Studies on Electron Escape Condition in Semiconductor Nanomaterials via Photodeposition Reaction
Abstract
:1. Introduction
2. Materials & Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fick, J. Chapter 8—Crystalline Nanoparticles in Glasses for Optical Applications. In Handbook of Surfaces and Interfaces of Materials; Nalwa, H.S., Ed.; Academic Press: Burlington, VT, USA, 2001; pp. 311–350. [Google Scholar]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. 9—Metal-based semiconductor nanomaterials for photocatalysis. In Multifunctional Photocatalytic Materials for Energy; Lin, Z., Ye, M., Wang, M., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 187–213. [Google Scholar]
- Ye, C.; Li, Z.; Ye, E. Chapter 4 Metal–Oxide Semiconductor Nanomaterials for Photothermal Catalysis. In Photothermal Nanomaterials; The Royal Society of Chemistry: Cambridge, UK, 2022; pp. 135–157. [Google Scholar]
- Regulacio, M.D. Chapter 5 Copper Sulfide-based Nanomaterials for Photothermal Applications. In Photothermal Nanomaterials; The Royal Society of Chemistry: Cambridge, UK, 2022; pp. 158–185. [Google Scholar]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Mohd Kaus, N.H.; Rithwan, A.F.; Adnan, R.; Ibrahim, M.L.; Thongmee, S.; Mohd Yusoff, S.F. Effective Strategies, Mechanisms, and Photocatalytic Efficiency of Semiconductor Nanomaterials Incorporating rGO for Environmental Contaminant Degradation. Catalysts 2021, 11, 302. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, W.; Li, Z.; Xiong, Y. Surface and Interface Engineering in Photocatalysis. ChemNanoMat 2015, 1, 223–239. [Google Scholar] [CrossRef]
- Li, J.; Lou, Z.; Li, B. Engineering plasmonic semiconductors for enhanced photocatalysis. J. Mater. Chem. A 2021, 9, 18818–18835. [Google Scholar] [CrossRef]
- Ren, H.; Yang, J.-L.; Yang, W.-M.; Zhong, H.-L.; Lin, J.-S.; Radjenovic, P.M.; Sun, L.; Zhang, H.; Xu, J.; Tian, Z.-Q.; et al. Core–Shell–Satellite Plasmonic Photocatalyst for Broad-Spectrum Photocatalytic Water Splitting. ACS Mater. Lett. 2021, 3, 69–76. [Google Scholar] [CrossRef]
- Su, H.; Wang, W. Dynamically Monitoring the Photodeposition of Single Cocatalyst Nanoparticles on Semiconductors via Fluorescence Imaging. Anal. Chem. 2021, 93, 11915–11919. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Li, J.; Li, J. Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties. Nanomaterials 2019, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Vinasco, J.A.; Radu, A.; Kasapoglu, E.; Restrepo, R.L.; Morales, A.L.; Feddi, E.; Mora-Ramos, M.E.; Duque, C.A. Effects of Geometry on the Electronic Properties of Semiconductor Elliptical Quantum Rings. Sci. Rep. 2018, 8, 13299. [Google Scholar] [CrossRef]
- Tavkhelidze, A.; Bibilashvili, A.; Jangidze, L.; Gorji, N.E. Fermi-Level Tuning of G-Doped Layers. Nanomaterials 2021, 11, 505. [Google Scholar] [CrossRef]
- Koochi, H.; Ebrahimi, F. Geometrical effects on the electron residence time in semiconductor nano-particles. J. Chem. Phys. 2014, 141, 094702. [Google Scholar] [CrossRef]
- Hemmerling, J.R.; Mathur, A.; Linic, S. Characterizing the Geometry and Quantifying the Impact of Nanoscopic Electrocatalyst/Semiconductor Interfaces under Solar Water Splitting Conditions. Adv. Energy Mater. 2022, 2103798. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Parwaz Khan, A.A.; Asiri, A.M.; Thakur, V.K.; Nguyen, V.-H. Surface defect engineering of metal oxides photocatalyst for energy application and water treatment. J. Mater. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, X.; Peng, S. Prospect Prediction of Terminal Clean Power Consumption in China via LSSVM Algorithm Based on Improved Evolutionary Game Theory. Energies 2020, 13, 2065. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Shao, Y.; Zhong, Y.; Lv, J.; Hao, X. 0D/2D nanocomposite visible light photocatalyst for highly stable and efficient hydrogen generation via recrystallization of CdS on MoS2 nanosheets. Nano Energy 2016, 27, 466–474. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Recent Development in Defects Engineered Photocatalysts: An Overview of the Experimental and Theoretical Strategies. Energy Environ. Mater. 2022, 5, 68–114. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Chen, W.; Chen, Z. Enhanced Charge Transport and Increased Active Sites on α-Fe2O3 (110) Nanorod Surface Containing Oxygen Vacancies for Improved Solar Water Oxidation Performance. ACS Omega 2018, 3, 14973–14980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Song, L.; Cen, J.; Liu, M. Mechanistic Insights into Defect-Assisted Carrier Transport in Bismuth Vanadate Photoanodes. J. Phys. Chem. C 2019, 123, 20730–20736. [Google Scholar] [CrossRef]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.-b.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen Vacancy Enhanced Photocatalytic Activity of Pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Li, Z.; Lou, Z.; Wang, Z.; Dai, Y.; Whangbo, M.-H. Synthesis and characterization of ZnS with controlled amount of S vacancies for photocatalytic H2 production under visible light. Sci. Rep. 2015, 5, 8544. [Google Scholar] [CrossRef]
- Cui, H.; Liu, H.; Shi, J.; Wang, C. Function of TiO2 Lattice Defects toward Photocatalytic Processes: View of Electronic Driven Force. Int. J. Photoenergy 2013, 2013, 364802. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Wang, S.; Xie, J.; Wang, L.; Zhang, X.; Zou, J.-J. Constructing TiO2 p-n homojunction for photoelectrochemical and photocatalytic hydrogen generation. Nano Energy 2016, 28, 296–303. [Google Scholar] [CrossRef]
- Chen, Y.; Crittenden, J.C.; Hackney, S.; Sutter, L.; Hand, D.W. Preparation of a Novel TiO2-Based p−n Junction Nanotube Photocatalyst. Environ. Sci. Technol. 2005, 39, 1201–1208. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Han, J.; Guo, R.; Xiong, H.; Yin, Y. TiO2/NiO hybrid shells: P–n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Park, Y.; Kim, J.; Ryu, W.; Rho, J.; Kim, K. Photodeposited metal-semiconductor nanocomposites and their applications. J. Mater. 2018, 4, 83–94. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Li, Q. Collagen Deposition and Fibrosis in the Lymphatic Tissues of HIV-1 Infected Individuals. In Encyclopedia of AIDS; Hope, T.J., Stevenson, M., Richman, D., Eds.; Springer: New York, NY, USA, 2021; pp. 1–6. [Google Scholar]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work Function of TiO2 (Anatase, Rutile, and Brookite) Single Crystals: Effects of the Environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]
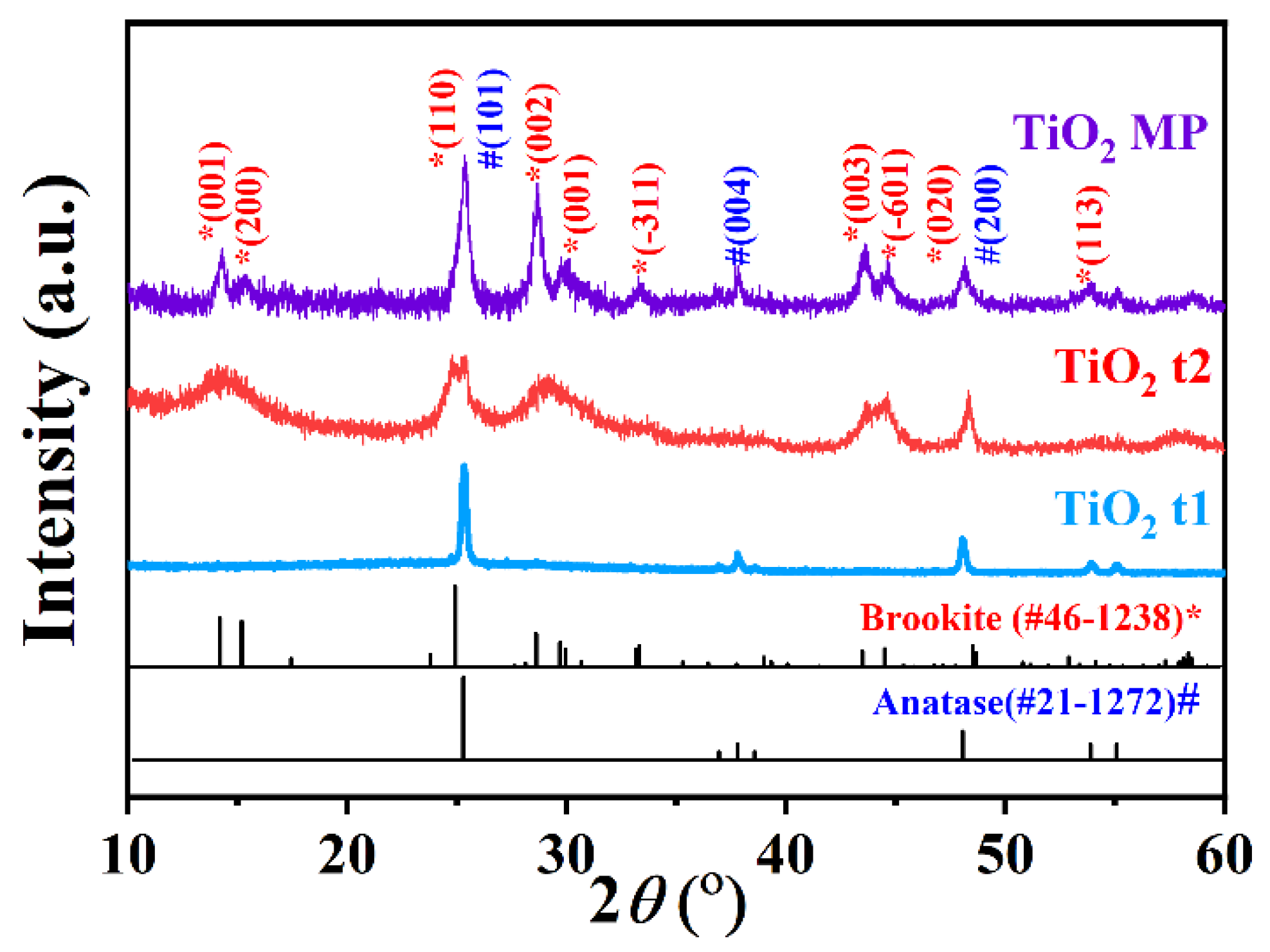
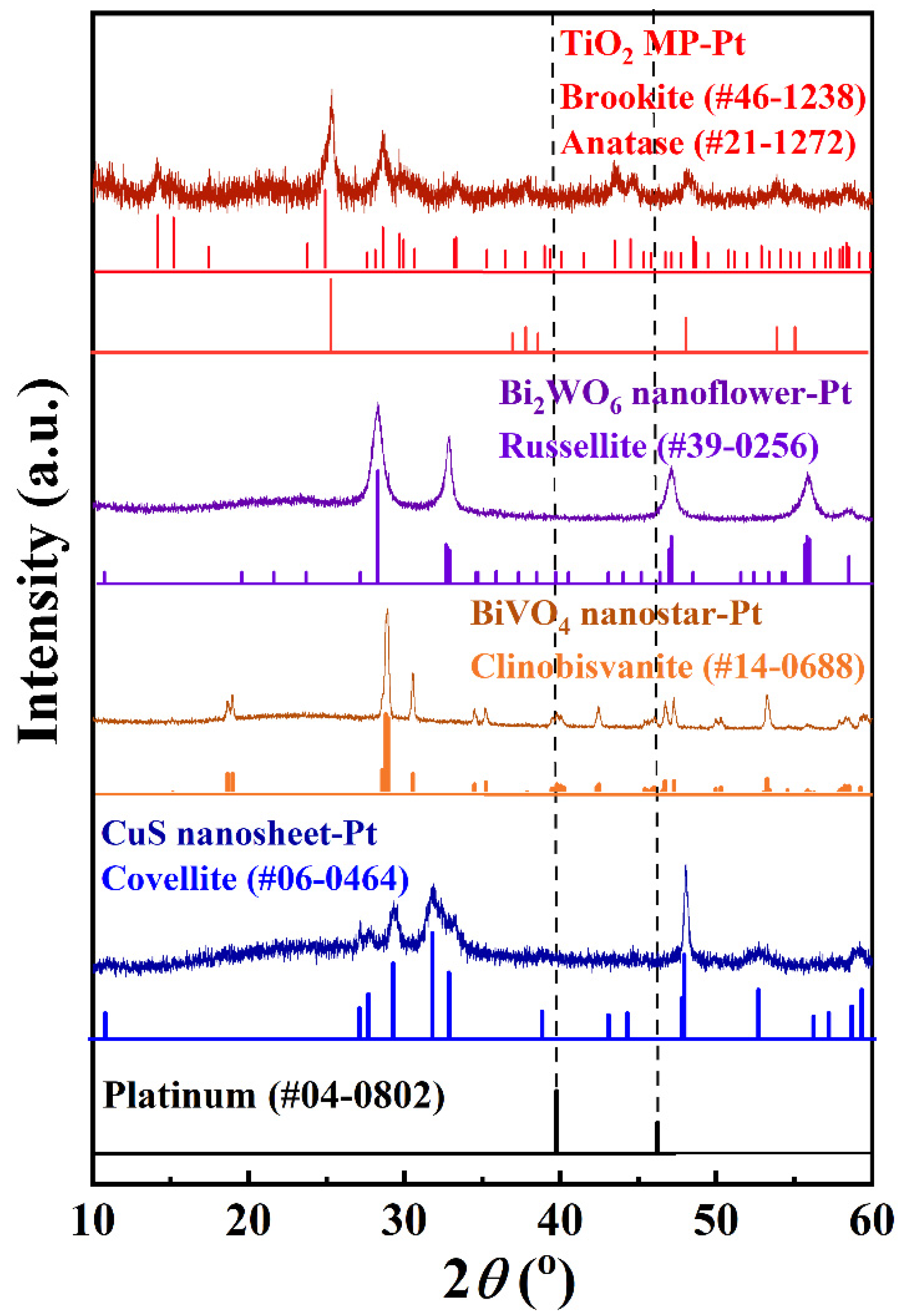


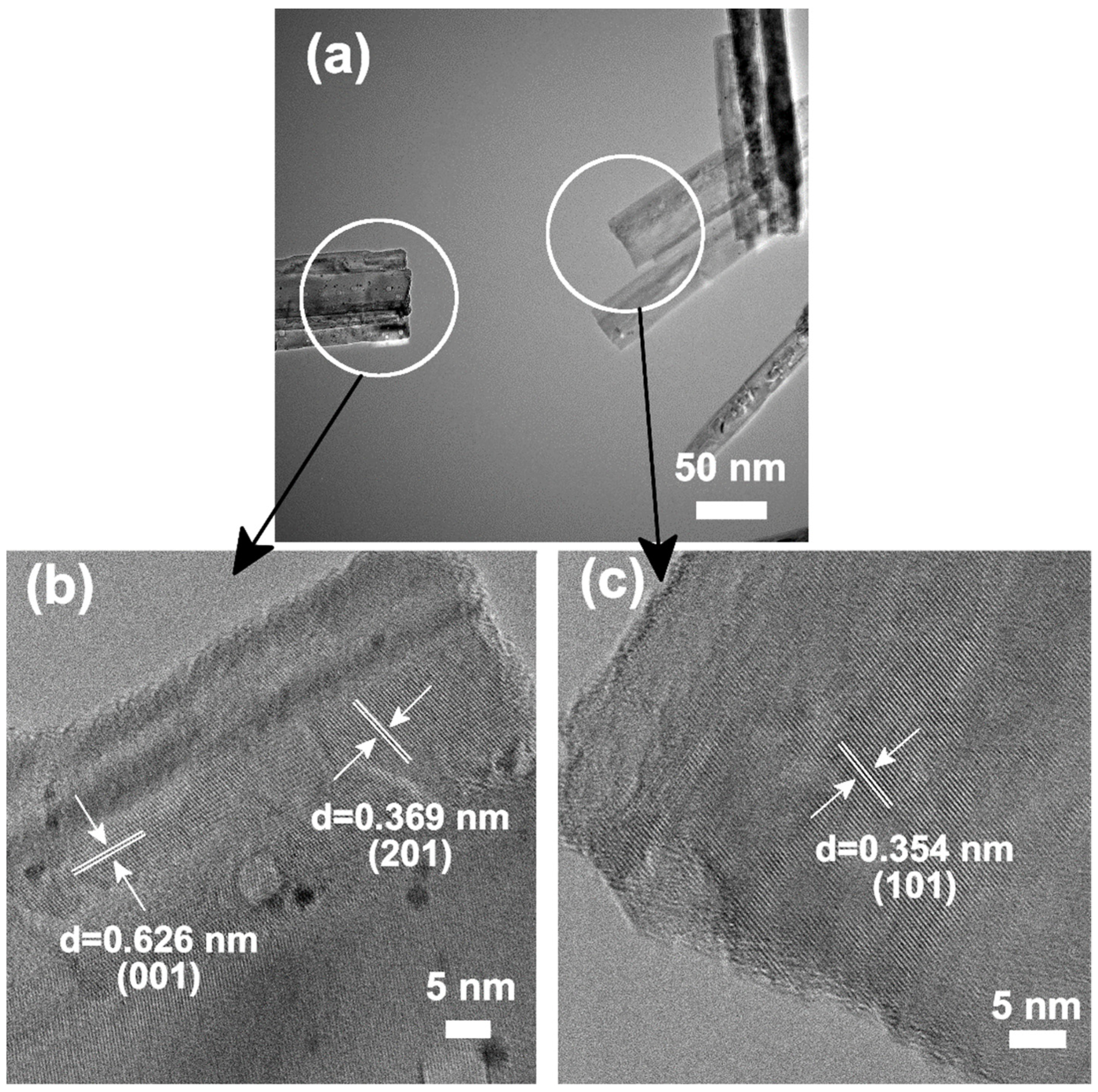


| Basic Materials | Crystal Structure | Pt4+ Loading Amount | Reaction Time |
|---|---|---|---|
| TiO2 t1 | Brookite | 1.2–12.0 wt% | 30 min |
| TiO2 t2 | Anatase | 1.2–12.0 wt% | 30 min |
| TiO2 MP | Brookite + Anatase | 0.23–12.0 wt% | 15 min–2 h |
| Bi2WO6 | Russellite | 2.9 wt% | 30 min |
| Bi2VO4 | Clinobisvanite | 2.9 wt% | 30 min |
| CuS | Covellite | 2.9 wt% | 30 min |
| Basic Materials | Pt4+ Loading Amount | Pt Deposited Amount (Analyzed by EDX) |
|---|---|---|
| TiO2 t1 | 1.2 wt% | 0.76 wt% |
| 2.3 wt% | 2.05 wt% | |
| 5.8 wt% | 4.07 wt% | |
| 12.0 wt% | 7.96 wt% | |
| TiO2 t2 | 1.2 wt% | 0.31 wt% |
| 2.3 wt% | 3.15 wt% | |
| 5.8 wt% | 6.03 wt% | |
| 12.0 wt% | 8.70 wt% | |
| TiO2 MP | 1.2 wt% | 0.37 wt% |
| 2.3 wt% | 2.30 wt% | |
| 5.8 wt% | 4.20 wt% | |
| 12.0 wt% | 7.26 wt% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Huan, Y. Studies on Electron Escape Condition in Semiconductor Nanomaterials via Photodeposition Reaction. Materials 2022, 15, 2116. https://doi.org/10.3390/ma15062116
Ye C, Huan Y. Studies on Electron Escape Condition in Semiconductor Nanomaterials via Photodeposition Reaction. Materials. 2022; 15(6):2116. https://doi.org/10.3390/ma15062116
Chicago/Turabian StyleYe, Chen, and Yu Huan. 2022. "Studies on Electron Escape Condition in Semiconductor Nanomaterials via Photodeposition Reaction" Materials 15, no. 6: 2116. https://doi.org/10.3390/ma15062116
APA StyleYe, C., & Huan, Y. (2022). Studies on Electron Escape Condition in Semiconductor Nanomaterials via Photodeposition Reaction. Materials, 15(6), 2116. https://doi.org/10.3390/ma15062116





