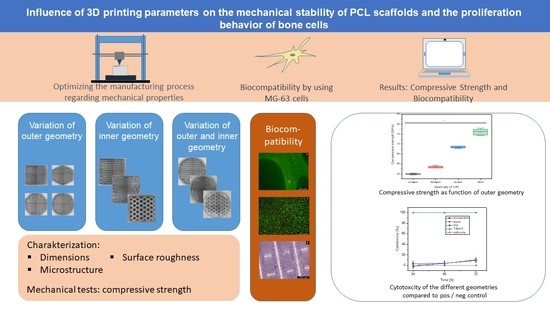
Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Manufacturing
2.2.1. Variation of the Strand Spacing
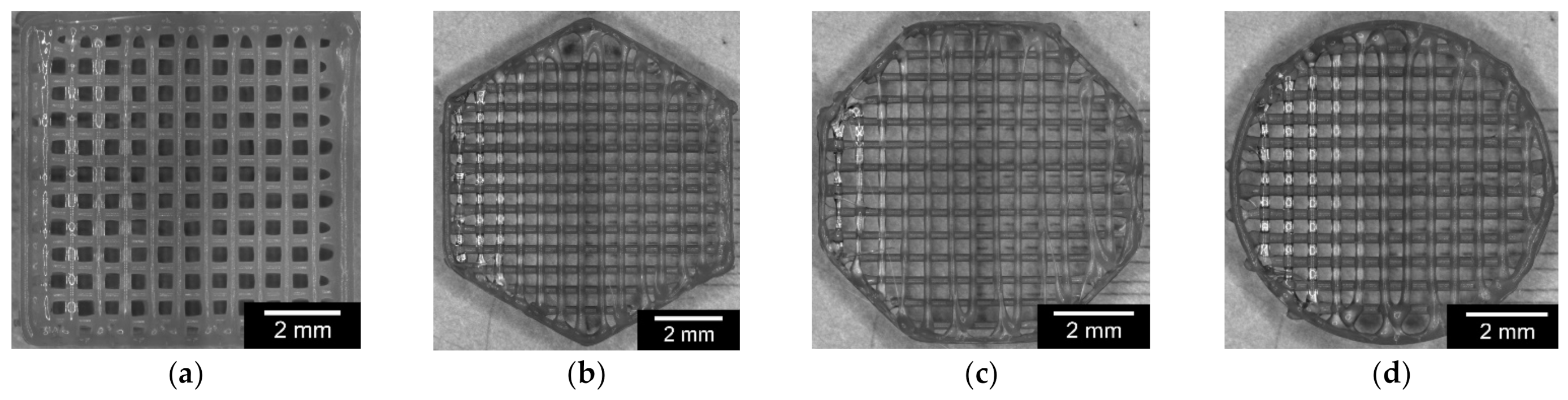
2.2.2. Variation of the Outer Geometry
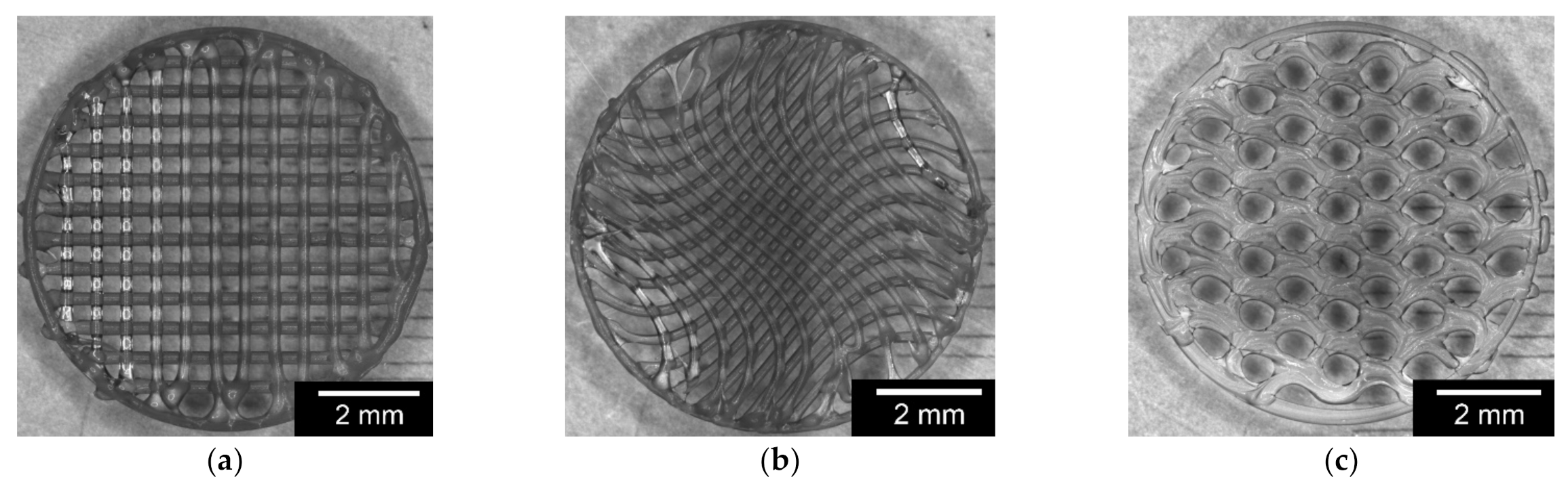
2.2.3. Variation of the Inner Geometry
2.2.4. Variation of Outer and Inner Geometry
2.3. Characterization of the 3D-Printed PCL Scaffolds
2.3.1. Characterization of Dimensions and Weight
2.3.2. Characterization of Surface Roughness
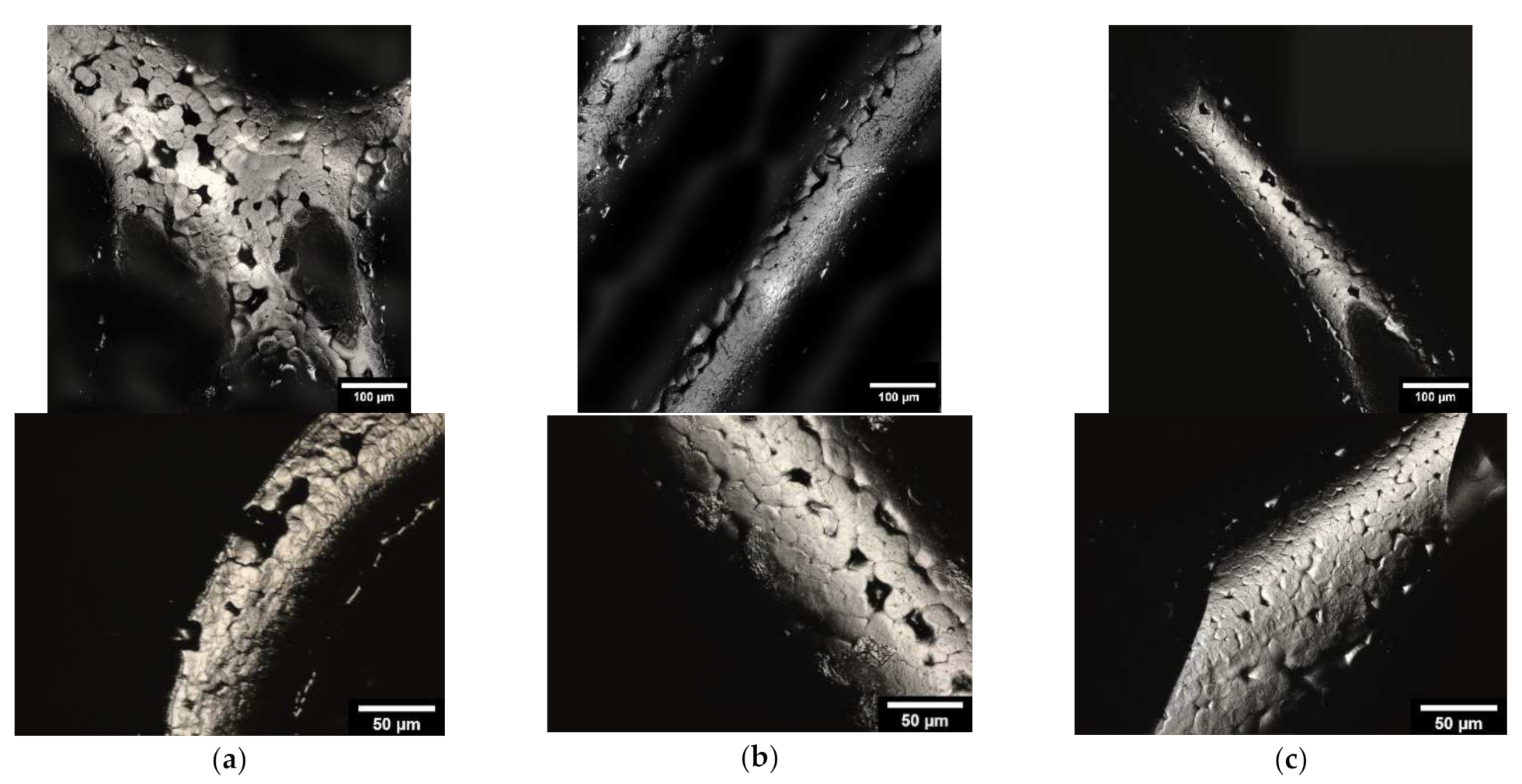
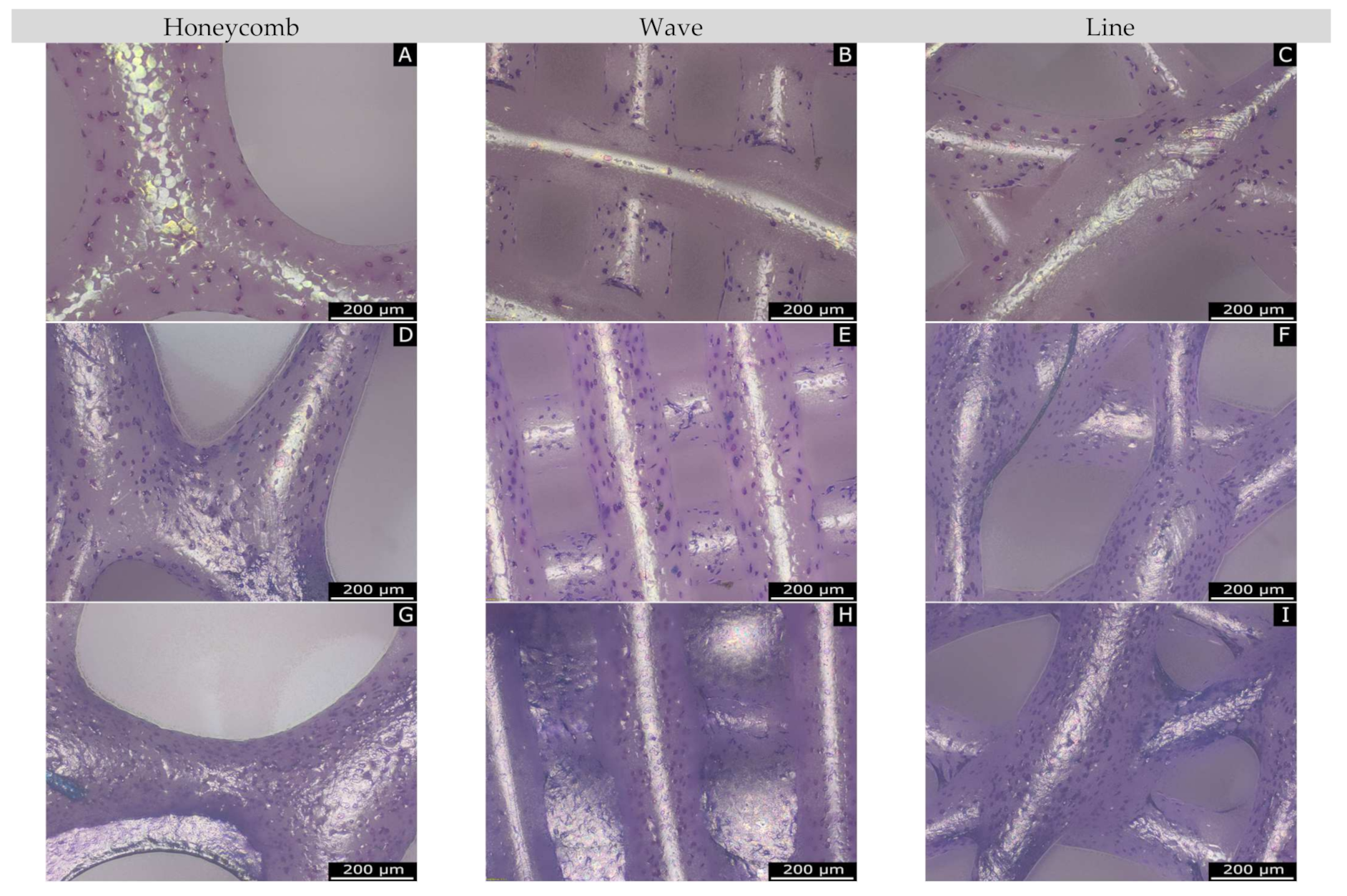
2.3.3. Characterization of the Microstructure by Means of ESEM
2.3.4. Measurement of Porosity
2.4. Mechanical Testing
2.5. Coating with Collagen-I
2.6. Biocompatibility
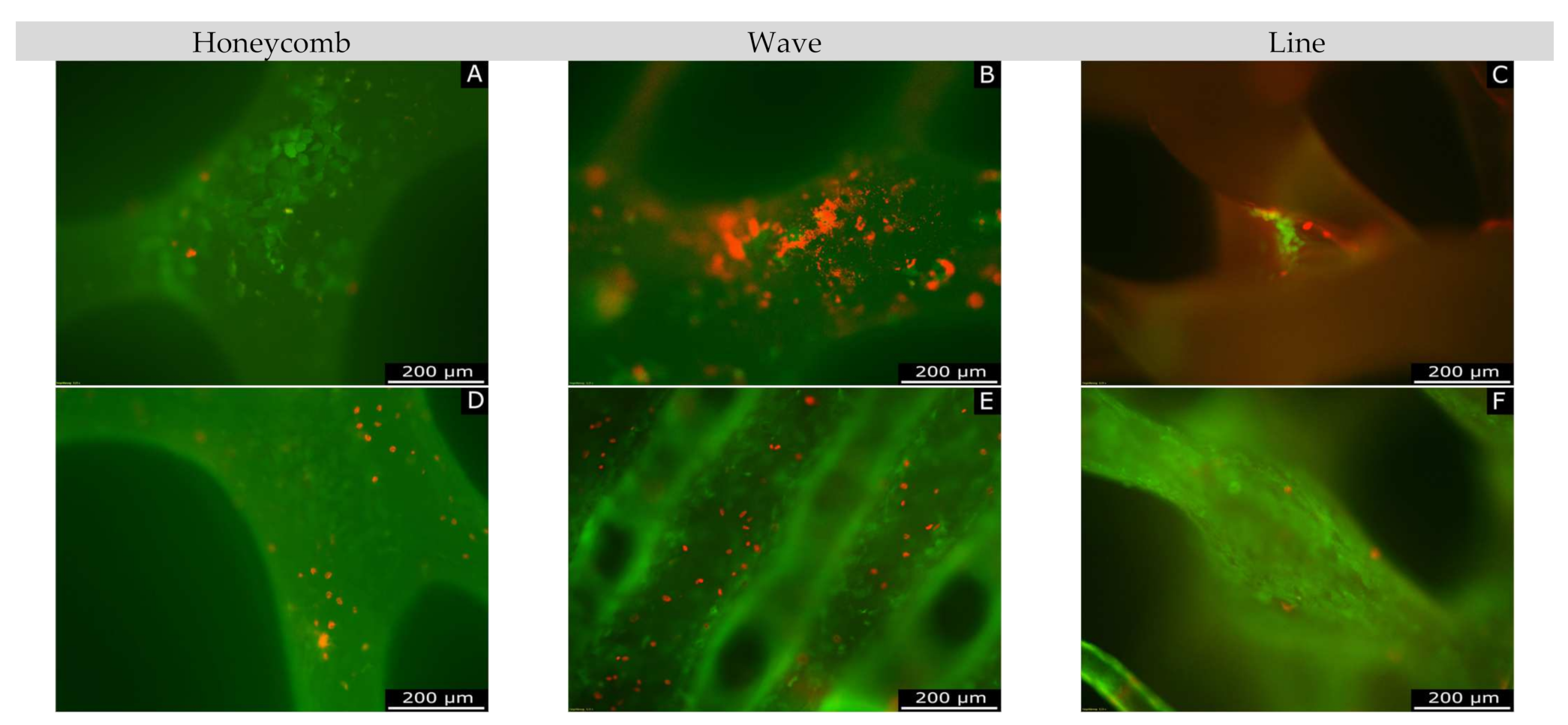
2.6.1. Live/Dead Assay
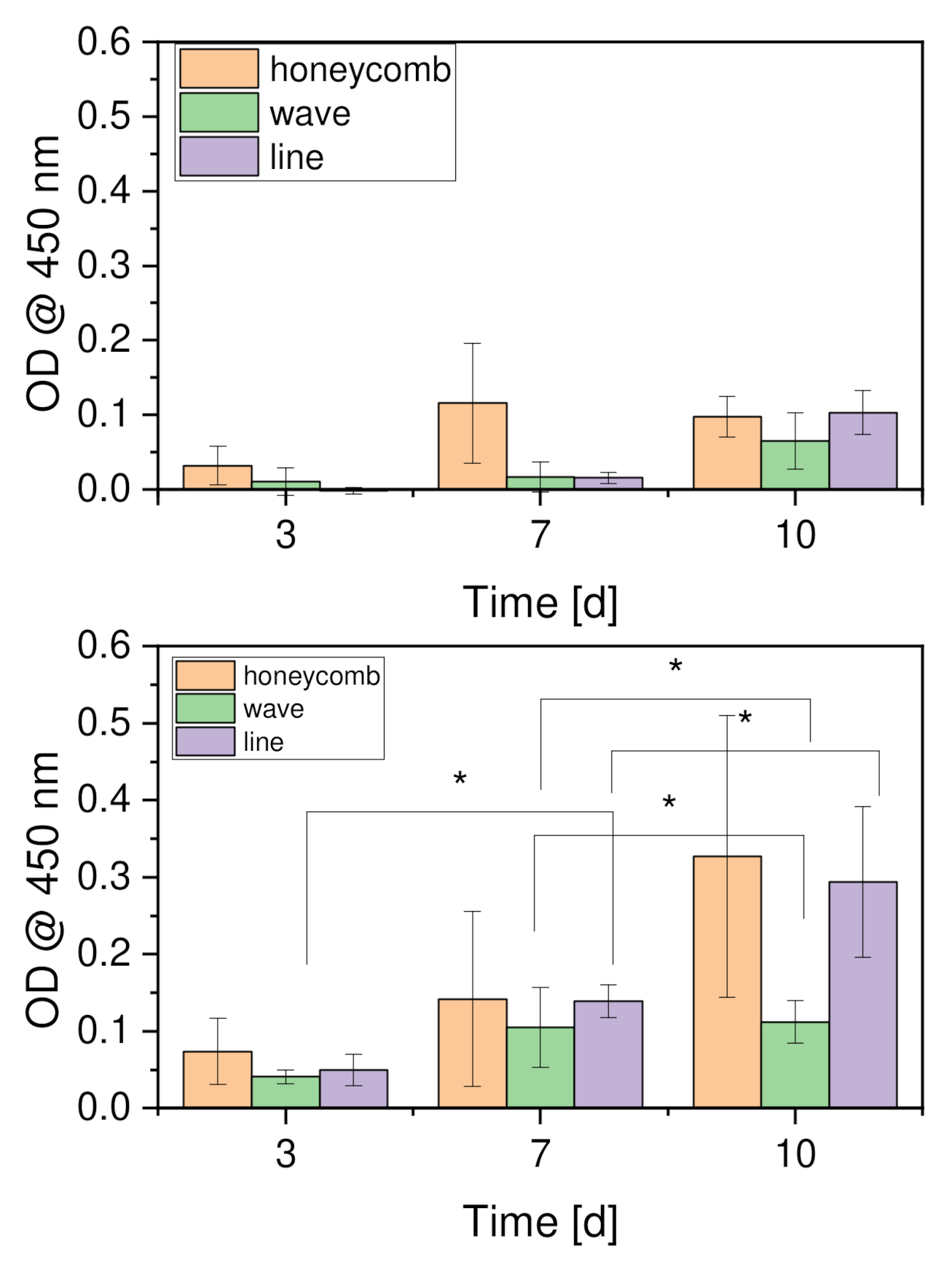
2.6.2. Cell Proliferation (WST-I)
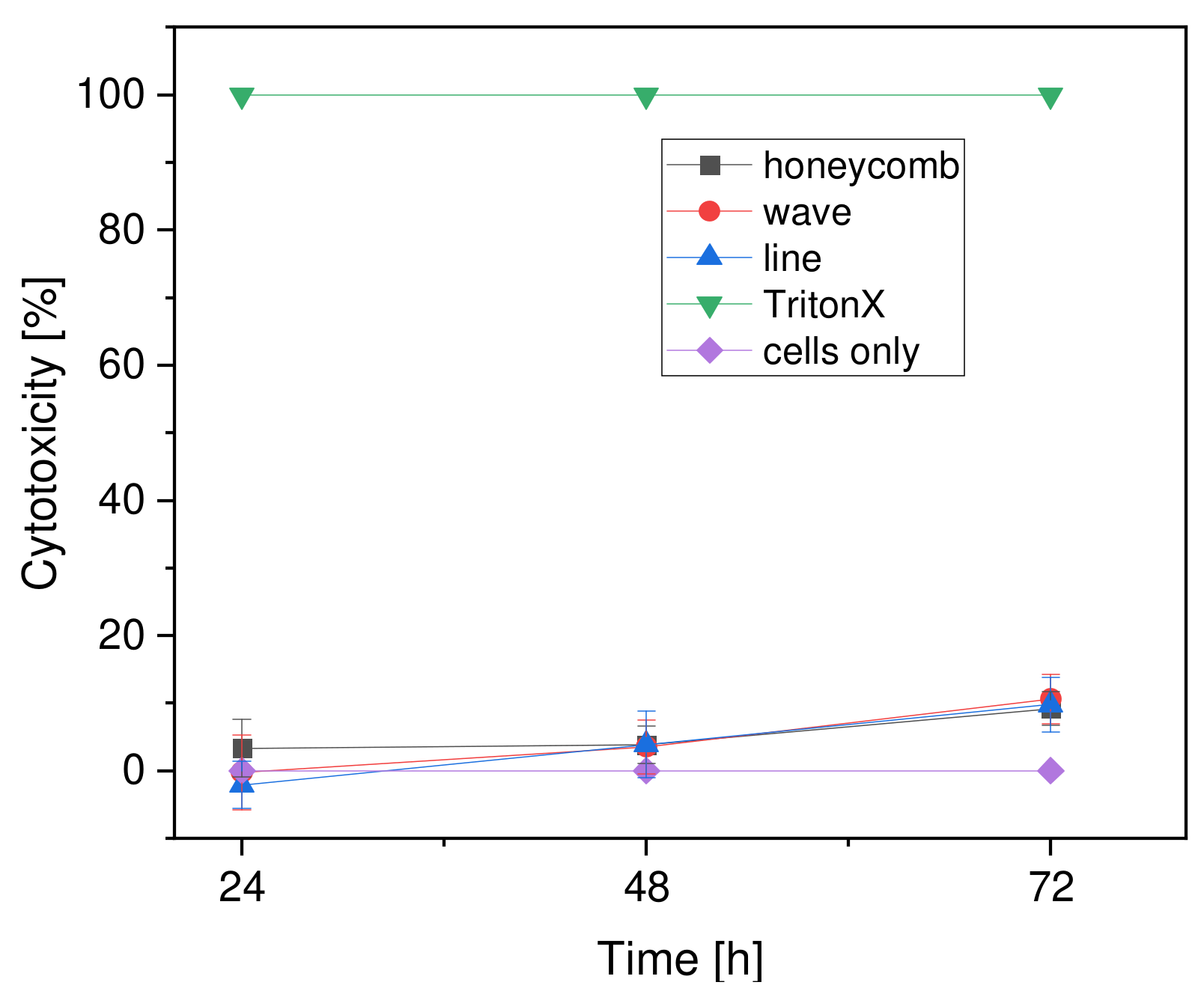
2.6.3. Cytotoxicity (LDH Assay)
2.7. Statistics
3. Results
3.1. Characterization of the 3D Printed Scaffolds
3.1.1. Characterization Dimensions and Weight
3.1.2. Characterization of Surface Roughness
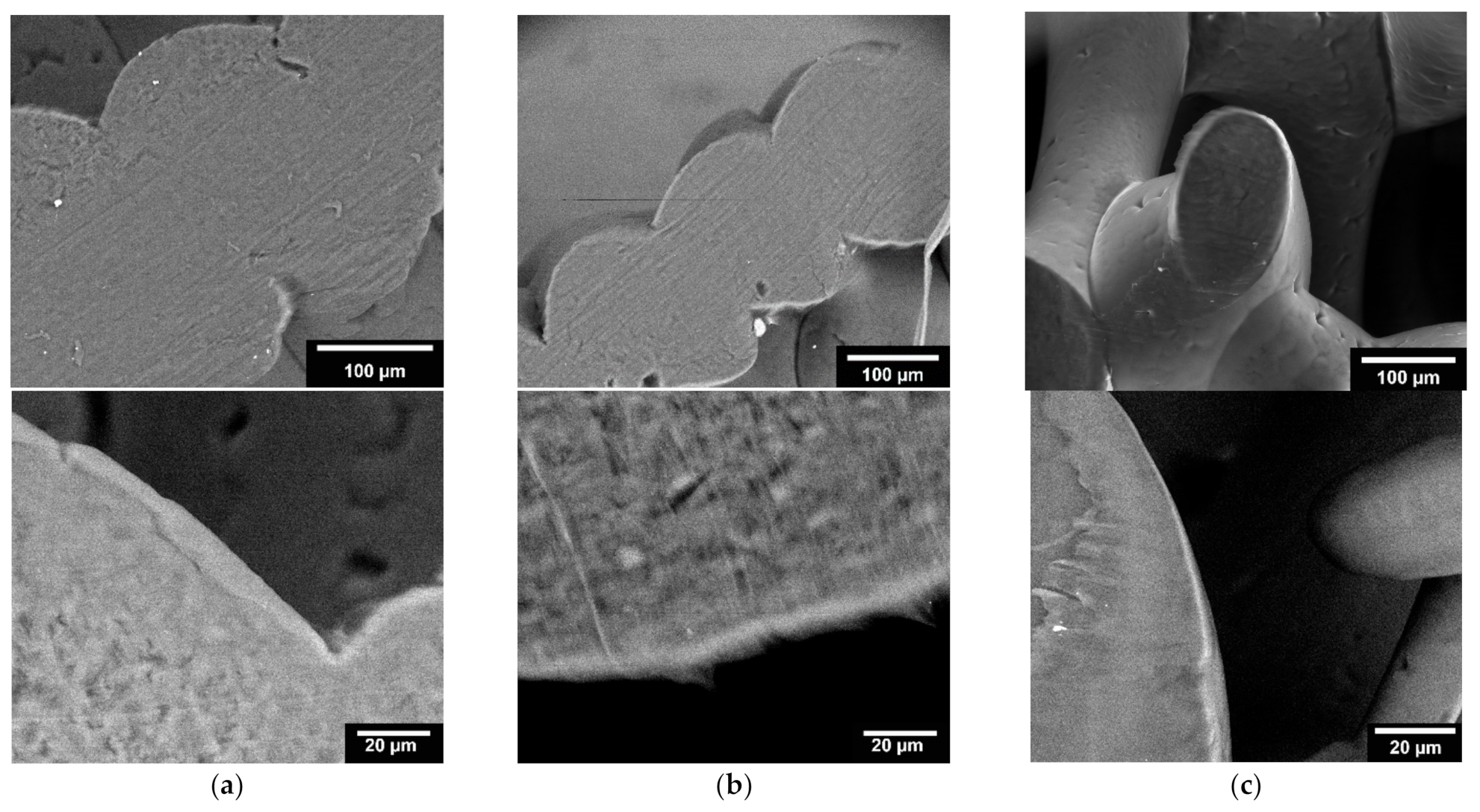
3.1.3. Microstructure Analysis by Means of ESEM
3.2. Mechanical Testing
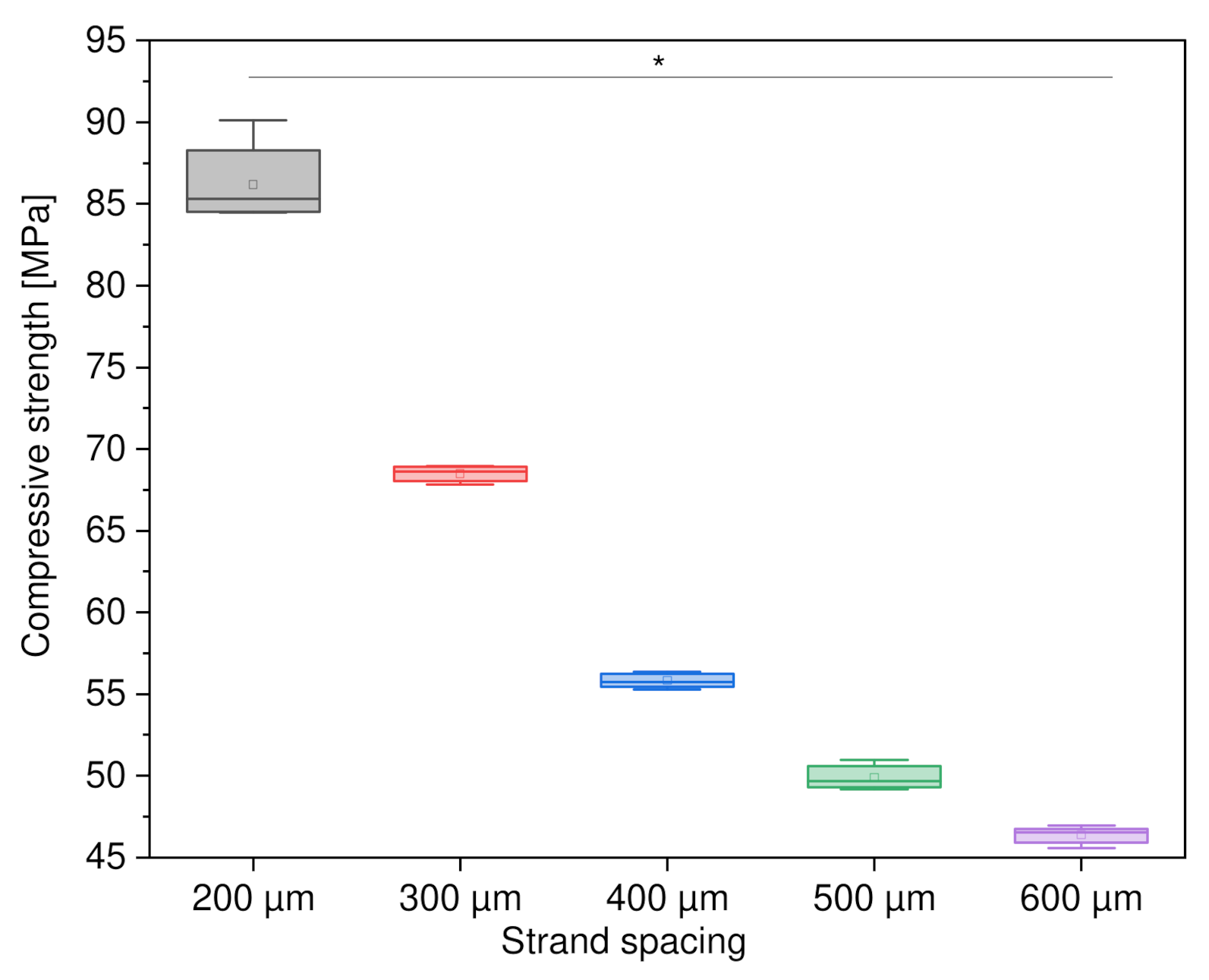
3.2.1. Variation of Strand Spacing
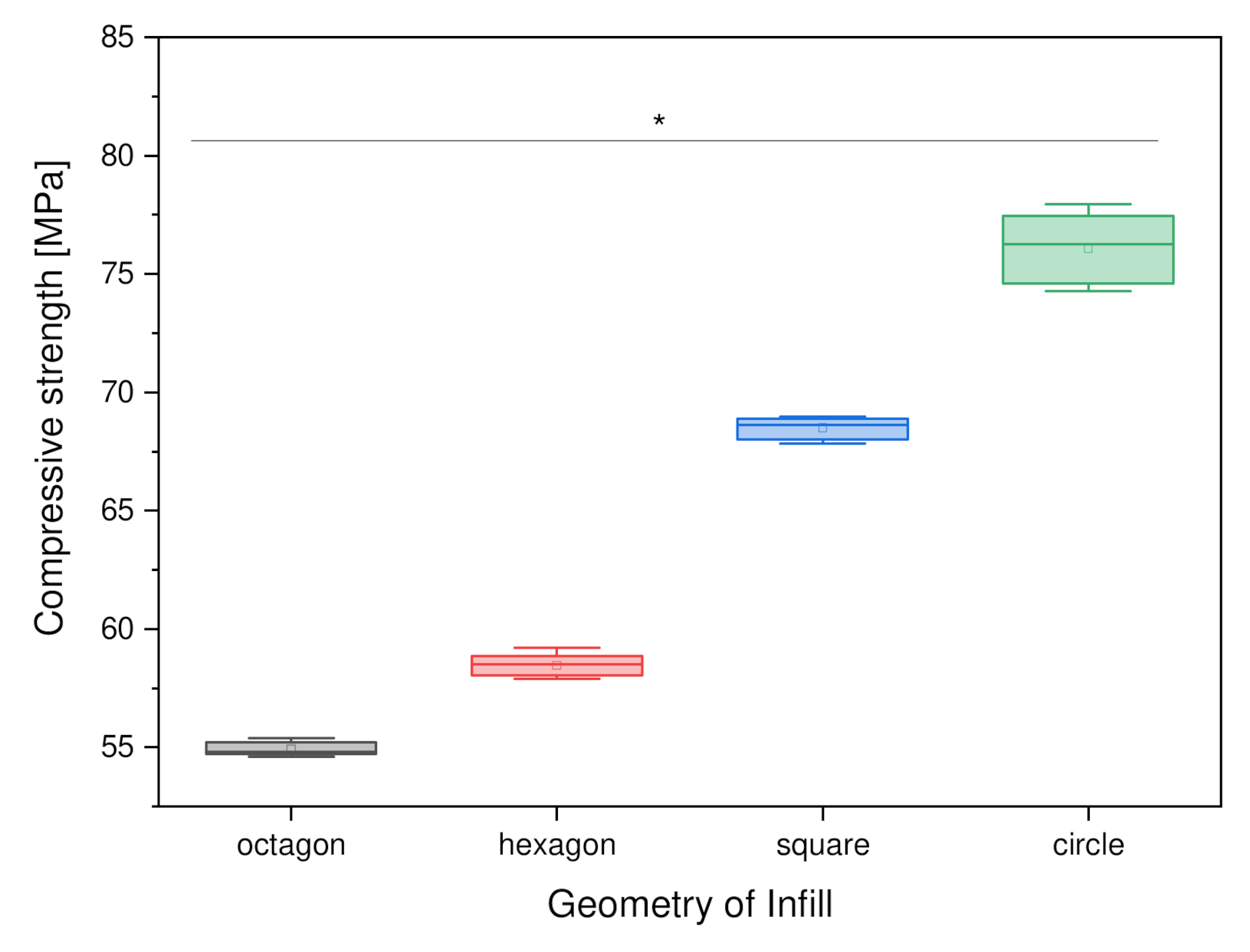
3.2.2. Variation of Outer Geometry
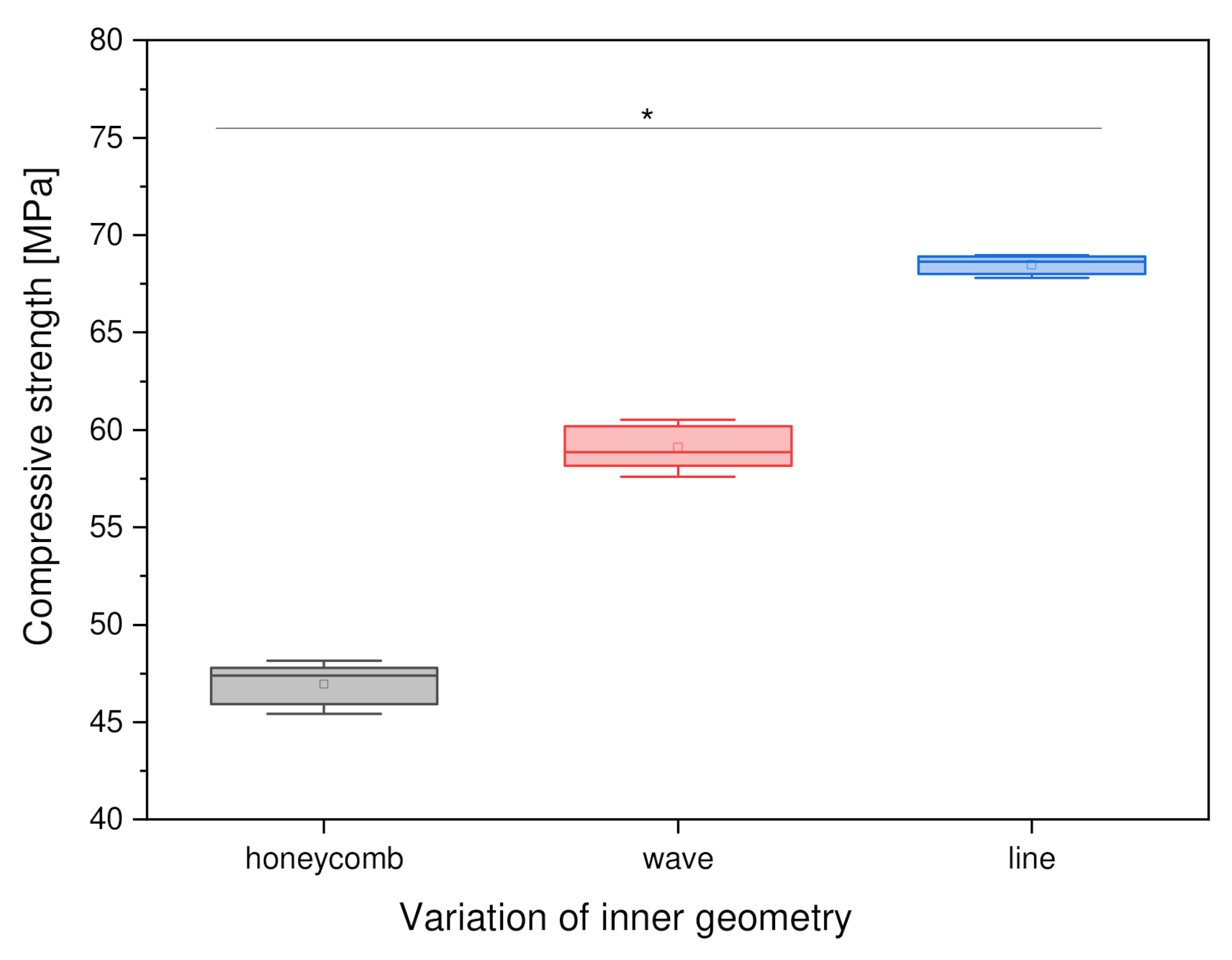
3.2.3. Variation of Inner Geometry
3.2.4. Variation of Outer and Inner Geometry
3.3. Biocompatibility
3.3.1. Live/Dead Assay
3.3.2. Cell Proliferation
3.3.3. Cytotoxicity
4. Discussion
4.1. Characterization of the Scaffolds
4.2. Surface Roughness
4.3. Microstructure by Means of ESEM
4.4. Compressive Strength
4.4.1. Variations of Strand Spacing
4.4.2. Variation of the Outer Geometry
4.4.3. Variation of the Inner Geometry
4.4.4. Variation of the Inner and Outer Geometry
4.5. Biocompatibility
4.5.1. Live/Dead Assay
4.5.2. Cell Proliferation
4.5.3. Cytotoxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, E.; Pritzkuleit, R.; Beske, F.; Katalinic, A. Demografischer wandel und krankheitshäufigkeiten. Bundesgesundheitsblatt-Gesundh. -Gesundh. 2010, 53, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. European Union: Age Structure in the Member States in 2019. Available online: https://de.statista.com/statistik/daten/studie/248981/umfrage/altersstruktur-in-den-eu-laendern/ (accessed on 21 March 2020).
- Schieker, M.; Mutschler, W. Die überbrückung von posttraumatischen knochendefekten. Unfallchirurg 2006, 109, 715–732. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destatis. Mitten im Demografischen Wandel. Available online: https://www.destatis.de/DE/Themen/Querschnitt/Demografischer-Wandel/demografie-mitten-im-wandel.html (accessed on 2 September 2020).
- Destatis. Gesundheit-Fallpauschalenbezogene Krankenhausstatistik (drg-Statistik) Operationen und Prozeduren der Vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller); Statistisches Bundesamt (Destatis): Wiesbaden, Germany, 2020. [Google Scholar]
- Huiskes, R.; Weinans, H.; Grootenboer, H.J.; Dalstra, M.; Fudala, B.; Slooff, T.J. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J. Biomech. 1987, 20, 1135–1150. [Google Scholar] [CrossRef] [Green Version]
- Moucha, C.S.; Urban, R.M.; Turner, T.M.; Jacobs, J.J.; Sumner, D.R. Fixation of implants. In Joint Replacement and Bone Resorption; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-42-911631-5. [Google Scholar]
- Sumner, D.R. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar]
- Charles, L.F.; Shaw, M.T.; Olson, J.R.; Wei, M. Fabrication and mechanical properties of plla/pcl/ha composites via a biomimetic, dip coating, and hot compression procedure. J. Mater. Sci. Mater. Med. 2010, 21, 1845–1854. [Google Scholar] [CrossRef]
- Kim, G.U.; Jang, J.M.; Kim, Y.D.; Lee, J.C.; Lee, W. Fabrication of open-pored titanium foam for biomedical implants. J. Korean Inst. Met. Mater. 2019, 57, 679–687. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [Green Version]
- Jafari Chashmi, M.; Fathi, A.; Shirzad, M.; Jafari-Talookolaei, R.-A.; Bodaghi, M.; Rabiee, S.M. Design and analysis of porous functionally graded femoral prostheses with improved stress shielding. Designs 2020, 4, 12. [Google Scholar] [CrossRef]
- Patel, P.; Bhingole, P.P.; Makwana, D. Manufacturing, characterization and applications of lightweight metallic foams for structural applications: Review. Mater. Today Proc. 2018, 5, 20391–20402. [Google Scholar] [CrossRef]
- Saad, M.; Akhtar, S.; Srivastava, S. Composite polymer in orthopedic implants: A review. Mater. Today Proc. 2018, 5, 20224–20231. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Liu, H.-C.; Di Trani, N.; Susnjar, A.; Ho, J.; Scorrano, G.; Rhudy, J.; Sizovs, A.; Lolli, G.; Hernandez, N.; et al. Carbon fiber reinforced polymers for implantable medical devices. Biomaterials 2021, 271, 120719. [Google Scholar] [CrossRef] [PubMed]
- Theivendran, K.; Arshad, F.; Hanif, U.-K.; Reito, A.; Griffin, X.; Foote, C.J. Response to letter: Carbon fibre reinforced peek versus traditional metallic implants for orthopaedic trauma surgery: A systematic review. J. Clin. Orthop. Trauma 2022, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet-rich plasma for bone regeneration. Materials 2017, 10, 831. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Moran, J.M.; Pazzano, D.; Bonassar, L.J. Characterization of polylactic acid–polyglycolic acid composites for cartilage tissue engineering. Tissue Eng. 2003, 9, 63–70. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Del. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. Pcl and pcl-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Domingo, C. Pore structure properties of scaffolds constituted by aggregated microparticles of pcl and pcl-ha processed by phase separation. J. Porous Mater. 2015, 22, 425–435. [Google Scholar] [CrossRef]
- Cheng, M.-Q.; Wahafu, T.; Jiang, G.-F.; Liu, W.; Qiao, Y.-Q.; Peng, X.-C.; Cheng, T.; Zhang, X.-L.; He, G.; Liu, X.-Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Platzer, W. Taschenatlas Anatomie, 1 Bewegungsapparat, 11th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2013; p. 467. ISBN 978-3-13-492011-6. [Google Scholar]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Choi, S.-W.; Zhang, Y.; Xia, Y. Three-dimensional scaffolds for tissue engineering: The importance of uniformity in pore size and structure. Langmuir 2010, 26, 19001–19006. [Google Scholar] [CrossRef] [Green Version]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Abdullah, A.; Muhammad, M.R.; Eylem, A. In Investigating compression strengths of 3D printed polymeric infill specimens of various geometries. In Proceedings of the SPIE (Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring), Denver, CO, USA, 23 March 2018. [Google Scholar]
- Weingärtner, L.; Latorre, S.H.; Velten, D.; Bernstein, A.; Schmal, H.; Seidenstuecker, M. The effect of collagen-i coatings of 3D printed pcl scaffolds for bone replacement on three different cell types. Appl. Sci. 2021, 11, 11063. [Google Scholar] [CrossRef]
- Woodfield, T.B.F.; Malda, J.; De Wijn, J.; Péters, F.; Riesle, J.; Van Blitterswijk, C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef]
- Roopavath, U.K.; Malferrari, S.; Van Haver, A.; Verstreken, F.; Rath, S.N.; Kalaskar, D.M. Optimization of extrusion based ceramic 3D printing process for complex bony designs. Mater. Des. 2019, 162, 263–270. [Google Scholar] [CrossRef]
- Sears, N.; Dhavalikar, P.; Whitely, M.; Cosgriff-Hernandez, E. Fabrication of biomimetic bone grafts with multi-material 3D printing. Biofabrication 2017, 9, 025020. [Google Scholar] [CrossRef] [PubMed]
- Soufivand, A.A.; Abolfathi, N.; Hashemi, A.; Lee, S.J. The effect of 3D printing on the morphological and mechanical properties of polycaprolactone filament and scaffold. Polym. Adv. Technol. 2020, 31, 1038–1046. [Google Scholar] [CrossRef]
- Cea, K.; Donoso, M.; Sérandour, G.; Martínez, G.; Alegría, L. Evaluation of parameters in pla and pcl scaffolds to be used in cartilaginous tissues. Mex. J. Biomed. Eng. 2021, 42, 149–159. [Google Scholar]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-L.; Kim, D.-E. Self-healing characteristics of collagen coatings with respect to surface abrasion. Sci. Rep. 2016, 6, 20563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beşkardeş, I.G.; Gümüşderelioğlu, M. Biomimetic apatite-coated pcl scaffolds: Effect of surface nanotopography on cellular functions. J. Bioact. Compatible Polym. 2009, 24, 507–524. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Lange, S.; Esslinger, S.; Latorre, S.H.; Krastev, R.; Gadow, R.; Mayr, H.O.; Bernstein, A. Inversely 3D-printed β-tcp scaffolds for bone replacement. Materials 2019, 12, 3417. [Google Scholar] [CrossRef] [Green Version]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Schilling, P.; Ritschl, L.; Lange, S.; Schmal, H.; Bernstein, A.; Esslinger, S. Inverse 3D printing with variations of the strand width of the resulting scaffolds for bone replacement. Materials 2021, 14, 1964. [Google Scholar] [CrossRef]
- Götz, L.-M.; Holeczek, K.; Groll, J.; Jüngst, T.; Gbureck, U. Extrusion-based 3D printing of calcium magnesium phosphate cement pastes for degradable bone implants. Materials 2021, 14, 5197. [Google Scholar] [CrossRef]
- Sajesh, K.M.; Jayakumar, R.; Nair, S.V.; Chennazhi, K.P. Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 62, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kieswetter, K.; Schwartz, Z.; Hummert, T.W.; Cochran, D.L.; Simpson, J.; Dean, D.D.; Boyan, B.D. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like mg-63 cells. J. Biomed. Mater. Res. 1996, 32, 55–63. [Google Scholar] [CrossRef]
- Fink, A.; Brückner, D.B.; Schreiber, C.; Röttgermann, P.J.F.; Broedersz, C.P.; Rädler, J.O. Area and geometry dependence of cell migration in asymmetric two-state micropatterns. Biophys. J. 2020, 118, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Guo, W.H.; Kim, Y.; Wang, Y.L. Guidance of cell migration by substrate dimension. Biophys. J. 2013, 104, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, K.; Hlady, V.; Tresco, P.A. Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2000, 49, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Kumar, G.; Co, C.C.; Ho, C.-C. Geometric control of cell migration. Sci. Rep. 2013, 3, 2827. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]




| Needle Inner Diameter (µm) | Temperature (°C) | Pressure (bar) | Speed (mm/s) | Needle Offset (mm) | Pre-/Post-Flow (s) | Platform Temperature (°C) |
|---|---|---|---|---|---|---|
| 550 | 80 | 6.0 | 1.0 | 0.3 | 0.4 | 11 |
| Parameter\Shape | Honeycomb | Wave Shape | Line Shape |
|---|---|---|---|
| Needle-Offset (mm) | 0.17 | 0.34 | 0.20 |
| Pre-Flow (s) | 0.70 | 0.55 | 0.20 |
| Post-Flow (s) | 0.58–0.70 | −0.25 | −0.10 |
| Parameter\Geometry | Square | Hexagon | Octagon | Circle |
|---|---|---|---|---|
| Weight (mg) | 120 ± 20 | 80.81 ± 2.17 | 44.71 ± 1.58 | 72.65 ± 12.87 |
| Length (mm) | 8.72 ± 0.05 | 8.55 ± 0.03 | 8.10 ± 0.06 | 8.04 ± 0.07 |
| Height (mm) | 2.85 ± 0.04 | 2.59 ± 0.01 | 2.14 ± 0.09 | 2.37 ± 0.4 |
| Pore size (µm) | 295.36 ± 9.78 | 295.36 ± 9.78 * | 295.36 ± 9.78 * | 295.36 ± 9.78 * |
| Strand width (µm) | 300 ± 12.56 | 300 ± 12.56 * | 300 ± 12.56 * | 300 ± 12.56 * |
| Porosity (%) | 31.9 | 28.9 | 34.9 | 26.4 |
| Parameter\Geometry | Honeycomb | Wave | Line |
|---|---|---|---|
| Weight (mg) | 139 ± 24 | 128 ± 26 | 120 ± 20 |
| Length (mm) | 8.71 ± 0.11 | 8.70 ± 0.08 | 8.72 ± 0.05 |
| Height (mm) | 2.84 ± 0.07 | 2.83 ± 0.09 | 2.85 ± 0.04 |
| Pore size (µm) | 354.54 ± 36.29 | 244.95 ± 78.22 | 295.36 ± 9.78 |
| Strand width (µm) | 336.40 ± 59.78 | 258.75 ± 32.59 | 300 ± 12.56 |
| Porosity (%) | 31.2 | 32.2 | 25 |
| Parameter\Geometry | Honeycomb | Wave | Line |
|---|---|---|---|
| Weight (mg) | 57.74 ± 4.00 | 54.77 ± 0.89 | 44.71 ± 1.58 |
| Diameter (mm) | 8.14 ± 0.11 | 8.06 ± 0.08 | 8.10 ± 0.06 |
| Height (mm) | 2.14 ± 0.07 | 2.19 ± 0.09 | 2.14 ± 0.09 |
| Pore size (µm) | 300.54 ± 36.29 | 244.95 ± 50.22 | 262.19 ± 44.53 |
| Strand width (µm) | 294.40 ± 48.61 | 258.75 ± 32.59 | 267.64 ± 45.59 |
| Porosity (%) | 31.8 | 29.3 | 26.4 |
| Surface Roughness (Sa) (µm) | |||
|---|---|---|---|
| Parameter\Geometry | Honeycomb | Wave | Line |
| uncoated | 4.52 ± 2.14 | 5.60 ± 2.22 | 6.13 ± 2.84 |
| coated | 3.31 ± 1.85 | 2.43 ± 1.19 | 2.52 ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber, F.; Vollmer, D.; Vinke, J.; Riedel, B.; Zankovic, S.; Schmal, H.; Seidenstuecker, M. Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells. Materials 2022, 15, 2091. https://doi.org/10.3390/ma15062091
Huber F, Vollmer D, Vinke J, Riedel B, Zankovic S, Schmal H, Seidenstuecker M. Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells. Materials. 2022; 15(6):2091. https://doi.org/10.3390/ma15062091
Chicago/Turabian StyleHuber, Fabian, David Vollmer, Johannes Vinke, Bianca Riedel, Sergej Zankovic, Hagen Schmal, and Michael Seidenstuecker. 2022. "Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells" Materials 15, no. 6: 2091. https://doi.org/10.3390/ma15062091
APA StyleHuber, F., Vollmer, D., Vinke, J., Riedel, B., Zankovic, S., Schmal, H., & Seidenstuecker, M. (2022). Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells. Materials, 15(6), 2091. https://doi.org/10.3390/ma15062091