Molecular Dynamics Simulation and Structure Changes of Polyester in Water and Non-Aqueous Solvents
Abstract
:1. Introduction
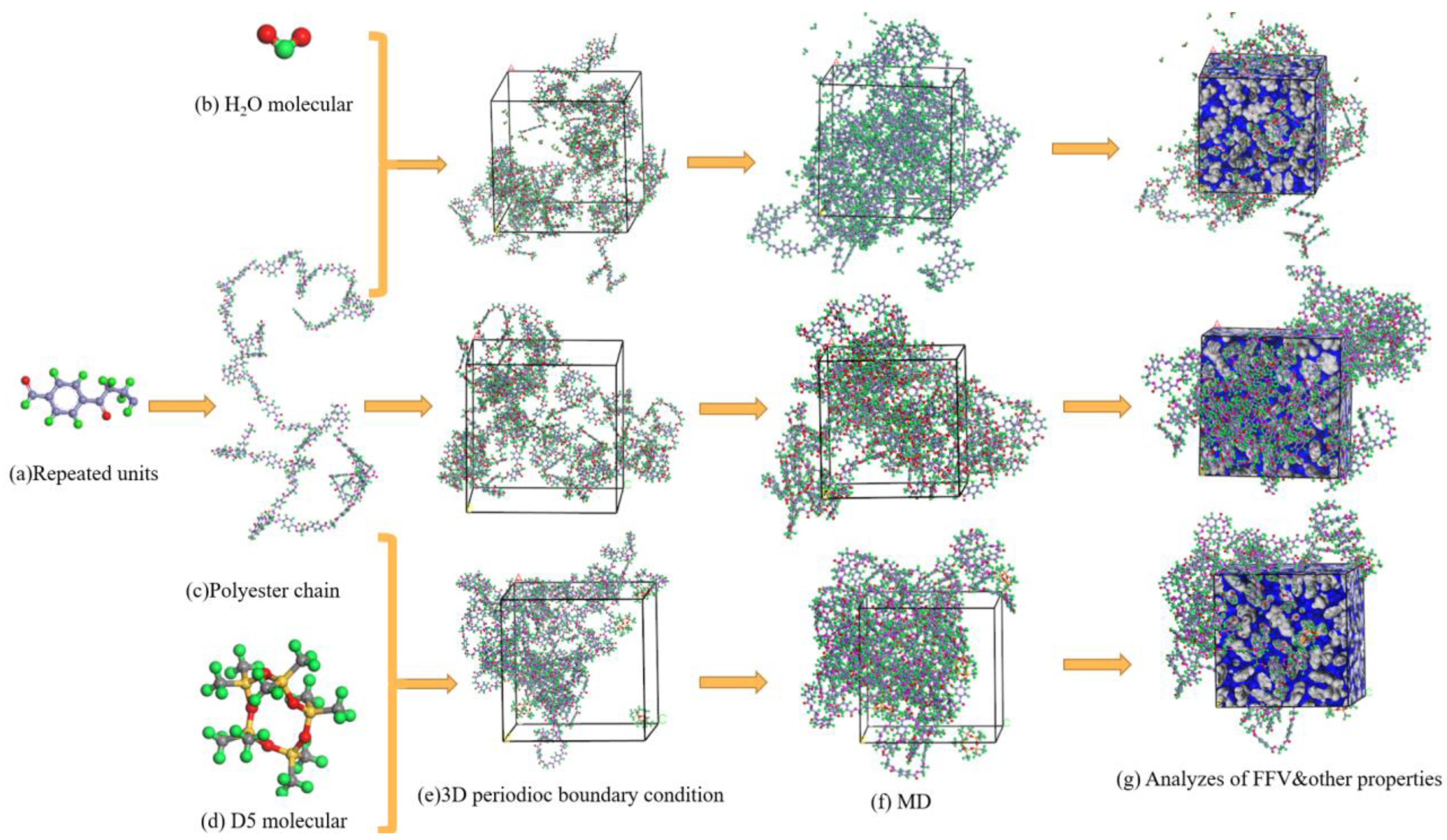
2. Materials and Methods
3. Results and Discussion
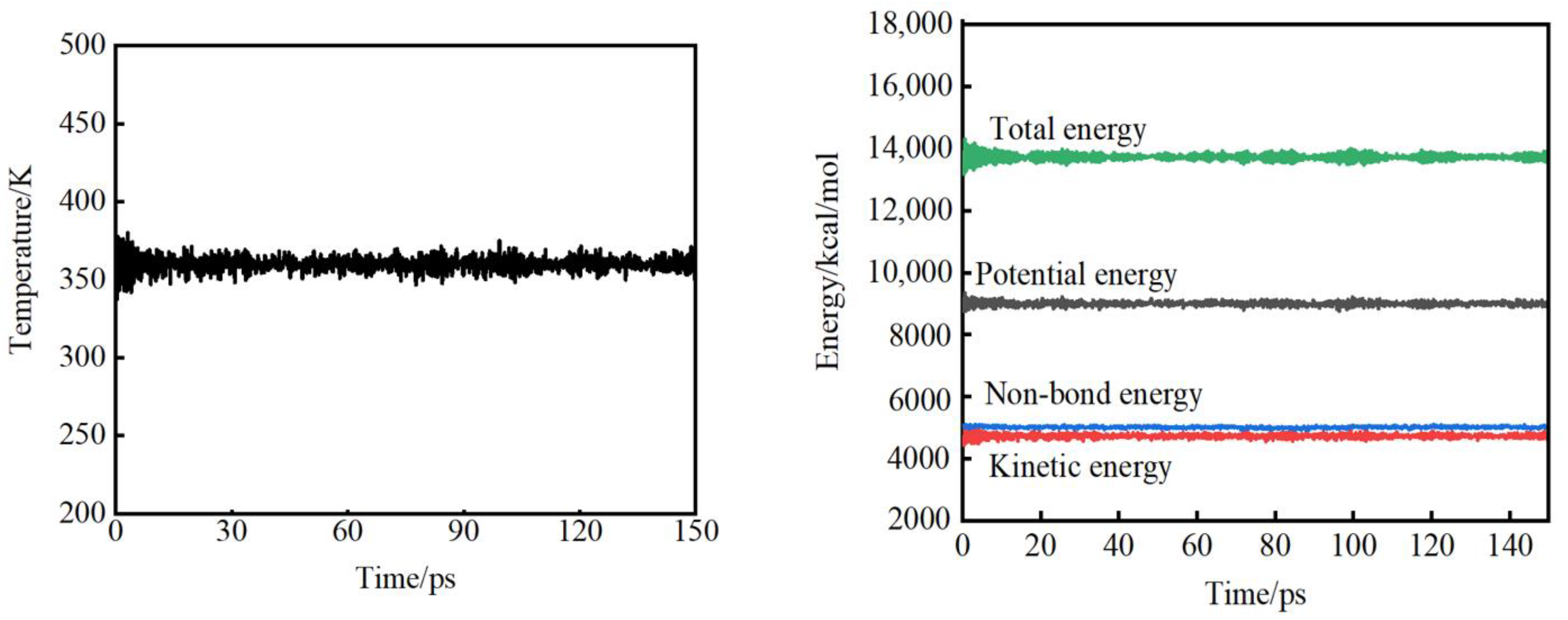
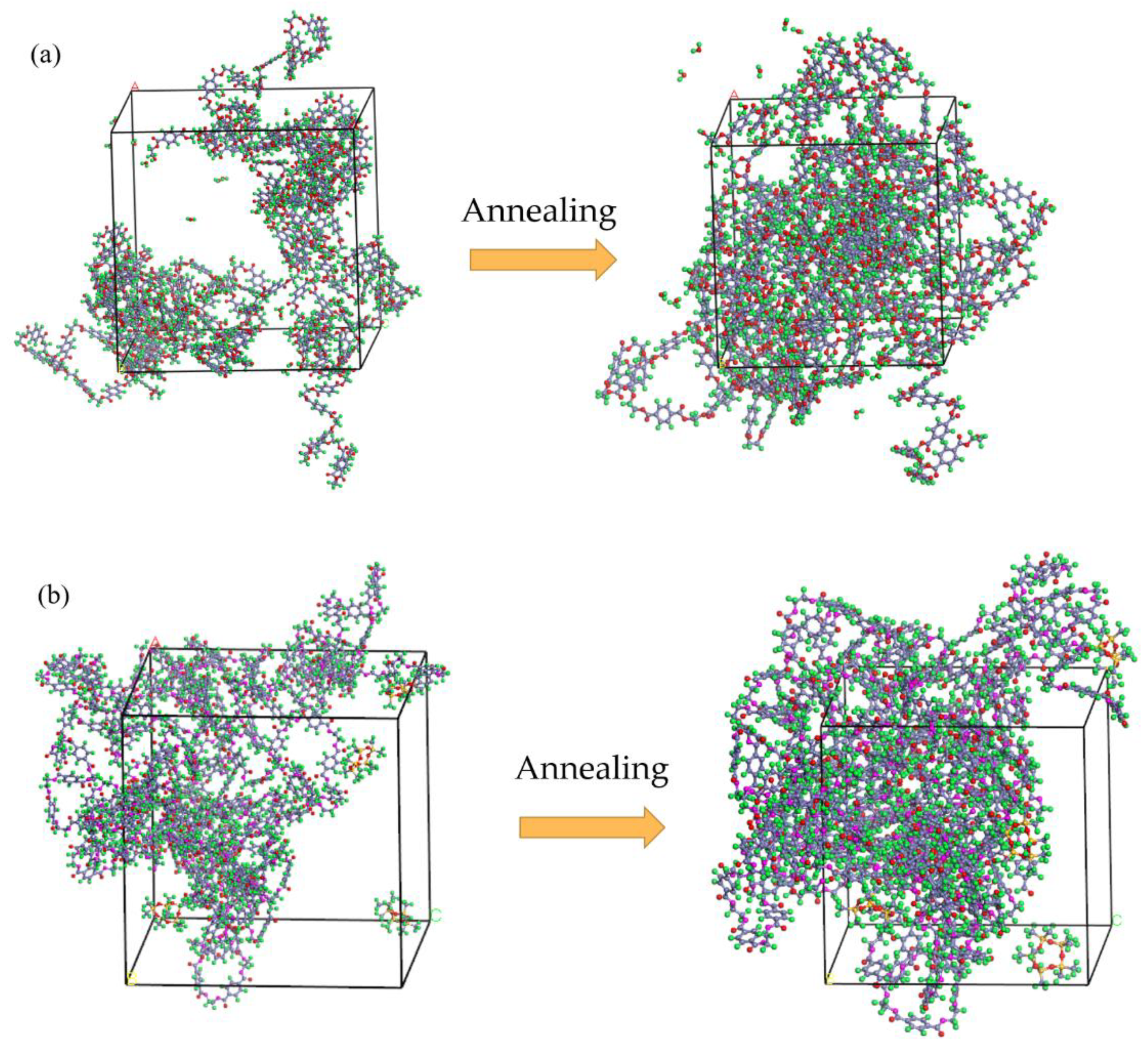
3.1. Evaluation of System Balance
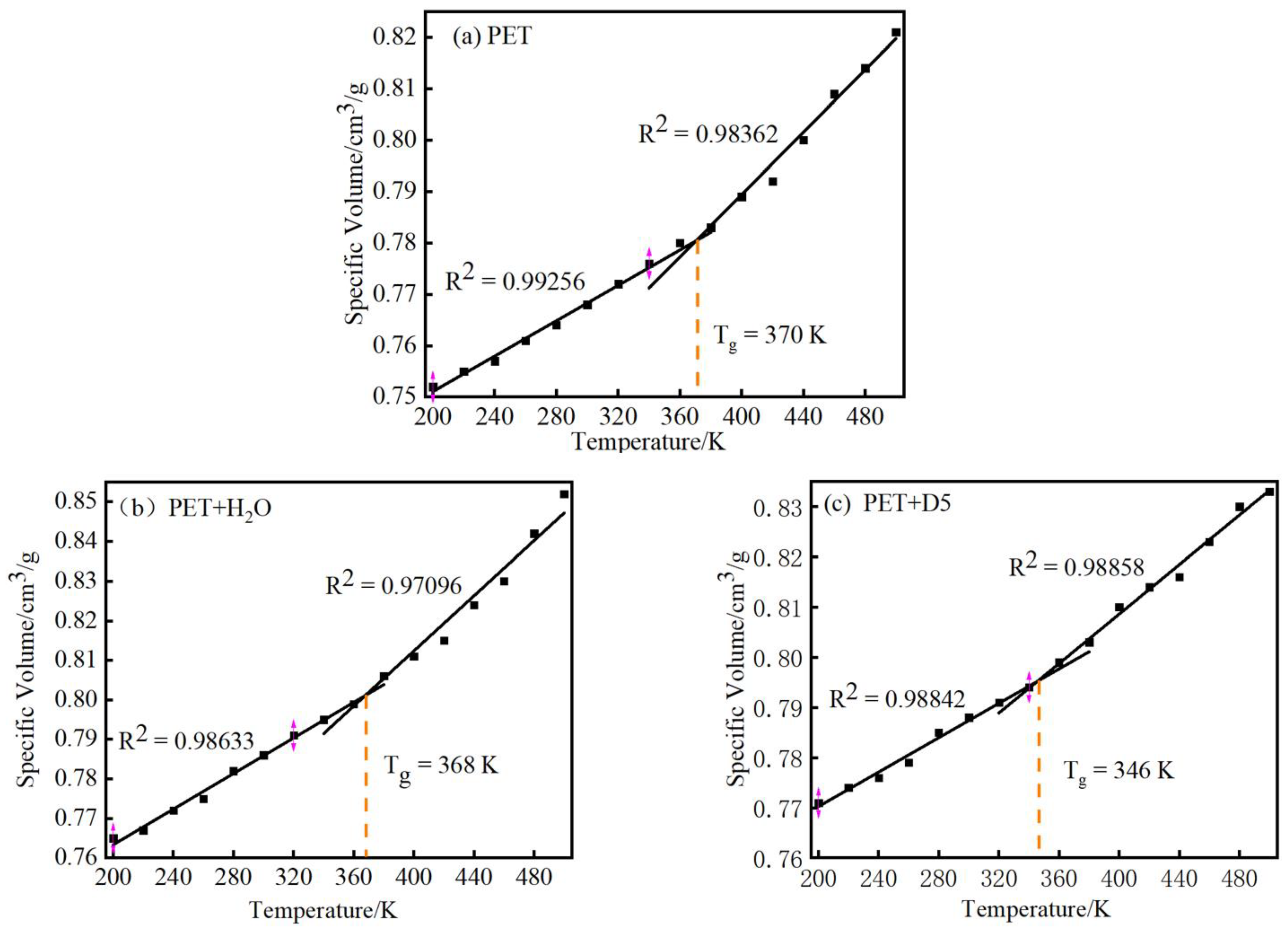
3.2. Simulation Analysis of Glass Transition Temperature of PET
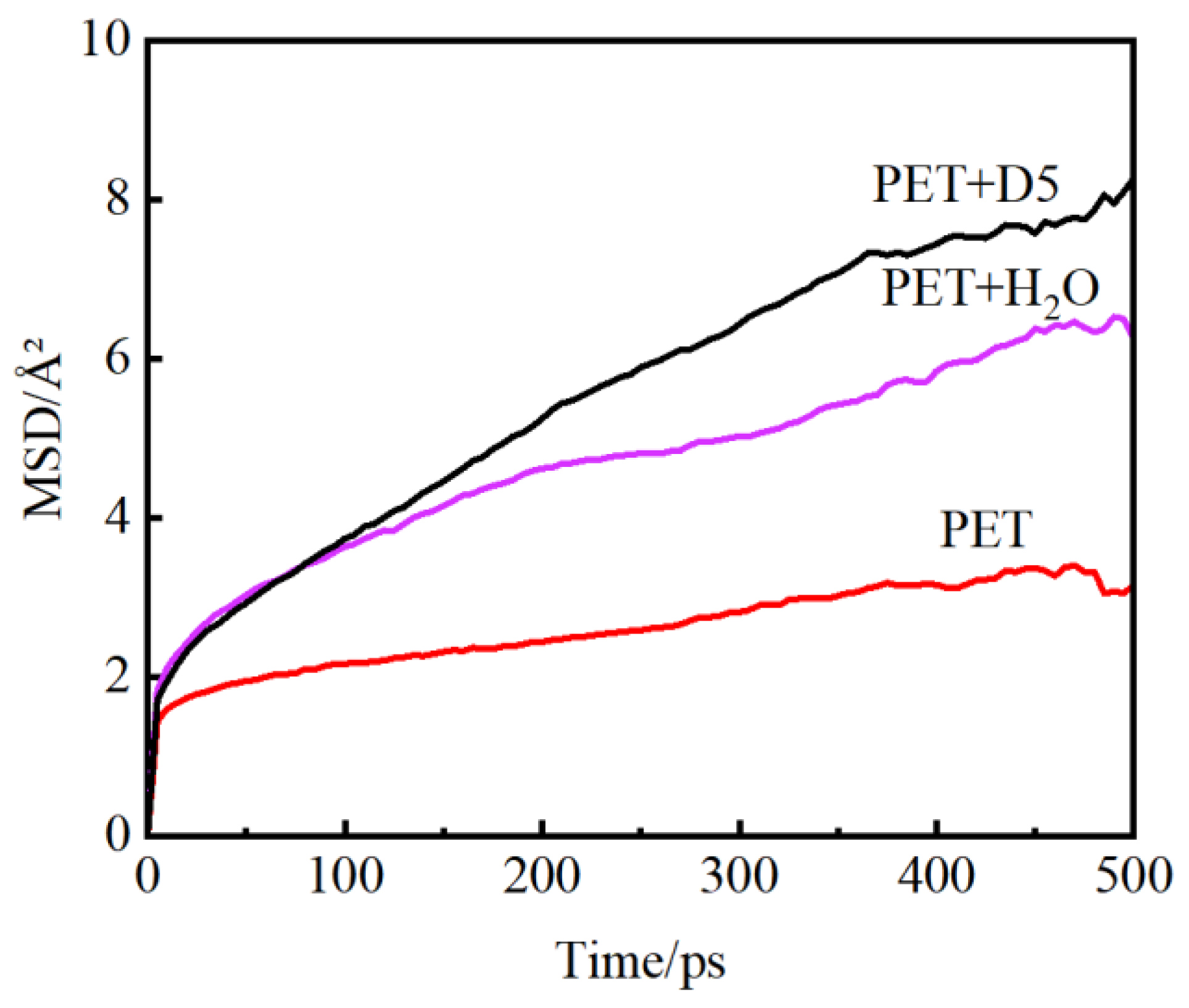
3.3. Simulation Analysis of Molecular Ability and Diffusion Characteristics of Polyester Molecular Chains
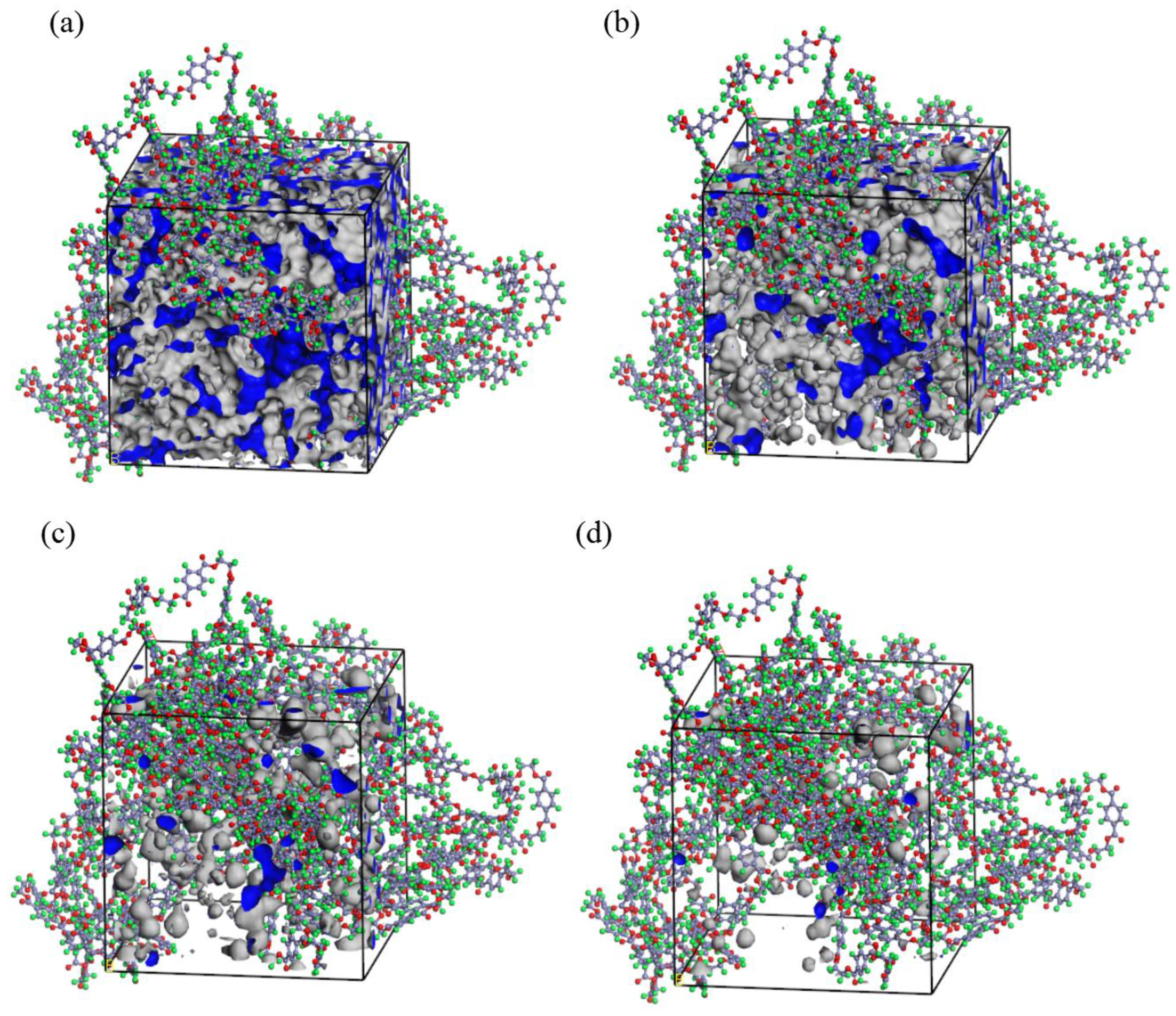
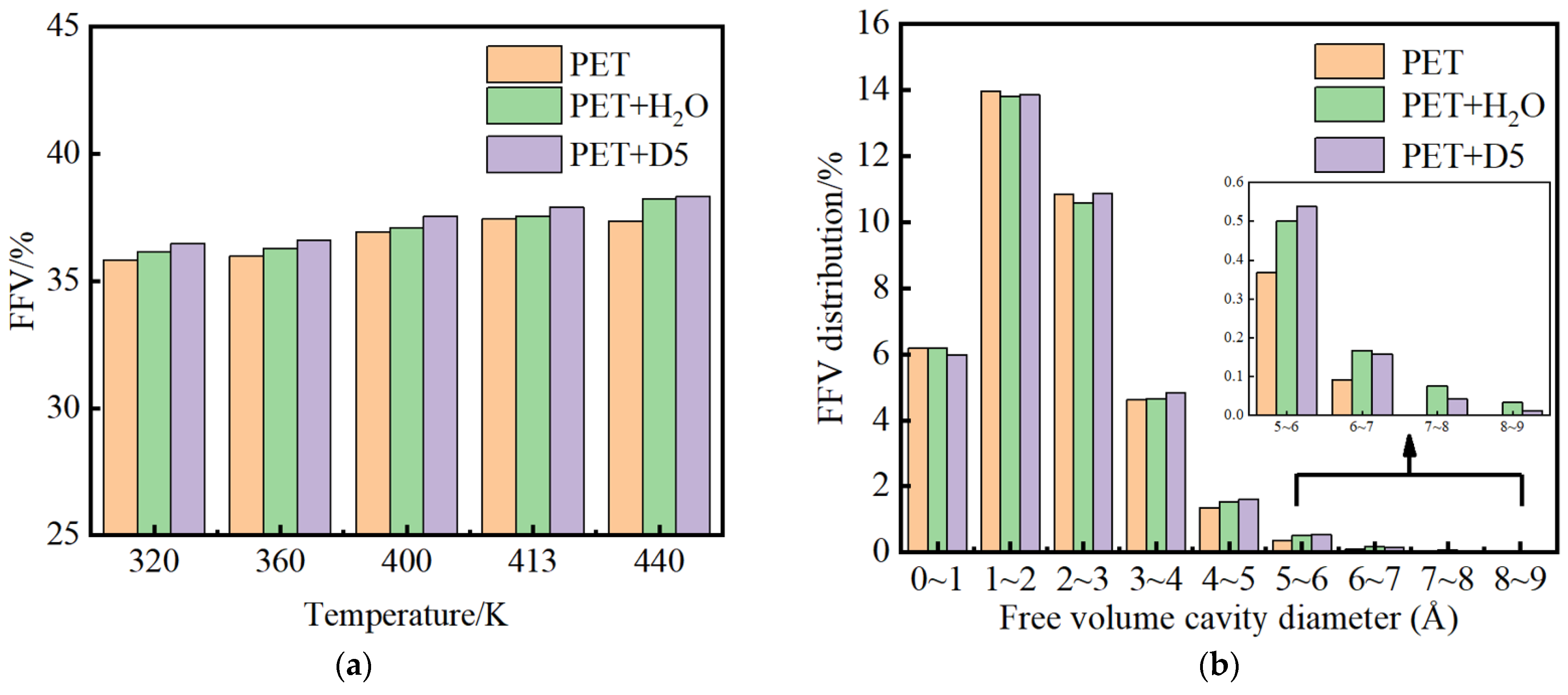
3.4. Simulation Analysis of PET Microvoids and Their Distribution
3.5. Interfacial Interaction Model and MD Simulation
3.6. Simulation Analysis of Solubility of PET Molecular Chains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Hashem, N.M.; Zhao, H.; Wang, J.; Sun, Y.; Xiong, X.; Zheng, L.; Sofan, M.; Abou Elmaaty, T. Effect of the degree of esterification of disperse dyes on the dyeing properties of polyethylene terephthalate in supercritical carbon dioxide. J. Supercrit. Fluids 2021, 175, 105270. [Google Scholar] [CrossRef]
- Aysha, T.; Zain, M.; Arief, M.; Youssef, Y. Alkali-stable solid state fluorescent pyrazolo/pyrrolinone disperse dyes: Synthesis and application for dyeing polyester fabric. J. Mol. Struct. 2022, 1249, 131623. [Google Scholar] [CrossRef]
- Li, M.; Ma, W.; Zhang, L.; Agbo, C.; Fu, S. Investigation from synthesis to crystal structure to application of ecofriendly disperse dyes on one-step dyeing of PET fabric. ACS Sustain. Chem. Eng. 2017, 5, 758–766. [Google Scholar] [CrossRef]
- Yan, L.; Hou, L.; Sun, S.; Wu, P. Dynamic diffusion of disperse dye in a polyethylene terephthalate film from an infrared spectroscopic perspective. Ind. Eng. Chem. Res. 2020, 59, 7398–7404. [Google Scholar] [CrossRef]
- Wang, S.; Gao, L.; Hou, A.; Xie, K.; Song, X. Design, synthesis of novel bisazo disperse dyes: Structure analysis and dyeing performance on PET. Dye. Pigment. 2021, 196, 109761. [Google Scholar] [CrossRef]
- Mahar, F.K.; Mehdi, M.; Qureshi, U.A.; Brohi, K.M.; Zahid, B.; Ahmed, F.; Khatri, Z. Dyeability of recycled electrospun polyethylene terephthalate (PET) nanofibers: Kinetics and thermodynamic study. J. Mol. Liq. 2017, 248, 911–919. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Z.; Jiang, G.; Wang, J.; Miao, X.; Wan, A. Effect of the dyeing process on thermal and dyeing properties of poly (butylene terephthalate) fibers. Text. Res. J. 2020, 91, 580–588. [Google Scholar] [CrossRef]
- Ketema, A.; Worku, A. Review Article Review on Intermolecular Forces between Dyes Used for Polyester Dyeing and Polyester Fiber. J. Chem. 2020, 2020, 6628404. [Google Scholar] [CrossRef]
- Hou, A.; Li, M.; Gao, F.; Xie, K.; Yu, X. One-step dyeing of polyethylene terephthalate fabric, combining pretreatment and dyeing using alkali-stable disperse dyes. Coloration Technol. 2013, 129, 438–442. [Google Scholar] [CrossRef]
- Kim, I.S.; Cho, H.M.; Koh, J.; Kim, J.P. Low-Temperature Carrier Dyeing of Poly(vinyl chloride)Fibers with Disperse Dyes. J. Appl. Polym. Sci. 2003, 90, 3896–3904. [Google Scholar] [CrossRef]
- Radei, S.; Carrión-Fité, F.J.; Ardanuy, M.; Canal, J.M. Kinetics of Low Temperature Polyester Dyeing with High Molecular Weight Disperse Dyes by Solvent Microemulsion and AgroSourced Auxiliaries. Polymers 2018, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, H. Textile Dyeing: Environmental Friendly Osage Orange Extract on Protein Fabrics. In Eco-Friendly Textile Dyeing and Finishing; InteachOpen: London, UK, 2013. [Google Scholar]
- HU, J.; Yan, J.; Peng, J.; Zheng, L.; Zheng, H.; Zhao, H.; Xiong, X. Study on supercritical carbon dioxide dyeing of polyester and brocade composite fabric. Shanghai Text. Sci. Technology. 2019, 47, 58–60. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.Q.; Li, Y.Q.; Li, L.M. Study on the Dyeing of PET Fiber with Disperse Dyes in D5 Media. Adv. Mater. Res. 2011, 331, 334–337. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, W.; Gao, Y.; Zhu, L.; Pei, L. Mechanism of accelerant on disperse dyeing for PET fiber in the silicone solvent dyeing system. Polymers 2019, 11, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.Z.; Liu, Y.Q.; Lan, Y.H. Molecular simulation on the glass transition of polypropylene. Polym. Mater. Sci. Eng. 2009, 23, 53–56. [Google Scholar]
- Zuo, Z.; Liang, L.; Bao, Q.; Yan, P.; Jin, X.; Yang, Y. Molecular Dynamics Calculation on the Adhesive Interaction Between the Polytetrafluoroethylene Transfer Film and Iron Surface. Front. Chem. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, D.; Yang, X.; Zhao, Y.; Liu, H.; Tang, H.; Cui, F. Molecular dynamics simulations of interfacial interactions between small nanoparticles during diffusion-limited aggregation. Appl. Surf. Sci. 2015, 357, 1114–1121. [Google Scholar] [CrossRef]
- Roussou, R.E.; Karatasos, K. Graphene/poly (ethylene glycol) nanocomposites as studied by molecular dynamics simulations. Mater. Des. 2016, 97, 163–174. [Google Scholar] [CrossRef]
- Sheng, Y.Z.; Yang, H.; Li, J.Y.; Sun, M. Predicting glass transition temperature of polyethylene/graphene nanocomposites by molecular dynamic simulation. Chem. Res. Chin. Univ. 2013, 29, 788–792. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Chen, Y.; Wu, J.Y.; Zhu, A.M. Microstructure dependent diffusion ofwater–ethanol in swollen poly (vinyl alcohol): A molecular dynamics simulation study. Chem. Eng. Sci. 2009, 64, 334–340. [Google Scholar] [CrossRef]
- Shanks, R.; Pavel, D. Simulation of diffusion of O2 and CO2 in amorphous poly (ethylene terephthalate) and related alkylene and isomeric polyesters. Mol. Simul. 2002, 28, 939–969. [Google Scholar] [CrossRef]
- Pavel, D.; Shanks, R. Molecular dynamics simulation of diffusion of O2 and CO2 in blends of amorphous poly (ethylene terephthalate) and related polyesters. Polymer 2005, 46, 6135–6147. [Google Scholar] [CrossRef]
- Orekhov, M.A. Improving molecular dynamics calculation of diffusivity in liquids with theoretical models. J. Mol. Liq. 2021, 322, 114554. [Google Scholar] [CrossRef]
- Gao, S.H.; Han, Y.T.; Li, F.X.; Yan, J.; Lu, Y.H.; Zheng, H.D. Structure and properties of polyethylene terephthalate treated by supercritical CO2. Therm. Sci. 2018, 22, 1645–1650. [Google Scholar] [CrossRef]
- Bao, Q.; Yang, Z.; Lu, Z. Molecular dynamics simulation of amorphous polyethylene (PE) under cyclic tensile-compressive loading below the glass transition temperature. Polymer 2020, 186, 121968. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, W.H.; Zhang, Z.; Xu, L.; Li, P. Study of the glass transition temperature and the mechanical properties of PET/modified silica nanocomposite by molecular dynamics simulation. Eur. Polym. J. 2016, 75, 36–45. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Wang, J. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study. Mar. Pollut. Bull. 2019, 145, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Grujicic, M.; Pandurangan, B. Mesoscale analysis of segmental dynamics in microphase-segregated polyurea. J. Mater. Sci. 2012, 47, 3876–3889. [Google Scholar] [CrossRef]
- Koopman, E.A.; Lowe, C.P. Advantages of a Lower-Andersen Thermostat in Molecular Dynamics Simulations. J. Chem. Phys. 2006, 124, 137. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Di Nola, A.; Haak, J.R. Molecular-Dynamics with Coupling to An External Bath. J. Chem. Phys. 1984, 81, 3684. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, J.; Veltzke, T.; Wells, B.; Schneider, J.; Meierhofer, F.; Ciacchi, L.C.; Chaffee, A.; Thöming, J. Molecular dynamics simulations on scattering of single Ar, N2, and CO2 molecules on realistic surfaces. Comput. Fluids 2014, 97, 31–39. [Google Scholar] [CrossRef]
- Ciu, H. How to set the polyester high temperature and high pressure dyeing temperature and temperature control temperature? Dye. Finish. 2014, 40, 58. (In Chinese) [Google Scholar]
- Müller-Plathe, F.; Rogers, S.C.; Gunsteren, W.F. Computational evidence for anomalous diffusion of small molecules in amorphous polymers. Chem. Phys. Lett. 1992, 199, 237–243. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ping, L.I.; Sun, Q.; Wang, W.H. Predition the Glass Transition Temperature of PET by Molecular Dynamics Simulation. J. Qufu Norm. Univ. 2014, 40, 74–77. [Google Scholar]
- Kailiang, Y.; Dinghui, Z.; Bo, Y.; Xuehong, Z.; Qing, X.; Duanjun, X. Investigation of H-bonding for the related force fields in materials studio software. Comput. Appl. Chem. 2006, 23, 1335. [Google Scholar]
- Wei, Q.; Zhang, Y.; Wang, Y.; Yang, M. A molecular dynamic simulation method to elucidate the interaction mechanism of nano-SiO2 in polymer blends. J. Mater. Sci. 2017, 52, 12889–12901. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gupta, J.; Nunes, C.; Vyas, S.; Jonnalagadda, S. Prediction of Solubility Parameters and Miscibility of Pharmaceutical Compounds by Molecular Dynamics Simulations. J. Phys. Chem. B 2011, 115, 2014–2023. [Google Scholar] [CrossRef] [PubMed]


| System | Cell Component | Weight Fraction of Components (w/%) Cell after Refinement | ||||
|---|---|---|---|---|---|---|
| PET | D5/H2O | Density (ρ/103 kg·m−3) | Volume (V/Å3) | Cell Size/Å3 | ||
| 1 | 4 PET chains | 100 | 0 | 1.241 | 50,839.980 | 36.713 |
| 2 | 4 PET chains, 60 H2O | 97.2 | 2.8 | 1.230 | 53,345.383 | 37.093 |
| 3 | 4 PET chains, 4 D5 | 96.3 | 3.7 | 1.239 | 53,495.574 | 37.283 |
| Temperature (T/K) | Diffusion Coefficients of PET Main Chains in Three Systems (D/10−8 cm2 s−1) | ||
|---|---|---|---|
| PET | PET + H2O | PET + D5 | |
| 320 | 0.98 × 10−3 | 0.94 × 10−3 | 0.99 × 10−3 |
| 360 | 0.99 × 10−3 | 1.07 × 10−3 | 1.79 × 10−3 |
| 400 | 1.15 × 10−3 | 1.16 × 10−3 | 2.36 × 10−3 |
| 413 | 1.22 × 10−3 | 1.37 × 10−3 | 2.09 × 10−3 |
| 440 | 1.23 × 10−3 | 1.75 × 10−3 | 2.56 × 10−3 |
| System | Energy (kcal/mol) | |||||
|---|---|---|---|---|---|---|
| Etotal | Epolymer | EH2O | ED5 | Einteraction | Ebinding | |
| PET/H2O | 9450.79 | 9774.79 | −366.21 | 0 | 42.21 | −42.21 |
| PET/D5 | 8168.45 | 9774.79 | 0 | −1567.567 | −38.77 | 38.77 |
| Components | Solubility Parameter (δ/Cal1/2/cm3/2) | |
|---|---|---|
| Simulated Results δMD | Experimental Results δExp | |
| PET | 19.5 | 19.9 |
| H2O | 50.7 | 47.8 |
| D5 | 12.17 | 12.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Wang, D.; Zhang, Q.; Song, M.; Jiao, M.; Zhang, Z. Molecular Dynamics Simulation and Structure Changes of Polyester in Water and Non-Aqueous Solvents. Materials 2022, 15, 2148. https://doi.org/10.3390/ma15062148
Zheng J, Wang D, Zhang Q, Song M, Jiao M, Zhang Z. Molecular Dynamics Simulation and Structure Changes of Polyester in Water and Non-Aqueous Solvents. Materials. 2022; 15(6):2148. https://doi.org/10.3390/ma15062148
Chicago/Turabian StyleZheng, Jin, Dongshuang Wang, Qi Zhang, Meng Song, Mingli Jiao, and Zhicheng Zhang. 2022. "Molecular Dynamics Simulation and Structure Changes of Polyester in Water and Non-Aqueous Solvents" Materials 15, no. 6: 2148. https://doi.org/10.3390/ma15062148
APA StyleZheng, J., Wang, D., Zhang, Q., Song, M., Jiao, M., & Zhang, Z. (2022). Molecular Dynamics Simulation and Structure Changes of Polyester in Water and Non-Aqueous Solvents. Materials, 15(6), 2148. https://doi.org/10.3390/ma15062148







