Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys
Abstract
:1. Introduction
2. Material and Test Methods
3. Test Results
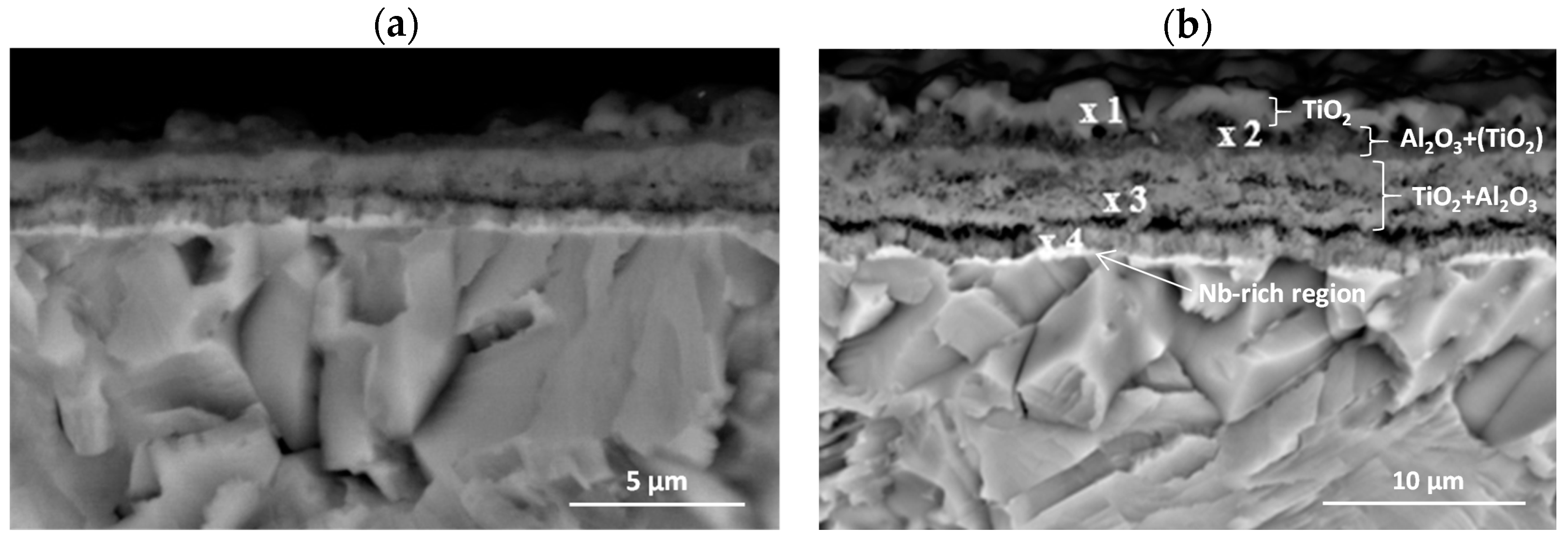
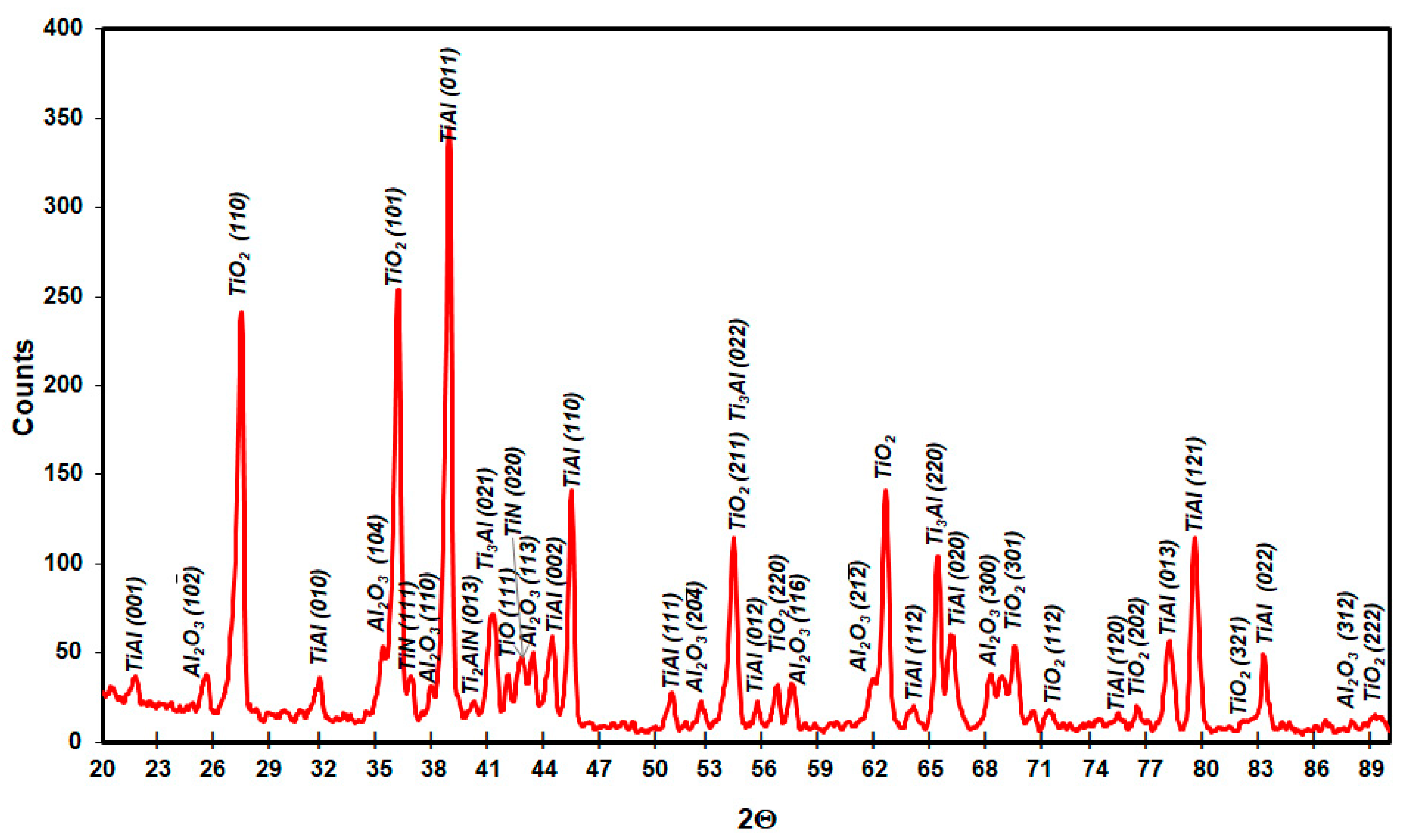
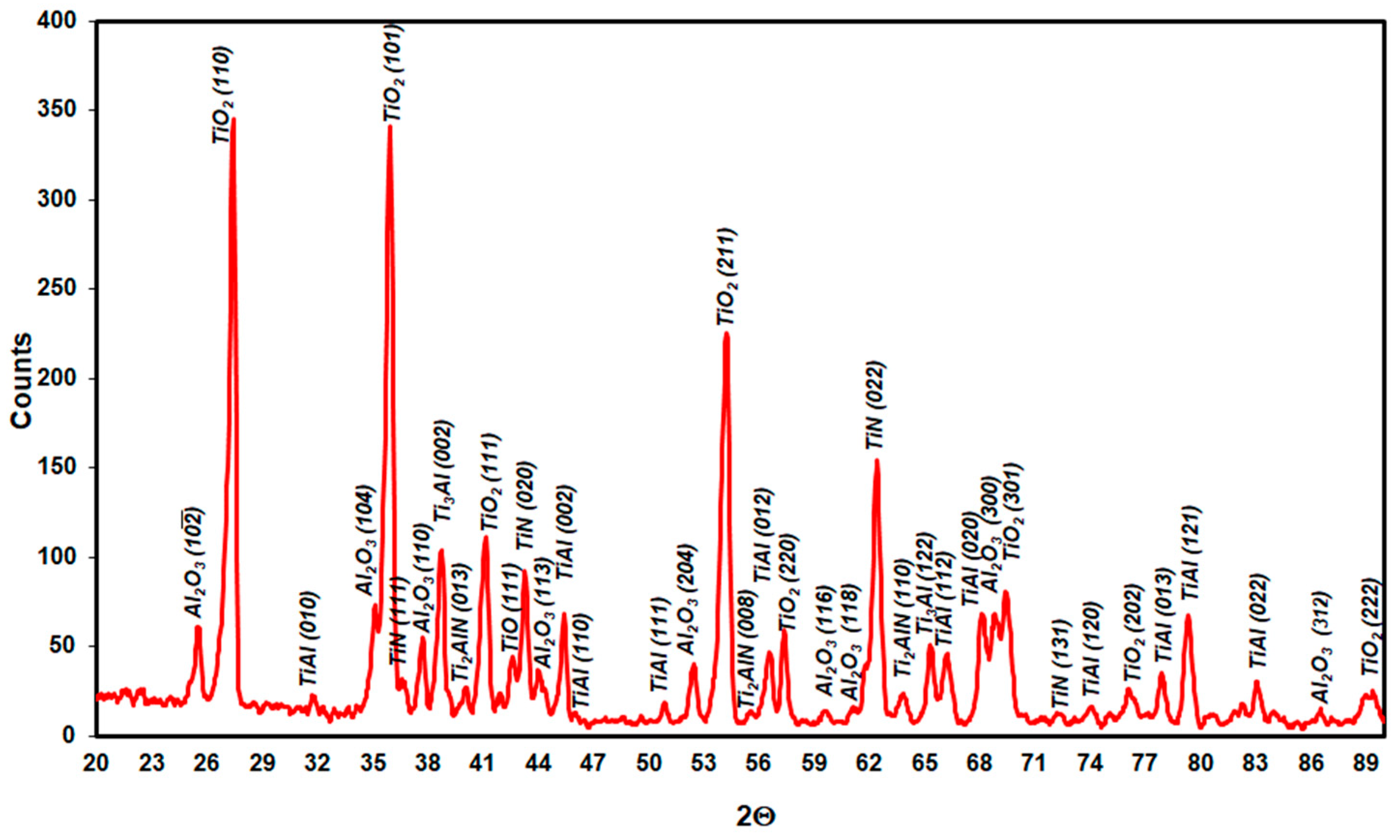
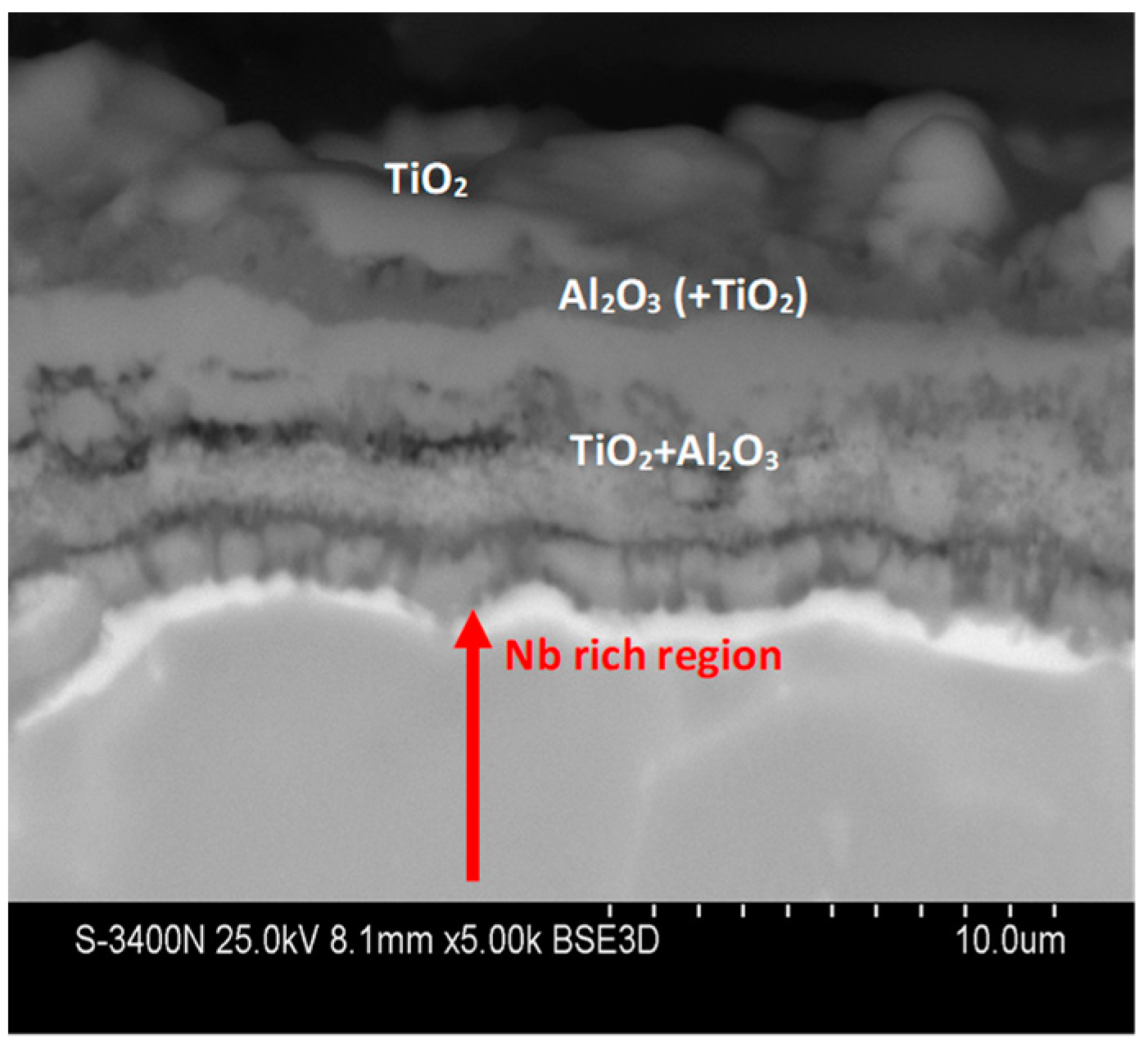
3.1. Microstructure Analysis
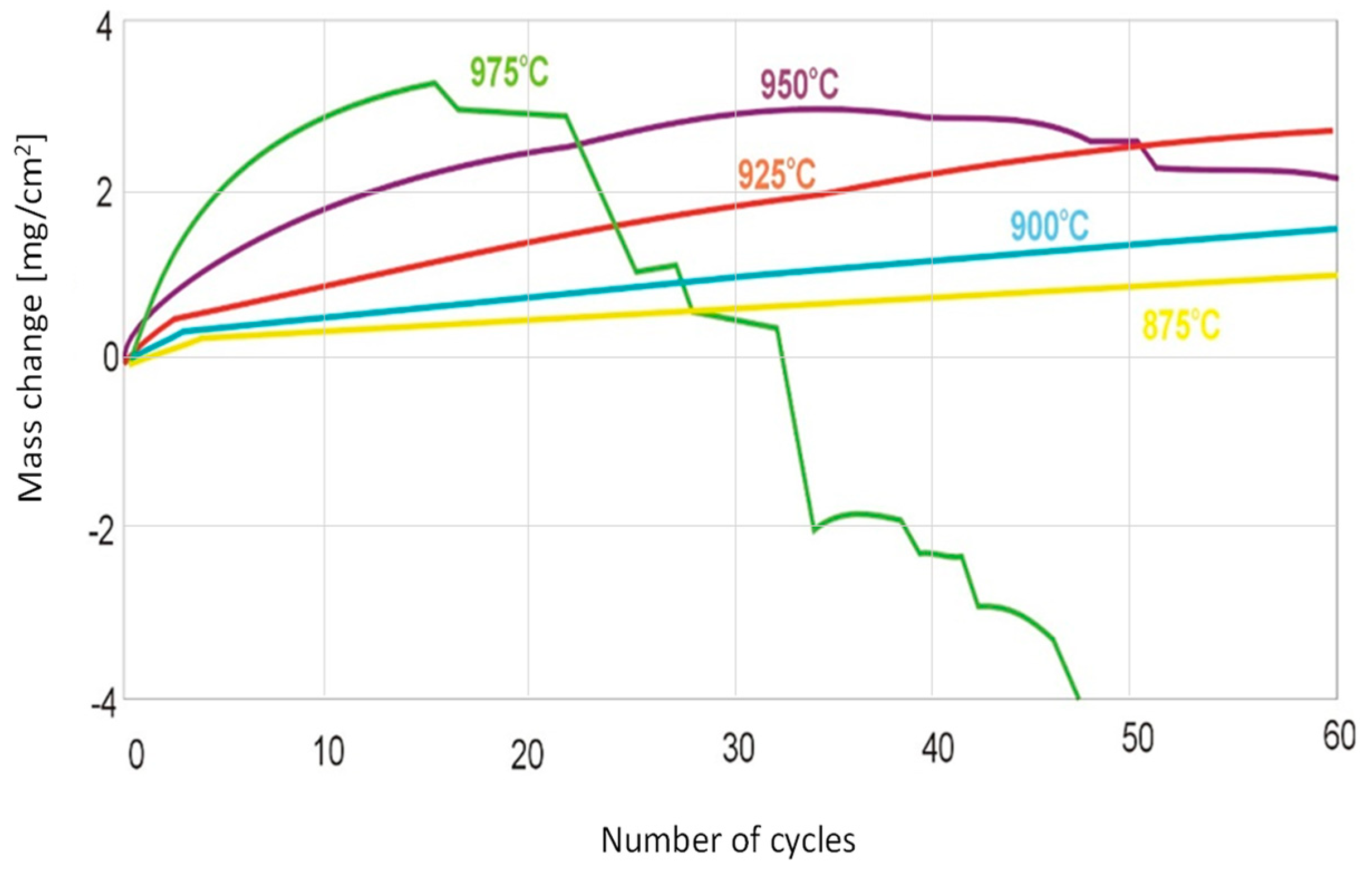
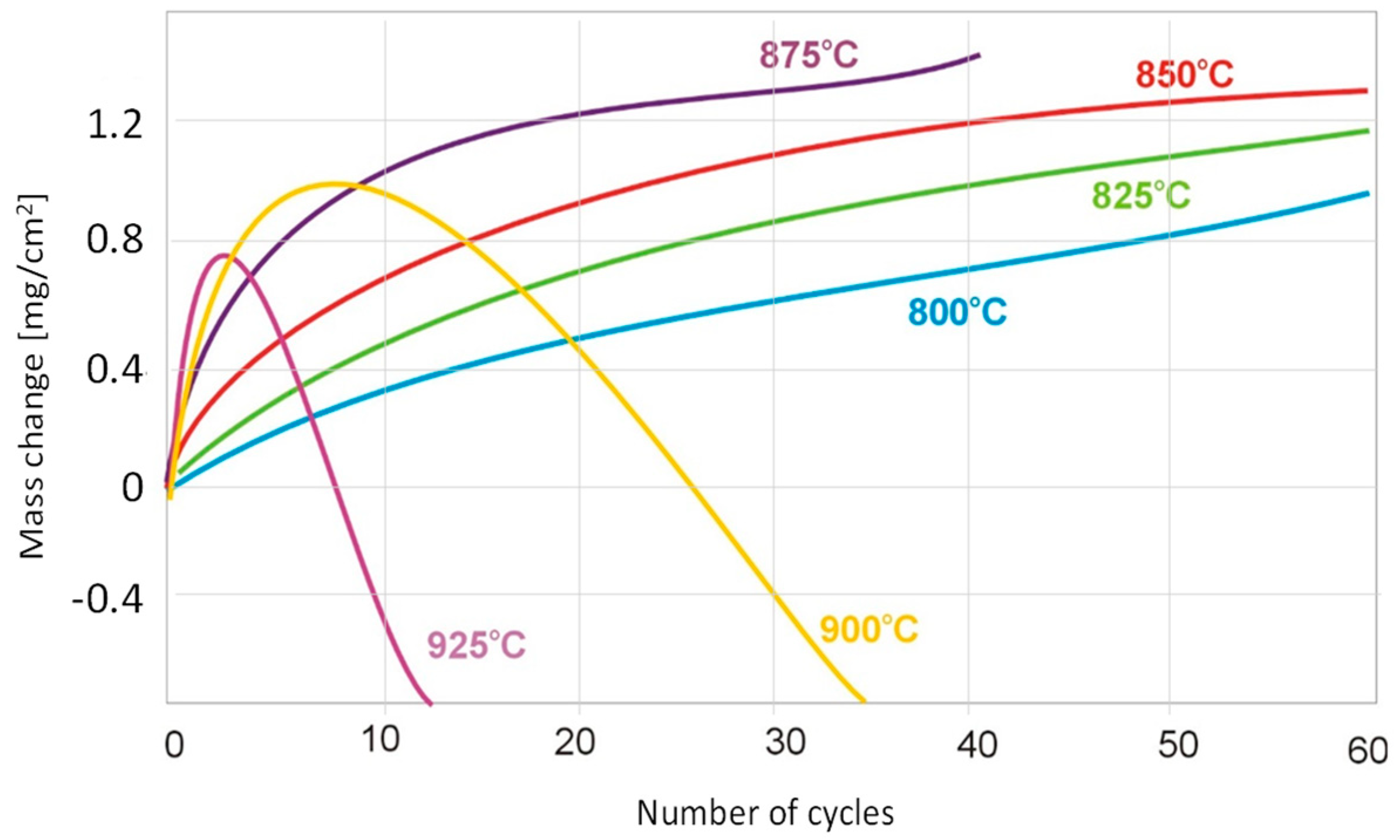
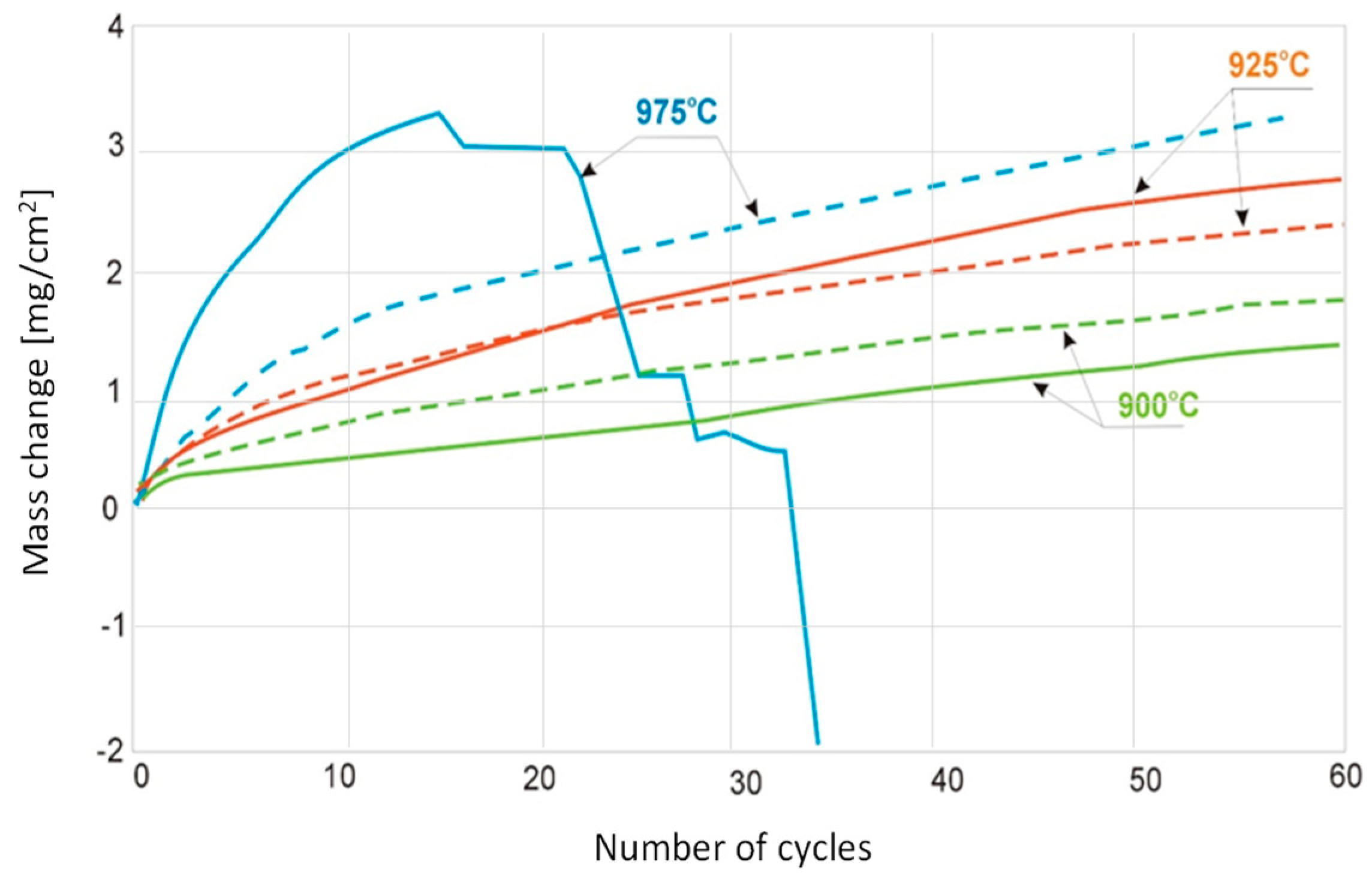
3.2. Determination of Kinetics of Cyclic Oxidation
3.3. Effect of Cyclic Oxidation on the Microstructure and Chemical Composition of the Scale
4. Discussion
- -
- Niobium inhibits film growth kinetics by decreasing titanium activity;
- -
- By reducing the activity of titanium, it increases the activity of aluminium, and supresses the growth of rutile TiO2;
- -
- Nb ions replace Ti ions, thus leading to a reduction in oxygen vacancies that reduce oxygen diffusion; and
- -
- Niobium hinders the mass transfer of TiO2.
5. Conclusions
- The addition of 7 at.% Nb causes a decrease in the oxidation rate, which is manifested by a 30% lower mass gain and better adhesion of the product layer compared to an alloy containing 2 at.% Nb.
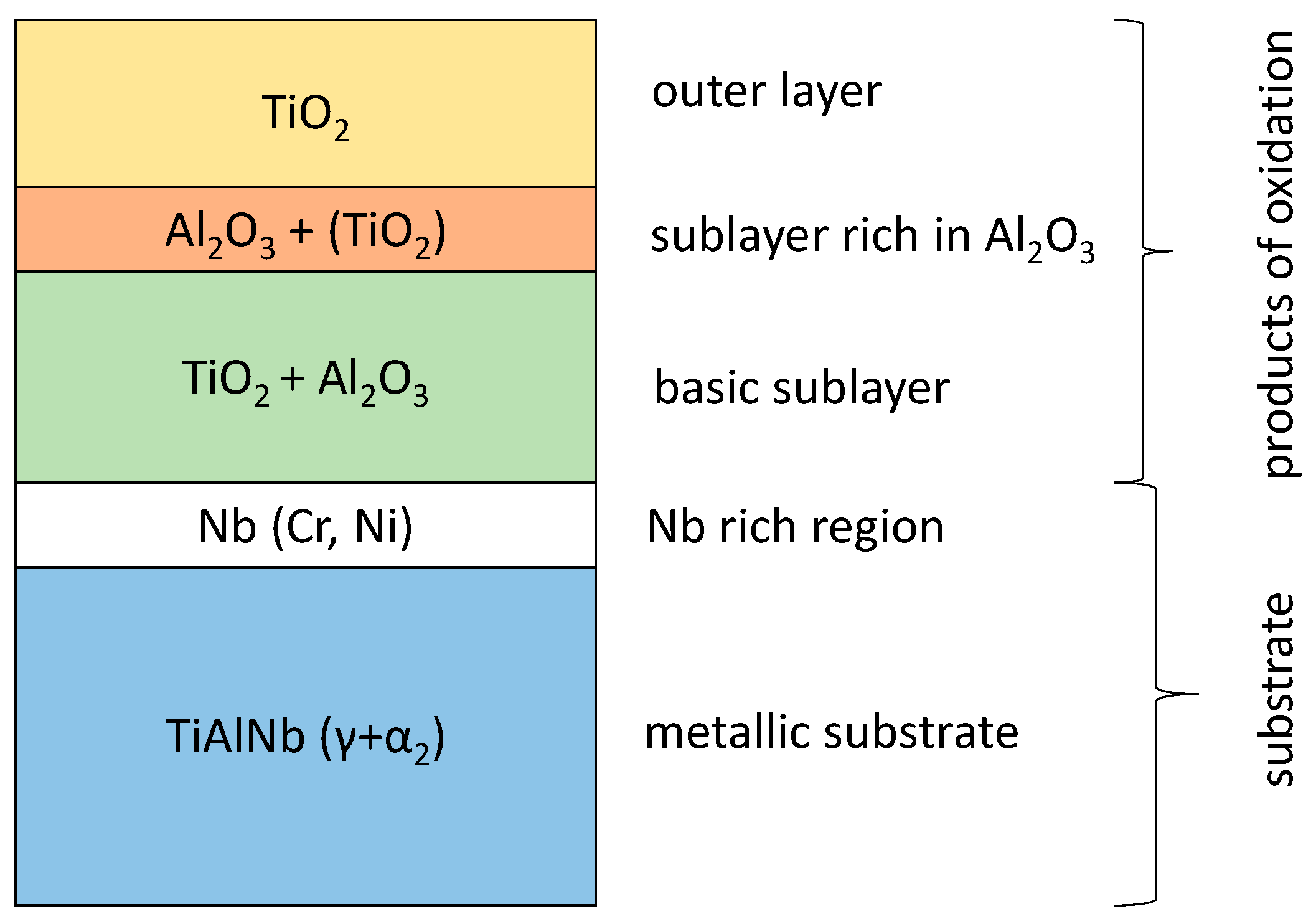
- Multiphase scale is formed, which is always characterised by a sequence of sublayers:
- -
- Outer rutile sublayer;
- -
- Sublayer rich in Al2O3 and containing TiO2 in smaller amounts; and
- -
- Sublayer containing Al2O3, TiO2, and oxides of Nb, Cr and Ni.
- In the oxidation tests, changes in the metallic substrate were observed, consisting of Al diffusion and the dominance at the oxidation temperature of the β-Ti(Nb,Al,N,O) phase with low amounts of dissolved oxygen and nitrogen.
- Nb forming a discontinuous band in the metallic substrate inhibits the diffusion processes taking place.
- The increase in surface roughness contributes to a decrease in the oxidation rate and inhibits oxidation product spallation processes due to a decrease in compressive stress and an increase in cohesion between the oxide and the metallic substrate.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, H.; Kestler, H. Processing and Applications of Intermetallics γ-TiAl—Based Alloys. Adv. Eng. Mater. 2000, 2, 551–570. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kim, Y.W. Oxidation behavior of gamma alloys designed for high temperature applications. Intermetallics 2005, 13, 952–958. [Google Scholar] [CrossRef]
- Bauer, P.; Laska, N.; Swadźba, R. Increasing the oxidation resistance of γ-TiAl by applying a magnetron sputtered aluminum and silicon based coating. Intermetallics 2021, 122, 107177. [Google Scholar] [CrossRef]
- Donchev, A.; Mengis, L.; Couret, A.; Mayer, S.; Clemens, H.; Galetz, M. Effects of tungsten alloying and fluorination on the oxidation behavior of intermetallic titanium aluminides for aerospace applications. Intermetallics 2021, 139, 107270. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.H.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Ostrovskaya, O.; Badini, C.; Baudana, G.; Padovano, E.; Biamino, S. Thermogravimetric investigation on oxidation kinetics of complex Ti-Al. Intermetallics 2018, 93, 244–250. [Google Scholar] [CrossRef]
- Park, S.Y.; Seo, D.Y.; Kim, S.W.; Kim, S.E.; Hong, J.K.; Lee, D.B. High temperature oxidation of Ti-46Al-6Nb-0.5W-0.5Cr-0.3Si-0.1C alloy. Intermetallics 2016, 74, 8–14. [Google Scholar] [CrossRef]
- Laska, N.; Braun, R.; Knittel, S. Oxidation behavior of protective Ti-Al-Cr based coatings applied on the γ-TiAl alloys Ti-48-2-2 and TNM-B1. Surf. Coat. Technol. 2018, 349, 347–356. [Google Scholar] [CrossRef]
- Durda, E.; Przybylski, K. Oxidation behavior of Ti-46Al-8Nb alloy with boron and carbon addition under thermal cycling conditions. Intermetallics 2016, 71, 51–56. [Google Scholar] [CrossRef]
- Mitoraj, M.; Godlewska, E.M. Oxidation of Ti-46Al-8Ta in air at 700 °C and 800 °C under thermal cycling conditions. Intermetallics 2013, 34, 112–121. [Google Scholar] [CrossRef]
- Narayana, P.L.; Kim, J.H.; Yun, D.W. High temperature isothermal oxidation behavior of electron beam melted multi-phase γ-TiAl alloy. Intermetallics 2022, 141, 107424. [Google Scholar] [CrossRef]
- Smialek, J.L.; Barrett, C.A.; Scheaffer, C. Design for oxidation resistance. In Materials Selection and Design, ASM Handbook; Dieter, G.E., Ed.; ASM International: Materials Park, OH, USA, 1997; pp. 589–602. [Google Scholar]
- Lowell, C.E.; Deadmore, D.L. The role of thermal shock in cyclic oxidation. Oxid. Met. 1980, 14, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Rakowski, J.M.; Pettit, I.S.; Meier, G.H. The effect of surface preparation on the oxidation behaviour of gamma Ti-Al-base intermetallic alloys. Scr. Mater. 1996, 35, 1417–1422. [Google Scholar] [CrossRef]
- Pérez, P.; Jiménez, J.A.; Frommeyer, G.; Adeva, P. The influence of the alloy microstructure on the oxidation behavior of Ti-46Al-1Cr-0.2Si alloy. Oxid. Met. 2000, 53, 99–124. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Schaaf, P.; Zheng, N.; Gil, A.; Wallura, E. Beneficial and detrimental effects on nitrogen on the oxidation behaviour of TiAl-based intermetalics. Mater. Corros. 1997, 48, 28–34. [Google Scholar] [CrossRef]
- Rakowski, J.M.; Pettit, F.S.; Meier, G.M. The effect of nitrogen on the oxidation of γ-TiAl. Scr. Metall. Mater. 1995, 33, 997–1003. [Google Scholar] [CrossRef]
- Zeller, A.; Dettenwanger, F.; Schütze, M. Influence of water vapor on the oxidation behaviour of titanium aluminides. Intermetallics 2002, 10, 59–72. [Google Scholar] [CrossRef]
- Brady, M.P.; Smialek, J.L.; Humphrey, D.L.; Smith, J. The role of Cr in promoting protective alumina scale formation by γ-based Ti-Al-Cr alloys. Part II: Oxidation behavior in air. Acta Mater. 1997, 45, 2371–2382. [Google Scholar] [CrossRef]
- Li, X.Y.; Taniguchi, S. Correlation of high temperature oxidation with tensile properties for Ti–48Al–2Cr–2Nb and Ti–48Al–2Cr–2Fe alloys. Intermetallics 2005, 13, 683–693. [Google Scholar] [CrossRef]
- Małecka, J.; Grzesik, W.; Hernas, A. An investigation on oxidation wear mechanisms of Ti-46Al-7Nb-0.7Cr-0.1Si-0.2Ni intermetallic-based alloys. Corossion Sci. 2010, 52, 263–272. [Google Scholar] [CrossRef]
- Terner, M.; Biamino, S.; Pelissero, F.; Sabbadini, S.; Fino, P.; Pavese, M.; Badini, C. Oxidation behavior of Ti-47Al-2Cr-2Nb and Ti-45Al-2Cr-8Nb produced by Electron Beam Melting. In Proceedings of the Conference: EuroPM, Volume 2: Full Density and Alternative Consolidation, Gothenburg, Sweden, 15–18 September 2013. [Google Scholar]
- Jang, Y.-D.; Lee, D.B.; Seo, D.Y. High temperature oxidation of thermomechanically treated Ti-47Al−2Cr−2Nb. Met. Mater. Int. 2002, 8, 503–506. [Google Scholar] [CrossRef]
- Król, S. Effect of tantalum on the oxidation resistance of Ti3Al + TiAl titanium intermetallic alloy. In Proceedings of the 14th International Metallurgical & Materials Conference, Brno, Czech Republic, 24–25 May 2005. [Google Scholar]
- Rhines, F.N.; Wolf, J.S. The role of oxide microstructure and growth stresses in the high-temperature scaling of nickel. Metall. Mater. Trans. 1970, 62, 1701–1718. [Google Scholar] [CrossRef]
- Małecka, J. Transformation and precipitation processes in a metal substrate of oxidized TiAl-based alloys. Oxid. Met. 2019, 91, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Jungling, T.; Rapp, R.A. High temperature oxidation of iron at 1200 °C in hot-stage environmental scanning electron microscope. Metall. Mater. Trans. 1982, 134A, 929–935. [Google Scholar] [CrossRef]
- Reynaud, G.M.; Clark, W.A.T.; Rapp, R.A. In situ observation of copper oxidation at high temperatures. Metall. Mater. Trans. 1984, 15A, 573–586. [Google Scholar] [CrossRef]
- Rapp, R.A. The high temperature oxidation of metals forming cation-diffusing scales. Metall. Mater. Trans. 1984, 15A, 765–782. [Google Scholar] [CrossRef]
- Małecka, J. Oxidation resistance of AlCrN coating deposited on Ti-46Al-7Nb-0.7Cr-0.1Si-0.2Ni alloy. Arch. Metall. Mater. 2014, 59, 621–627. [Google Scholar] [CrossRef]
- Roy, T.K.; Balasubramaniam, R.; Ghosh, A. Effect of nitrogen on the oxidation behavior of Ti3Al-based intermetallic alloys. Metall. Mater. Trans. 1996, 27A, 4003–4010. [Google Scholar] [CrossRef]
- ASM Handbook. Vol. 3: Alloy Phase Diagrams, Metal Treatment, Structure and Joining Collection. In Section: Binary Alloy Phase Diagrams—Standard Content; ASM International: Materials Park, OH, USA, 1998. [Google Scholar]
- Taniguchi, S.; Shibata, T. Influence of additional elements on the oxidation behaviour of TiAl. Intermetallics 1996, 4, 85–93. [Google Scholar] [CrossRef]
- Haanappel, V.A.C.; Clemens, H.; Stroosnijder, M.F. The high temperature oxidation behaviour of high and low alloyed TiAl-based intermetallics. Intermetallics 2002, 10, 293–305. [Google Scholar] [CrossRef]
- Varma, S.K.; Chan, A.; Mahapatra, B.N. Static and cyclic oxidation of Ti–44Al and Ti–44Al–xNb alloys. Oxid. Met. 2001, 55, 423–435. [Google Scholar] [CrossRef]
- Fergus, J.W. Review of the effect of alloy composition on the growth rates of scales formed during oxidation of gamma titanium aluminide alloys. Mater. Sci. Eng. 2002, 338A, 108–125. [Google Scholar] [CrossRef]
- Jung, H.G.; Kim, K.Y. Effect of ternary elements on the oxidation behavior of aluminized TiAl alloys. Oxid. Met. 2002, 58, 197–216. [Google Scholar] [CrossRef]
- Becker, S.; Rahmel, A.; Schorr, M. Mechanism of isothermal oxidation of the intel-metallic TiAl and of TiAl alloys. Oxid. Met. 1992, 38, 425–464. [Google Scholar] [CrossRef]
- Jiang, H.; Hirohasi, M.; Lu, Y.; Imanari, H. Effect of Nb on the high temperature oxidation of Ti-(0–50at.%)Al. Scr. Mater. 2002, 46, 639–643. [Google Scholar] [CrossRef]






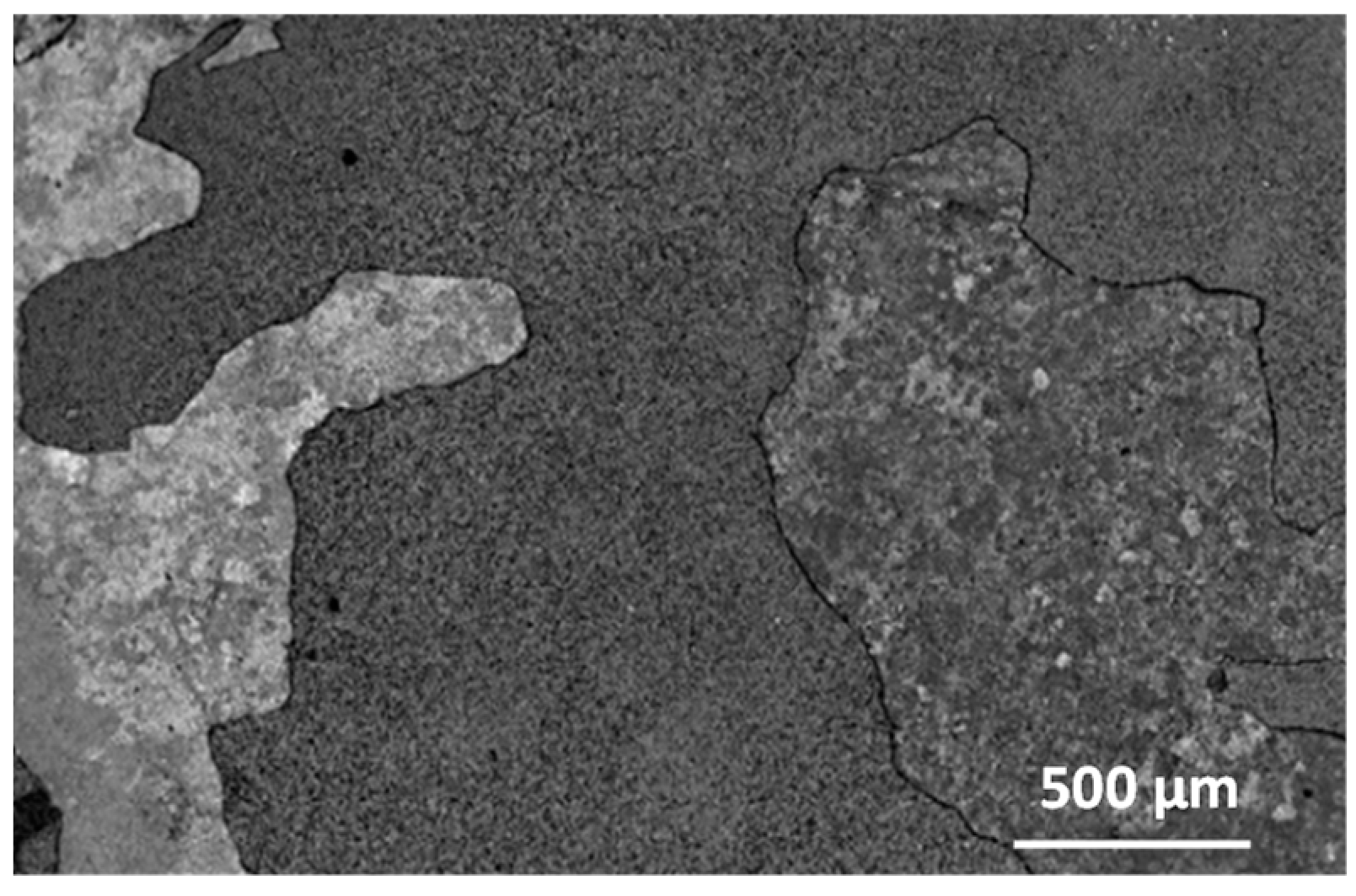



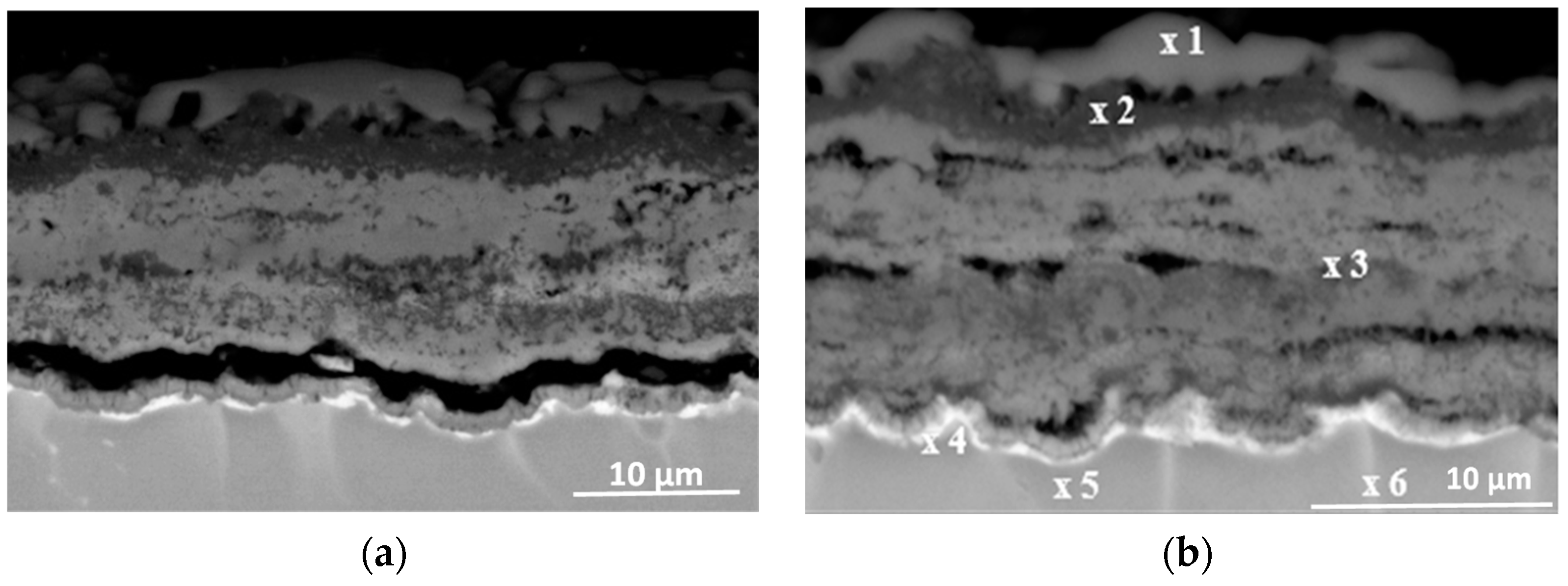




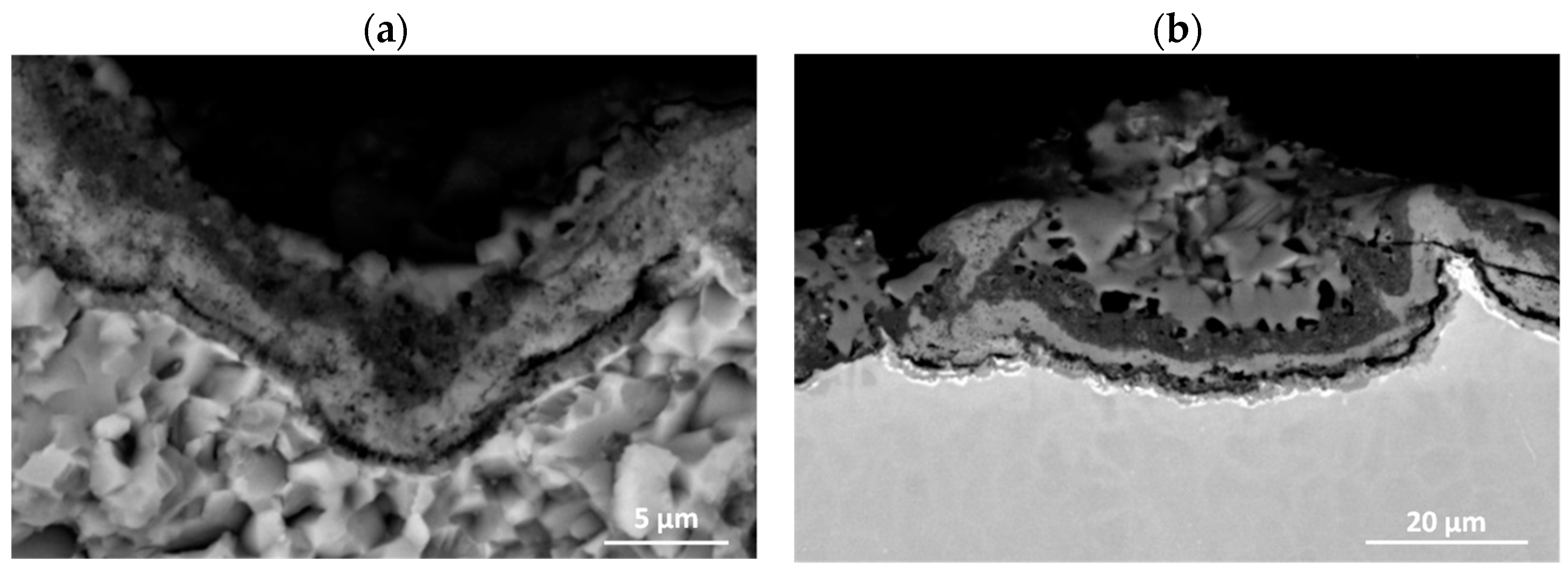
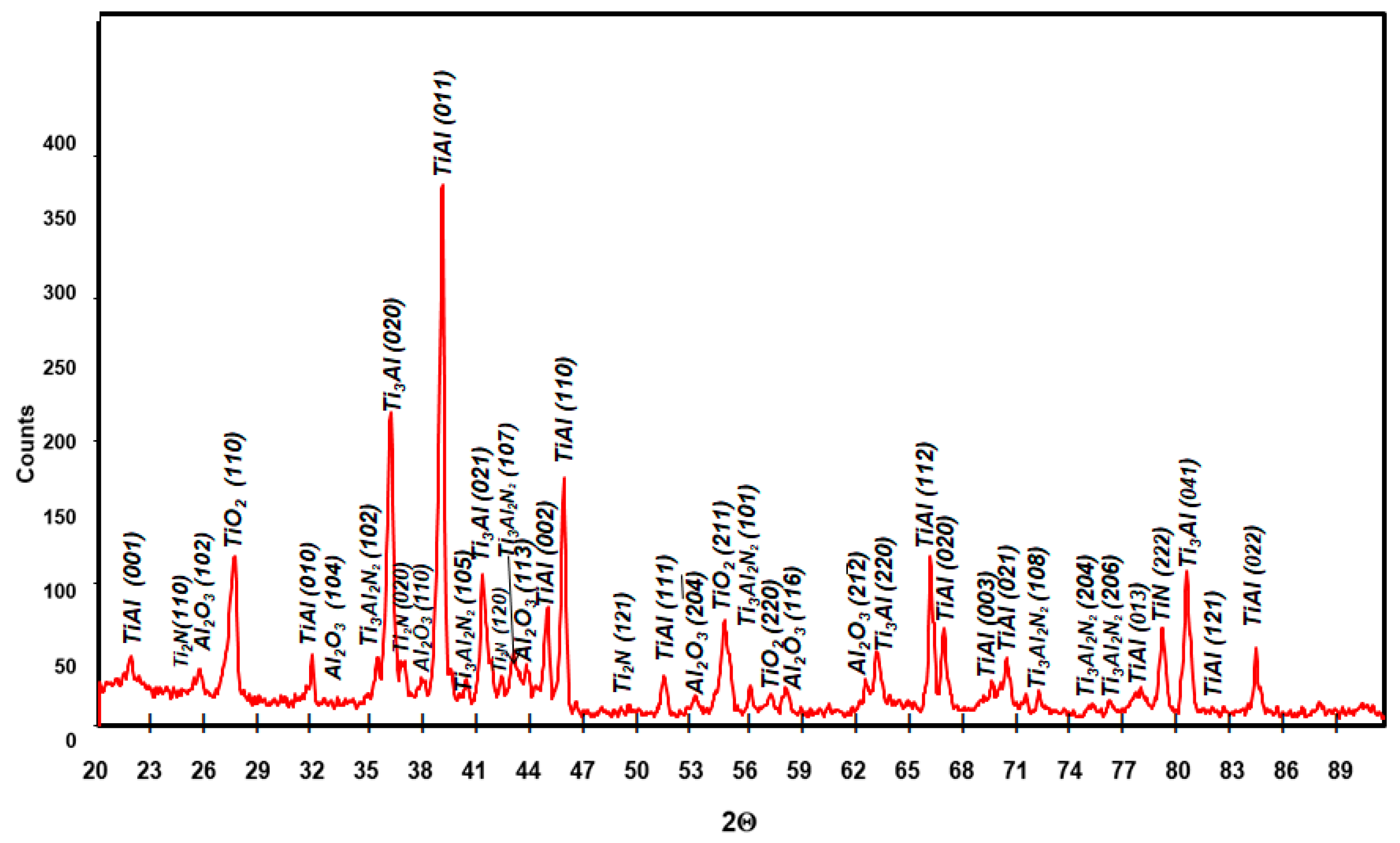
| Element | Ti | Al | Nb | Cr | Ni | Si | |
|---|---|---|---|---|---|---|---|
| Chemical composition from the location #1 | %mass | 75.9 ± 2.16 | 23.1 ± 0.67 | - | - | - | 1.0 ± 0.12 |
| %at. | 64.0 ± 1.69 | 34.6 ± 0.85 | - | - | - | 1.4 ± 0.16 | |
| Chemical composition from the location #2 | %mass | 43.3 ± 1.01 | 50.0 ± 1.13 | 6.7 ± 0.7 | - | - | - |
| %at. | 32.0 ± 0.92 | 65.5 ± 2.03 | 2.5 ± 0.28 | - | - | - | |
| Chemical composition from the location #3 | %mass | 73.1 ± 2.61 | 17.1 ± 0.53 | 9.8 ± 0.62 | - | - | - |
| %at. | 67.4 ± 1.72 | 27.0 ± 0.84 | 5.6 ± 0.76 | - | - | - | |
| Chemical composition from the location #4 | %mass | 44.5 ± 1.12 | 25.2 ± 0.64 | 26.1 ± 0.69 | 1.3 ± 0.13 | 2.9 ± 0.33 | - |
| %at. | 42.9 ± 0.97 | 41.5 ± 0.86 | 12.7 ± 0.42 | 0.6 ± 0.04 | 2.3 ± 0.26 | - |
| Element | Ti | Al | Nb | Cr | Ni | |
|---|---|---|---|---|---|---|
| Chemical composition from the location #A | %mass | 85.4 ± 3.01 | 14.6 ± 0.43 | - | - | - |
| %at. | 76.7 ± 2.18 | 23.3 ± 0.71 | - | - | - | |
| Chemical composition from the location #B | %mass | 61.7 ± 1.72 | 10.5 ± 0.73 | 27.8 ± 0.91 | - | - |
| %at. | 65.1 ± 1.79 | 19.7 ± 0.43 | 15.2 ± 0.42 | - | - | |
| Chemical composition from the location #C | %mass | 61.5 ± 1.45 | 22.3 ± 0.58 | 14.0 ± 0.40 | 0.9 ± 0.05 | 1.3 ± 0.15 |
| %at. | 55.8 ± 1.12 | 35.9 ± 1.01 | 6.5 ± 0.06 | 0.8 ± 0.48 | 1.0 ± 0.13 |
| Element | Ti | Al | Nb | Cr | Ni | |
|---|---|---|---|---|---|---|
| Chemical composition from the location #1 | %mass | 92.8 ± 2.96 | 7.2 ± 0.48 | - | - | - |
| %at. | 87.9 ± 3.01 | 12.1 ± 1.01 | - | - | - | |
| Chemical composition from the location #2 | %mass | 39.1 ± 1.21 | 55.2 ± 1.23 | 5.7 ± 0.7 | - | - |
| %at. | 27.9 ± 1.03 | 70.0 ± 1.76 | 2.1 ± 0.27 | - | - | |
| Chemical composition from the location #3 | %mass | 61.3 ± 1.71 | 24.9 ± 0.74 | 13.8 ± 0.56 | - | - |
| %at. | 54.4 ± 1.56 | 39.3 ± 0.83 | 6.3 ± 0.23 | - | - | |
| Chemical composition from the location #4 | %mass | 29.6 ± 0.98 | 27.4 ± 0.85 | 37.2 ± 0.81 | 2.3 ± 0.28 | 3.5 ± 0.33 |
| %at. | 28.9 ± 0.99 | 47.5 ± 1.13 | 18.7 ± 0.53 | 2.1 ± 0.29 | 2.8 ± 0.21 | |
| Chemical composition from the location #5 | %mass | 49.3 ± 1.15 | 32.3 ± 0.89 | 17.8 ± 0.54 | 0.6 ± 0.05 | - |
| %at. | 42.3 ± 0.98 | 49.3 ± 1.02 | 7.9 ± 0.61 | 0.5 ± 0.02 | - | |
| Chemical composition from the location #6 | %mas | 45.6 ± 1.03 | 33.2 ± 0.76 | 20.3 ± 0.44 | 0.9 ± 0.05 | - |
| %at. | 39.4 ± 0.78 | 50.8 ± 1.02 | 9.0 ± 0.32 | 0.7 ± 0.03 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małecka, J. Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys. Materials 2022, 15, 2137. https://doi.org/10.3390/ma15062137
Małecka J. Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys. Materials. 2022; 15(6):2137. https://doi.org/10.3390/ma15062137
Chicago/Turabian StyleMałecka, Joanna. 2022. "Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys" Materials 15, no. 6: 2137. https://doi.org/10.3390/ma15062137
APA StyleMałecka, J. (2022). Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys. Materials, 15(6), 2137. https://doi.org/10.3390/ma15062137






