The Cytocompatibility of Silver Diamine Fluoride on Mesenchymal Stromal Cells from Human Exfoliated Deciduous Teeth: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of SDF Eluates
2.2. Isolation and Culture of Mesenchymal Stromal Cells from Deciduous Teeth
2.3. IC50 and MTT Assays
2.4. Cell Migration
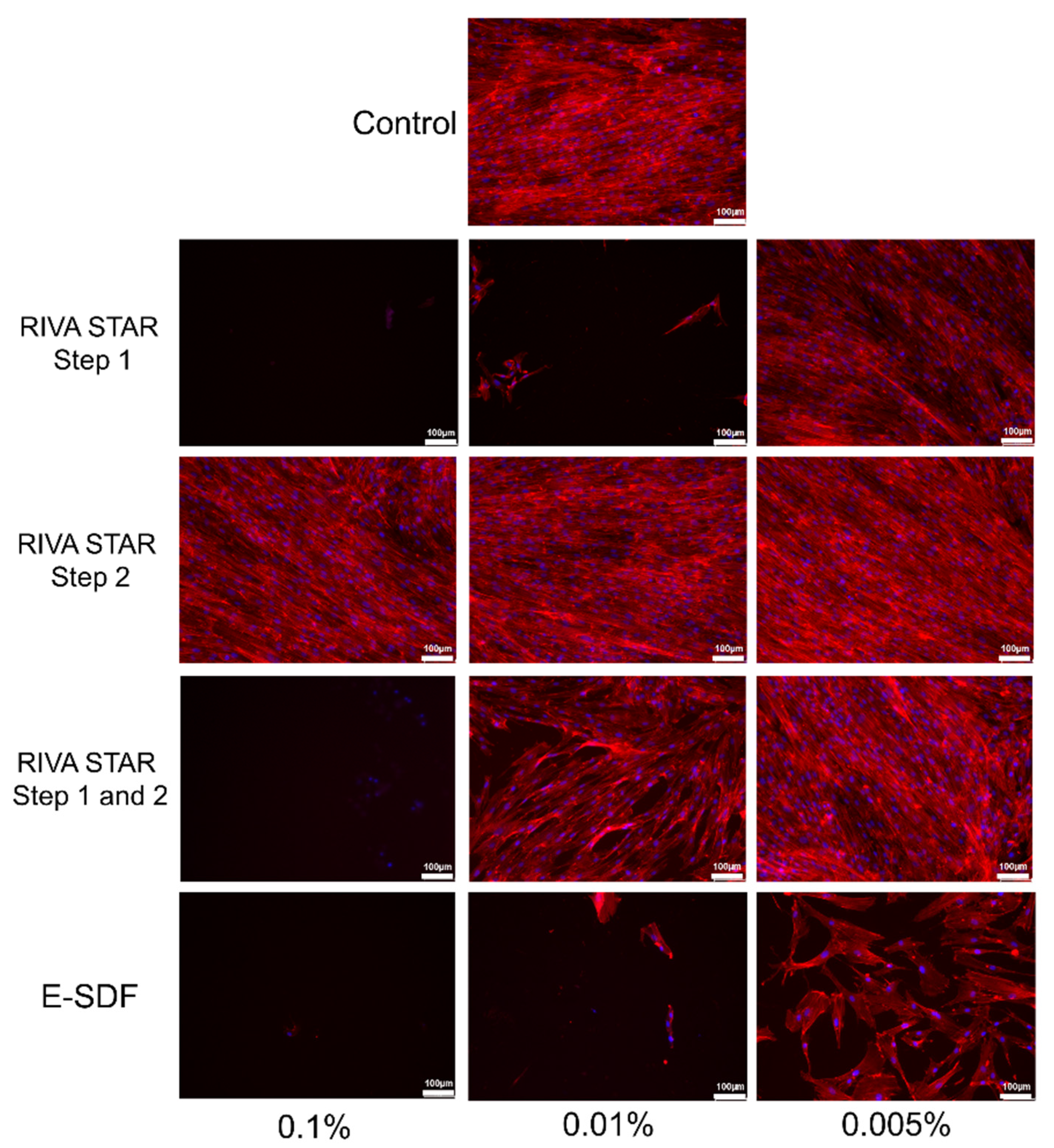
2.5. Cell Cytoskeleton Staining
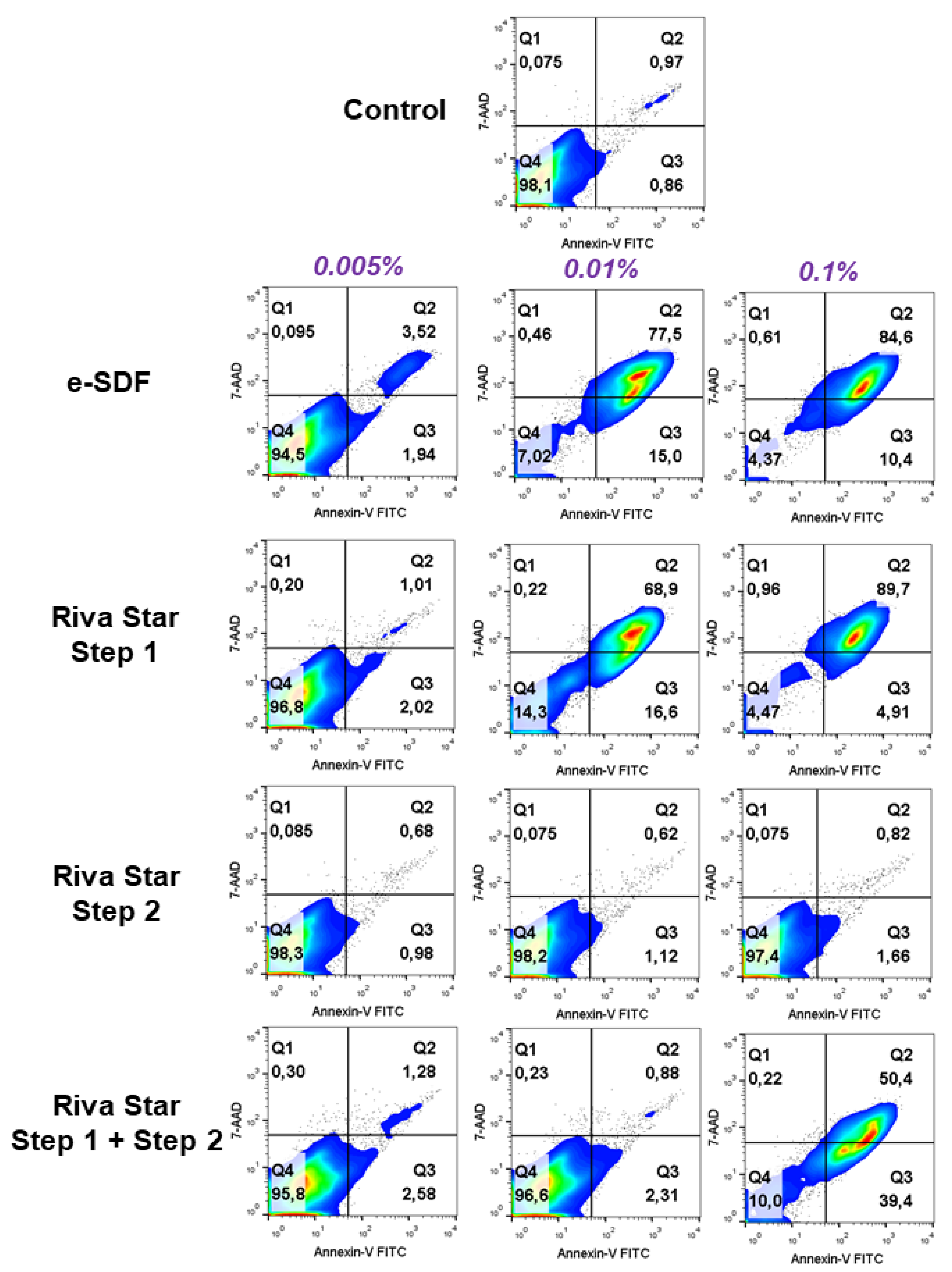
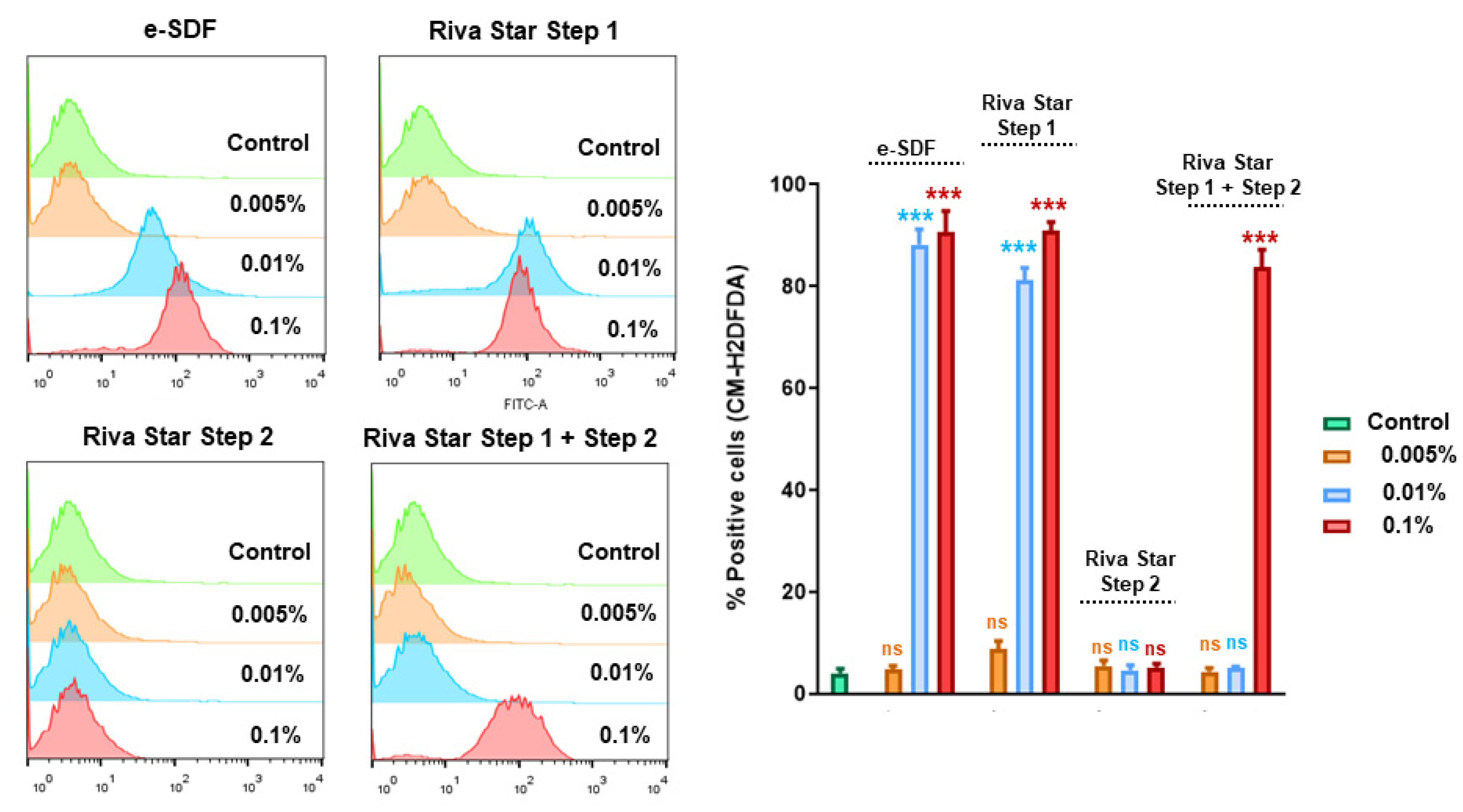
2.6. Apoptosis/Necrosis and ROS Assay
2.7. Ion Chromatography
2.8. Statistical Analysis
3. Results
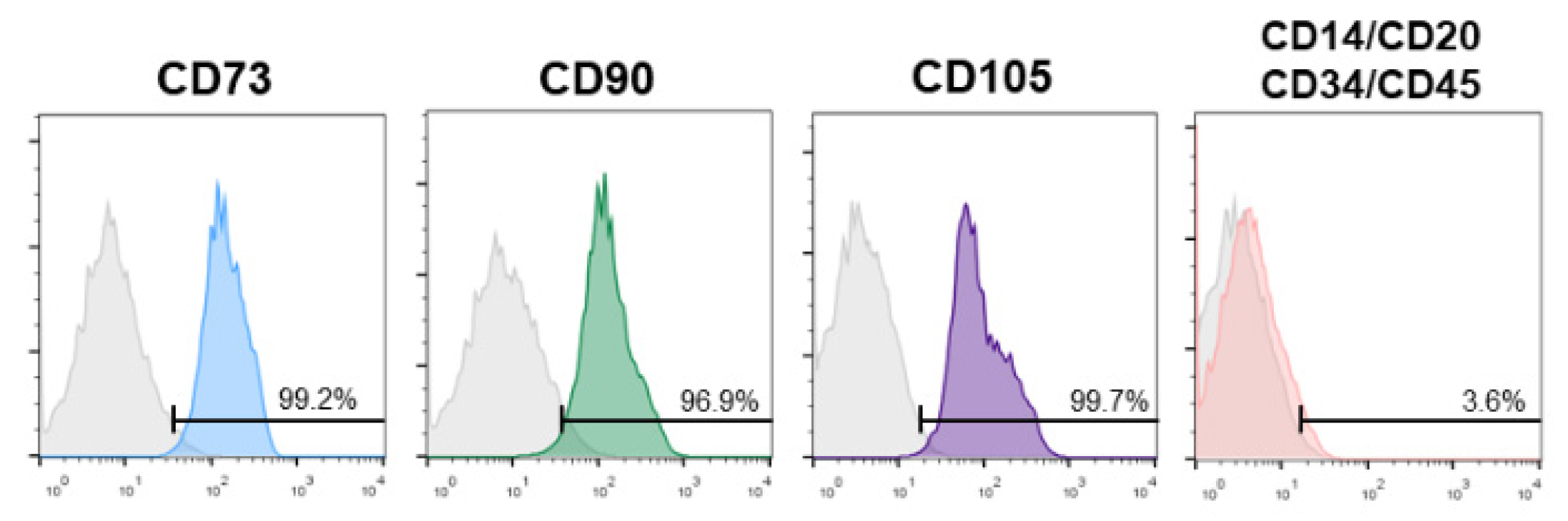
3.1. SHEDs Phenotypic Characterization
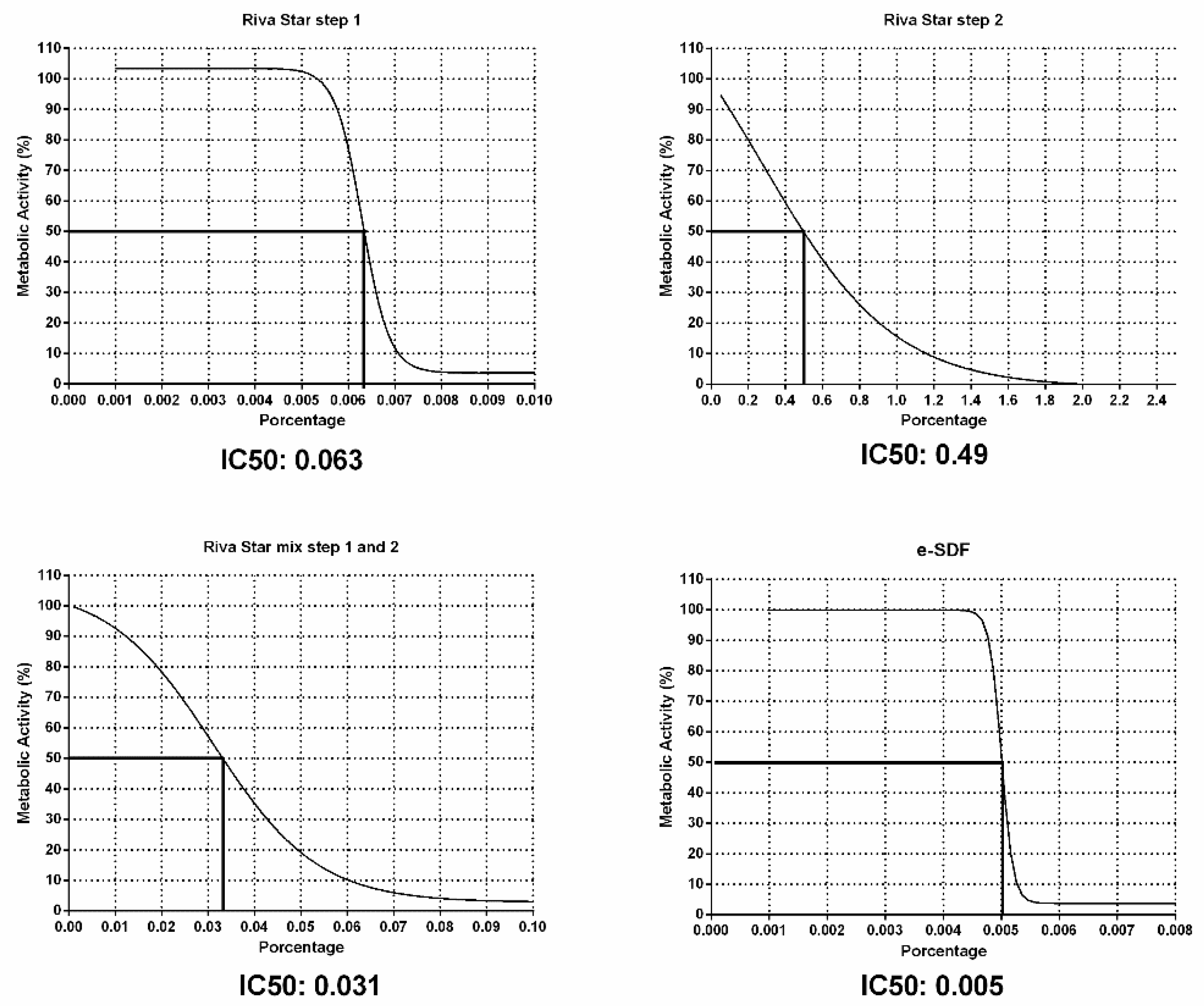
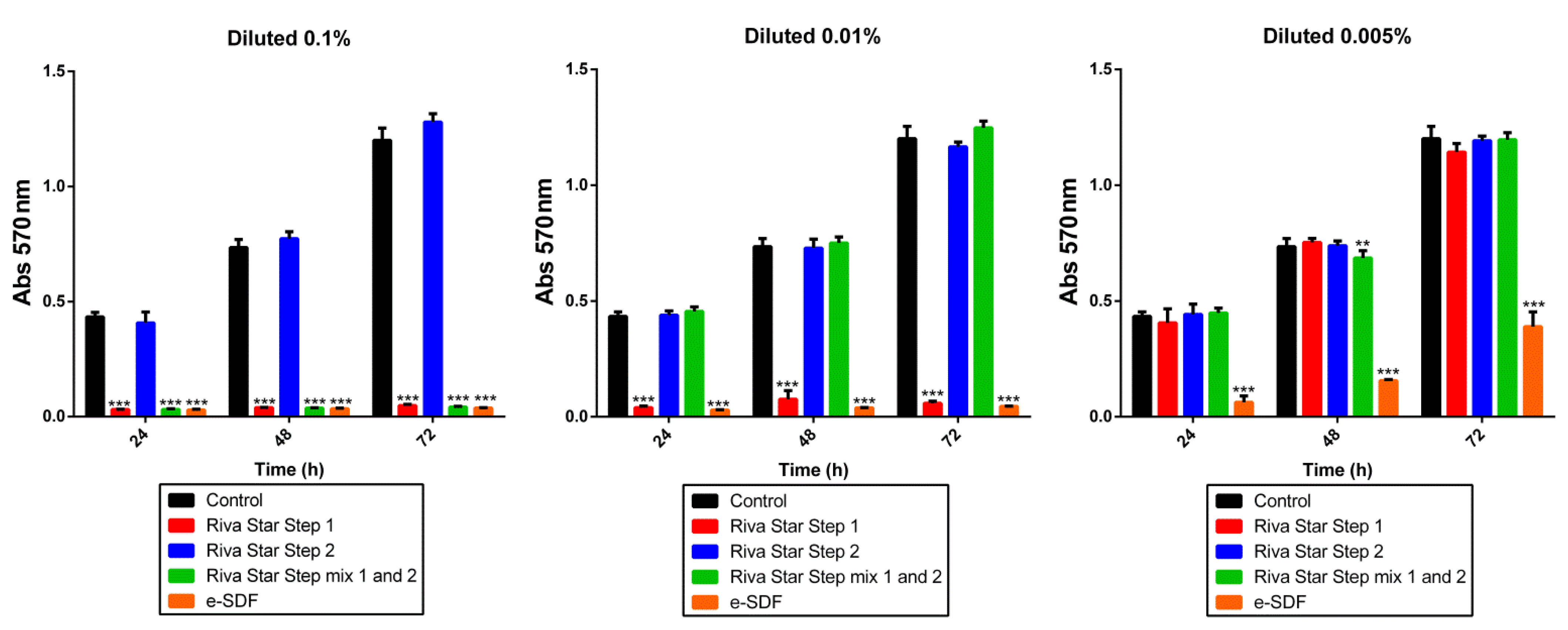
3.2. IC50 and MTT
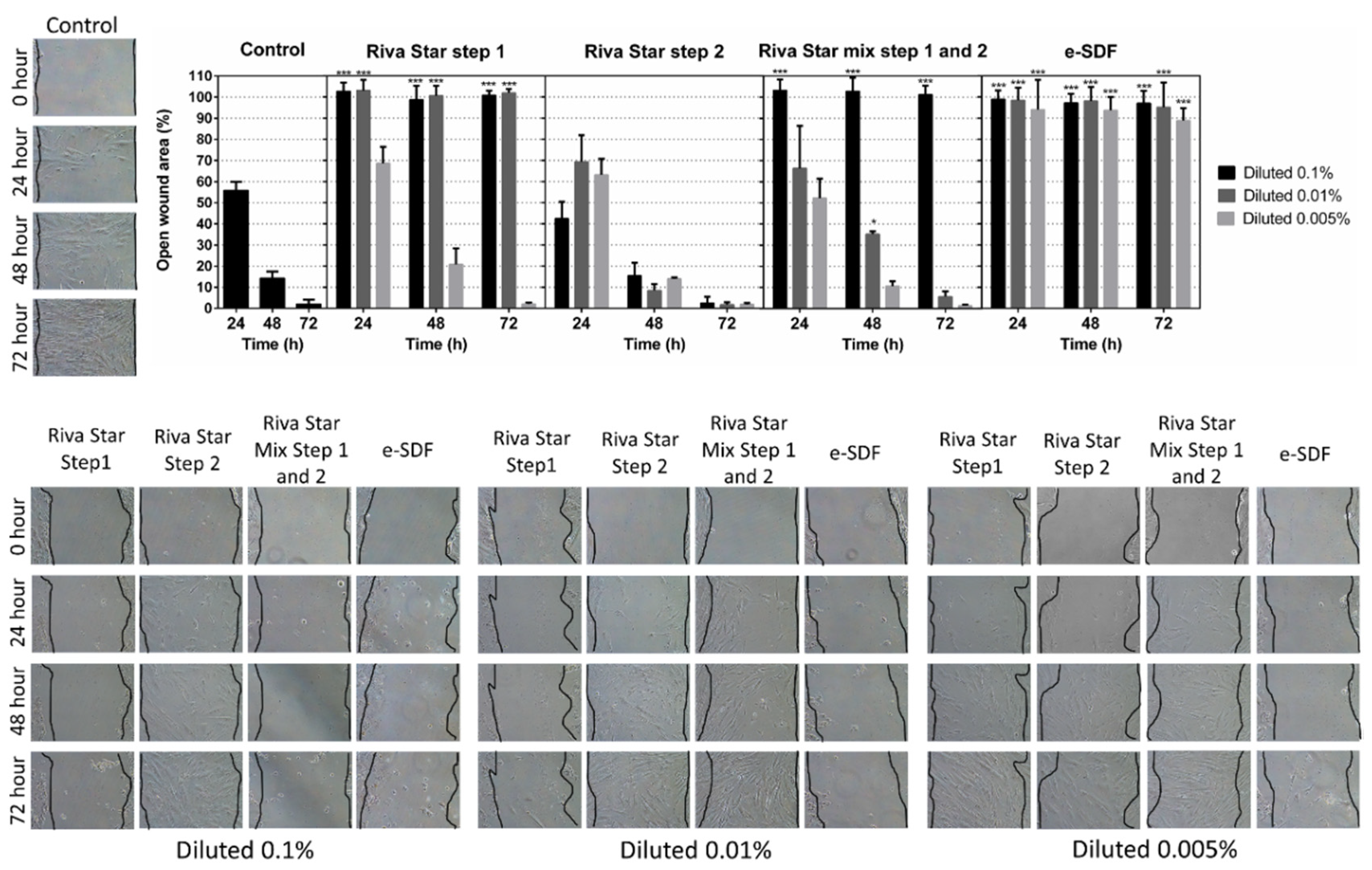
3.3. Cell Migration
3.4. Cell Cytoskeleton Staining
3.5. Apoptosis/Necrosis Assay and ROS Assay
3.6. Ion Chromatography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.; Frencken, J.E.; Schwendicke, F.; Innes, N.P.T. Contemporary operative caries management: Consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Kurokawa, H.; Okuwaki, T.; Takamizawa, T.; Tsujimoto, A.; Shiratsuchi, K.; Ishii, R.; Miyazaki, M. Ultrasonic measurement of dentin remineralization effects of dentifrices and silver diamine fluoride. Acta Odontol. Scand. 2021, 79, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Lo, E.C.M.; Chu, C.H. Arresting Dentine Caries with Silver Diamine Fluoride: What’s Behind It? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Paglia, L. COVID-19 and Paediatric Dentistry after the lockdown. Eur. J. Paediatr. Dent. 2020, 21, 89. [Google Scholar] [PubMed]
- Eden, E.; Frencken, J.; Gao, S.; Horst, J.A.; Innes, N. Managing dental caries against the backdrop of COVID-19: Approaches to reduce aerosol generation. Br. Dent. J. 2020, 229, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Generali, L.; Generali, P. Isolation through Rubber Dam to Prevent COVID-19 Exposure during Flapless Trans-Crestal Sinus Lift Procedures. J. Oral Implantol. 2021, 47, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Seifo, N.; Robertson, M.; MacLean, J.; Blain, K.; Grosse, S.; Milne, R.; Seeballuck, C.; Innes, N. The use of silver diamine fluoride (SDF) in dental practice. Br. Dent. J. 2020, 228, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hammersmith, K.J.; DePalo, J.R.; Casamassimo, P.S.; MacLean, J.K.; Peng, J. Silver Diamine Fluoride and Fluoride Varnish May Halt Interproximal Caries Progression in the Primary Dentition. J. Clin. Pediatr. Dent. 2020, 44, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Vollú, A.L.; Rodrigues, G.F.; Teixeira, R.V.R.; Cruz, L.R.; Dos Santos Massa, G.; de Lima Moreira, J.P.; Luiz, R.R.; Barja-Fidalgo, F.; Fonseca-Goncalves, A. Efficacy of 30% silver diamine fluoride compared to atraumatic restorative treatment on dentine caries arrestment in primary molars of preschool children: A 12-months parallel randomized controlled clinical trial. J. Dent. 2019, 88, 103165. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Janal, M.N.; Hamilton, D.S.; Niederman, R. Parental perceptions and acceptance of silver diamine fluoride staining. J. Am. Dent. Assoc. 2017, 148, 510–518.e4. [Google Scholar] [CrossRef]
- Gao, S.S.; Amarquaye, G.; Arrow, P.; Bansal, K.; Bedi, R.; Campus, G.; Chen, K.J.; Chibinski, A.C.R.; Chinzorig, T.; Crystal, Y.O.; et al. Global Oral Health Policies and Guidelines: Using Silver Diamine Fluoride for Caries Control. Front. Oral Health 2021, 2, 685557. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, H.M.; Ali, A.M.; Baghdady, S.I.; ElKateb, M.A. Caries arrest effectiveness of silver diamine fluoride compared to alternative restorative technique: Randomized clinical trial. Eur. Arch. Paediatr. Dent. 2021, 22, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.; Gold, J.; Chaffin, J. Effect and acceptance of silver diamine fluoride treatment on dental caries in primary teeth. J. Public Health Dent. 2018, 78, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Y.O.; Niederman, R. Evidence-Based Dentistry Update on Silver Diamine Fluoride. Dent. Clin. N. Am. 2019, 63, 45–68. [Google Scholar] [CrossRef]
- Hendre, A.D.; Taylor, G.W.; Chávez, E.M.; Hyde, S. A systematic review of silver diamine fluoride: Effectiveness and application in older adults. Gerodontology 2017, 34, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.H.T.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Randomized Clinical Trial of 12% and 38% Silver Diamine Fluoride Treatment. J. Dent. Res. 2018, 97, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Meyer, B.; Duggal, M. A silver renaissance in dentistry. Eur. Arch. Paediatr. Dent. 2018, 19, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Chen, K.J.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Managing Early Childhood Caries with Atraumatic Restorative Treatment and Topical Silver and Fluoride Agents. Int. J. Environ. Res. Public Health 2017, 14, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifo, N.; Cassie, H.; Radford, J.R.; Innes, N.P.T. Silver diamine fluoride for managing carious lesions: An umbrella review. BMC Oral Health 2019, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Jaamela, H.K.; Zohaib, S.; Ruth, M.; Abudrya, M.; Schmoeckel, J.; Zafar, M.S.; Splieth, C.H. Silver diamine fluoride: A magic bullet for caries management. Fluoride 2021, 54, 210–218. [Google Scholar]
- Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Ortolani-Seltenerich, P.S.; Álvarez-Muro, T.; Lozano, A.; Forner, L.; Llena, C.; Moraleda, J.M.; Rodriguez-Lozano, F.J. Cytotoxicity and bioactivity of various pulpotomy materials on mesenchymal stromal cells from human exfoliated primary teeth. Int. Endod. J. 2017, 50 (Suppl. 2), e19–e30. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, S.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Onate-Sanchez, R.E.; Garcia-Bernal, D.; Castelo-Baz, P.; Rodriguez-Lozano, F.J.; Guerrero-Girones, J. In Vitro Evaluation of the Biological Effects of ACTIVA Kids BioACTIVE Restorative, Ionolux, and Riva Light Cure on Human Dental Pulp Mesenchymal stromal cells. Materials 2019, 12, 3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-García, S.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Guerrero-Gironés, J.; Rodríguez-Lozano, F.J.; García-Bernal, D. Topical fluoride varnishes promote several biological responses on human gingival cells. Ann. Anat. 2021, 237, 151723. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, S.; Pecci-Lloret, M.P.; Garcia-Bernal, D.; Guerrero-Girones, J.; Pecci-Lloret, M.R.; Rodriguez-Lozano, F.J. Are Denture Adhesives Safe for Oral Cells? J. Prosthodont. 2021, 30, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fancher, M.E.; Fournier, S.; Townsend, J.; Lallier, T.E. Cytotoxic effects of silver diamine fluoride. Am. J. Dent. 2019, 32, 152–156. [Google Scholar] [PubMed]
- Kim, S.; Nassar, M.; Tamura, Y.; Hiraishi, N.; Jamleh, A.; Nikaido, T.; Tagami, J. The effect of reduced glutathione on the toxicity of silver diamine fluoride in rat pulpal cells. J. Appl. Oral Sci. 2021, 29, e20200859. [Google Scholar] [CrossRef] [PubMed]
- Collado-Gonzalez, M.; Pecci-Lloret, M.P.; Garcia-Bernal, D.; Aznar-Cervantes, S.; Onate-Sanchez, R.E.; Moraleda, J.M.; Cenis, J.L.; Rodriguez-Lozano, F.J. Biological effects of silk fibroin 3D scaffolds on mesenchymal stromal cells from human exfoliated deciduous teeth (SHEDs). Odontology 2018, 106, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; García-Bernal, D. Influence of dual-cure and self-cure abutment cements for crown implants on human gingival fibroblasts biological properties. Ann. Anat. 2022, 239, 151829. [Google Scholar] [CrossRef] [PubMed]
- Johns, C.; Shellie, R.A.; Potter, O.G.; O’Reilly, J.W.; Hutchinson, J.P.; Guijt, R.M.; Breadmore, M.; Hilder, E.; Dicinoski, G.W.; Haddad, P.R. Identification of homemade inorganic explosives by ion chromatographic analysis of post-blast residues. J. Chromatogr. A 2008, 1182, 205–214. [Google Scholar] [CrossRef]
- Illeperuma, R.P.; Park, Y.J.; Kim, J.M.; Bae, J.Y.; Che, Z.M.; Son, H.K.; Han, M.R.; Kim, K.-M.; Kim, J. Immortalized gingival fibroblasts as a cytotoxicity test model for dental materials. J. Mater. Sci. Mater. Med. 2012, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Serrano-Belmonte, I.; Pérez Calvo, J.C.; Coronado-Parra, M.T.; Bernabeu-Esclapez, A.; Moraleda, J.M. Effects of two low-shrinkage composites on dental mesenchymal stromal cells (viability, cell damaged or apoptosis and mesenchymal markers expression). J. Mater. Sci. Mater. Med. 2013, 24, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Foster, D.; Smirk, M.; Turton, B.; Anthonappa, R. Acidity, fluoride and silver ion concentrations in silver diamine fluoride solutions: A pilot study. Aust. Dent. J. 2021, 66, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Soares-Yoshikawa, A.L.; Cury, J.A.; Tabchoury, C.P.M. Fluoride Concentration in SDF Commercial Products and Their Bioavailability with Demineralized Dentine. Braz. Dent. J. 2020, 31, 257–263. [Google Scholar] [CrossRef]
| Material | Manufacturer | Composition | Lot Number |
|---|---|---|---|
| Riva Star | SDI, 3-15 Brunsdon Street, Victoria 3153, Bayswater, Australia | Step 1 (Silver capsule): 30–35% silver fluoride and >60% ammonia solution. Step 2 (Green capsule): saturated potassium iodide solution | 11508613 |
| e-SDF | Kids-e-Dental, Akruti Arcade, Headquarters 411, JP Rd, opp. A.H. Wadia school, Azad Nagar, Andheri West, Mumbai, Maharashtra, 400053, India | Silver diamine fluoride contains approximately 24–28% (weight/volume) silver and 5–6% (weight/volume), ammonia solution | ESDF JK 122 |
| Product | Fluoride Concentration |
|---|---|
| e-SDF 0.005% | 3.4657 mg/L |
| e-SDF 0.01% | 6.9901 mg/L |
| e-SDF 0.1% | 73.7795 mg/L |
| Riva Star Step 1 0.005% | 3.185 mg/L |
| Riva Star Step 1 0.01% | 6.5263 mg/L |
| Riva Star Step 1 0.1% | 70.3579 mg/L |
| Riva Star Step 2 0.005% | <0.3 ppm |
| Riva Star Step 2 0.01% | <0.3 ppm |
| Riva Star Step 2 0.1% | <0.3 ppm |
| Riva Star Mix Step 1 and Step 2 0.005% | 0.779 mg/L |
| Riva Star Mix Step 1 and Step 2 0.01% | 1.9342 mg/L |
| Riva Star Mix Step 1 and Step 2 0.1% | 20.0438 mg/L |
| e-SDF | 7.38 gr/L |
| Riva Star Step 1 | 7.04 gr/L |
| Riva Star Step 2 | <0.3 ppm |
| Riva Star Mix Step 1 and Step 2 | 2 gr/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Bernal, D.; Pecci-Lloret, M.P.; López-García, S. The Cytocompatibility of Silver Diamine Fluoride on Mesenchymal Stromal Cells from Human Exfoliated Deciduous Teeth: An In Vitro Study. Materials 2022, 15, 2104. https://doi.org/10.3390/ma15062104
García-Bernal D, Pecci-Lloret MP, López-García S. The Cytocompatibility of Silver Diamine Fluoride on Mesenchymal Stromal Cells from Human Exfoliated Deciduous Teeth: An In Vitro Study. Materials. 2022; 15(6):2104. https://doi.org/10.3390/ma15062104
Chicago/Turabian StyleGarcía-Bernal, David, Maria Pilar Pecci-Lloret, and Sergio López-García. 2022. "The Cytocompatibility of Silver Diamine Fluoride on Mesenchymal Stromal Cells from Human Exfoliated Deciduous Teeth: An In Vitro Study" Materials 15, no. 6: 2104. https://doi.org/10.3390/ma15062104
APA StyleGarcía-Bernal, D., Pecci-Lloret, M. P., & López-García, S. (2022). The Cytocompatibility of Silver Diamine Fluoride on Mesenchymal Stromal Cells from Human Exfoliated Deciduous Teeth: An In Vitro Study. Materials, 15(6), 2104. https://doi.org/10.3390/ma15062104







