Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications
Abstract
:1. Introduction
2. Dealloying for Fabricating Nanoporous Materials
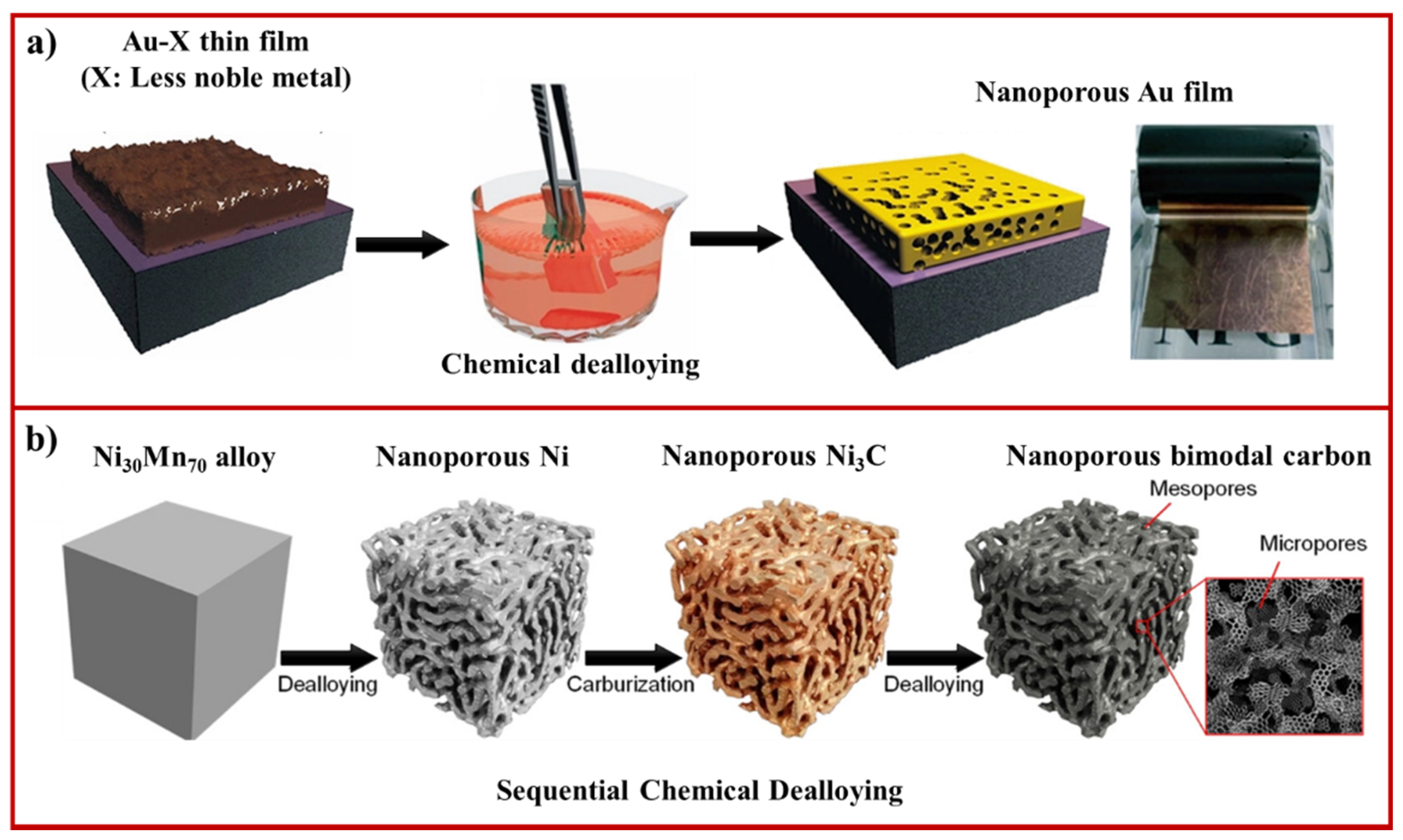
2.1. Chemical Dealloying
2.2. Electrochemical Dealloying
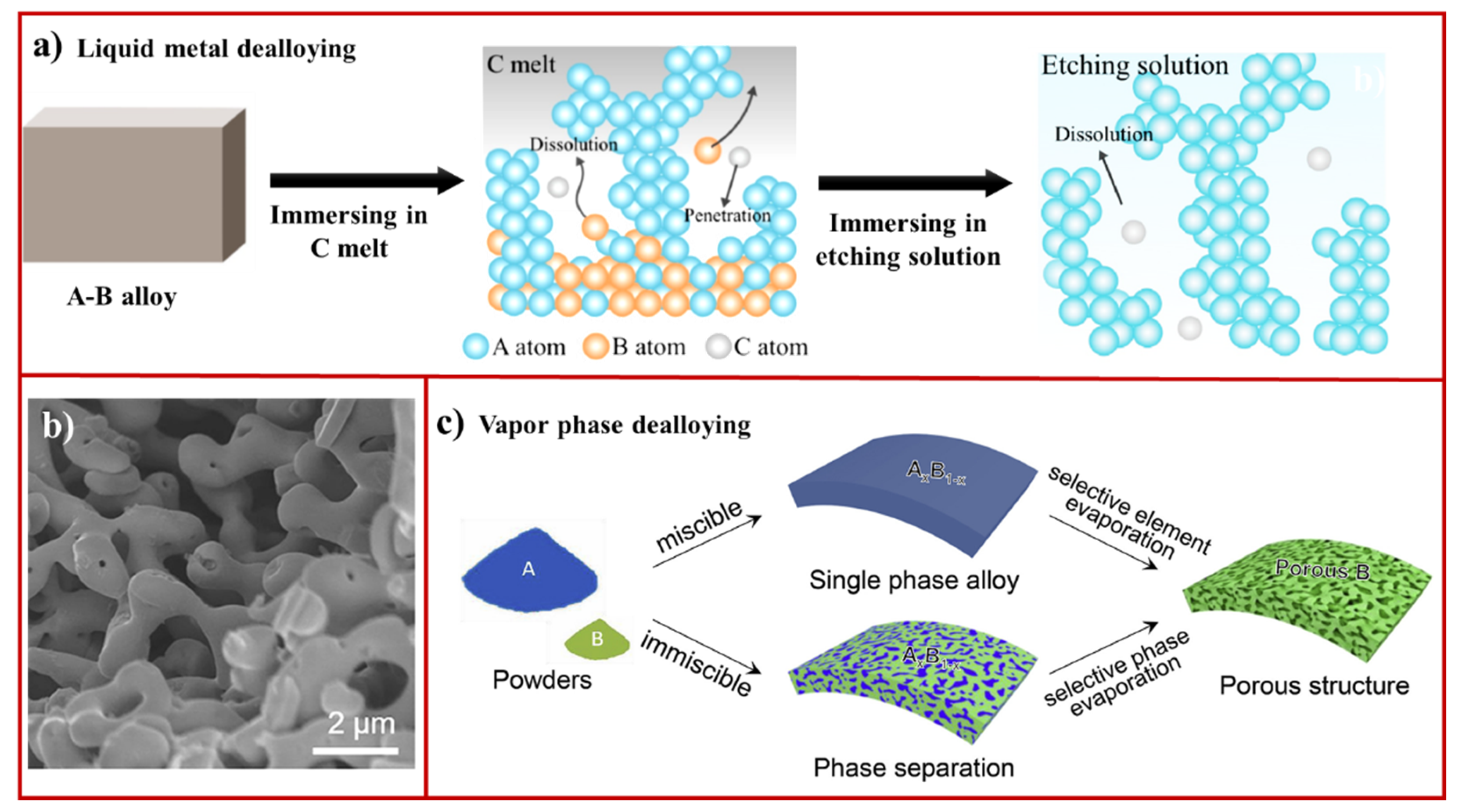
2.3. Liquid Metal Dealloying
2.4. Vapor Phase Dealloying
3. Templating
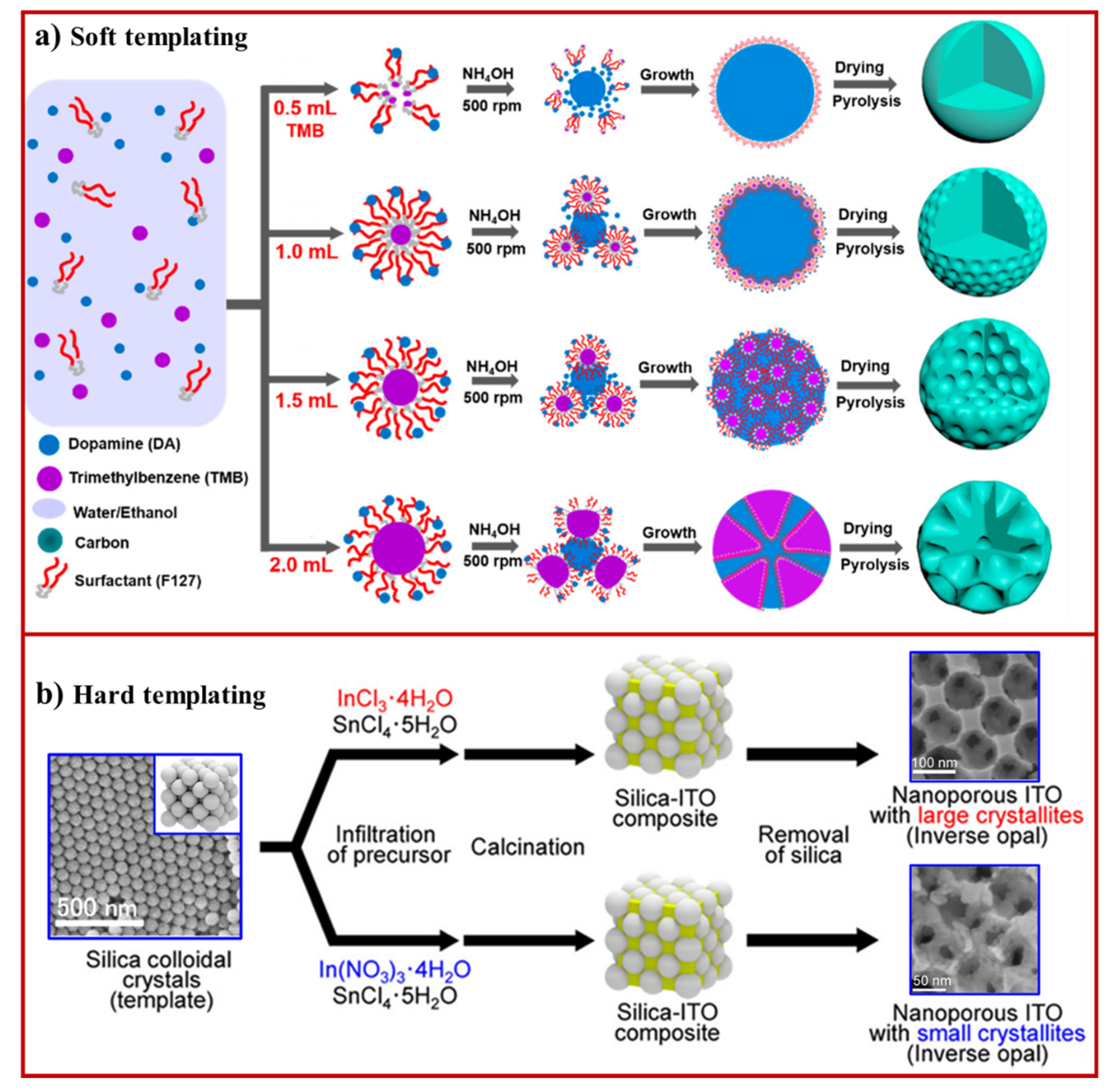
3.1. Soft Template Method
3.2. Hard Template Method
4. Microwave-Based Fabrication of Nanoporous Materials
5. Additive Manufacturing of Nanoporous Materials
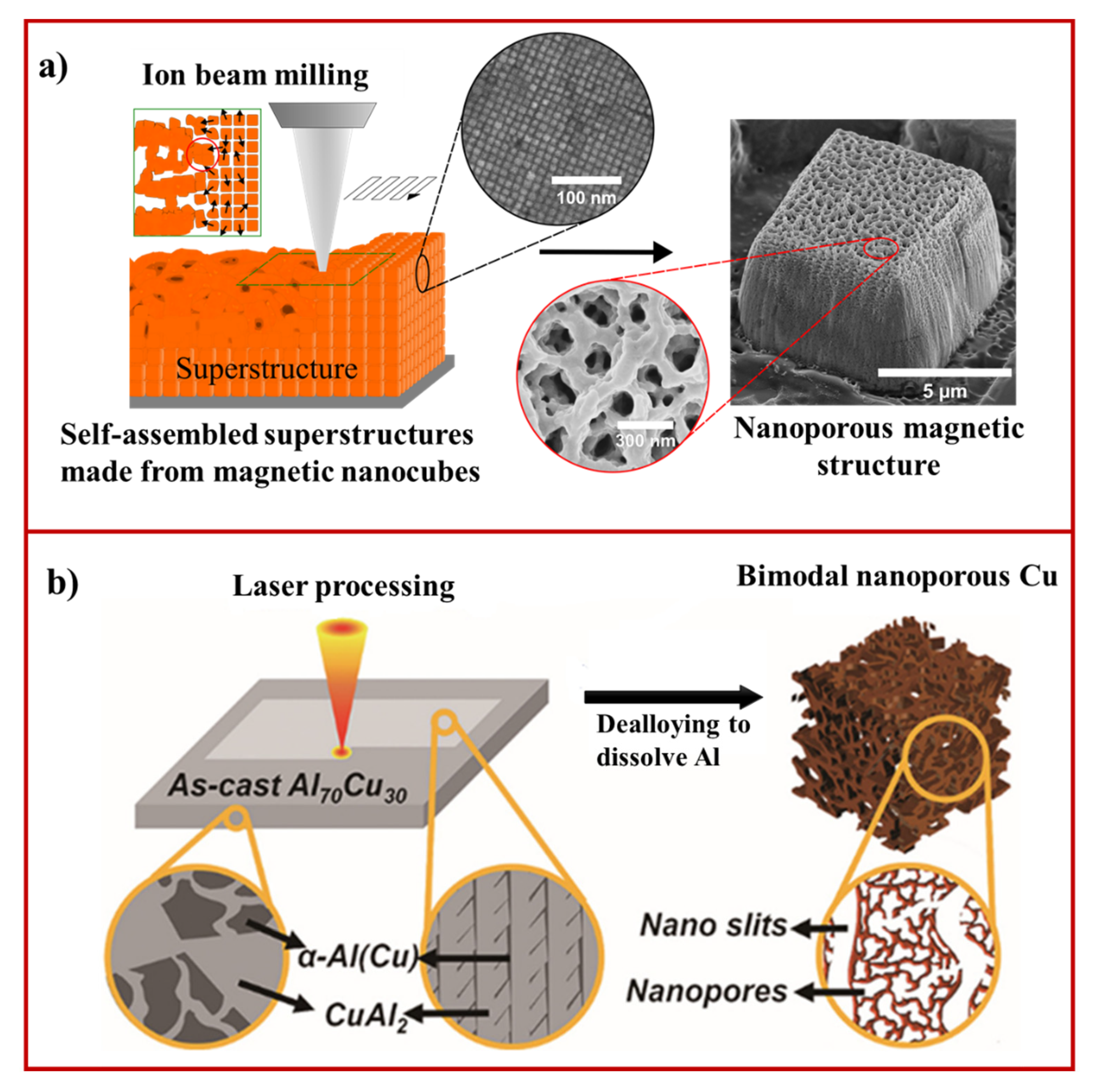
6. Ion Beam-Induced Fabrication of Nanoporous Materials
7. Laser-Induced Fabrication of Nanoporous Materials
8. Biomedical Applications of Nanoporous Materials
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noori, Y.; Akhbari, K. Post-synthetic ion-exchange process in nanoporous metal–organic frameworks; an effective way for modulating their structures and properties. RSC Adv. 2017, 7, 1782–1808. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, X.; Tan, H.; Qi, W.; Wang, L.; Ali, M.C.; Zhang, H.; Chen, J.; Hu, P.; Fan, C.; et al. Combustion Fabrication of Nanoporous Graphene for Ionic Separation Membranes. Adv. Funct. Mater. 2018, 28, 1805026. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, V.; Baranwal, A.; Chandra, P.; Pandey, L.M. Engineered nanoporous materials mediated heterogeneous catalysts and their implications in biodiesel production. Mater. Sci. Energy Technol. 2018, 1, 11–21. [Google Scholar] [CrossRef]
- Wong, T.S.B.; Newman, R. Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Networks. Sensors 2020, 20, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Colombi Ciacchi, L.; Wei, G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, G.; Greenaway, A.; Yiu, H.H.P. The Potential Applications of Nanoporous Materials for the Adsorption, Separation, and Catalytic Conversion of Carbon Dioxide. Adv. Energy Mater. 2014, 4, 1301873. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef]
- Fu, J.; Detsi, E.; De Hosson, J.T.M. Recent advances in nanoporous materials for renewable energy resources conversion into fuels. Surf. Coat. Technol. 2018, 347, 320–336. [Google Scholar] [CrossRef]
- Kapruwan, P.; Ferré-Borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Platforms for Drug Delivery Applications: Recent Advances and Perspective. Adv. Mater. Inter. 2020, 7, 2001133–2001150. [Google Scholar] [CrossRef]
- Davoodi, E.; Zhianmanesh, M.; Montazerian, H.; Milani, A.S.; Hoorfar, M. Nano-porous anodic alumina: Fundamentals and applications in tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 60. [Google Scholar] [CrossRef]
- Guo, T.; Oztug, N.A.K.; Han, P.; Ivanovski, S.; Gulati, K. Influence of sterilization on the performance of anodized nanoporous titanium implants. Mater. Sci. Eng. C 2021, 130, 112429. [Google Scholar] [CrossRef] [PubMed]
- Wonanke, A.D.D.; Bennett, P.; Caldwell, L.; Addicoat, M.A. Role of Host-Guest Interaction in Understanding Polymerisation in Metal-Organic Frameworks. Front. Chem. 2021, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Ritter, H.; Ramm, J.H.; Brühwiler, D. Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules. Materials 2010, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Grommet, A.B.; Feller, M.; Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 2020, 15, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, N.; Yu, Y.; Yan, Y.; Zhang, H.; Li, J.; Yu, J. Carbon dots in zeolites: A new class of thermally activated delayed fluorescence materials with ultralong lifetimes. Sci. Adv. 2017, 3, 1603171–1603179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Ron, R.; Haleva, E.; Salomon, A. Nanoporous Metallic Networks: Fabrication, Optical Properties, and Applications. Adv. Mater. 2018, 30, 1706755. [Google Scholar] [CrossRef]
- Sayari, A. Catalysis by Crystalline Mesoporous Molecular Sieves. Chem. Mater. 1996, 8, 1840–1852. [Google Scholar] [CrossRef]
- Barton, T.J.; Bull, L.M.; Klemperer, W.G.; Loy, D.A.; McEnaney, B.; Misono, M.; Monson, P.A.; Pez, G.; Scherer, G.W.; Vartuli, J.C.; et al. Tailored Porous Materials. Chem. Mater. 1999, 11, 2633–2656. [Google Scholar] [CrossRef] [Green Version]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger Robert, J.; Chmelka Bradley, F.; Ryoo, R. Directing Zeolite Structures into Hierarchically Nanoporous Architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, R.; Kaleeswaran, D.; Haldar, S.; Chakraborty, D.; Mullangi, D.; Borah, A.; Vinod, C.P.; Murugavel, R.; Vaidhyanathan, R. Nanoporous Covalent Organic Framework Embedded with Fe/Fe3O4 Nanoparticles as Air-Stable Low-Density Nanomagnets. ACS Appl. Nano Mater. 2020, 3, 9088–9096. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Huang, Y.; Guo, Z.; Shao, L. Building Nanoporous Metal–Organic Frameworks “Armor” on Fibers for High-Performance Composite Materials. ACS Appl. Mater. Inter. 2017, 9, 5590–5599. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-K. Metal–organic framework membranes: Unprecedented opportunities for gas separations. AIChE J. 2021, 67, e17258. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhou, S.; Wang, J.; Xin, H.; Wei, S.; Liu, S.; Wang, Z.; Lu, X. Tracking CO2 capture and separation over N2 in a flexible metal–organic framework: Insights from GCMC and DFT simulations. J. Mater. Sci. 2021, 56, 10414–10423. [Google Scholar] [CrossRef]
- Chakraborty, D.; Bej, S.; Sahoo, S.; Chongdar, S.; Ghosh, A.; Banerjee, P.; Bhaumik, A. Novel Nanoporous Ti-Phosphonate Metal–Organic Framework for Selective Sensing of 2,4,6-Trinitrophenol and a Promising Electrode in an Energy Storage Device. ACS Sust. Chem. Eng. 2021, 9, 14224–14237. [Google Scholar] [CrossRef]
- Noor, T.; Pervaiz, S.; Iqbal, N.; Nasir, H.; Zaman, N.; Sharif, M.; Pervaiz, E. Nanocomposites of NiO/CuO Based MOF with rGO: An Efficient and Robust Electrocatalyst for Methanol Oxidation Reaction in DMFC. Nanomaterials 2020, 10, 8. [Google Scholar] [CrossRef]
- Ahmad, R.; Iqbal, N.; Baig, M.M.; Noor, T.; Ali, G.; Gul, I.H. ZIF-67 derived nitrogen doped CNTs decorated with sulfur and Ni(OH)2 as potential electrode material for high-performance supercapacitors. Electrochim. Acta 2020, 364, 137147. [Google Scholar] [CrossRef]
- Rizvi, S.A.M.; Iqbal, N.; Haider, M.D.; Noor, T.; Anwar, R.; Hanif, S. Synthesis and Characterization of Cu-MOF Derived Cu@AC Electrocatalyst for Oxygen Reduction Reaction in PEMFC. Catal. Lett. 2020, 150, 1397–1407. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Li, Z.; Goyal, N.; Du, T.; He, J.; Li, G.K. Shaping of Metal–Organic Frameworks: A Review. Energy Fuels 2022. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef] [Green Version]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Luc, W.; Jiao, F. Synthesis of Nanoporous Metals, Oxides, Carbides, and Sulfides: Beyond Nanocasting. Acc. Chem. Res. 2016, 49, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Fechete, I.; Vedrine, J.C. Nanoporous Materials as New Engineered Catalysts for the Synthesis of Green Fuels. Molecules 2015, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Glowniak, S.; Szczesniak, B.; Choma, J.; Jaroniec, M. Advances in Microwave Synthesis of Nanoporous Materials. Adv. Mater. 2021, 33, e2103477. [Google Scholar] [CrossRef] [PubMed]
- Malgras, V.; Tang, J.; Wang, J.; Kim, J.; Torad, N.L.; Dutta, S.; Ariga, K.; Hossain, M.S.A.; Yamauchi, Y.; Wu, K.C.W. Fabrication of Nanoporous Carbon Materials with Hard- and Soft-Templating Approaches: A Review. J. Nanosci. Nanotechnol. 2019, 19, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis Strategies of Porous Carbon for Supercapacitor Applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, C.; Tian, Q.; Yu, D. Liquid metals dealloying as a general approach for the selective extraction of metals and the fabrication of nanoporous metals: A review. Mater. Today Commun. 2021, 26, 102007. [Google Scholar] [CrossRef]
- Chuang, A.; Erlebacher, J. Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing. Materials 2020, 13, 17. [Google Scholar] [CrossRef]
- Thommes, M.; Schlumberger, C. Characterization of Nanoporous Materials. Ann. Rev. Chem. Biomol. Eng. 2021, 12, 137–162. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Qi, Z.; Zhang, W.; Qin, J.; Frenzel, J. Generalized Fabrication of Nanoporous Metals (Au, Pd, Pt, Ag, and Cu) through Chemical Dealloying. J. Phys. Chem. C 2009, 113, 12629–12636. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, R.; Li, Z.; Wang, X.; Wang, H.; Wu, Y.; Jiang, S.; Lu, Z. Formation mechanism and characterization of immiscible nanoporous binary Cu–Ag alloys with excellent surface-enhanced Raman scattering performance by chemical dealloying of glassy precursors. Inorg. Chem. Front. 2020, 7, 1127–1139. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered Mesoporous Cobalt Oxide as Highly Efficient Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-W.; Liu, X.; Le, G.-M.; Li, J.-F.; Qu, F.-S.; Lu, S.-Y.; Qi, L. Morphology evolution and SERS activity of the nanoporous Au prepared by dealloying sputtered Au-Ag film. Phys. B Cond. Matter 2019, 558, 49–53. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L. Robust Metallic Actuators Based on Nanoporous Gold Rapidly Dealloyed from Gold–Nickel Precursors. Adv. Funct. Mater. 2021, 31, 2107241. [Google Scholar] [CrossRef]
- Mahr, C.; Schowalter, M.; Mitterbauer, C.; Lackmann, A.; Fitzek, L.; Mehrtens, T.; Wittstock, A.; Rosenauer, A. Nanoporous gold dealloyed from AuAg and AuCu: Comparison of structure and chemical composition. Materialia 2018, 2, 131–137. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Ann. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L. Improving the chemical de-alloying of amorphous Au alloys. Corr. Sci. 2017, 127, 141–146. [Google Scholar] [CrossRef]
- Vega, A.A.; Newman, R.C. Nanoporous Metals Fabricated through Electrochemical Dealloying of Ag-Au-Pt with Systematic Variation of Au:Pt Ratio. J. ElectroChem. Soc. 2013, 161, C1–C10. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, Y.; Zhang, J.; Ding, Y.; Peng, Z.; Zhang, Z. Hierarchically nanoporous nickel-based actuators with giant reversible strain and ultrahigh work density. J. Mater. Chem. C 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Song, T.; Yan, M.; Webster, N.A.S.; Styles, M.J.; Kimpton, J.A.; Qian, M. In-situ and ex-situ synchrotron X-ray diffraction studies of microstructural length scale controlled dealloying. Acta Mater. 2019, 168, 376–392. [Google Scholar] [CrossRef]
- Duan, H.; Hao, Q.; Xu, C. Hierarchical nanoporous PtTi alloy as highly active and durable electrocatalyst toward oxygen reduction reaction. J. Power Source 2015, 280, 483–490. [Google Scholar] [CrossRef]
- Lu, Q.; Hutchings, G.S.; Yu, W.; Zhou, Y.; Forest, R.V.; Tao, R.; Rosen, J.; Yonemoto, B.T.; Cao, Z.; Zheng, H.; et al. Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2015, 6, 6567. [Google Scholar] [CrossRef] [Green Version]
- Hyun, J.-I.; Noh, H.-S.; Kong, K.; Kim, W.T.; Kim, D.H. Formation of different types nanoporous composite structure by dealloying of Ag and Ni containing Al-Cu-Ti base amorphous precursor alloys. Mater. Charact. 2021, 175, 111093. [Google Scholar] [CrossRef]
- El Mel, A.-A.; Boukli-Hacene, F.; Molina-Luna, L.; Bouts, N.; Chauvin, A.; Thiry, D.; Gautron, E.; Gautier, N.; Tessier, P.-Y. Unusual Dealloying Effect in Gold/Copper Alloy Thin Films: The Role of Defects and Column Boundaries in the Formation of Nanoporous Gold. ACS Appl. Mater. Inter. 2015, 7, 2310–2321. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Lu, Z.; Huang, G.; Johnson, I.; Watanabe, K.; Chen, M. 3D Bimodal Porous Amorphous Carbon with Self-Similar Porosity by Low-Temperature Sequential Chemical Dealloying. Chem. Mater. 2021, 33, 1013–1021. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Han, J.; Cheng, C.; Ning, S.; Hirata, A.; Fujita, T.; Chen, M. Engineering the internal surfaces of three-dimensional nanoporous catalysts by surfactant-modified dealloying. Nat. Commun. 2017, 8, 1066. [Google Scholar] [CrossRef]
- Lan, J.; Peng, M.; Liu, P.; Chen, D.; Xu, X.; Luo, M.; Tan, Y.; Chen, M. Scalable synthesis of nanoporous boron for high efficiency ammonia electrosynthesis. Mater. Today 2020, 38, 58–66. [Google Scholar] [CrossRef]
- Jiao, W.; Liu, P.; Lin, H.; Zhou, W.; Wang, Z.; Fujita, T.; Hirata, A.; Li, H.-W.; Chen, M. Tunable Nanoporous Metallic Glasses Fabricated by Selective Phase Dissolution and Passivation for Ultrafast Hydrogen Uptake. Chem. Mater. 2017, 29, 4478–4483. [Google Scholar] [CrossRef]
- Rysiakiewicz-Pasek, E.; Ciżman, A.; Antropova, T.; Gorokhovatsky, Y.; Pshenko, O.; Fomicheva, E.; Drozdova, I. An insight into inorganic glasses and functional porous glass-based nanocomposites. Mater. Chem. Phys. 2020, 243, 122585. [Google Scholar] [CrossRef]
- Maruo, Y.Y. Measurement of ambient ozone using newly developed porous glass sensor. Sens. Actuators B Chem. 2007, 126, 485–491. [Google Scholar] [CrossRef]
- Sun, L.; Chien, C.-L.; Searson, P.C. Fabrication of Nanoporous Nickel by Electrochemical Dealloying. Chem. Mater. 2004, 16, 3125–3129. [Google Scholar] [CrossRef]
- Lu, X.; Yu, T.; Wang, H.; Qian, L.; Lei, P. Electrochemical Fabrication and Reactivation of Nanoporous Gold with Abundant Surface Steps for CO2 Reduction. ACS Catal. 2020, 10, 8860–8869. [Google Scholar] [CrossRef]
- Luo, J.; Han, P.; Dan, Z.; Qin, F.; Tang, T.; Dong, Y. Bimodal nanoporous silver fabricated from dual-phase Ag10Zn90 precursor via electrochemical dealloying for direct ammonia-borane electrooxidation. Micropor. Mesopor. Mater. 2020, 308, 110532. [Google Scholar] [CrossRef]
- Steyskal, E.-M.; Qi, Z.; Pölt, P.; Albu, M.; Weissmüller, J.; Würschum, R. Electrochemically Tunable Resistance of Nanoporous Platinum Produced by Dealloying. Langmuir 2016, 32, 7757–7764. [Google Scholar] [CrossRef]
- Shi, S.; Markmann, J.; Weissmüller, J. Actuation by hydrogen electrosorption in hierarchical nanoporous palladium. Philos. Mag. 2017, 97, 1571–1587. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Abbott, A.P.; Yang, C. Electrochemical fabrication of nanoporous copper films in choline chloride–urea deep eutectic solvent. Phys. Chem. Chem. Phys. 2015, 17, 14702–14709. [Google Scholar] [CrossRef]
- Zeng, Y.; Gaskey, B.; Benn, E.; McCue, I.; Greenidge, G.; Livi, K.; Zhang, X.; Jiang, J.; Elebacher, J. Electrochemical dealloying with simultaneous phase separation. Acta Mater. 2019, 171, 8–17. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Liu, Y.-C.; Hsieh, Y.-T.; Sun, I.W. Some Aspects on the One-Pot Fabrication of Nanoporous Pd–Au Surface Films by Electrochemical Alloying/Dealloying of (Pd–Au)–Zn from a Chlorozincate Ionic Liquid. ACS Omega 2017, 2, 4911–4919. [Google Scholar] [CrossRef]
- Fu, J.; Corsi, J.S.; Welborn, S.S.; Basile, V.; Wang, L.; Ng, A.K.; Detsi, E. Eco-friendly Synthesis of Nanoporous Magnesium by Air-Free Electrolytic Dealloying with Recovery of Sacrificial Elements for Energy Conversion and Storage Applications. ACS Sustain. Chem. Eng. 2021, 9, 2762–2769. [Google Scholar] [CrossRef]
- Yaghoobnejad Asl, H.; Fu, J.; Kumar, H.; Welborn, S.S.; Shenoy, V.B.; Detsi, E. In Situ Dealloying of Bulk Mg2Sn in Mg-Ion Half Cell as an Effective Route to Nanostructured Sn for High Performance Mg-Ion Battery Anodes. Chem. Mater. 2018, 30, 1815–1824. [Google Scholar] [CrossRef]
- Chen, Q.; Sieradzki, K. Spontaneous evolution of bicontinuous nanostructures in dealloyed Li-based systems. Nat. Mater. 2013, 12, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, J.; Wang, Z.; Wang, L.; Corsi, J.S.; Detsi, E. Perspective—Reversible Magnesium Storage in Silicon: An Ongoing Challenge. J. Electrochem. Soc. 2020, 167, 050514. [Google Scholar] [CrossRef]
- Corsi, J.S.; Fu, J.; Wang, Z.; Lee, T.; Ng, A.K.; Detsi, E. Hierarchical Bulk Nanoporous Aluminum for On-Site Generation of Hydrogen by Hydrolysis in Pure Water and Combustion of Solid Fuels. ACS Sustain. Chem. Eng. 2019, 7, 11194–11204. [Google Scholar] [CrossRef]
- Song, T.; Tang, H.P.; Li, Y.; Qian, M. Liquid metal dealloying of titanium-tantalum (Ti-Ta) alloy to fabricate ultrafine Ta ligament structures: A comparative study in molten copper (Cu) and Cu-based alloys. Corr. Sci. 2020, 169, 108600. [Google Scholar] [CrossRef]
- Han, J.; Li, C.; Lu, Z.; Wang, H.; Wang, Z.; Watanabe, K.; Chen, M. Vapor phase dealloying: A versatile approach for fabricating 3D porous materials. Acta Mater. 2019, 163, 161–172. [Google Scholar] [CrossRef]
- Harrison, J.D.; Wagner, C. The attack of solid alloys by liquid metals and salt melts. Acta Metall. 1959, 7, 722–735. [Google Scholar] [CrossRef]
- Wada, T.; Yubuta, K.; Inoue, A.; Kato, H. Dealloying by metallic melt. Mater. Lett. 2011, 65, 1076–1078. [Google Scholar] [CrossRef]
- Tsuda, M.; Wada, T.; Kato, H. Kinetics of formation and coarsening of nanoporous α-titanium dealloyed with Mg melt. J. Appl. Phys. 2013, 114, 113503. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Volegov, A.S.; Markmann, J. Tuning microstructure and mechanical properties of open porous TiNb and TiFe alloys by optimization of dealloying parameters. Scr. Mater. 2018, 154, 68–72. [Google Scholar] [CrossRef]
- Berger, S.A.; Okulov, I.V. Open Porous α + β Titanium Alloy by Liquid Metal Dealloying for Biomedical Applications. Metals 2020, 10, 11. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Soldatov, I.V.; Luthringer, B.; Willumeit-Römer, R.; Wada, T.; Kato, H.; Weissmüller, J.; Markmann, J. Open porous dealloying-based biomaterials as a novel biomaterial platform. Mater. Sci. Eng. C 2018, 88, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.-H.; Wada, T.; Kato, H. Development of porous FeCo by liquid metal dealloying: Evolution of porous morphology and effect of interaction between ligaments and melt. Mater. Des. 2019, 180, 107908. [Google Scholar] [CrossRef]
- Kim, J.W.; Tsuda, M.; Wada, T.; Yubuta, K.; Kim, S.G.; Kato, H. Optimizing niobium dealloying with metallic melt to fabricate porous structure for electrolytic capacitors. Acta Mater. 2015, 84, 497–505. [Google Scholar] [CrossRef]
- Yu, S.-G.; Yubuta, K.; Wada, T.; Kato, H. Three-dimensional bicontinuous porous graphite generated in low temperature metallic liquid. Carbon 2016, 96, 403–410. [Google Scholar] [CrossRef]
- Okulov, A.V.; Joo, S.-H.; Kim, H.S.; Kato, H.; Okulov, I.V. Nanoporous High-Entropy Alloy by Liquid Metal Dealloying. Metals 2020, 10, 10. [Google Scholar] [CrossRef]
- Joo, S.-H.; Bae, J.W.; Park, W.-Y.; Shimada, Y.; Wada, T.; Kim, H.S.; Takeuchi, A.; Konno, T.J.; Kato, H.; Okulov, I.V. Beating Thermal Coarsening in Nanoporous Materials via High-Entropy Design. Adv. Mater. 2020, 32, 1906160. [Google Scholar] [CrossRef]
- Balluffi, R.W.; Alexander, B.H. Development of Porosity during Diffusion in Substitutional Solid Solutions. J. Appl. Phys. 1952, 23, 1237–1244. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.; Han, J.; Zhang, F.; Liu, P.; Wang, H.; Wang, Z.; Cheng, C.; Chen, L.; Hirata, A.; et al. Three-dimensional bicontinuous nanoporous materials by vapor phase dealloying. Nat. Commun. 2018, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Mandal, J.; Ye, Q.; Li, A.; Cheng, Q.; Gong, T.; Jin, T.; He, Y.; Yu, N.; Yang, Y. A Scalable Dealloying Technique To Create Thermally Stable Plasmonic Nickel Selective Solar Absorbers. ACS Appl. Energy Mater. 2019, 2, 6551–6557. [Google Scholar] [CrossRef]
- Pinna, A.; Pia, G.; Casula, M.F.; Delogu, F.; Sogne, E.; Falqui, A.; Pilia, L. Fabrication of Nanoporous Al by Vapor-Phase Dealloying: Morphology Features, Mechanical Properties and Model Predictions. Appl. Sci. 2021, 11, 14. [Google Scholar] [CrossRef]
- Moriguchi, I.; Ozono, A.; Mikuriya, K.; Teraoka, Y.; Kagawa, S.; Kodama, M. Micelle-Templated Mesophases of Phenol-Formaldehyde Polymer. Chem. Lett. 1999, 28, 1171–1172. [Google Scholar] [CrossRef]
- Saito, Y.; Matsuno, T.; Guo, Q.; Mori, T.; Kashiwagi, M.; Shimojima, A.; Wada, H.; Kuroda, K. Preparation of Ordered Nanoporous Indium Tin Oxides with Large Crystallites and Individual Control over Their Thermal and Electrical Conductivities. ACS Appl. Mater. Inter. 2021, 13, 15373–15382. [Google Scholar] [CrossRef]
- Peng, L.; Hung, C.T.; Wang, S.; Zhang, X.; Zhu, X.; Zhao, Z.; Wang, C.; Tang, Y.; Li, W.; Zhao, D. Versatile Nanoemulsion Assembly Approach to Synthesize Functional Mesoporous Carbon Nanospheres with Tunable Pore Sizes and Architectures. J. Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, H.; Sun, H.; Hua, W.; Wang, H.; Liu, X.; Wei, B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, L.; Lou, X.W. Nanowire-templated formation of SnO2/carbon nanotubes with enhanced lithium storage properties. Nanoscale 2016, 8, 8384–8389. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Kiyomura, T.; Kurata, H.; Abe, T.; Nakanishi, K. Hierarchically Porous Carbon Monoliths Comprising Ordered Mesoporous Nanorod Assemblies for High-Voltage Aqueous Supercapacitors. Chem. Mater. 2016, 28, 3944–3950. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Chien, Y.C.; Cheng, C.F.; Ho, R.M. Fabrication of Mesoporous Polystyrene Films with Controlled Porosity and Pore Size by Solvent Annealing for Templated Syntheses. Langmuir 2017, 33, 8428–8435. [Google Scholar] [CrossRef]
- Fei, H.-F.; Li, W.; Nuguri, S.; Yu, H.-J.; Yavitt, B.M.; Fan, W.; Watkins, J.J. One-Step Synthesis of Hierarchical, Bimodal Nanoporous Carbons via Co-templating with Bottlebrush and Linear Block Copolymers. Chem. Mater. 2020, 32, 6055–6061. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wei, W.; Wu, Z.-S.; Feng, X.; Müllen, K. Mesoporous Metal–Nitrogen-Doped Carbon Electrocatalysts for Highly Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Zhang, C.; Du, C.; Cheng, C.; Chen, W. High rate-performance supercapacitor based on nitrogen-doped hollow hexagonal carbon nanoprism arrays with ultrathin wall thickness in situ fabricated on carbon cloth. J. Power Source 2019, 434, 226701. [Google Scholar] [CrossRef]
- Fang, Y.; Lv, Y.; Che, R.; Wu, H.; Zhang, X.; Gu, D.; Zheng, G.; Zhao, D. Two-Dimensional Mesoporous Carbon Nanosheets and Their Derived Graphene Nanosheets: Synthesis and Efficient Lithium Ion Storage. J. Am. Chem. Soc. 2013, 135, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Wan, Z.; Wang, X.; Zhang, C.; Zhang, Y. Recent Advances in Binary Colloidal Crystals for Photonics and Porous Material Fabrication. J. Phys. Chem. B 2021, 125, 6012–6022. [Google Scholar] [CrossRef]
- Håkonsen, V.; Singh, G.; Normile, P.S.; De Toro, J.A.; Wahlström, E.; He, J.; Zhang, Z. Magnetically Enhanced Mechanical Stability and Super-Size Effects in Self-Assembled Superstructures of Nanocubes. Adv. Funct. Mater. 2019, 29, 1904825. [Google Scholar] [CrossRef] [Green Version]
- Muramoto, N.; Matsuno, T.; Wada, H.; Kuroda, K.; Shimojima, A. Preparation of an Ordered Nanoporous Silicone-based Material Using Silica Colloidal Crystals as a Hard Template. Chem. Lett. 2021, 50, 1038–1040. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Y.; Yan, J.; Ning, G.; Wang, Q.; Wei, T.; Zhi, L.; Wei, F. Template-Directed Synthesis of Pillared-Porous Carbon Nanosheet Architectures: High-Performance Electrode Materials for Supercapacitors. Adv. Energy Mater. 2012, 2, 419–424. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Shan, X.; Liu, G.; Chen, Y. Co-nanocasting synthesis of mesoporous Cu–Mn composite oxides and their promoted catalytic activities for gaseous benzene removal. Appl. Catal. B: Environ. 2015, 162, 110–121. [Google Scholar] [CrossRef]
- Markoulaki Ι, V.; Papadas, I.T.; Kornarakis, I.; Armatas, G.S. Synthesis of Ordered Mesoporous CuO/CeO2 Composite Frameworks as Anode Catalysts for Water Oxidation. Nanomaterials 2015, 5, 4. [Google Scholar] [CrossRef]
- Liu, H.; Lv, H.; Kan, K.; Liu, Y.; Zhang, W.; Wang, Y.; Ikram, M.; Du, L.; Shi, K.; Yu, H.-t. Biocarbon-templated synthesis of porous Ni–Co-O nanocomposites for room-temperature NH3 sensors. N. J. Chem. 2018, 42, 17606–17614. [Google Scholar] [CrossRef]
- Ergün, A.N.; Kocabaş, Z.Ö.; Baysal, M.; Yürüm, A.; Yürüm, Y. Synthesis Of Mesoporous Mcm-41 Materials With Low-Power Microwave Heating. Chem. Eng. Commun. 2013, 200, 1057–1070. [Google Scholar] [CrossRef]
- Dündar-Tekkaya, E.; Yürüm, Y. Synthesis of palladium incorporated MCM-41 via microwave irradiation and investigation of its hydrogen storage properties. Int. J. Hydrogen Energy 2016, 41, 9828–9833. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Ma, X. Microwave synthesis, characterization and transesterification activities of Ti-MCM-41. Micropor. Mesopor. Mater. 2012, 156, 22–28. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Qian, E.W. Synthesis of mesoporous Ti-inserted SBA-15 and CoMo/Ti-SBA-15 catalyst for hydrodesulfurization and hydrodearomatization. Micropor. Mesopor. Mater. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Hung, C.-T.; Yang, C.-F.; Lin, J.-S.; Huang, S.-J.; Chang, Y.-C.; Liu, S.-B. Capture of carbon dioxide by polyamine-immobilized mesostructured silica: A solid-state NMR study. Micropor. Mesopor. Mater. 2017, 238, 2–13. [Google Scholar] [CrossRef]
- Chen, W.; Luo, M.; Yang, K.; Zhou, X. Microwave-assisted KOH activation from lignin into hierarchically porous carbon with super high specific surface area by utilizing the dual roles of inorganic salts: Microwave absorber and porogen. Micropor. Mesopor. Mater. 2020, 300, 110178. [Google Scholar] [CrossRef]
- Salem, S.; Salem, A.; Parni, M.H.; Jafarizad, A. Microwave-assisted pyrolysis of organometallic gel prepared through ternary combination of surfactants for fabrication of nano-porous gamma alumina: Adsorptive properties, characterization. J. Chem. Technol. Biotechnol. 2021, 96, 1187–1196. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Jaramillo-Páez, C.; Sánchez-Cid, P.; Hidalgo, M.C. Microwave-assisted sol-gel synthesis of TiO2 in the presence of halogenhydric acids. Characterization and photocatalytic activity. J. Photochem. Photobiol. A Chem. 2020, 394, 112457. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Tan, H.W.; Patel, D.C.; Choong, W.T.N.; Chen, C.-H.; Low, H.Y.; Tan, M.J.; Patel, C.D.; Chua, C.K. The global rise of 3D printing during the COVID-19 pandemic. Nat. Rev. Mater. 2020, 5, 637–639. [Google Scholar] [CrossRef]
- Mooraj, S.; Welborn, S.S.; Jiang, S.; Peng, S.; Fu, J.; Baker, S.; Duoss, E.B.; Zhu, C.; Detsi, E.; Chen, W. Three-dimensional hierarchical nanoporous copper via direct ink writing and dealloying. Scr. Mater. 2020, 177, 146–150. [Google Scholar] [CrossRef]
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Worsley, M.A.; Ye, J.; Duoss, E.B.; Spadaccini, C.M.; et al. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Sci. Adv. 2018, 4, eaas9459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.; Guo, S.; Li, B.; Tian, Y.; Dong Qiu, J.C.; Sun, C.-N.; Yan, C.; Qi, H.J.; Zhou, K. 3D Printing and Chemical Dealloying of a Hierarchically Micro- and Nanoporous Catalyst for Wastewater Purification. ACS Appl. Mater. Inter. 2021, 13, 48709–48719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Nomura, N.; Fujita, T. Hierarchical Nanoporous Copper Architectures via 3D Printing Technique for Highly Efficient Catalysts. Small 2019, 15, e1805432. [Google Scholar] [CrossRef]
- Dong, Z.; Cui, H.; Zhang, H.; Wang, F.; Zhan, X.; Mayer, F.; Nestler, B.; Wegener, M.; Levkin, P.A. 3D printing of inherently nanoporous polymers via polymerization-induced phase separation. Nat. Commun. 2021, 12, 247. [Google Scholar] [CrossRef]
- Bischoff, L.; Pilz, W.; Schmidt, B. Amorphous solid foam structures on germanium by heavy ion irradiation. Appl. Phys. A 2011, 104, 1153–1158. [Google Scholar] [CrossRef]
- Miyaji, T.; Nitta, N. Nanoporous Structure Formation on the Surface of InSb by Ion Beam Irradiation. Nanomaterials 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Håkonsen, V.; Singh, G.; He, J.; Zhang, Z. Focused ion beam milling of self-assembled magnetic superstructures: An approach to fabricate nanoporous materials with tunable porosity. Mater. Horiz. 2018, 5, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Chan, H.; Baskin, A.; Gelman, E.; Repnin, N.; Kral, P.; Klajn, R. Self-assembly of magnetite nanocubes into helical superstructures. Science 2014, 345, 1149–1153. [Google Scholar] [CrossRef]
- Cui, M.; Huang, T.; Xu, J.; Xiao, R. Homogeneously bimodal nanoporous copper by laser processing-dealloying approach. J. Manufact. Processes 2022, 73, 815–821. [Google Scholar] [CrossRef]
- Yang, L.; Wei, J.; Ma, Z.; Song, P.; Ma, J.; Zhao, Y.; Huang, Z.; Zhang, M.; Yang, F.; Wang, X. The Fabrication of Micro/Nano Structures by Laser Machining. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Cheng, Y. Femtosecond Laser 3D Fabrication in Porous Glass for Micro- and Nanofluidic Applications. Micromachines 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Dong, C.; Zhong, M.; Ma, M.; Li, L.; Liu, W. Fabrication of nanoporous manganese by laser cladding and selective electrochemical de-alloying. Appl. Surf. Sci. 2011, 257, 3211–3215. [Google Scholar] [CrossRef]
- Huang, T.; Lu, J.; Zhang, X.; Xiao, R.; Yang, W.; Wu, Q. Femtosecond Laser Fabrication of Anatase TiO2 Micro-nanostructures with Chemical Oxidation and Annealing. Sci. Rep. 2017, 7, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiga, S.P.; Jin, C.; Curtiss, L.A.; Monteiro-Riviere, N.A.; Narayan, R.J. Nanoporous membranes for medical and biological applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 568–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Jiang, X. Osteogenic TiO2 composite nano-porous arrays: A favorable platform based on titanium alloys applied in artificial implants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128301. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Ding, X.; Xu, B.; Wang, J.; Li, Y.; Chen, F.; Meng, F.; Song, W.; Zhang, Y. Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin β1/FAKpY397/MAPK pathway. Bioact. Mater. 2022, 8, 109–123. [Google Scholar] [CrossRef]
- Shuang, F.; Deng, H.; Shafique, A.B.; Marsh, S.; Treiman, D.; Tsakalis, K.; Aifantis, K.E. A first study on nanoporous tungsten recording electrodes for deep brain stimulation. Mater. Lett. 2020, 260, 126885. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Lu, W.; Tian, G.; Zhao, Q.; Yang, D.; Sun, J.; Qi, D. Strategies for interface issues and challenges of neural electrodes. Nanoscale 2022, 14, 3346–3366. [Google Scholar] [CrossRef]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/nano-structured TiO2 surface with dual-functional antibacterial effects for biomedical applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef]
- Bae, I.; Lim, K.S.; Park, J.K.; Song, J.H.; Oh, S.H.; Kim, J.W.; Zhang, Z.; Park, C.; Koh, J.T. Evaluation of cellular response and drug delivery efficacy of nanoporous stainless steel material. Biomater. Res. 2021, 25, 30. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous materials for pesticide formulation and delivery in the agricultural sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef] [PubMed]

| Fabrication Technique | Advantages | Disadvantages | |
|---|---|---|---|
| Dealloying | Chemical |
|
|
| Electrochemical |
|
| |
| Liquid Metal |
|
| |
| Vapor Phase |
|
| |
| Templating | Soft |
|
|
| Hard |
|
| |
| Microwave-Based Fabrication |
|
| |
| Additive Manufacturing |
|
| |
| Ion Beam-Induced Fabrication |
|
| |
| Laser-Induced Fabrication |
|
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadden, M.; Martinez-Martin, D.; Yong, K.-T.; Ramaswamy, Y.; Singh, G. Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications. Materials 2022, 15, 2111. https://doi.org/10.3390/ma15062111
Hadden M, Martinez-Martin D, Yong K-T, Ramaswamy Y, Singh G. Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications. Materials. 2022; 15(6):2111. https://doi.org/10.3390/ma15062111
Chicago/Turabian StyleHadden, Matthew, David Martinez-Martin, Ken-Tye Yong, Yogambha Ramaswamy, and Gurvinder Singh. 2022. "Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications" Materials 15, no. 6: 2111. https://doi.org/10.3390/ma15062111
APA StyleHadden, M., Martinez-Martin, D., Yong, K.-T., Ramaswamy, Y., & Singh, G. (2022). Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications. Materials, 15(6), 2111. https://doi.org/10.3390/ma15062111








