zn–Ca alloys through Pd-alloying. J. Mech. Behav. Biomed. 2012, 6, 53–62. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Liaw, P.K.; Zhang, T. In vitro investigation of Mg–Zn–Ca–Ag bulk metallic glasses for biomedical applications. J. Non Cryst. Solids 2015, 427, 134–138. [Google Scholar] [CrossRef]
- Chen, M. Mechanical behavior of metallic glasses: Microscopic understanding of strength and ductility. Ann. Rev. Mater. Res. 2008, 38, 445–469. [Google Scholar] [CrossRef]
- Wong, P.C.; Lee, T.H.; Tsai, P.H.; Cheng, C.K.; Li, C.; Jang, J.S.C.; Huang, J.C. Enhanced mechanical properties of MgZnCa bulk metallic glass composites with Ti-particle dispersion. Metals 2016, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.C.; Song, S.M.; Nien, Y.Y.; Wang, W.R.; Tsai, P.H.; Wu, J.L.; Jang, J.S.C. Mechanical properties enhanced by the dispersion of porous Mo particles in the biodegradable solid and bi-phase core–shell structure of Mg-based bulk metallic glass composites for applications in orthopedic implants. J. Alloy. Compd. 2021, 877, 160233. [Google Scholar] [CrossRef]
- Qiao, J. In-situ dendrite/metallic glass matrix composites: A review. J. Mater. Sci. Technol. 2013, 29, 685–701. [Google Scholar] [CrossRef]
- Chen, S.; Tu, J.; Hu, Q.; Xiong, X.; Wu, J.; Zou, J.; Zeng, X. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Hu, L.; Ye, F. Crystallization kinetics of Ca65Mg15Zn20 bulk metallic glass. J. Alloy. Compd. 2013, 557, 160–165. [Google Scholar] [CrossRef]
- Sun, Y.D.; Shen, P.; Li, Z.Q.; Liu, J.S.; Cong, M.Q.; Jiang, M. Kinetics of crystallization process of Mg–Cu–Gd based bulk metallic glasses. J. Non Cryst. Solids 2012, 358, 1120–1127. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Rocher, G.J.; Briccoli, B.; Kevorkov, D.; Liu, X.B.; Altounian, Z.; Medraj, M. Crystallization characteristics of the Mg-rich metallic glasses in the Ca–Mg–Zn system. J. Alloy. Compd. 2013, 552, 88–97. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Li, S.; Xu, L.; Zhao, B.; Zhai, Q.; Gao, Y. Revealing the crystallization kinetics and phase transitions in Mg65Zn30Ca5 metallic glass by nanocalorimetry. J. Alloy. Compd. 2022, 899, 163353. [Google Scholar] [CrossRef]
- Opitek, B.; Lelito, J.; Szucki, M.; Piwowarski, G.; Gondek, L.; Rogal, L. Analysis of the Crystallization Kinetics and Thermal Stability of the Amorphous Mg72Zn24Ca4 Alloy. Materials 2021, 14, 3583. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Zhong, S.; Xi, T.; Wang, J.; Wang, W. Corrosion of, and cellular responses to Mg–Zn–Ca bulk metallic glasses. Biomaterials 2010, 31, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xie, G.; Dan, Z.; Zhu, S.; Seki, I. Corrosion behavior and mechanical properties of Mg–Zn–Ca amorphous alloys. Intermetallics 2013, 42, 9–13. [Google Scholar] [CrossRef]
- Yu, H.J.; Wang, J.Q.; Shi, X.T.; Louzguine-Luzgin, D.V.; Wu, H.K.; Perepezko, J.H. Ductile biodegradable Mg-based metallic glasses with excellent biocompatibility. Adv. Funct. Mater. 2013, 23, 4793–4800. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, H.; Liaw, P.K. Metallic glass matrix composites. Mater. Sci. Eng. R 2016, 100, 1–69. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Chan, K.C.; Guo, S.F.; Yu, P. Plasticity improvement of an Fe-based bulk metallic glass by geometric confinement. Mater. Lett. 2011, 65, 1172–1175. [Google Scholar] [CrossRef]
- Chen, W.; Chan, K.C.; Yu, P.; Wang, G. Encapsulated Zr-based bulk metallic glass with large plasticity. Mater. Sci. Eng. A 2011, 528, 2988–2994. [Google Scholar] [CrossRef]
- Chen, W.; Chan, K.C.; Chen, S.H.; Guo, S.F.; Li, W.H.; Wang, G. Plasticity enhancement of a Zr-based bulk metallic glass by an electroplated Cu/Ni bilayered coating. Mater. Sci. Eng. A 2012, 552, 199–203. [Google Scholar] [CrossRef]
- Miskovic, D.M.; Pohl, K.; Birbilis, N.; Laws, K.J.; Ferry, M. Formation of a phosphate conversion coating on bioresorbable Mg-based metallic glasses and its effect on corrosion performance. Corros. Sci. 2017, 129, 214–225. [Google Scholar] [CrossRef]
- Wong, P.C.; Tsai, P.H.; Li, T.H.; Cheng, C.K.; Jang, J.S.C.; Huang, J.C. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloy. Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, L.; Li, Y.; Wang, X.; Qin, C.; Zhao, W.; Yu, H.; Wang, Z. Effects of Ag, Nd, and Yb on the microstructures and mechanical properties of Mg–Zn–Ca metallic glasses. Metals 2018, 8, 856. [Google Scholar] [CrossRef] [Green Version]
- Zberg, B.; Arata, E.R.; Uggowitzer, P.J.; Löffler, J.F. Tensile properties of glassy MgZnCa wires and reliability analysis using Weibull statistics. Acta Mater. 2009, 57, 3223–3231. [Google Scholar] [CrossRef]
- He, M.; Wang, H.; Zhou, K.; Pan, D.; Liu, F. Effects of Li addition on the corrosion behaviour and biocompatibility of Mg(Li)–Zn–Ca metallic glasses. J. Mater. Sci. 2018, 53, 9928–9942. [Google Scholar]
- Wang, J.; Huang, S.; Wei, Y.; Guo, S.; Fusheng, P. Enhanced mechanical properties and corrosion resistance of a Mg–Zn–Ca bulk metallic glass composite by Fe particle addition. Mater. Lett. 2013, 91, 311–314. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Pang, S.; Liaw, P.K.; Zhang, T. Corrosion fatigue behavior of a Mg-based bulk metallic glass in a simulated physiological environment. Intermetallics 2016, 73, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liang, Z.; Yang, L.; Zhao, W.; Wang, Y.; Yu, H.; Qin, C.; Wang, Z. Surface morphologies and mechanical properties of Mg-Zn-Ca amorphous alloys under chemistry-mechanics interactive environments. Metals 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Vojtěch, D.; Kubásek, J.; Čapek, J. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Hermawan, H.; Dube, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.; Gebert, A.; Ghinea, A.C.; Gostin, P.F.; Abdi, S.; Mickel, C.; Eckert, J. Designing biocompatible Ti-based metallic glasses for implant applications. Mater. Sci. Eng. C 2013, 33, 875–883. [Google Scholar] [CrossRef]
- Cesarz-Andraczke, K.; Nowosielski, R.; Babilas, R. Corrosion properties of Mg-Zn-Ca-(Cu,Au) metallic glasses in artificial physiological fluid. Arch. Civ. Mech. Eng. 2019, 19, 716–723. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, X.; Etim, I.P.; Siddiqui, M.A.; Su, X. Comparative study of the effects of MAO coating and Ca-P coating on the biodegradation and biocompatibility of Mg69Zn27Ca4 metal glass. Mater. Technol. 2022, 37, 21–27. [Google Scholar] [CrossRef]
- Miskovic, D.M.; Pohl, K.; Birbilis, N.; Laws, K.J.; Ferry, M. Examining the elemental contribution towards the biodegradation of Mg–Zn–Ca ternary metallic glasses. J. Mater. Chem. B 2016, 4, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.F.; Chan, K.C.; Jiang, X.Q.; Zhang, H.J.; Zhang, D.F.; Wang, J.F.; Jiang, B.; Pan, F.S. Atmospheric RE-free Mg-based bulk metallic glass with high bio-corrosion resistance. J. Non Cryst. Solids 2013, 379, 107–111. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Subramanian, S.; Raj, B.; Ravi, K.R. Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg–Zn–Ca bulk metallic glass for biomedical applications. Mater. Des. 2015, 86, 829–835. [Google Scholar] [CrossRef]
- Chlewicka, M.; Cieślak, G.; Dobkowska, A.; Mizera, J. The impact of different volume fractions of crystalline structures on the electrochemical behaviour of Mg67Zn29Ca4 alloys for biomedical applications. Corros. Eng. Sci. Technol. 2019, 54, 659–665. [Google Scholar] [CrossRef]
- Ford, D.C.; Hicks, D.; Oses, C.; Toher, C.; Curtarolo, S. Metallic glasses for biodegradable implants. Acta Mater. 2019, 176, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Wan, Y.; Ma, Z.J.; Guo, Y.C.; Yang, Z.; Wang, P.; Li, J.P. Glass-forming ability and corrosion performance of Mn-doped Mg–Zn–Ca amorphous alloys for biomedical applications. Rare Met. 2018, 37, 579–586. [Google Scholar] [CrossRef]
- Sun, S.J.; Ju, S.P.; Yang, C.C.; Chang, K.C.; Lee, I.J. Effects of Strontium incorporation to Mg-Zn-Ca biodegradable bulk metallic glass investigated by molecular dynamics simulation and density functional theory calculation. Sci. Rep. 2020, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications. Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Stormer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Chan, W.Y.; Chian, K.S.; Tan, M.J. In vitro metal ion release and biocompatibility of amorphous Mg67Zn28Ca5 alloy with/without gelatin coating. Mater. Sci. Eng. C 2013, 33, 5019–5027. [Google Scholar] [CrossRef] [PubMed]
- Buehler, J.; Chappuis, P.; Saffar, J.L.; Tsouderos, Y.; Vignery, A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis). Bone 2001, 29, 176–179. [Google Scholar] [CrossRef]
- Monfared, A.; Ghaee, A.; Ebrahimi-Barough, S. Fabrication of tannic acid/poly(N-vinylpyrrolidone) layer-by-layer coating on Mg-based metallic glass for nerve tissue regeneration application. Colloid Surf. B 2018, 170, 617–626. [Google Scholar] [CrossRef]
- Dong, J.; Tan, L.; Yang, J.; Wang, Y.; Chen, J.; Wang, W.; Zhao, D.; Yang, K. In vitro and in vivo studies on degradation and bone response of Mg-Sr alloy for treatment of bone defect. Mater. Technol. 2018, 33, 387–397. [Google Scholar] [CrossRef]
- Wong, C.C.; Wong, P.C.; Tsai, P.H.; Jang, J.S.; Cheng, C.K.; Chen, H.H.; Chen, C.H. Biocompatibility and osteogenic capacity of Mg-Zn-Ca bulk metallic glass for rabbit tendon-bone interference fixation. Int. J. Mol. Sci. 2019, 20, 2191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ouyang, D.; Pauly, S.; Liu, L. 3D printing of bulk metallic glasses. Mat. Sci. Eng. R 2021, 145, 100625. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental theory of biodegradable metals—Definition, criteria, and design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Smith, M.R.; Atkinson, P.; White, D.; Piersma, T.; Gutierrez, G.; Rossini, G.; Desai, S.; Wellinghoff, S.; Yu, H.; Cheng, X. Design and assessment of a wrapped cylindrical Ca-P AZ31 Mg alloy for critical-size ulna defect repair. J. Biomed. Mater. Res. B 2012, 100, 206–216. [Google Scholar] [CrossRef]
- Huang, L.; Tang, X.; Jiang, G.; Fang, K.; Yao, K.; Zhang, Z.; Chen, N.; Shan, Z. A high-strength Co–Fe–Ta–B metallic-glass phase enabled tensile plasticity in Co–Fe–Ta–B–O oxide glass matrix nanocomposites. Appl. Phys. Lett. 2020, 116, 081903. [Google Scholar] [CrossRef]
- Wang, X.L.; Jiang, F.; Hahn, H.; Li, J.; Gleiter, H.; Sun, J.; Fang, J.X. Plasticity of a scandium-based nanoglass. Scr. Mater. 2015, 98, 40–43. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Han, B.; Luan, J.; Kai, J.J.; Liu, C.T.; Wu, G.; Lu, J. Second phase effect on corrosion of nanostructured Mg-Zn-Ca dual-phase metallic glasses. J. Magnes. Alloy. 2021, 9, 1546–1555. [Google Scholar] [CrossRef]

| Materials (at.%) | dc (mm) | σf (MPa) | Rm (MPa) | σy (MPa) | A (%) | Hv (HV) | Ref |
|---|---|---|---|---|---|---|---|
| Mg60Zn34Ca6 | 3 | — | — | 888 | — | 296 ± 25 | [55] |
| Mg62Zn32Ca6 | 2 | — | 110 | — | 0 | 218 | [28] |
| Mg63Zn32Ca5 | 3 | — | 156 | — | 0.2 | 261 | [28] |
| Mg64Zn30Ca6 | 2 | — | 160 | — | 0.2 | 244 | [28] |
| Mg64Zn32Ca4 | 3 | — | 166 | — | 0.6 | 263 | [28] |
| Mg65Zn30Ca5 | 3 | — | 191 | — | 0 | 284 | [28] |
| Mg65Zn32Ca3 | 1 | — | 175 | — | 0.2 | 272 | [28] |
| Mg66Zn28Ca6 | 2 | — | 90 | — | 0 | 212 | [28] |
| Mg66Zn30Ca4 | 5 | 716–854 | — | — | — | — | [63] |
| Mg66Zn30Ca4 | 4 | 787 ± 22 | — | — | — | 2.45 ± 0.01 (GPa) | [58] |
| Mg66Zn30Ca4 | 4 | — | 191 | — | 0.3 | 291 | [28] |
| Mg67Zn28Ca5 | 2 | 622 | — | 662 | 0.2 1 | — | [27] |
| Mg67Zn28Ca5 | 3 | — | 117 | — | 0.6 | 233 | [28] |
| Mg67Zn30Ca3 | 1 | — | 195 | — | 0.2 | 305 | [28] |
| Mg68Zn28Ca4 | 3 | 671 | — | 540 | 0.43 1 | — | [27] |
| Mg68Zn28Ca4 | 4 | — | 125 | — | 0.1 | 235 | [28] |
| Mg69Zn28Ca3 | 2 | 675 | — | 591 | 0.5 1 | — | [27] |
| Mg69Zn28Ca3 | 1 | — | 128 | — | 0.1 | 237 | [28] |
| Mg71Zn25Ca4 | ≥2 | 672–752 | — | — | — | — | [63] |
| Mg73Zn23Ca4 | 2 | — | — | 636 | — | 212 ± 19 | [55] |
| Mg80−xCa5Zn15+x (x = 5–20) | 1–4 | 700 | — | — | — | 2.16 (GPa) | [21] |
| Materials (at.%) | D (mm) | σf (MPa) | A (%) | E (GPa) | Hv (GPa) | Ref |
|---|---|---|---|---|---|---|
| Desirable materials for orthopedic implants | Large size | ≥230 | ≥15,10 | 17–22 | — | [45] |
| Mg60Zn35Ca5 | 2 | 571 | — | — | — | [90] |
| Mg62.9Zn32.3Ca4.8 | 2 | 590 ± 5.1 | — | — | — | [91] |
| Mg65Zn30Ca5 | 2 | 722 | — | 49 | — | [83] |
| Mg66Zn30Ca4 | 4 | 787 ± 22 | — | 48.8 ± 0.1 | 2.45 ± 0.01 | [58] |
| Mg66.2Zn28.8Ca5 | 2 | 787 | — | — | — | [69] |
| Mg67Zn28Ca5 | 100 μm (thin wires) | 675–894 1 | 3–5 | — | 2.16 | [92] |
| Mg69Zn27Ca4 | 1.5 | 550 2 | 1.3 | — | — | [59] |
| Mg70Zn25Ca5 | 2 | 565.8 ± 23.2 | — | — | — | [82] |
| Mg72Zn23Ca5 | 2 | — | — | 50.38 | 2.71 | [70] |
| Mg64Li2Zn30Ca4 | 30 μm in thickness | — | — | 42.893 | 1.64 | [93] |
| Mg63Li3Zn30Ca4 | 30 μm in thickness | — | — | 54.357 | 1.98 | [93] |
| Mg62Li4Zn30Ca4 | 30 μm in thickness | — | — | 51.541 | 2.26 | [93] |
| Mg61Li5Zn30Ca4 | 30 μm in thickness | — | — | 62.451 | 3.05 | [93] |
| Mg68.5Zn27Ca4Mn0.5 | 1.5 | 475 | — | — | — | [68] |
| Mg68Zn27Ca4Mn1 | 1.5 | 364 | — | — | — | [68] |
| (Mg66.2Zn28.8Ca5)99Cu1 | 2 | 811 | — | — | — | [69] |
| (Mg66.2Zn28.8Ca5)97Cu3 | 2 | 979 | — | — | — | [69] |
| (Mg66.2Zn28.8Ca5)95Cu5 | 2 | 583 | — | — | — | [69] |
| Mg66Zn30Ca3.5Sr0.5 | 6 | 827 ± 21 | — | 48.5 ± 0.2 | 2.49 ± 0.01 | [58] |
| Mg66Zn30Ca3Sr1 | 6 | 848 ± 21 | — | 49.1 ± 0.2 | 2.51 ± 0.02 | [58] |
| Mg66Zn30Ca2.5Sr1.5 | 4 | 841 ± 24 | — | 49.4 ± 0.2 | 2.51 ± 0.02 | [58] |
| Mg68Zn27Ca4Y1 | 1.5 | 1012 2 | 3.1 | — | — | [59] |
| Mg67Zn27Ca4Y2 | 1.5 | 770 2 | 2.0 | — | — | [59] |
| Mg65Zn30Ca4Ag1 | 2 | 759 | — | 50 | 2.35 ± 0.03 | [71,83] |
| Mg63Zn30.2Ca4.5Ag2.3 | 2 | 506.5 ± 7.5 | — | — | — | [91] |
| 3 | 347.6 ± 8.2 | — | — | — | ||
| Mg63Zn30Ca4Ag3 | 2 | 540 | — | 63 | 2.35 ± 0.03 | [71,83] |
| Mg59.8Zn33.1Ca4.7Nd2.4 | 2 | 465.5 ± 6.4 | — | — | — | [91] |
| 3 | 298.4 ± 9.3 | — | — | — | ||
| Mg66Zn30Ca2Yb2 | 40 μm in thickness | 500 | — | 35 | — | [84] |
| Mg59.3Zn32.4Ca4.8Yb3.5 | 2 | 606.2 ± 4.9 | — | — | — | [91] |
| 3 | 540.8 ± 5.2 | — | — | — | ||
| Mg66Zn30Yb4 | 40 μm in thickness | 500 | — | 35 | — | [84] |
| Mg70Zn23Ca5Pd2 | 2 | — | — | 64.20 | 3.56 | [70] |
| Mg66Zn23Ca5Pd6 | 2 | — | — | 72.98 | 3.90 | [70] |
| Mg60Zn35Ca5 MGMC with 50 vol % 20–75 µm Ti particles | 2 | 1190 | — | — | — | [73] |
| Mg60Zn35Ca5 MGMC with 40 vol% spherical Ti particles of 75–105 μm in diameter | 2 | 807 | — | — | — | [90] |
| Mg67Zn28Ca5 MGMC with 40% volume fraction Ti particles of 75–105 μm diameter | 2 | 690 | — | — | — | [90] |
| Mg67Zn29Ca4/NiTi composite | 2 | ~592 ± 22 | — | — | — | [60] |
| Mg69Zn27Ca4/Fe (3 wt% Fe) | 1.5 | 648 | 1.5 | — | — | [94] |
| Mg70Zn25Ca5 (MGMC) | 2 | 642 | — | — | — | [27] |
| Mg80Zn15Ca5 (MGMC) | 3 | 513 | — | — | — | [27] |
| Mg66Zn30Ca4 with phosphate conversion coated | 2 | 671 | — | — | — | [89] |
| Materials (at.%) | Electrolyte | Ecorr (VSCE) | icorr (μA/cm2) | Corrosion Rate (mm y−1) | Ref |
|---|---|---|---|---|---|
| Mg69Zn25Ca5Au1 (after 1 h immersion) | Artificial physiological fluid | −1.318 | 25 | — | [100] |
| Mg69Zn25Ca5Cu1 (after 1 h immersion) | Artificial physiological fluid | −1.314 | 63 | — | [100] |
| Mg69Zn25Ca5Au0.5Cu0.5 (after 1 h immersion) | Artificial physiological fluid | −1.311 | 57 | — | [100] |
| Mg | Hank’s solution | −1.700 ± 0.050 | 4.410 ± 0.300 | 0.100 ± 0.006 | [54] |
| Mg65Zn30Ca5 (ribbon) | Hank’s solution | — | 6.6 1 | — | [83] |
| Mg69Zn27Ca4 | Hank’s solution | −1.300 ± 0.040 | 0.440 ± 0.150 | 0.010 ± 0.003 | [54] |
| Mg72Zn23Ca5 | Hank’s solution | — | 1.7 (mA cm−2) | — | [70] |
| Mg70Zn23Ca5Pd2 | Hank’s solution | — | 2.1 (mA cm−2) | — | [70] |
| Mg66Zn23Ca5Pd6 | Hank’s solution | — | 2.7 (mA cm−2) | — | [70] |
| Mg65Zn30Ca4Ag1 (ribbon) | Hank’s solution | — | 3.5 1 | — | [83] |
| Mg63Zn30Ca4Ag3 (ribbon) | Hank’s solution | — | 19 1 | — | [83] |
| MAO-coated Mg69Zn27Ca4 | Hank’s solution | −1.33 | 0.95 | 0.31 | [101] |
| Ca-P-coated Mg69Zn27Ca4 | Hank’s solution | −1.28 | 0.31 | 0.1 | [101] |
| Mg | MEM | −1700 (mVSHE) | 11.0 (± 6.0) | — | [102] |
| Mg65Zn30Ca5 | MEM | −1.27 | 6.9 | — | [26] |
| Mg66Zn30Ca4 | MEM | –1107 ± 6 (mVSHE) | 13.1 ± 1.8 | — | [102] |
| Mg69Zn26Ca5 | MEM | −1110 ± 6 (mVSHE) | 16.5 ± 2.3 | — | [102] |
| Mg69Zn27Ca4 | MEM | −1083 ± 24 (mVSHE) | 13.2 ± 2.6 | — | [102] |
| Mg69Zn28Ca3 | MEM | −1123 ± 11 (mVSHE) | 14.4 ± 2.2 | — | [102] |
| Mg72Zn24Ca4 | MEM | −1126 ± 25 (mVSHE) | 19.9 ± 6.0 | — | [102] |
| Mg66Zn30Ca4 | PBS | — | — | 0.340 ± 0.043 (after the 3-day immersion) | [71] |
| Mg66.2Zn28.8Ca5 | PBS | — | 7.41 | — | [69] |
| Mg69Zn27Ca4 | PBS | −1.33 | 10−4.38 A/cm2 | — | [103] |
| Mg65.2Zn30Ca4Mn0.8 | PBS | −1.219 | 104 | 2.40 | [66] |
| Mg64.9Zn30Ca4Mn0.8Sr0.3 | PBS | −1.1174 | 34.6 | 1.32 | [66] |
| Mg64.7Zn30Ca4Mn0.8Sr0.5 | PBS | −1.1173 | 16.1 | 0.36 | [66] |
| Mg64.4Zn30Ca4Mn0.8Sr0.8 | PBS | −1.1175 | 71.8 | 1.81 | [66] |
| (Mg66.2Zn28.8Ca5)99Cu1 | PBS | — | 5.37 | — | [69] |
| (Mg66.2Zn28.8Ca5)97Cu3 | PBS | — | 6.91 | — | [69] |
| (Mg66.2Zn28.8Ca5)95Cu5 | PBS | — | 60.2 | — | [69] |
| Mg66Zn29Ca4Ag1 (after the 3-day immersion) | PBS | — | — | 0.308 ± 0.029 | [71] |
| Mg66Zn27Ca4Ag3 (after the 3-day immersion) | PBS | — | — | 0.265 ± 0.042 | [71] |
| Mg62Zn32Ca6 (after 15 min immersion) | Ringer’s solution | −1.18 (NEK) | 40 | 0.85 | [28] |
| Mg63Zn32Ca5 (after 15 min immersion) | Ringer’s solution | −1.27 (NEK) | 24.7 | 0.51 | [28] |
| Mg64Zn30Ca6 (after 15 min immersion) | Ringer’s solution | −1.20 (NEK) | 33 | 0.73 | [28] |
| Mg64Zn32Ca4 (after 15 min immersion) | Ringer’s solution | −1.32 (NEK) | 24.6 | 0.51 | [28] |
| Mg65Zn30Ca5 (after 15 min immersion) | Ringer’s solution | −1.21 (NEK) | 28 | 0.63 | [28] |
| Mg65Zn32Ca3 (after 15 min immersion) | Ringer’s solution | –1.32 (NEK) | 21 | 0.43 | [28] |
| Mg66Zn28Ca6 (after 15 min immersion) | Ringer’s solution | –1.21 (NEK) | 76 | 1.67 | [28] |
| Mg66Zn30Ca4 (after 15 min immersion) | Ringer’s solution | –1.34 (NEK) | 29 | 0.64 | [28] |
| Mg67Zn28Ca5 (after 15 min immersion) | Ringer’s solution | −1.26 (NEK) | 55 | 1.17 | [28] |
| Mg67Zn30Ca3 (after 15 min immersion) | Ringer’s solution | −1.26 (NEK) | 30 | 0.64 | [28] |
| Mg68Zn28Ca4 (after 15 min immersion) | Ringer’s solution | −1.35 (NEK) | 41 | 0.88 | [28] |
| Mg69Zn28Ca3 (after 15 min immersion) | Ringer’s solution | −1.27 (NEK) | 62 | 1.33 | [28] |
| Mg | SBF | −1.636 | 10−3.96 (A/cm2) | — | [59] |
| Mg60Zn34Ca6 | SBF | — | — | 0.06 | [55] |
| Mg60Zn35Ca5 (completely crystalline) | SBF | −1.360 | 222 | 331.8 (mpy) | [104] |
| Mg60Zn35Ca5 (partially amorphous) | SBF | −1.240 | 4.1 | 0.1554 | [104] |
| Mg65.2Zn28.8Ca6 | SBF | –1.345 ± 0.031 | 7.50 ± 0.45 | — | [76] |
| Mg66Zn30Ca4 | SBF | — | 3.53 | — | [82] |
| Mg66Zn30Ca4 (completely crystalline) | SBF | –1.510 | 1530 | 2286 (mpy) | [104] |
| Mg66Zn30Ca4 (partially amorphous) | SBF | –1.270 | 8.490 | 12.69 (mpy) | [104] |
| Mg67Zn29Ca4 | SBF | −1.18 (Ref) | 18.9 | 0.21 | [105] |
| Mg69Zn27Ca4 | SBF | −1.12 | 10−5.81 A/cm2 | — | [103] |
| Mg70Zn25Ca5 | SBF | — | 11.2 | — | [82] |
| Mg73Zn23Ca4 | SBF | — | — | 0.21 | [55] |
| Mg68.5Zn27Ca4Mn0.5 | SBF | −1.235 | — | — | [68] |
| Mg68Zn27Ca4Mn1 | SBF | −1.254 | — | — | [68] |
| Mg68Zn27Ca4Y1 | SBF | −1.246 | 10−4.96 (A/cm2) | — | [59] |
| Mg67Zn27Ca4Y2 | SBF | −1.283 | 10−4.78 (A/cm2) | — | [59] |
| MAO-treated Mg65.2Zn28.8Ca6 | SBF | –1.244 ± 0.016 | (7.23 ± 0.13) × 10−2 | — | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Liu, Z.; Yu, W.; Qin, C.; Yu, H.; Wang, Z. Biodegradable Mg–Zn–Ca-Based Metallic Glasses. Materials 2022, 15, 2172. https://doi.org/10.3390/ma15062172
Jin C, Liu Z, Yu W, Qin C, Yu H, Wang Z. Biodegradable Mg–Zn–Ca-Based Metallic Glasses. Materials. 2022; 15(6):2172. https://doi.org/10.3390/ma15062172
Chicago/Turabian StyleJin, Chao, Zhiyuan Liu, Wei Yu, Chunling Qin, Hui Yu, and Zhifeng Wang. 2022. "Biodegradable Mg–Zn–Ca-Based Metallic Glasses" Materials 15, no. 6: 2172. https://doi.org/10.3390/ma15062172
APA StyleJin, C., Liu, Z., Yu, W., Qin, C., Yu, H., & Wang, Z. (2022). Biodegradable Mg–Zn–Ca-Based Metallic Glasses. Materials, 15(6), 2172. https://doi.org/10.3390/ma15062172







